Abstract
Oxidative stress caused by reactive oxygen species (ROS) affects the aging process and increases the likelihood of several diseases. A new frontier in its prevention includes bioactive foods and natural extracts that can be introduced by the diet in combination with specific probiotics. Among the natural compounds that we can introduce by the diet, Panax ginseng extract is one of the most utilized since it contains a vast number of bioactive molecules such as phenolic acids, flavonoids, and polysaccharides that have been shown to possess antioxidant, anti-ageing, anti-cancer, and immunomodulatory activity. In this work, the ability of a P. ginseng extract in combination with a probiotic formulation was taken into consideration to evaluate its effects on the modulation of in vitro reconstructed human gut microbiota (HGM). After evaluating the growth of the individual strains on the ginseng extract, we tested the in vitro reconstructed HGM setup (probiotics, minimal core, and whole community) using 2% w/v ginseng as the only carbon and energy source. The probiotic strains reached the highest growth, while the minimal core and the whole community showed almost the same growth. Specifically, the presence of the ginseng extract favors L. plantarum and B. animalis subsp. lactis among the probiotics, while B. cellulosilyticus prevails over the other strains in the minimal core condition. In the presence of both probiotics and minimal core strains, L. plantarum, B. animalis subsp. lactis, and B. cellulosilyticus reach the highest growth values. The bacterial metabolites produced during ginseng extract fermentation in the three conditions were administered to human intestinal epithelial cells (HT-29) to investigate a potential antioxidant effect. Remarkably, our results highlighted a significant reduction in the total ROS and a slightly reduction in the cytosolic superoxide anion content in HT-29 cells treated with bacterial metabolites deriving from ginseng extract fermentation by the whole community.
1. Introduction
The human intestine is inhabited by high microbial biodiversity constituted by hundreds of bacterial species [1]. Analysis of 16S rRNA gene sequences highlighted that a healthy human colon is dominated by two bacterial phyla, the Bacillota (previously Firmicutes) and Bacteroidota (previously Bacteroidetes), representing up to 90% of the total gut microbiome [2,3]. The remaining 10% of the human gut microbiota (HGM) is composed of Pseudomonadota (Proteobacteria), Actinomycetota (Actinobacteria), and Verrucomicrobiota (Verrucomicrobia) [1]. This complex bacterial community plays a critical role in several host physiological processes, such as playing a fundamental role in digestion and metabolism, protection from pathogens, modulation of the immune system, controlling epithelial cell proliferation and antioxidant responses, and influencing brain–gut communication [4]. All of the primary functions carried out by HGM result from the complexity of the interactions established between the different bacterial populations. Indeed, microorganisms can communicate with the host and each other by producing diverse metabolites and molecules [5]. Among these, short-chain and branched-chain fatty acids (SCFAs and BCFAs) stand out for their beneficial effects on the host [5,6,7].
Diet exerts a significant impact on the composition of the intestinal microbiota and consequently can affect human health [1]. Components of the diet, principally polysaccharides, such as pectin, inulin, fructooligosaccharides (FOS), or xylan, are not altered or absorbed in the gut and can be degraded by different bacterial populations through a cross-feeding mechanism [8,9]. For example, members of the phylum Bacteroidota can break down inaccessible plant-derived carbohydrates introduced by the diet, mucin-associated glycans, and host polysaccharides, making them more available to the intestinal community thanks to the highest range of polysaccharide utilization loci (PUL) [10,11]. Furthermore, the Bifidobacterium genus is capable of utilizing simpler oligosaccharides that are readily available and releasing sugar monomers that could be used by other bacteria [12].
Single strains have been used to study the ability of gut microbes to degrade complex polysaccharides [1]. However, the intricate interplay between human gut microbiota bacteria is fundamental in maintaining intestinal homeostasis and beneficial effects. Indeed, recently, an increasing number of studies have focused on the development of in vitro models of the HGM to assess the effects of various dietary components. The HGM models available range from deep-well plates to super-controlled bioreactors, which can operate in batch, semi-continuous, and continuous modes, with single or multi-stages [13]. Their dependability is deeply associated with the viability and the performance of the bacterial inoculum. In particular, synthetic defined communities are utilized to understand the cause-and-effect relationships between the administration of a carbon source and the modulation of the human gut microbiota [14]. Steimle et al. [15] employed a 14-member synthetic human gut microbiome (composed of Bacteroides ovatus, Bacteroides uniformis, Bacteroides thetaiotaomicron, Bacteroides caccae, Barnesiella intestinihominis, Roseburia intestinalis, Eubacterium rectale, Faecalibacterium praustnizii, Marvinbryantia formatexigens, Clostridium symbiosum, Colinsella aerofaciens, Escherichia coli SH, Akkermantia muciniphila, and Desulfovibrio piger) to study the effects of dietary concentrated raw fibers. Shetty et al. [16] designed a synthetic microbiome termed the Mucin and Diet-based Minimal Microbiome (MDb-MM) to identify human gut microbes’ dynamic metabolic interactions and trophic roles. De Giani et al. [17] reconstructed a minimal core model of the gut microbiota composed of four strains (Bacteroides cellulosilyticus, Clostridium symbiosum, Flavonifractor plautii, and Escherichia coli ATCC 25922) to assess the effects of a prebiotic Maitake extract and three probiotic strains (Lactiplantibacillus plantarum, Lactobacillus acidophilus, and Bifidobacterium animalis subsp. lactis).
Among the compounds that we can introduce by the diet, Panax ginseng is one of the most studied since it contains different bioactive components such as phenolic acids (gallic acid, caffeic acid, coumaric acid, salicylic acid, and cinnamic acid), flavonoids, polysaccharides, amino acids, phytosterol, ginseng oil, and specific vitamins [18]. Each part of the ginseng plant has a unique ginsenoside profile, meaning the various parts likely exert different pharmacological effects [19]. The roots of this plant are traditionally used as herbal medicines for the treatment of human diseases [19,20]. Indeed, root extracts possess anti-stress, anti-diabetic, anti-inflammatory, antioxidative, anti-ageing, anti-cancer, and immunomodulatory activity [20]. Recently, some studies have shown that the berry of Panax ginseng has a much stronger pharmacological activity than its root [19,21]. Ginseng polysaccharides have attracted much attention recently because of their abundance and diverse known bioactivities, such as antioxidant activity [22]. Reactive oxygen species (ROS) are chemically active compounds that can cause cellular damage. Usually, ROS are produced during cellular metabolism, mainly from the oxidative phosphorylation process that occurs in the mitochondrial electron transport chain. Oxidative stress accumulation can lead to metabolic disorders such as cancer, ageing, inflammation, and neurodegeneration [23]. The cells have enzymatic and non-enzymatic antioxidative defense systems to compensate for the ROS concentration and maintain equilibrium [21,24]. Plant-derived polysaccharides are shown to possess free radical scavenging activities [20].
The goal of the present work is to evaluate the effects of a ginseng extract as a prebiotic [21] and a probiotic formulation on the modulation of an in vitro reconstructed synthetic gut microbiota [17]. The activity of the metabolites from microbial ginseng extract fermentation was then evaluated on human intestinal epithelial cells to investigate a potential antioxidant effect.
2. Materials and Methods
2.1. Extraction and Characterisation of Ginseng Bioactive Molecules
The Panax ginseng berries extract (P. ginseng C.A Meyer, sold as “Panaxolyde”) was supplied by Flanat Research Italia Srl (Rho, Italy). The method for obtaining the extract from the berries of Panax ginseng shown in Figure 1 is reported in De Giani et al. [21].
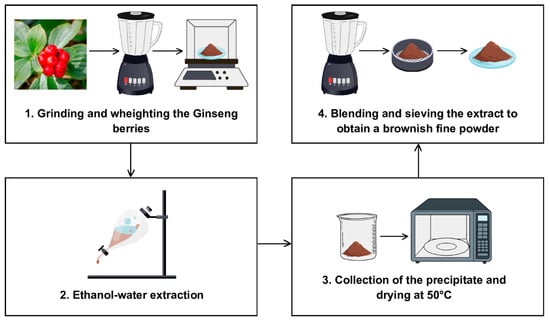
Figure 1.
The extraction process for obtaining the extract from the berries of Panax ginseng.
The principal components of the ginseng powder were characterized. The protein content, polyphenol content, ginsenoside content, and polysaccharides content were determined with the methods described in De Giani et al. [21]. The components are reported in Table 1.

