RNA Sequencing of Immune Response-Related Gene Expression Characteristics in Bovine Mammary Glands Infected with Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Sample Collection
2.3. HE Staining
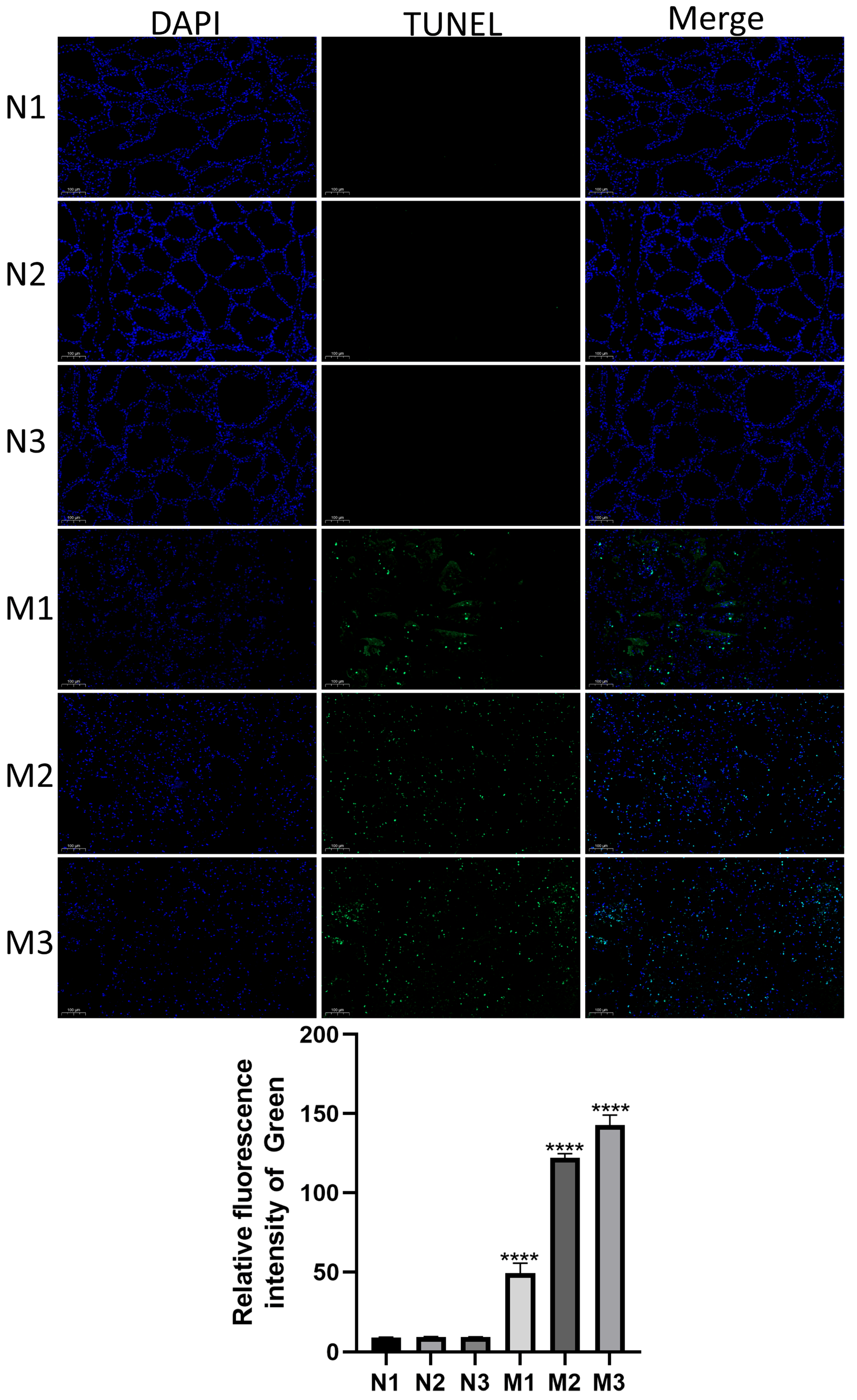
2.4. TUNEL Staining
2.5. RNA-Seq Analysis
2.6. Real-Time-PCR Analysis
2.7. Statistical Analysis
3. Results
3.1. HE Staining Results
3.2. Detection of Apoptosis in Mastitic Mammary Glands by TUNEL Staining
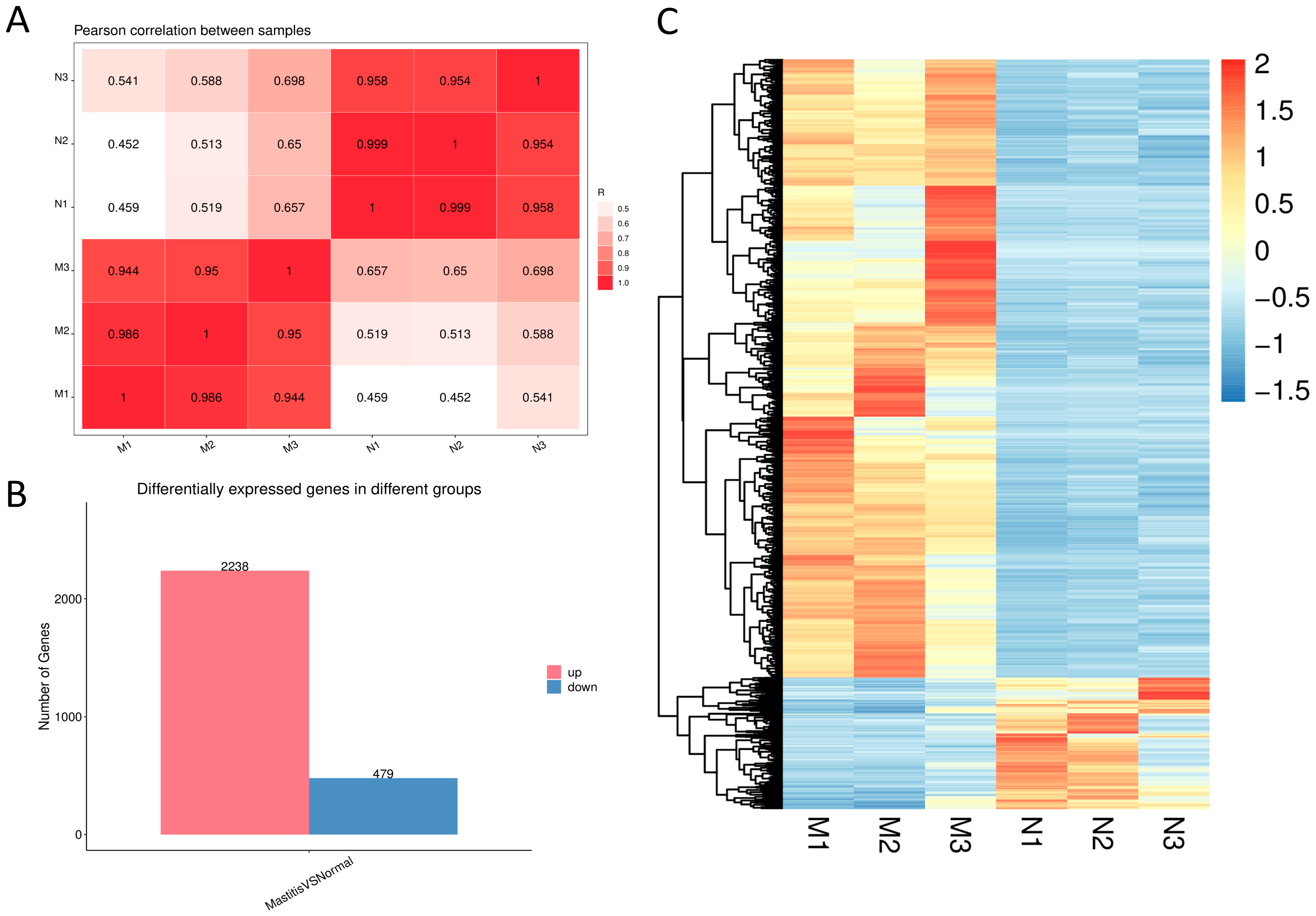
3.3. Assessment of Sample Correlation and DEG Analysis
3.4. DEG Screening
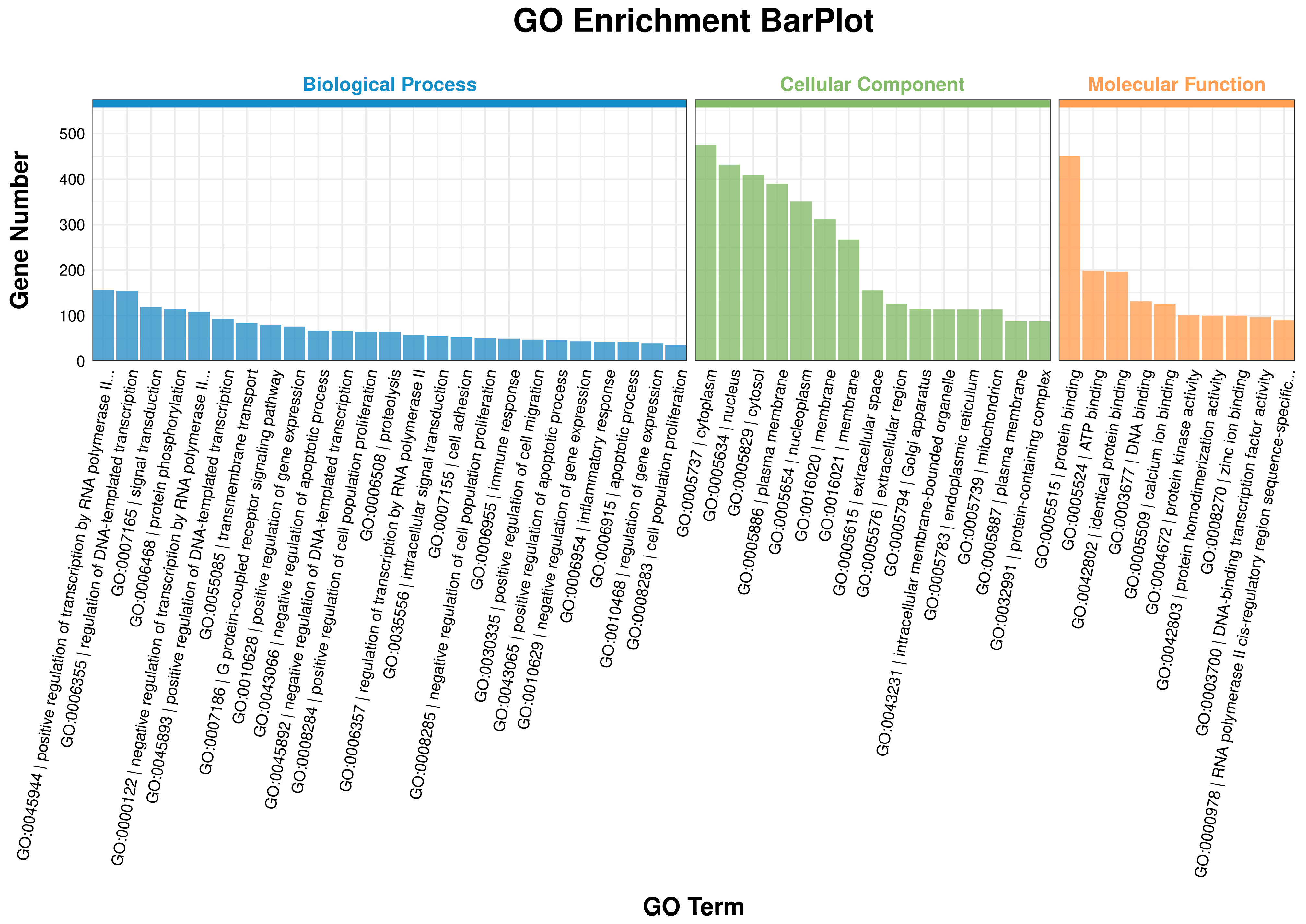
3.5. GO Enrichment Analysis
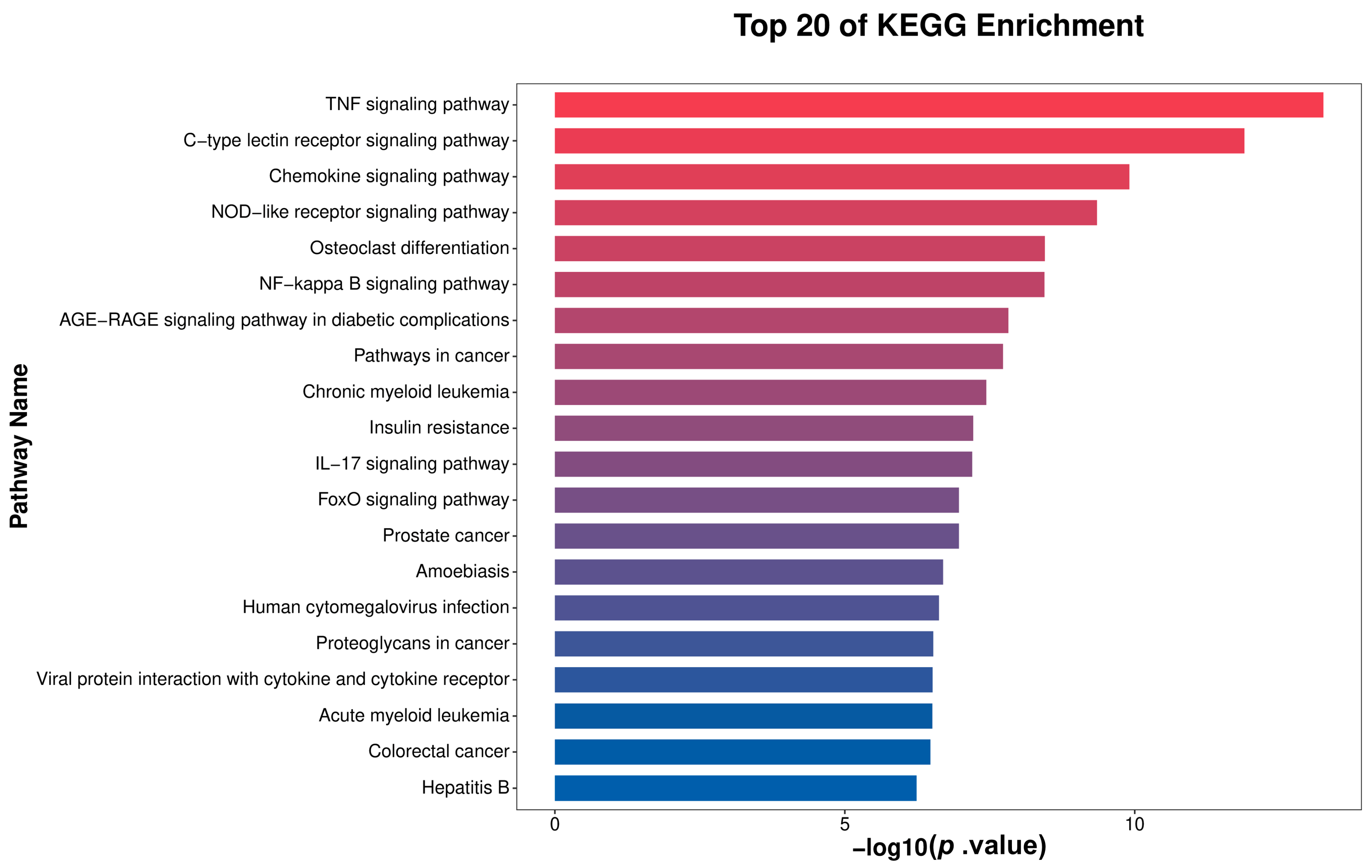
3.6. KEGG Enrichment Analysis
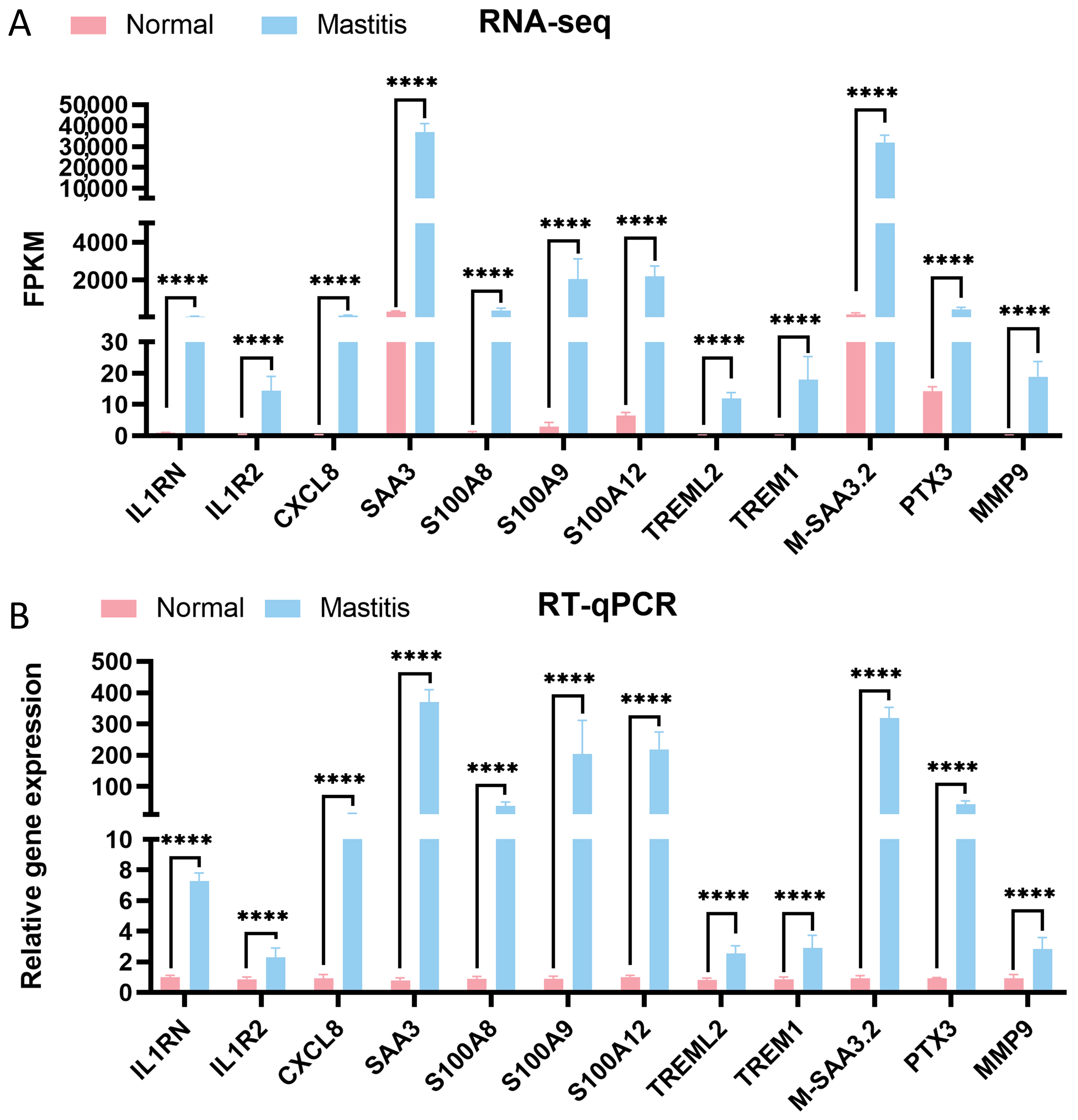
3.7. Validation of DEGs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| E. coli | Escherichia coli |
| HE | Hematoxylin-eosin |
| RNA-seq | RNA sequencing |
| DEGs | Differentially expressed genes |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| BP | Biological Process |
| CC | Cellular Component |
| MF | Molecular Function |
| TdT | Terminal Deoxynucleotidyl Transferase |
| DAB | Diaminobenzidine |
References
- Kabelitz, T.; Aubry, E.; van Vorst, K.; Amon, T.; Fulde, M. The Role of Streptococcus Spp. in Bovine Mastitis. Microorganisms 2021, 9, 1497. [Google Scholar] [CrossRef]
