Anti-Methanogenic Potential of Seaweeds and Impact on Feed Fermentation and Rumen Microbiome In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Seaweeds
2.1.1. Chemical Composition
2.1.2. Microbial Inoculum, Buffer, and Total Gas Production
2.1.3. CH4 Production
2.2. Experiment II
2.2.1. Chemical Composition
2.2.2. Total Gas and CH4 Production
2.2.3. In Vitro Dry Matter Digestibility (IVDMD)
2.2.4. In Vitro Organic Matter Digestibility (IVOMD)
2.2.5. Volatile Fatty Acid (VFA) and Ammonia-N
2.2.6. Protozoa Enumeration
2.3. Statistical Analysis
2.4. DNA Isolation
Bioinformatic Analysis
3. Results
3.1. Chemical Composition
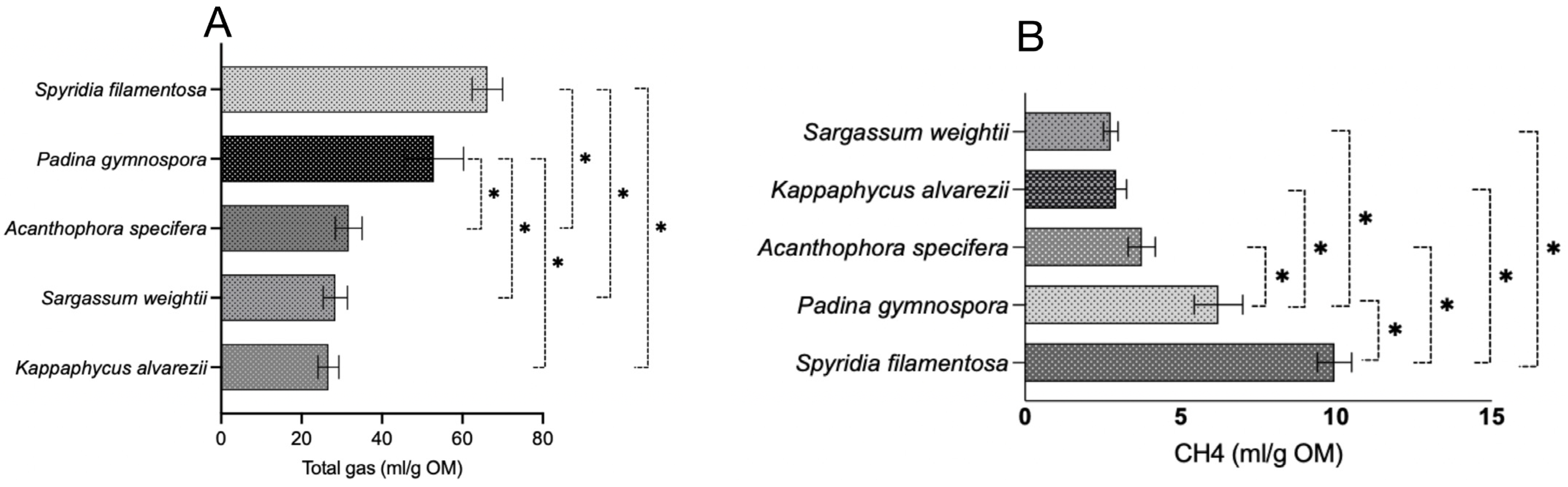
3.1.1. Total Gas
3.1.2. CH4 Production
3.2. Effect of Graded Levels of Selected Seaweeds
3.2.1. Chemical Composition
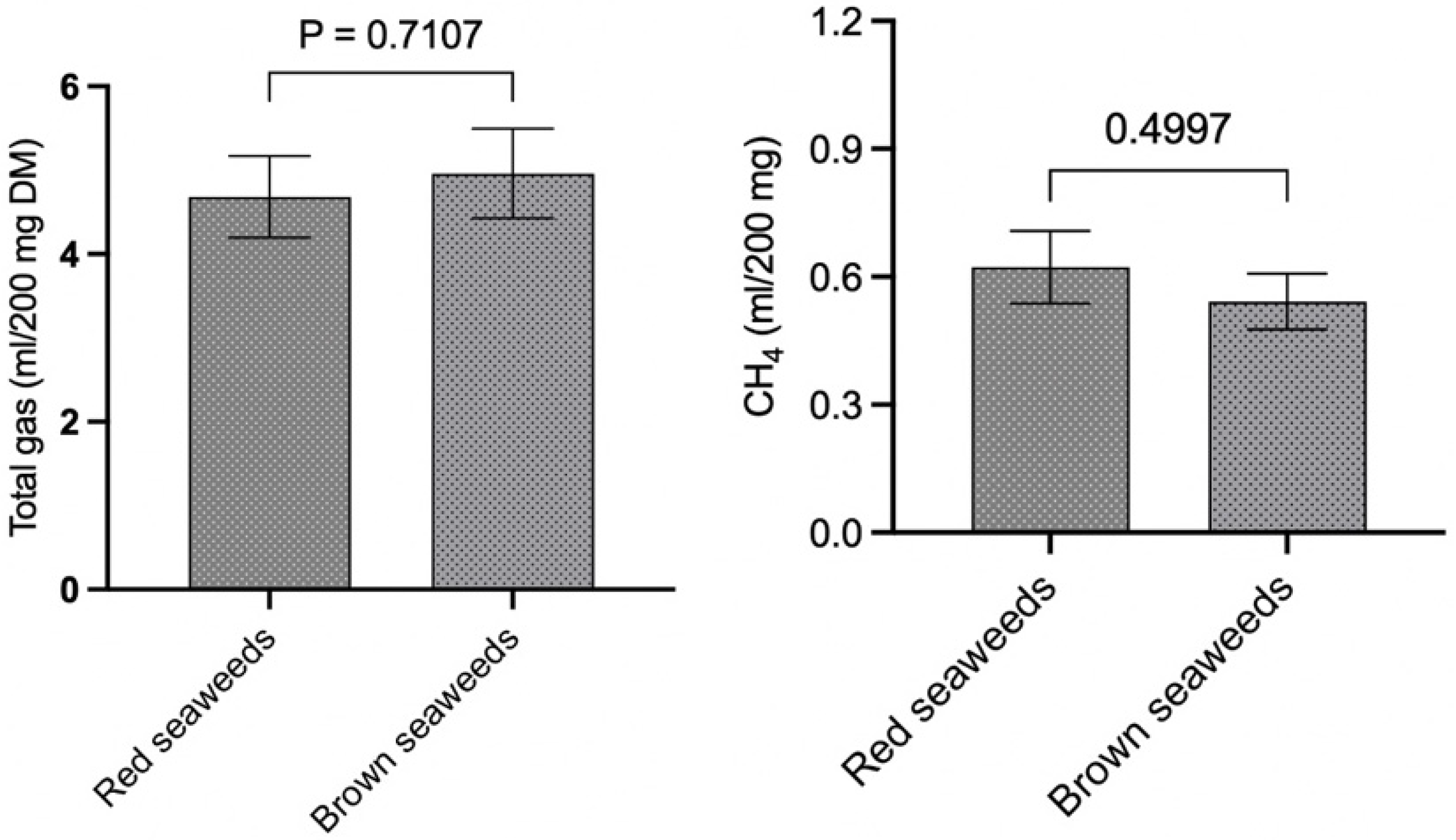
3.2.2. Total Gas Production
3.2.3. CH4 Production
3.2.4. In Vitro Digestibility
3.2.5. VFA Production
3.2.6. Rumen Protozoa