Table 1.
Characterization of ginseng extract.
2.2. Bacterial Strains and Culture Conditions
The bacterial strains used in this study are listed in Table 2. The probiotics (Lactiplantibacillus plantarum, Lactobacillus acidophilus, and Bifidobacterium animalis subsp. lactis) were supplied by Roelmi HPC (Origgio, Italy) [25]. The minimal core microbiota strains Bacteroides cellulosilyticus, Clostridium symbiosum, and Flavonifractor plautii belong to BEI Resources (NIAID, NIH collection, as part of the Human Microbiome Project), and Escherichia coli ATCC 25922 derives from the American Type Culture Collection (ATCC, Manassas, VA, USA).

Table 2.
Bacterial strains used in the study.
Lactobacillus and Bifidobacterium strains were maintained in De Man, Rogosa, and Sharp medium (MRS) (Conda Lab, Madrid, Spain) with the addition of 0.03% L-cysteine (Merk, Milano, Italy); minimal core microbiota strains were maintained in Reinforced Clostridia Medium (RCM) (Conda Lab, Madrid, Spain), with the addition of 0.03% L-cysteine and 0.01 g/L of hemin (Hemin chloride, Cayman Chemical, Ann Arbor, MI, USA). All the strains were grown at 37 °C in anaerobic conditions obtained by Anaerocult GasPack System (Merck, Darmstadt, Germany).
The experiments were performed in a modified MRS as mMRS supplemented with 2%, 1.08%, 0.18%, and 0.1% w/v of ginseng extract, pectin (pectin from apple, poly-D-galacturonic acid methyl ester, Merck, Milano, Italy), galactose (D-(+)-Galactose, Merck, Milano, Italy), and galacturonic acid (D-(+)-Galacturonic acid monohydrate, Merck, Milano, Italy), respectively [21]. The substrates were prepared as described by De Giani et al. [21]. The growth experiments with single strains were conducted in a final volume of 1 mL in 24 multiwells (24 wells, SPL Lifesciences, Pocheonsi, Korea). Each strain’s initial optical density at 600 nm (OD600 nm) was 0.1. All of the strains were grown statically in anaerobiosis (Anaerocult GasPack System, Merck, Darmstadt, Germany) at 37 °C for 48 h, and the OD600 nm was measured.
2.3. Experiments in Batch Fermentation System
Pre-cultures of 72 h for the minimal core microbiota strains and of 48 h for the probiotics were prepared at 37 °C in anaerobic conditions before the setup of the experiments [17].
The three consortia setups (probiotics, minimal core, and whole community) were cultured in a 400 mL batch reactor (Colaver, Vimodrone, Italy), using mMRS (negative control) or mMRS supplemented with 2% ginseng extract as a carbon source. In all of the experimental conditions, the batch reactor was stirred at 60 rpm and maintained under anaerobic conditions by fluxing nitrogen 5.0 (Sapio, Monza, Italy) for 48 h at 37 °C. All of the bacterial strains were added with an initial OD600 nm of 0.05 [17].
The different experimental setups were followed, as reported by De Giani et al. [17]. Briefly, 10 mL samples were taken every 8 h (T0, T8, T16, T24, T32, and T48). Nitrogen was flushed for 10 min before and after the sampling to maintain anaerobiotic conditions. Overall microbial growth was evaluated by OD600 nm. Culture broth was analyzed for microbial metabolites’ production. Bacterial cells were stored for DNA extraction and bacterial species quantification.
2.4. Total DNA Extraction
Total DNA was extracted from single bacterial cultures at a concentration of 108 CFU/mL to construct a standard curve to title the strains by qPCR analyses [26,27]. Total DNA was obtained using the Ultraclean Microbial DNA Isolation Kit (Qiagen, Milano, Italy) following the protocol provided by the manufacturer.
The total DNA of the three fermentation setups was obtained using the Stool Nucleic Acid Isolation Kit (Norgen Biotek Corp., Thorold, ON, Canada) following the protocol provided by the manufacturer with some modifications [27].
The concentrations and purity of the DNA were analyzed spectrophotometrically (NanoDrop One Microvolume UV–Vis Spectrophotometer, ThermoFisher Scientific, Monza, Italy).
2.5. Modulation of the Strain Abundances of the In Vitro Reconstructed HGM Through qPCR
The single strain modulation was followed through qPCR reactions using PCR Real-Time StepOne Plus (Applied Biosystems, Monza, Italy) and the PowerUp SYBR Green Master Mix (Applied Biosystems, Monza, Italy) with species-specific primers. The bacterial counts/mL of each strain were calculated using the absolute quantification method, which utilizes a calibration curve. The average slope and y-intercept of each standard curve were determined by regression analyses and used to calculate the bacterial counts/mL for each bacterial target. The species-specific primer sets utilized in this study are reported by De Giani et al. [17] and in Table S1 [26,27,28,29,30,31]. Different qPCR programs were employed, as described by De Giani et al. [17]. Each DNA sample was analyzed in triplicate.
2.6. Extraction and Characterisation of Microbial Metabolites
After acidification at pH 2 (6 M HCl) of the cultured broth, bacterial metabolites were extracted as reported by De Giani et al. [21]. A 1:1 ratio of Ethyl Acetate (Merck, Milano, Italy) was employed for the extraction.
The metabolite analysis was carried out with a gas chromatograph (Technologies 6890 N Network GC System, Agilent Technologies, Santa Clara, CA, USA) equipped with a mass selective detector (5973 Network, Agilent Technologies, Santa Clara, CA, USA). The analyses were performed as described by De Giani et al. [21]. All of the samples were injected three times. The obtained chromatograms and mass spectra were interpreted by comparison with the National Institute of Standards and Technology (NIST) library and with injected standard molecules (Merck, Milano, Italy).
2.7. In Vitro Experiments on Human Cell Lines
2.7.1. HT-29 Cell Line Maintenance and Viability Assay
The colon cancer cell line HT-29 (ATCC® HTB-38™) was cultured in DMEM medium supplemented with heat-inactivated 10% FBS, 2 mM L-glutamine, 100 U/mL of penicillin, and 100 µg/mL of streptomycin and maintained at 37 °C in a humidified 5% CO2 incubator. The HT-29 cell line was verified by short tandem repeat profiles generated by the simultaneous amplification of multiple short tandem repeat loci and amelogenin (used for gender determination).
For the evaluation of cell viability in the presence of the extracts, cells were seeded in 96-well microtiter plates at a density of 1 × 104 cells/well and 24 h later treated with 0.1 and 0.5 mg/mL for each extract. After 48 h, cell viability was assessed through an in vitro MTT-based toxicology assay kit (Merck KGaA, Darmstadt, Germany), by replacing the medium with a complete medium without phenol red, and adding 10 µL of 5 mg/mL MTT (3-(4,5-dimethylthiazol-2)-2,5-diphenyltetrazolium bromide) solution to each well. After 2 h of incubation, the resulting formazan crystals were solubilized with 10% Triton-X-100 in acidic isopropanol (0.1 N HCl) and absorbance was recorded at 570 nm with a micro plate reader. The results were expressed as the mean values ± ES of at least three independent experiments.
All of the reagents for cell culture were supplied by EuroClone (EuroClone S.p.A, Pero, Italy).
2.7.2. ROS Detection
Intracellular reactive oxygen species (ROS) were detected via the oxidation of 2′,7′-dichlorofluorescin diacetate (H2DCFDA) and dihydroethidium (DHE) (Merck KGaA, Darmstadt, Germany). Cells were seeded in 96-well black microtiter plates at a density of 1 × 104 cells/well and cultured in complete medium for 24 h.
For total cytosolic ROS, seeded cells were incubated with 5 µM H2DCFDA in PBS (10 mM K2HPO4, 150 mM NaCl, pH 7.2) for 30 min in the dark at 37 °C; then, they were rinsed in PBS and treated for 4 h with 0.1 and 0.5 mg/mL for each extract or with TBHP 100 µM, as a positive control. Fluorescence (λem = 485 nm/λex = 535 nm) was measured using a fluorescence microtiter plate reader (VICTOR X3, PerkinElmer, Akron, OH, USA), whereas for the cytosolic superoxide anion (O2•−) content, seeded cells were first incubated with 10 µM DHE in complete medium for 30 min in the dark at 37 °C and then 0.1 and 0.5 mg/mL for each extract were added to perform a 4 h treatment. Subsequently, cells were washed with PBS and fluorescence (λex = 480–520 nm/λem = 570–600 nm) was measured using a fluorescence microtiter plate reader (VICTOR X3, PerkinElmer, Akron, OH, USA). Antimycin A at a final concentration of 150 µM was used as a positive control.