- Kaisa, K. Importance of Bovine Mastitis Associated Gene Expression Analysis—A Review. J. Anim. Res. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Shaheen, M.; Tantary, T.A.; Nabi, S.U. A Treatise on Bovine Mastitis: Disease and Disease Economics, Etiological Basis, Risk Factors, Impact on Human Health, Therapeutic Management, Prevention and Control Strategy. J. Adv. Dairy Res. 2015, 4, 1–10. [Google Scholar] [CrossRef]
- Hogeveen, H.; Huijps, K.; Lam, T.J.G.M. Economic Aspects of Mastitis: New Developments. New Zealand Vet. J. 2011, 59, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Petzl, W.; Zerbe, H.; Günther, J.; Yang, W.; Seyfert, H.-M.; Nürnberg, G.; Schuberth, H.-J. Escherichia Coli, but Not Staphylococcus Aureus Triggers an Early Increased Expression of Factors Contributing to the Innate Immune Defense in the Udder of the Cow. Vet. Res. 2008, 39, 18. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, R.N.; Middleton, J.R.; McDougall, S.; Katholm, J.; Schukken, Y.H. Molecular Epidemiology of Mastitis Pathogens of Dairy Cattle and Comparative Relevance to Humans. J. Mammary Gland. Biol. Neoplasia 2011, 16, 357–372. [Google Scholar] [CrossRef]