All of the fluorescence measurements were normalized against the total protein amount, determined by a Bradford assay [32].
2.8. Statistical Analyses
The data derived from the bacteria growth are shown as the mean values ± standard error. The statistical significance was assessed through Student’s t test and was defined as * p-value < 0.05, ** p-value < 0.01, or *** p-value < 0.001. Bacterial counts/mL resulting from real-time q-PCR analysis are reported as the mean values ± standard error. The statistical significance was assessed through the Wilcoxon Signed Rank test and was defined as * p-value < 0.05, ** p-value < 0.01.
The data derived from oxidative stress analysis on the intestinal human cell line are represented as the mean ± standard error. Statistically significant results are highlighted with **** p-value < 0.0001, *** p-value < 0.001, ** p-value < 0.01, and * < 0.05, calculated employing one-way ANOVA with Dunnett’s multiple comparisons tests.
3. Results
3.1. Single Strain Growths on the Ginseng Extract and Its Components
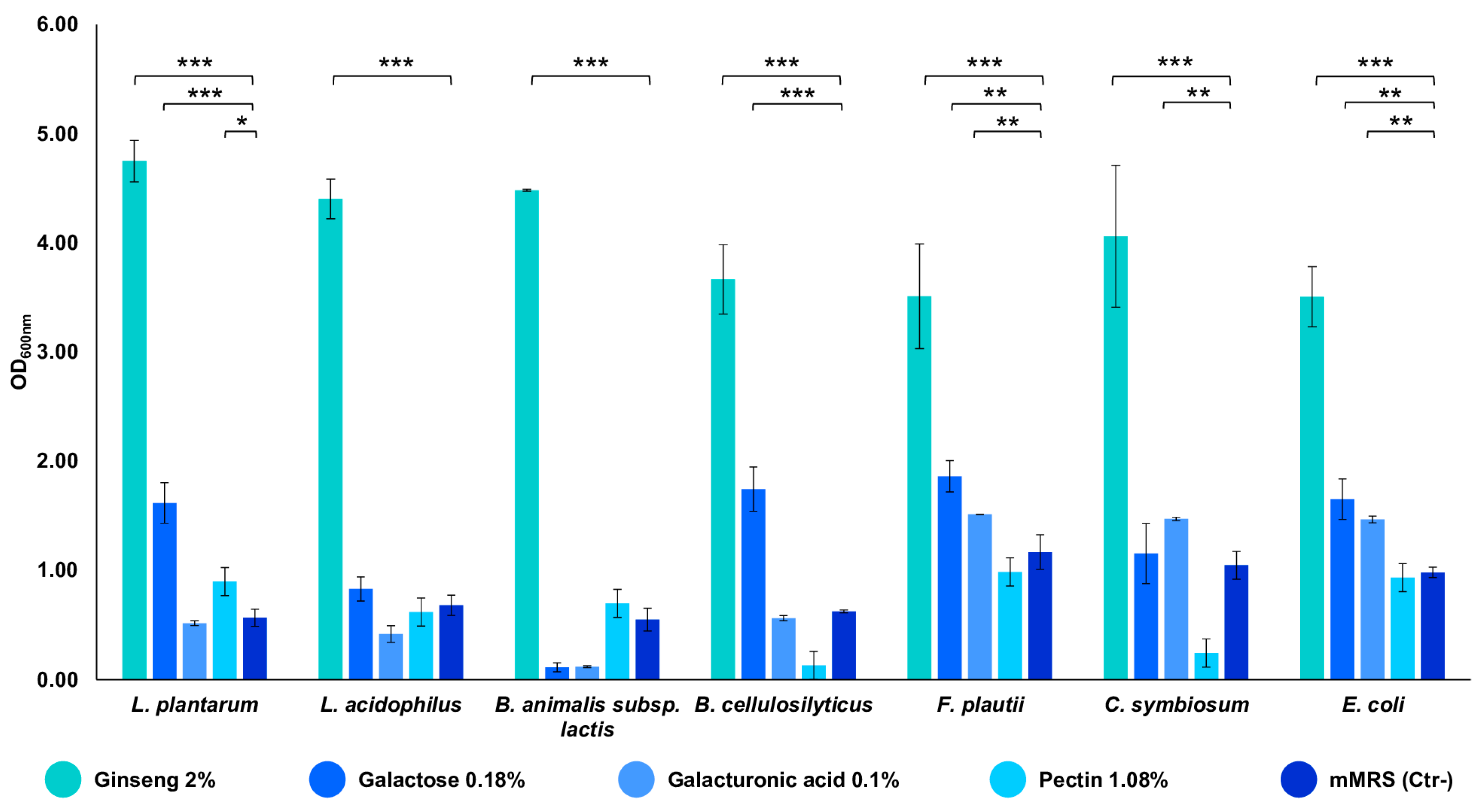
The strains composing the in vitro gut microbiota model demonstrated a remarkable ability to grow on the ginseng extract at a final concentration of 2% w/v. All of the strains showed an OD600 nm between 3.5 and 4.8, indicating that the extract can sustain their growth (p-value < 0.001 vs. mMRS) (Figure 2). Subsequently, the seven strains were also tested for their ability to utilize the single components of the characterized extract. The bacteria exploited the substrates differently, but all of the strains demonstrated their ability to utilize them, reaching an OD600 nm near 1.5 (p-value < 0.001 and < 0.01 vs. mMRS) (Figure 2). In particular, galactose is used by the majority of the seven strains; the minimal core strains, except for B. cellulosilyticus, are able to grow on galacturonic acid (p-value < 0.01); pectin significantly sustains the growth of L. plantarum only (p-value < 0.05 vs. mMRS) (Figure 2).
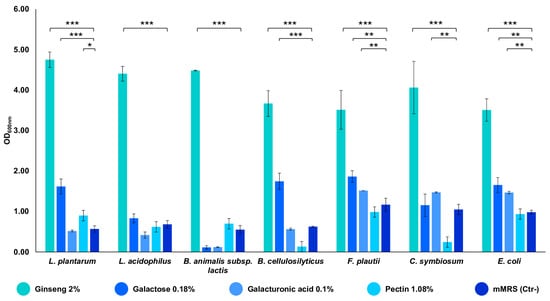
Figure 2.
Growths of the single bacterial strains on ginseng extract and its components. The growth is presented as the mean value of OD600 nm ± SE in the presence of 2% ginseng extract, 0.18% galactose, 0.1% galacturonic acid, 1.08% pectin, and mMRS (Ctr- medium). The statistical significance was calculated via Student’s t test: * p-value < 0.05, ** p < 0.01, *** p < 0.001.