- Srinivasan, V.; Gillespie, B.E.; Lewis, M.J.; Nguyen, L.T.; Headrick, S.I.; Schukken, Y.H.; Oliver, S.P. Phenotypic and Genotypic Antimicrobial Resistance Patterns of Escherichia Coli Isolated from Dairy Cows with Mastitis. Vet. Microbiol. 2007, 124, 319–328. [Google Scholar] [CrossRef]
- Kvist, L.J.; Larsson, B.W.; Hall-Lord, M.L.; Steen, A.; Schalén, C. The Role of Bacteria in Lactational Mastitis and Some Considerations of the Use of Antibiotic Treatment. Int. Breastfeed. J. 2008, 3, 6. [Google Scholar] [CrossRef]
- Rainard, P.; Riollet, C. Innate Immunity of the Bovine Mammary Gland. Vet. Res. 2006, 37, 369–400. [Google Scholar] [CrossRef]
- Wellnitz, O.; Bruckmaier, R.M. The Innate Immune Response of the Bovine Mammary Gland to Bacterial Infection. Vet. J. 2012, 192, 148–152. [Google Scholar] [CrossRef]
- Costa, V.; Aprile, M.; Esposito, R.; Ciccodicola, A. RNA-Seq and Human Complex Diseases: Recent Accomplishments and Future Perspectives. Eur. J. Hum. Genet. 2013, 21, 134–142. [Google Scholar] [CrossRef]
- Liu, B.; Fang, J.; Chen, H.; Sun, Y.; Yang, S.; Gao, Q.; Zhang, Y.; Chen, C. GcvB Regulon Revealed by Transcriptomic and Proteomic Analysis in Vibrio Alginolyticus. Int. J. Mol. Sci. 2022, 23, 9399. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhou, X.; Yin, Y.; Luo, B.; Liu, Y.; Yang, C. Inflammatory Microenvironment Accelerates Bone Marrow Mesenchymal Stem Cell Aging. Front. Bioeng. Biotechnol. 2022, 10, 870324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Jia, Y.; Qian, Y.; Jiang, X.; Zhang, S.; Liu, B.; Cao, J.; Song, Y.; Mao, W. Staphylococcus Aureus Increases Prostaglandin E2 Secretion in Cow Neutrophils by Activating TLR2, TLR4, and NLRP3 Inflammasome Signaling Pathways. Front. Microbiol. 2023, 14, 1163261. [Google Scholar] [CrossRef]
- Aksentijevich, I.; Masters, S.L.; Ferguson, P.J.; Dancey, P.; Frenkel, J.; van Royen-Kerkhoff, A.; Laxer, R.; Tedgård, U.; Cowen, E.W.; Pham, T.-H.; et al. An Autoinflammatory Disease with Deficiency of the Interleukin-1-Receptor Antagonist. N. Engl. J. Med. 2009, 360, 2426–2437. [Google Scholar] [CrossRef]
- Supino, D.; Minute, L.; Mariancini, A.; Riva, F.; Magrini, E.; Garlanda, C. Negative Regulation of the IL-1 System by IL-1R2 and IL-1R8: Relevance in Pathophysiology and Disease. Front. Immunol. 2022, 13, 804641. [Google Scholar] [CrossRef]
- Sreejit, G.; Flynn, M.C.; Patil, M.; Krishnamurthy, P.; Murphy, A.J.; Nagareddy, P.R. S100 Family Proteins in Inflammation and Beyond. Adv. Clin. Chem. 2020, 98, 173–231. [Google Scholar] [CrossRef]