3.2. Modulation of the In Vitro Reconstructed Human Gut Microbiota in Presence of the Ginseng Extract
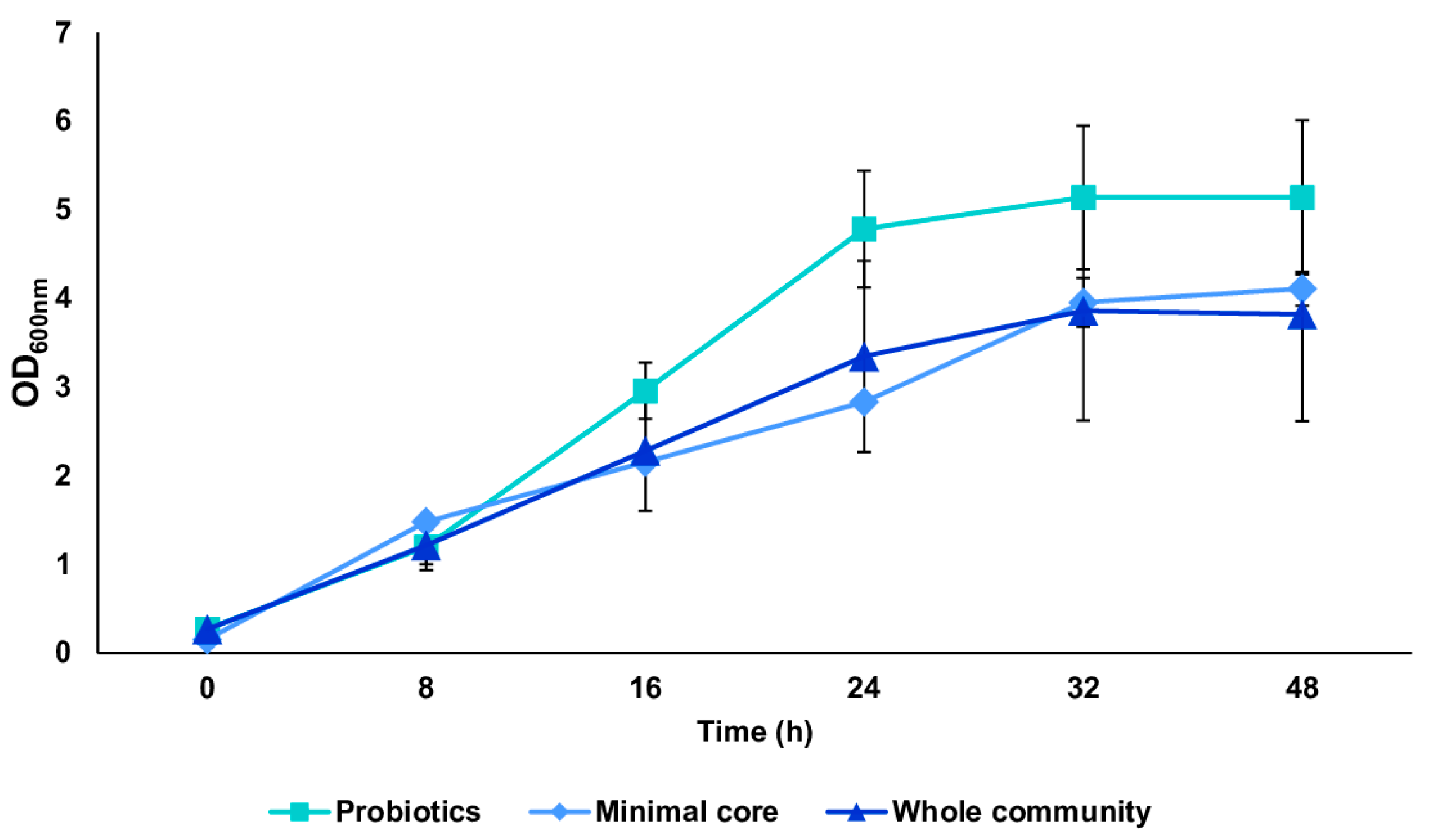
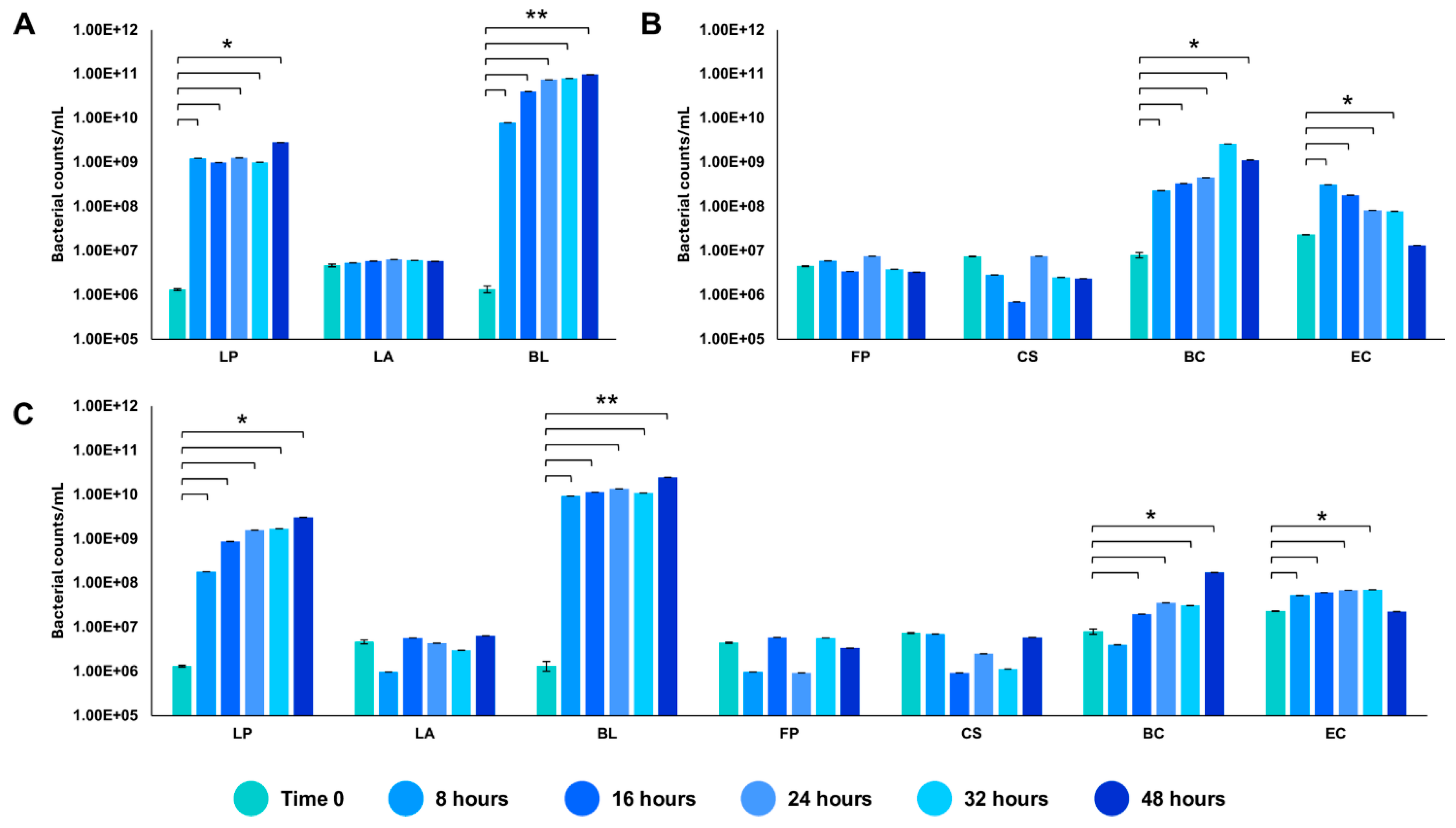
We tested the in vitro reconstructed human gut microbiota (HGM) setups (previously validated by De Giani et al., 2024 [17]) in the presence of 2% w/v ginseng as the sole carbon and energy source. The probiotic bacteria reached a maximum OD600 nm of 5.14 ± 0.81 at 32 h, as shown in Figure 3. The minimal core achieved the highest OD600 nm at 48 h of fermentation (4.11 ± 0.19). The growth of the whole community showed the maximum growth at 32 h, reaching an OD600 nm of 3.86 ± 1.02. The modulation of the single strains composing the different configurations showed the prevalence of LP (109 bacterial counts/mL) and BL (1010 bacterial counts/mL) among the probiotics (p-value < 0.05 vs. time 0) (Figure 4A). Considering the minimal core, BC prevails over the other strains, reaching 109 bacterial counts/mL at 32 h of fermentation (Figure 4B) (p-value < 0.05 vs. time 0). In the whole community setup, LP, BL, and BC reached values of bacterial counts/mL in the order of 109, 1010, and 108, respectively (Figure 4C). EC is also able to significantly grow when in the presence of all the other strains of the bacterial community, reaching counts of 108 bacteria/mL. However, the growth of the probiotic strains (LP, LA, and BL) is favored overall.
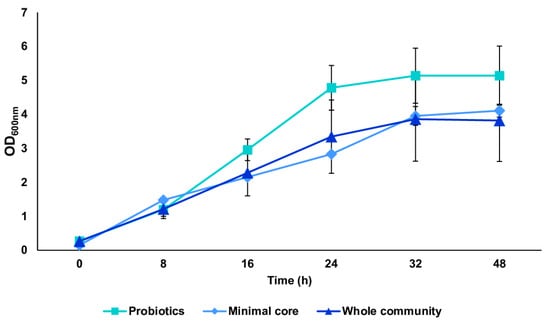
Figure 3.
Growth curves of probiotics, minimal core, and the whole community. Growth curves (mean value of OD600 nm ± SE during time) of the three conditions: probiotics, minimal core, and the whole community, in the presence of 2% ginseng extract in batch fermentation.
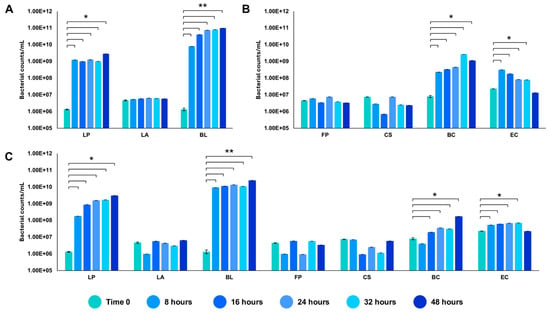
Figure 4.
Modulation of the single strains through qRT-PCR. Values of bacterial counts/mL reached by the probiotics (A), the minimal core strains (B), and the whole community (C) during growth on 2% ginseng extract. The statistical significance was calculated using Student’s t test: * p-value < 0.05, ** p < 0.01.
3.3. Production of Microbial Metabolites and SCFAs by the In Vitro Reconstructed Human Gut Microbiota
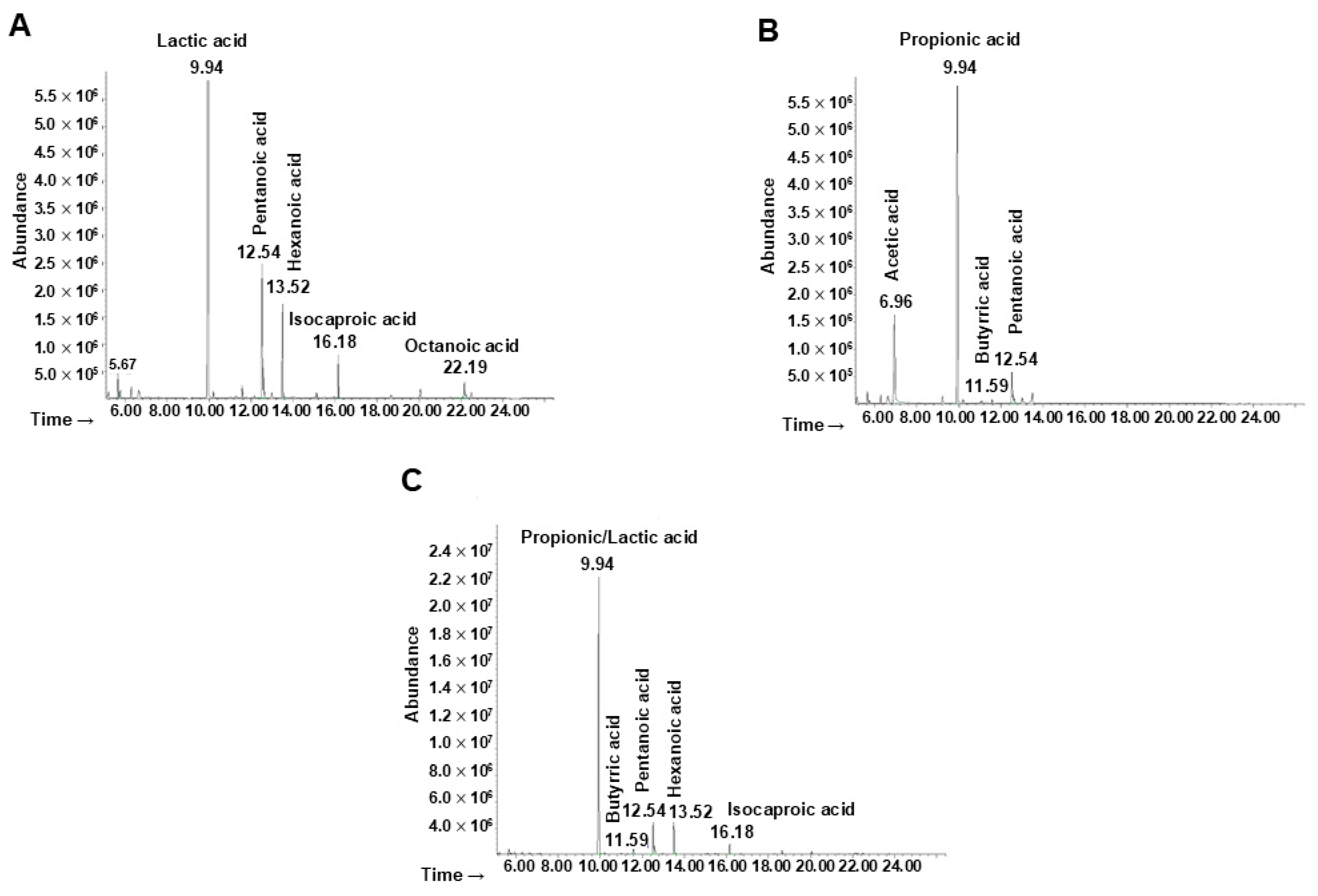
The metabolites derived from the fermentation of the ginseng extract by the probiotics, the minimal core, and the whole bacterial community were extracted and identified. Referring to the probiotic strains (Figure 5A), the first pick (Rt = 9.94 min) was identified as lactic acid. The two picks at Rt = 12.54 and Rt = 13.52 were associated with pentanoic and hexanoic acid, respectively. The other metabolites identified were isocaproic acid (Rt = 16.18) and octanoic acid (Rt = 22.19).
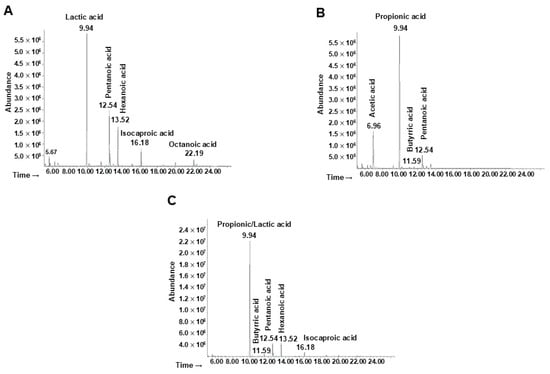
Figure 5.
Analyses of bacterial metabolites by GC-MSD after ginseng extract fermentation. Chromatogram of metabolites produced by (A) probiotics, (B) minimal core strains, and (C) the whole community.
The main metabolites produced by the minimal core in the presence of ginseng extract (Figure 5B) were identified as acetic acid (Rt = 6.96), propionic acid (Rt = 9.94), butyric acid (Rt = 11.59), and pentanoic acid (Rt = 12.54).
Finally, the production of metabolites by the whole bacterial community was analyzed (Figure 5C) showing at the Rt of 9.94 the presence of both lactic and propionic acid (or a mixture of both). The other molecules identified were butyric, pentanoic, hexanoic, and isocaproic acid, associated with an Rt of 11.59, 12.54, 13.52, and 16.18 respectively.
3.4. Biological Effects of Bacterial Metabolites and SCFAs on HT-29 Cell Line
The human colorectal cancer HT-29 cell line represents a valuable tool for the evaluation of the beneficial role of probiotics in the human host, as well as for the molecular mechanisms underpinning host–microbe interactions [33].