- Thomas, F.C.; Waterston, M.; Hastie, P.; Haining, H.; Eckersall, P.D. Early Post Parturient Changes in Milk Acute Phase Proteins. J. Dairy. Res. 2016, 83, 352–359. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Fu, X.-X.; Duan, R.; Wei, B.; Cao, H.-M.; Yan, E.; Chen, S.-Y.; Zhang, Y.-D.; Jiang, T. The Alzheimer′s Disease-Associated Gene TREML2 Modulates Inflammation by Regulating Microglia Polarization and NLRP3 Inflammasome Activation. Neural Regen. Res. 2023, 18, 434–438. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, A.; Mo, L.; Wan, G.; Lu, F.; Chen, L.; Fu, S.; Chen, H.; Fu, T.; Deng, H. Higher Expression of PLEK and LY86 as the Potential Biomarker of Carotid Atherosclerosis. Medicine 2023, 102, e34445. [Google Scholar] [CrossRef]
- Pei, S.; Xu, C.; Pei, J.; Bai, R.; Peng, R.; Li, T.; Zhang, J.; Cong, X.; Chun, J.; Wang, F.; et al. Lysophosphatidic Acid Receptor 3 Suppress Neutrophil Extracellular Traps Production and Thrombosis During Sepsis. Front. Immunol. 2022, 13, 844781. [Google Scholar] [CrossRef]
- Mao, Y.; Su, C.; Yang, H.; Ma, X.; Zhao, F.; Qu, B.; Yang, Y.; Hou, X.; Zhao, B.; Cui, Y. PI3K/AKT/mTORC1 Signalling Pathway Regulates MMP9 Gene Activation via Transcription Factor NF-κB in Mammary Epithelial Cells of Dairy Cows. Anim. Biotechnol. 2024, 35, 2314100. [Google Scholar] [CrossRef]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen Recognition by the Innate Immune System. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef]
- De Simone, V.; Franzè, E.; Ronchetti, G.; Colantoni, A.; Fantini, M.C.; Di Fusco, D.; Sica, G.S.; Sileri, P.; MacDonald, T.T.; Pallone, F.; et al. Th17-Type Cytokines, IL-6 and TNF-α Synergistically Activate STAT3 and NF-kB to Promote Colorectal Cancer Cell Growth. Oncogene 2015, 34, 3493–3503. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, M.; Su, H.; Zhao, F.; Wang, D.; Zhang, Y.; Cao, G.; Zhang, Y. Regulation of Inflammatory Responses of Cow Mammary Epithelial Cells through MAPK Signaling Pathways of IL-17A Cytokines. Animals 2024, 14, 1572. [Google Scholar] [CrossRef]

| Gene Name | Sequences (5′-3′) | Accession Number |
|---|---|---|
| β-actin | F:5′-CCAAGGCCAACCGTGAGAAGAT-3′ R:5′-CCACGTTCCGTGAGGATCTTCA-3′ | NM_173979.3 |
| IL1RN | F:5′-AGAGGCCGTGAACATCACTG-3′ R:5′-CCTCCAGTGATGTGCAGAGG-3′ | NM_174357.3 |
| IL1R2 | F: 5′-CCAGGCTGACAATCCCATGT-3′ R:5′-AAGGTGTTGTTGGCTGTCCA-3 | NM_001046210.2 |
| CXCL8 | F: 5′-GCTGGCTGTTGCTCTCTTGG-3′ R: 5′-GGGTGGAAAGGTGTGGAATGTG-3′ | NM_173925.2 |
| SAA3 | F: 5′-AAACTATGACGCTGCCCGAA-3′ R:5′-CCCATTCGTTGGCAAACTGG-3′ | NM_181016.3 |
| S100A8 | F: 5′-TGTGCCATTAACTCCCTGATTGAC-3′ R: 5′-TTGAACCAAGTGTCCGCATCC-3′ | NM_001113725.2 |