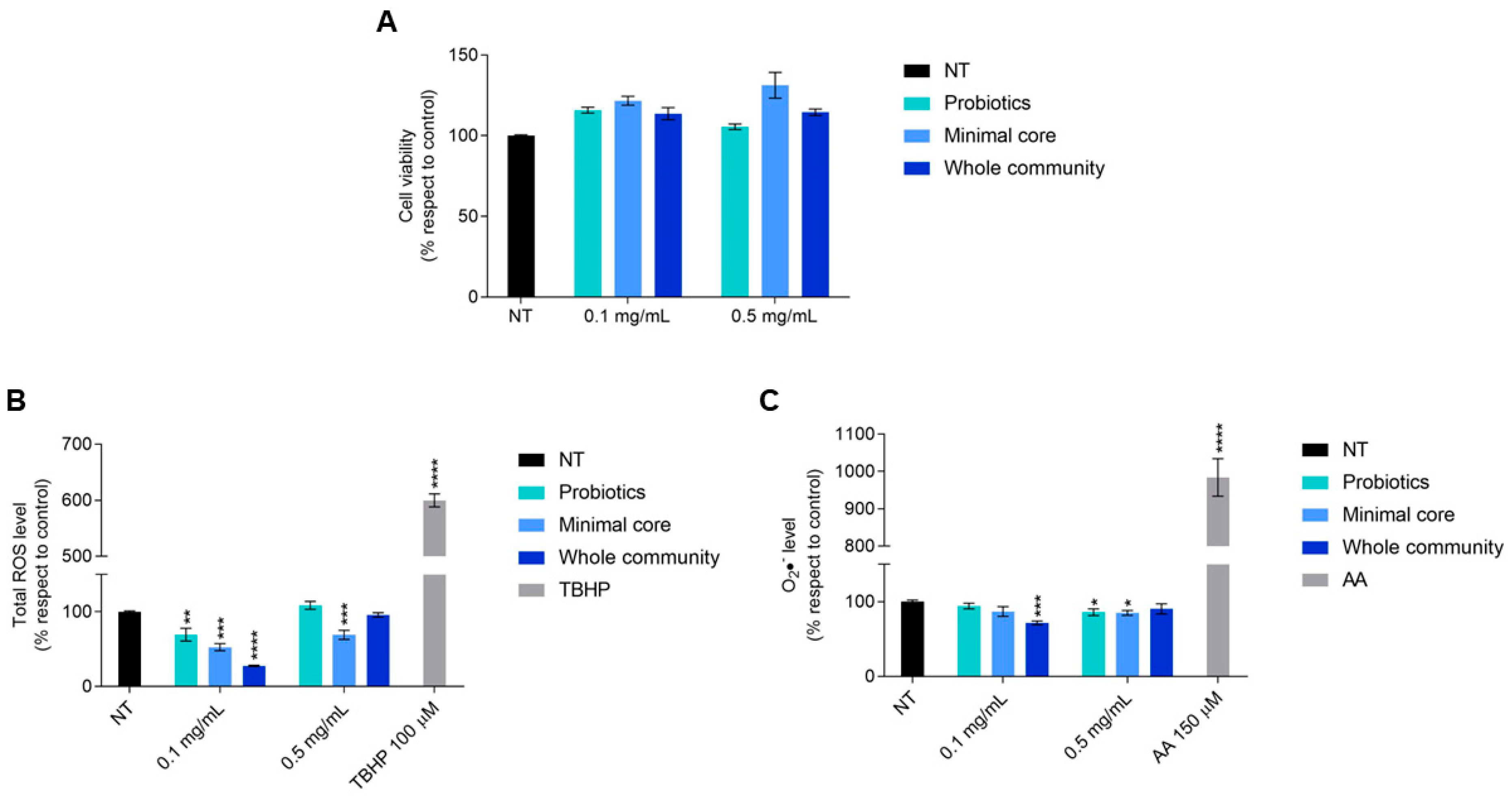
Intestinal cells showed no difference in their viability after a 48 h treatment with each extract taken into consideration; indeed, at both 0.1 and 0.5 mg/mL, a cellular viability slightly higher than in untreated cells could be observed (Figure 6A).
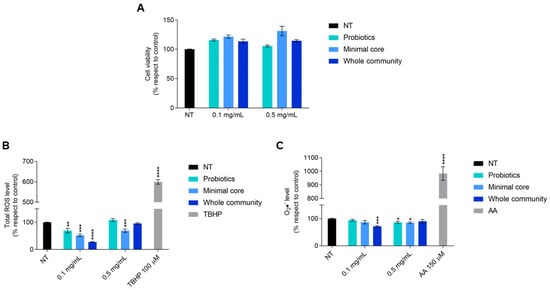
Figure 6.
Viability of HT-29 cells exposed for 48 h to the extracts derived from probiotics, minimal core bacteria, and the whole community fermented on a 2% ginseng extract. (A) Oxidative stress response of HT-29 after a 4 h exposure to metabolites deriving from probiotics, minimal core bacteria, and the whole community fermented on ginseng extracts. (B) Panel shows the total ROS level measured by H2DCFDA. (C) Panel shows the cytosolic superoxide anions (O2•−) content measured by DHE. TBHP 100 µM and antimycin A (AA) 150 µM were used as a positive control for H2DCFDA and DHE, respectively. Data are represented as the mean ± standard error (SE). Statistically significant results are highlighted with **** p-value < 0.0001, *** p-value < 0.001, ** p-value < 0.01, and * p < 0.05.
Given this result, we decided to investigate a possible antioxidant effect, due to the known antioxidant potential derived from ginseng fermentation by probiotics [21]. Cells treated for 4 h with the extract derived from probiotics fermented on ginseng showed a significant reduction in the total reactive oxygen species (ROS) level at 0.1 mg/mL and in cytosolic superoxide anion (O2•−) content at 0.5 mg/mL.
With regard to the effect of the minimal core bacteria fermented on a 2% ginseng solution, it is possible to observe a statistically significant reduction in the total ROS level at both doses and of the cytosolic superoxide anion at only 0.5 mg/mL. Interestingly, the extracts obtained from the whole community fermented on ginseng caused a 70% reduction in the total ROS level and a 30% reduction in the cytosolic superoxide anion level once given to the cells at the dose of 0.1 mg/mL (Figure 6B,C).
4. Discussion
In vitro gut microbiota reconstruction models have improved greatly over time, mainly in the rebuilding of digestion stage complexity, the different experimental conditions, and the multitude of ecological parameters to monitor. These models are a useful tool to study the impact of a given diet compound, e.g., prebiotics, or bioactive compound or the addition of probiotics on the human gut microbiota [10,16]. In particular, they can be used to analyze the changes in core microbial groups and selected species together with their metabolites, assaying their diversity and richness in the whole microbial community over time.
In a previous work, we developed a setup of an in vitro reconstructed HGM [17], demonstrating the possibility to assay several ecological parameters.
In this paper, we challenged our HGM reconstructed model using a ginseng berry extract, containing bioactive molecules and enriched in polysaccharides, resulting in pectin-based polysaccharides, which we have already demonstrated to possess a prebiotic potential [21].
The in vitro HMG setup is based on the findings of a previous study conducted by the same authors of this paper [17]. E. coli, B. cellulosilyticus, F. plautii, and C. symbiosum are included in the minimal core due to their classification within the Pseudomonadota, Bacteroidota, and Bacillota phyla, respectively. The minimal core was then implemented by adding the three selected probiotic strains (L. plantarum, L. acidophilus, and B. animalis subsp. lactis). The selected configuration reflects the current knowledge about gut microbiota composition in vivo [6,34,35]. The majority of the HGM microorganisms belong to the Bacillota and Bacteroidota phyla, while the remaining fall into the Pseudomonadota, Actinomycetota, and Verrucomicrobiota [6].
The novelty of the paper lies in the use of a synthetic human gut in vitro microbial community to enhance the understanding of gut bacteria interactions in the presence of a complex extract. The work focuses on analyzing the relationships established within the bacterial community in the presence of complex carbohydrates, such as those found in ginseng extract. The metabolic products derived from the fermentation of the ginseng extract that support the cross-feeding process have been examined.
The ability of the seven synthetic community strains to grow individually on the ginseng extract at 2% concentration was evaluated. The ginseng extract sustained the growth of all of the strains, which showed an OD600 nm between 3.5 and 4.8, with the three probiotic strains showing the highest growth. These data confirm the results of a previous work by De Giani et al. [27]. To better understand which carbohydrate component could promote bacterial growth, we tested the individual polysaccharides found in the extract, including pectin, D-galacturonic acid, and D-galactose. Pectin is considered a next-generation prebiotic due to its beneficial effects, such as regulating glucose and lipid metabolism [36,37] and supporting the growth of beneficial bacteria [38]. The activities of pectin are closely related to its structural characteristics, including monosaccharide composition, molecular weight, degree of esterification (DE), number of glycosidic bonds, ratio of rhamnogalacturonan-I (RG-I) to homogalacturonan (HG), and conformation [39]. Interestingly, of all the strains tested, pectin significantly sustained the growth of L. plantarum. This result is in line with previous studies indicating that the survival and growth of Lactobacillus sp. is enhanced by pectin [21,40,41]. Notably, D-galacturonic acid significantly sustains the growth of all the minimal core strains except for B. cellulosilitycus. Most of the seven strains showed the ability to use D-galactose as a carbon source.