| S100A9 | F: 5′-ATCATGGAGGATCTGGACACAAATG-3′ R: 5′-CGTGGGAGGCTACCGTCAG-3′ | NM_001046328.2 |
| S100A12 | F: 5′-GCTGGAAGATCACCTGGAGG-3′ R:5′-GCTTCAGCTCACGCTTGTTG-3′ | NM_174651.3 |
| TREML2 | F: 5′-CAGCTGGACTCCTCACCTTG-3′ R:5′-AGTGCTGGAGAAACTGGAGC-3′ | XM_015459834.3 |
| TREM1 | F: 5′-ACATCCCCAGTGAAGGCATG-3′ R:5′-GGACAGGGTGGAACAGGATG-3′ | NM_206970.1 |
| M-SAA3.2 | F: 5′-ACCTTTCCACGGGCATCATT-3′ R:5′-CGCGGGCATGGAAGTATTTG-3′ | NM_001242573.1 |
| PTX3 | F: 5′-CGGCGGAGAACTCAGATGATTATG-3′ R: 5′-CCAGCATGGTGAAGAGCTTGTC-3′ | NM_001076259.2 |
| MMP9 | F: 5′-GGTGCTGGCTTGCTGCTCTG-3′ R: 5′-TTGGTGAGGTTGGTTCGTGGTTC-3′ | NM_174744.2 |
| Gene ID | Gene Name |
|---|---|
| ENSBTAG00000012638 | S100A12 |
| ENSBTAG00000019665 | IL1RN |
| ENSBTAG00000019761 | CXCL8 |
| ENSBTAG00000049589 | SAA3 |
| ENSBTAG00000006505 | S100A9 |
| ENSBTAG00000009658 | PLEK |
| ENSBTAG00000006343 | IL1R2 |
| ENSBTAG00000003791 | LPAR3 |
| ENSBTAG00000015707 | TREML2 |
| ENSBTAG00000054278 | M-SAA3.2 |
| ENSBTAG00000012640 | S100A8 |
| ENSBTAG00000011465 | MYBPH |
| ENSBTAG00000020859 | SERPINF2 |
| ENSBTAG00000009012 | PTX3 |
| ENSBTAG00000006354 | HP |
| ENSBTAG00000020676 | MMP9 |
| ENSBTAG00000020580 | TCN1 |