After evaluating individual strains on the ginseng extract, we tested the in vitro reconstructed HGM setups [17] using 2% w/v ginseng as the only carbon source. The exponential growth phase of the three configurations (probiotics, minimal core, and whole community) started at 8 h of incubation and reached the stationary phase at around 32 h. Probiotic bacteria reached the highest growth, while the minimal core and the whole community showed almost the same growth. The modulation of the single strains showed the prevalence of L. plantarum and B. animalis subsp. lactis among the probiotics, while B. cellulosilyticus prevailed over the other strains in the minimal core setup. In the presence of both probiotics and minimal core strains, L. plantarum, B. animalis subsp. lactis, and B. cellulosilyticus reached the highest values of bacterial counts/mL. Interestingly, E. coli was able to grow significantly when in the presence of all the other strains of the bacterial community.
The Bacteroides genus is known as a primary degrader in the metabolism of complex carbohydrates producing several metabolites such as acetate and propionate [2,41].
In contrast, the Bifidobacterium genus more commonly metabolizes smaller oligosaccharides, producing lactate as the main metabolite [2,42]. As expected, due to the presence of both B. animalis subsp. lactis and L. plantarum, lactic acid was detected in the presence of probiotics, both alone and in combination with the minimal core strains. Indeed, L. plantarum is an heterofermenter [43]. Acetate was detected in the minimal core setup, while propionate is present in the minimal core and the whole community. Acetate and lactate can then be metabolized to produce butyrate by butyrate-producing bacteria such as Clostridia of the cluster IV and XIV species like Clostridium symbiosum and Flavonifractor plautii [2]. Indeed, in our work, butyrate has been identified in the presence of the minimal core strains alone and with the probiotics. L. plantarum and E. coli are also able to degrade the polysaccharides present in the extract and the oligosaccharides produced during fermentation.
All of the metabolites produced by the bacteria have an important effect on the host, in addition to their role in the metabolic interplay of the HGM. Antitumoral, anti-inflammatory, and antioxidant effects are some of the properties of the beneficial metabolites produced by the intestinal bacterial community [6]. The metabolites obtained from ginseng extract fermentation by probiotics, the minimal core, or the whole community do not affect intestinal cell viability. Since ginseng extract is well known for its antioxidant properties [20,21,22], we focused our attention on the antioxidant effect. Our results highlighted a significant reduction in total ROS when intestinal cells were exposed to 0.1 mg/mL of metabolites derived from ginseng extract fermentation by probiotics and the minimal core. Moreover, these two experimental groups caused a slight reduction in superoxide anion content when given to the cells at a final concentration of 0.5 mg/mL. Remarkably, 0.1 mg/mL of bacterial metabolites produced from the whole community in the presence of ginseng determined a major reduction in both total ROS and cytosolic superoxide anion levels. Indeed, short-chain fatty acids (SCFAs), in particular butyrate and acetate, are able to modulate oxidative stress [44]. Human colonocytes exposed to butyrate showed a notable decrease in DNA damage caused by H2O2 [45]. In addition, butyrate increased the activity of glutathione S-transferase in human colon carcinoma HT-29 cells [46].
This research represents an example of creating a rationally designed microbiota-based model for understanding gut microbial interactions in the presence of different prebiotic compounds to complement missing or underrepresented functions that, in in vivo situations, cannot be deeply studied. However, this kind of study is characterized by several limitations. In fact, there are several aspects that should be reinforced such as the stability of the whole microbial community during the time of the experiment or the technology used to include host cells. Another limitation is the use of a few strains, showing a reductionistic approach with respect to in vivo conditions where there is a more complex ecosystem.
On the other hand, this approach permits us to investigate the influence on gut microbiota of a vast variety of factors such as compounds introduced by the diet, microbial pathogens, bioactive compounds, and pharmaceutical substances and to better mimic this ecosystem for the development of strategies aimed to reduce the imbalance of the gut microbial community with probiotic supplementation.
5. Conclusions
In conclusion, this study shows that the fermentation of ginseng extract with probiotic bacteria in the presence of a reconstructed minimal HGM mediates an antioxidant effect on the HT-29 human intestinal cell line. Therefore, these results highlight a synergism between the host and its own intestinal microbiota and the significant role of administering a probiotic treatment alongside a suitable prebiotic to enhance beneficial antioxidant effects.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13010192/s1, Table S1: Bacterial primer sets utilized in the study.
Author Contributions
Experimental activities and analysis of the results, M.F. (Margherita Finazzi) and F.B.; original draft preparation, M.F. (Matilde Forcella), M.F. (Margherita Finazzi), F.B. and P.D.G.; review and editing, P.D.G. and P.F.; supervision of the chemical methodology and of the chemical result analysis, M.L.; conceptualization and supervision, P.D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “FoodNET, Food Social Sensor Network” 2018-2022 funded by Lombardy Region, Italy, grant number 2251551.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors wish to thank the technicians for their technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sankar, S.A.; Lagier, J.C.; Pontarotti, P.; Raoult, D.; Fournier, P.E. The human gut microbiome, a taxonomic conundrum. Syst. Appl. Microbiol. 2015, 38, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Thomson, P.; Medina, D.A.; Ortúzar, V.; Gotteland, M.; Garrido, D. Anti-inflammatory effect of microbial consortia during the utilization of dietary polysaccharides. Int. Food Res. 2018, 109, 14–23. [Google Scholar] [CrossRef]
- Isenring, J.; Bircher, L.; Geirnaert, A.; Lacroix, C. In vitro human gut microbiota fermentation models: Opportunities, challenges, and pitfalls. Microbiome Res. Rep. 2023, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- Krautkramer, K.A.; Fan, J.; Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 2021, 19, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Shetty, S.A.; Kuipers, B.; Atashgahi, S.; Aalvink, S.; Smidt, H.; de Vos, W.M. Inter-species metabolic interactions in an In-Vitro minimal human gut microbiome of core bacteria. npj Biofilms Microbiomes 2022, 8, 21. [Google Scholar]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef]
- Golisch, B.; Lei, Z.; Tamura, K.; Brumer, H. Configured for the human gut microbiota: Molecular mechanisms of dietary β-glucan utilization. ACS Chem. Biol. 2011, 16, 2087–2102. [Google Scholar] [CrossRef]
- Tamura, K.; Hemsworth, R.; Déjean, G.; Davies, G.J.; Martens, E.C.; Brumer, H. Molecular mechanism by which prominent human gut bacteroidetes utilize mixed-linkage beta-glucans, major health-promoting cereal polysaccharides. Cell Rep. 2017, 21, 417–430. [Google Scholar] [CrossRef]
- Khoroshkin, M.S.; Leyn, S.A.; Van Sinderen, D.; Rodionov, A.D. Transcriptional regulation of carbohydrate utilization pathways in the Bifidobacterium genus. Front. Microbiol. 2016, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Nissen, L.; Casciano, F.; Gianotti, A. Intestinal fermentation In Vitro models to study food-induced gut microbiota shift: An updated review. FEMS Microbiol. Lett. 2020, 367, 12. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, P.T.; Brul, S.; Zhang, J.; Wortel, M.T. Synthetic microbial communities (SynComs) of the human gut: Design, assembly, and applications. FEMS Microbiol. Rev. 2023, 47, 2. [Google Scholar] [CrossRef] [PubMed]
- Steimle, A.; Neumann, M.; Grant, E.T.; Turner, J.D.; Desai, M.S. Concentrated raw fibers enhance the fiber-degrading capacity of a synthetic human gut microbiome. Int. J. Mol. Sci. 2021, 22, 6855. [Google Scholar] [CrossRef]
- Shetty, S.A.; Kostopoulos, I.; Geerlings, S.Y.; Smidt, H.; de Vos, W.M.; Belzer, C. Dynamic metabolic interactions and trophic roles of human gut microbes identified using a minimal microbiome exhibiting ecological properties. ISME J. 2022, 16, 2144–2159. [Google Scholar] [CrossRef]
- De Giani, A.; Perillo, F.; Baeri, A.; Finazzi, M.; Facciotti, F.; Di Gennaro, P. Positive modulation of a new reconstructed human gut microbiota by Maitake extract helpfully boosts the intestinal environment In Vitro. PLoS ONE 2024, 19, e0301822. [Google Scholar] [CrossRef]
- Morshed, M.N.; Ahn, J.C.; Mathiyalagan, R.; Rupa, E.J.; Karim, M.R.; Jung, D.H.; Yang, D.U.; Yang, D.C.; Jung, S.K. Antioxidant activity of Panax ginseng to regulate ROS in various chronic diseases. Appl. Sci. 2023, 13, 2893. [Google Scholar] [CrossRef]
- Lee, D.Y.; Park, C.W.; Lee, S.J.; Park, H.R.; Kim, S.H.; Son, S.U.; Park, J.; Shin, K.S. Anti-cancer effects of Panax ginseng berry polysaccharides via activation of immune-related cells. Front. Pharmacol. 2019, 10, 1411. [Google Scholar] [CrossRef]
- Kim, C.J.; Ryu, E.Y.; Lee, S.; Lee, H.J.; Chun, Y.S.; Kim, J.K.; Yu, C.Y.; Ghimire, B.K.; Lee, J.G. Neuroprotective effect and antioxidant potency of fermented cultured wild ginseng root extracts of Panax ginseng CA meyer in mice. Molecules 2021, 26, 3001. [Google Scholar] [CrossRef]
- De Giani, A.; Oldani, M.; Forcella, M.; Lasagni, M.; Fusi, P.; Di Gennaro, P. Synergistic antioxidant effect of prebiotic ginseng berries extract and probiotic strains on healthy and tumoral colorectal cell lines. Int. J. Mol. Sci. 2022, 24, 373. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Hou, C.; Shi, M.; Yan, Y.; Liu, Y. An insight into the research concerning Panax ginseng CA Meyer polysaccharides: A review. Food Rev. Int. 2022, 38, 1149–1165. [Google Scholar] [CrossRef]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Presti, I.; D’ Orazio, G.; Labra, M.; La Ferla, B.; Mezzasalma, V.; Bizzaro, G.; Giardina, S.; Michelotti, A.; Tursi, M.; Vassallo, M.; et al. Evaluation of the probiotic properties of new Lactobacillus and Bifidobacterium strains and their In Vitro effect. Appl. Microbiol. Biotechnol. 2015, 99, 5613–5626. [Google Scholar] [CrossRef]
- Mezzasalma, V.; Manfrini, E.; Ferri, E.; Sandionigi, A.; La Ferla, B.; Schiano, I.; Michelotti, A.; Nobile, V.; Labra, M.; Di Gennaro, P. A randomized, double-blind, placebo-controlled trial: The efficacy of multispecies probiotic supplementation in alleviating symptoms of irritable bowel syndrome associated with constipation. Biomed. Res. Int. 2016, 26, 1. [Google Scholar] [CrossRef]
- De Giani, A.; Sandionigi, A.; Zampolli, J.; Michelotti, A.; Tursi, F.; Labra, M.; Di Gennaro, P. Effects of inulin-based prebiotics alone or in combination with probiotics on human gut microbiota and markers of immune system: A randomized, double-blind, placebo-controlled study in healthy subjects. Microorganisms 2022, 10, 1256. [Google Scholar] [CrossRef]
- Dejean, G.; Tamura, K.; Cabrera, A.; Jain, N.; Pudlo, N.A.; Pereira, G.; Viborg, A.H.; Petegem, F.V.; Martens, E.C.; Brumer, H. Synergy between cell surface glycosidases and glycan-binding proteins dictates the utilization of specific beta(1,3)-glucans by human gut Bacteroides. mBio 2020, 11, 2. [Google Scholar] [CrossRef]
- Alauzet, C.; Cunat, L.; Wack, M.; Lozniewski, A.; Busby, H.; Agrinier, N.; Cailliez-Grimal, C.; Frippiat, J.P. Hypergravity disrupts murine intestinal microbiota. Sci. Rep. 2019, 9, 9410. [Google Scholar] [CrossRef]
- Xie, Y.H.; Gao, Q.Y.; Cai, G.X.; Sun, X.M.; Zou, T.H.; Chen, H.M.; Yu, S.Y.; Qiu, Y.W.; Gu, W.Q.; Chen, X.Y.; et al. Fecal Clostridium symbiosum for noninvasive detection of early and advanced colorectal cancer: Test and validation studies. EBioMedicine 2017, 25, 32–40. [Google Scholar] [CrossRef]
- Huijsdens, X.W.; Linskens, R.K.; Mak, M.; Meuwissen, S.G.; Vandenbroucke-Grauls, C.M.; Savelkoul, P.H. Quantification of bacteria adherent to gastrointestinal mucosa by real-time PCR. J. Clin. Microbiol. 2002, 40, 4423–4427. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Maqueda, D.; Miralles, B.; Recio, I. HT29 Cell Line. In The Impact of Food Bioactives on Health; Verhoeckx, K., Cotter, P., Lopez-Exposito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., et al., Eds.; Springer: Cham, Switzerland, 2015; Chapter 11. [Google Scholar]
- Koliada, A.; Vladislav, M.; Romanenko, M.; Lushchak, O.; Kryzhanovska, N.; Guryanov, V.; Vaiserman, A. Sex differences in the phylum-level human gut microbiota composition. BMC Microbiol. 2021, 21, 131. [Google Scholar] [CrossRef]
- Graf, D.; Di Cagno, R.; Fåk, F.; Flint, H.J.; Nyman, M.; Saarela, M.; Watzl, B. Contribution of diet to the composition of the human gut microbiota. Microb. Ecol. Health Dis. 2015, 26, 26164. [Google Scholar] [CrossRef]
- Jiang, T. MON-P214: Apple-Derived Pectin Protects Lipid Metabolism in Diet-Induced Obese Rats. Clin. Nutr. 2016, 1, 35. [Google Scholar] [CrossRef]
- Samout, N.; Bouzenna, H.; Dhibi, S.; Ncib, S.; ElFeki, A.; Hfaiedh, N. Therapeutic effect of apple pectin in obese rats. Biomed. Pharmacother. 2016, 83, 1233–1238. [Google Scholar] [CrossRef]
- Sun, R.; Niu, Y.; Li, M.; Liu, Y.; Wang, K.; Gao, Z.; Wang, Z.; Yue, T.; Yuan, Y. Emerging trends in pectin functional processing and its fortification for synbiotics: A review. Trends Food Sci. 2023, 134, 80–97. [Google Scholar] [CrossRef]
- Ren, T.; Xu, M.; Zhou, S.; Ren, J.; Li, B.; Jiang, P.; Li, H.; Wu, W.; Chen, C.; Fan, M.; et al. Structural characteristics of mixed pectin from ginseng berry and its anti-obesity effects by regulating the intestinal flora. Int. J. Biol. Macromol. 2023, 242, 124687. [Google Scholar] [CrossRef]
- Larsen, N.; Cahú, T.B.; Saad, S.M.I.; Blennow, A.; Jespersen, L. The effect of pectins on survival of probiotic Lactobacillus spp. in gastrointestinal juices is related to their structure and physical properties. Food Microbiol. 2018, 74, 11–20. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Orlando, P.; Coppola, R. Biochemical traits, survival and biological properties of the probiotic Lactobacillus plantarum grown in the presence of prebiotic inulin and pectin as energy source. Pharmaceuticals 2012, 5, 481–492. [Google Scholar] [CrossRef]
- Fernandez-Julia, P.J.; Commane, D.; van Sinderen, D.; Munoz, J. Cross-feeding interactions between human gut commensals belonging to the Bacteroides and Bifidobacterium genera when grown on dietary glycans. Microbiome Res. Rep. 2022, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, N.; Garrido, D. Species deletions from microbiome consortia reveal key metabolic interactions between gut microbes. mSystems 2019, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Z.; Niu, J.; Dang, K.; Zhang, S.; Wang, S.; Wang, Z. Changes in physicochemical properties, enzymatic activities, and the microbial community of soil significantly influence the continuous cropping of Panax quinquefolius L. (American ginseng). Plant Soil 2021, 463, 427–446. [Google Scholar] [CrossRef]
- Rosignoli, P.; Fabiani, R.; De Bartolomeo, A.; Spinozzi, F.; Agea, E.; Pelli, M.A.; Morozzi, G. Protective activity of butyrate on hydrogen peroxide-induced DNA damage in isolated human colonocytes and HT29 tumour cells. Carcinogenesis 2001, 22, 1675–1680. [Google Scholar] [CrossRef]
- Ebert, M.N.; Klinder, A.; Peters, W.H.M.; Schäferhenrich, A.; Sendt, W.; Scheele, J.; Pool-Zobel, B.L. Expression of glutathione S-transferases (GSTs) in human colon cells and inducibility ofSTM2 by butyrate. Carcinogenesis 2003, 24, 1637–1644. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).