| ENSBTAG00000017593 | TREM1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Zhang, Y.; Su, H.; Zhang, M.; Zhao, F.; Wang, D.; Cao, G.; Zhang, Y.; Wang, C. RNA Sequencing of Immune Response-Related Gene Expression Characteristics in Bovine Mammary Glands Infected with Escherichia coli. Microorganisms 2025, 13, 2226. https://doi.org/10.3390/microorganisms13102226
Zhang K, Zhang Y, Su H, Zhang M, Zhao F, Wang D, Cao G, Zhang Y, Wang C. RNA Sequencing of Immune Response-Related Gene Expression Characteristics in Bovine Mammary Glands Infected with Escherichia coli. Microorganisms. 2025; 13(10):2226. https://doi.org/10.3390/microorganisms13102226
Chicago/Turabian StyleZhang, Kai, Yuanyuan Zhang, Hong Su, Min Zhang, Feifei Zhao, Daqing Wang, Guifang Cao, Yong Zhang, and Caiyun Wang. 2025. "RNA Sequencing of Immune Response-Related Gene Expression Characteristics in Bovine Mammary Glands Infected with Escherichia coli" Microorganisms 13, no. 10: 2226. https://doi.org/10.3390/microorganisms13102226
APA StyleZhang, K., Zhang, Y., Su, H., Zhang, M., Zhao, F., Wang, D., Cao, G., Zhang, Y., & Wang, C. (2025). RNA Sequencing of Immune Response-Related Gene Expression Characteristics in Bovine Mammary Glands Infected with Escherichia coli. Microorganisms, 13(10), 2226. https://doi.org/10.3390/microorganisms13102226