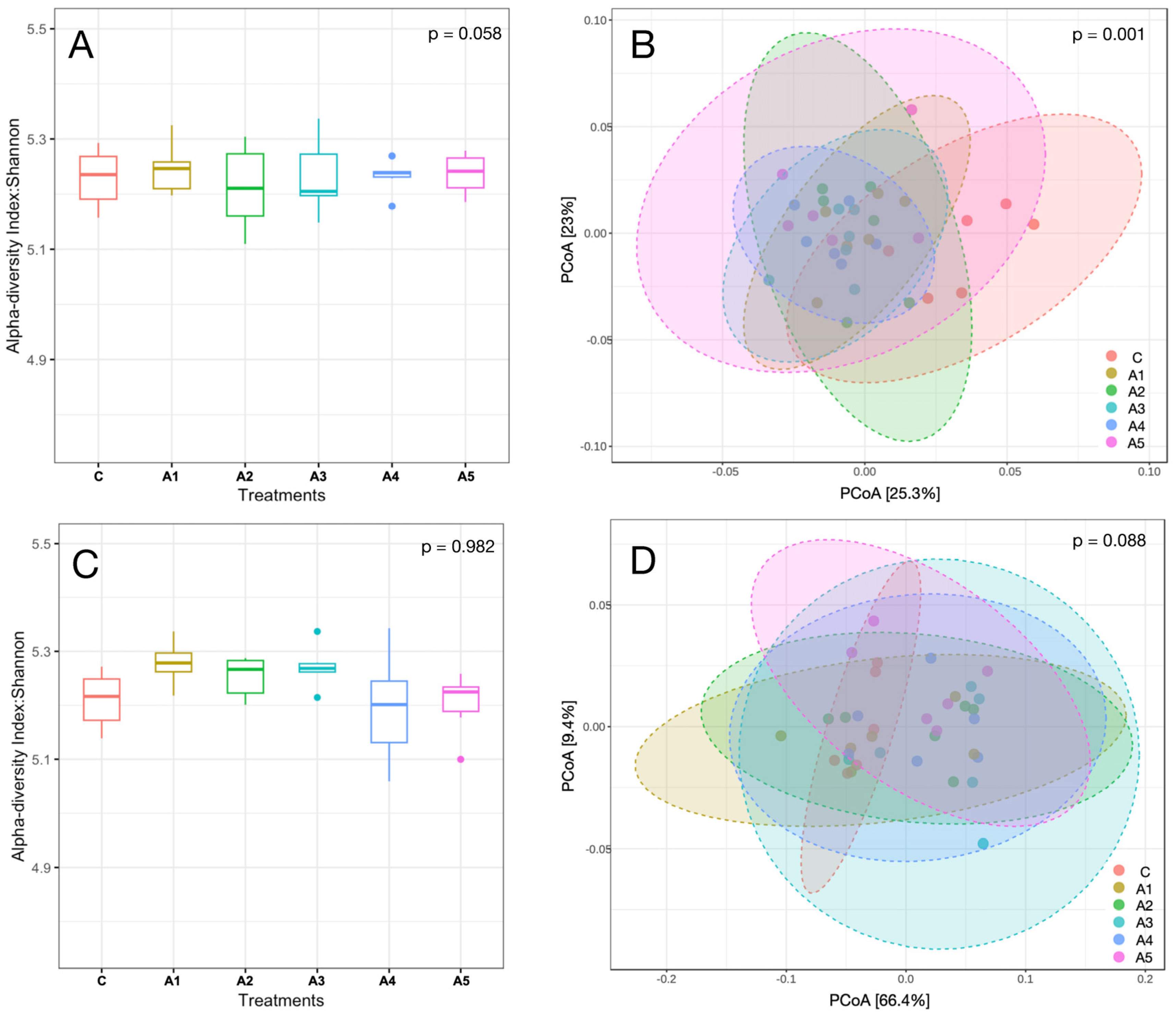
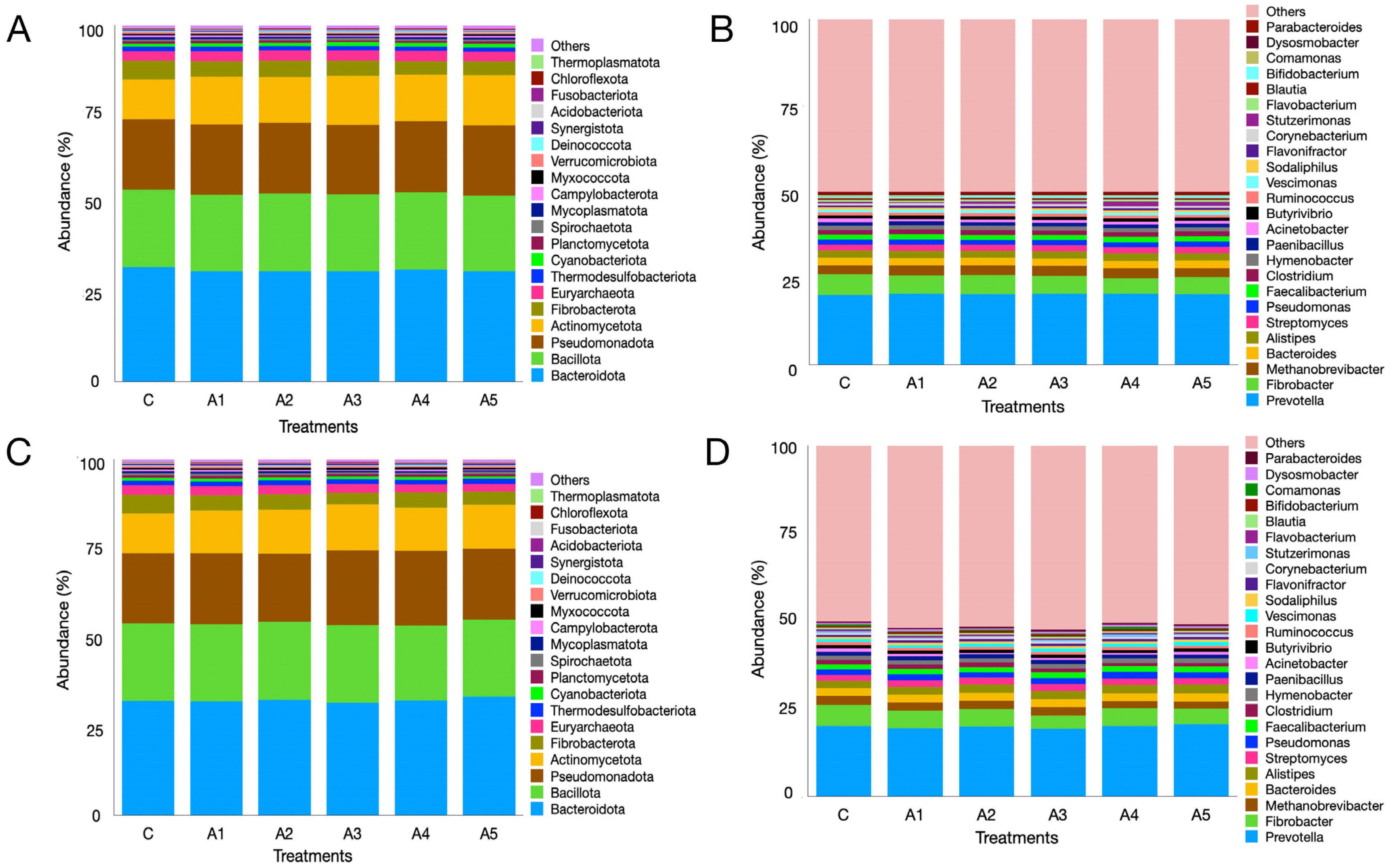
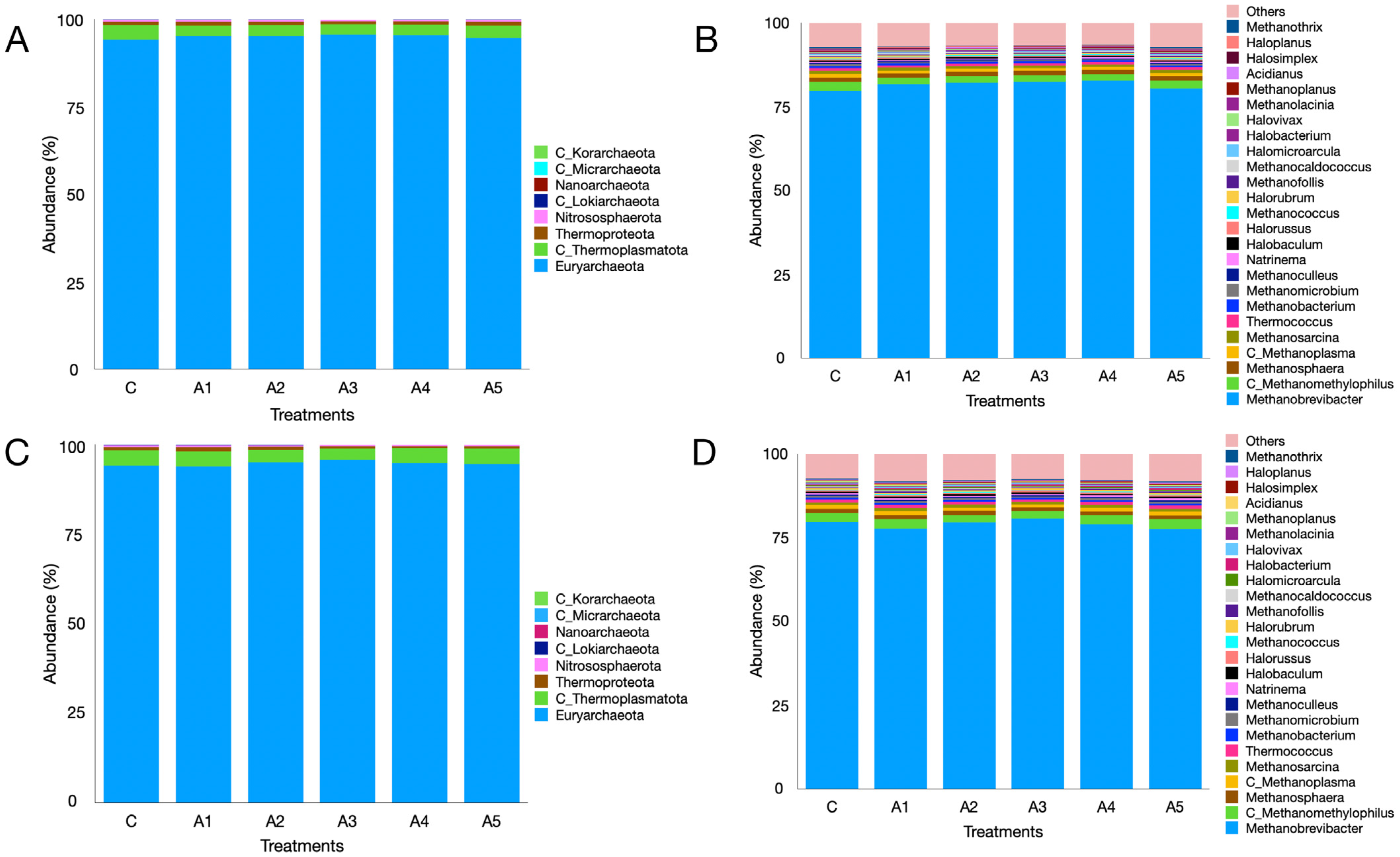
3.2.7. Microbial Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EPA Global Methane Initiative. Importance of Methane. 2023. Available online: https://www.epa.gov/gmi/learn-about-global-methane-initiative (accessed on 19 September 2023).
- Friedlingstein, P.; O’sullivan, M.; Jones, M.W.; Andrew, R.M.; Hauck, J.; Olsen, A.; Peters, G.P.; Peters, W.; Pongratz, J.; Sitch, S.; et al. Global Carbon Budget 2020. Earth Syst. Sci. Data Discuss. 2020, 12, 1–3. [Google Scholar] [CrossRef]
- Global Methane Hub. 2024. Available online: https://www.globalmethanehub.org (accessed on 6 November 2024).
- Bhatta, R. Issue of Enteric Methane Emissions from Indian Livestock. Curr. Sci. 2023, 125, 227–228. [Google Scholar]
- Bhatta, R.; Malik, P.K.; Sejian, V. Enteric Methane Emission and Reduction Strategies in Sheep. In Sheep Production Adapting to Climate Change; Sejian, V., Bhatta, R., Gaughan, J., Malik, P.K., Naqvi, S.M.K., Lal, R., Eds.; Springer Nature: Singapore, 2017; pp. 291–305. [Google Scholar]
- Guan, H.; Wittenberg, K.M.; Ominski, K.H.; Krause, D.O. Efficacy of Ionophores in Cattle Diets for Mitigation of Enteric Methane. J. Anim. Sci. 2006, 84, 1896–1906. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Ungerfeld, E.M.; Eckard, R.J.; Wang, M. Review: Fifty Years of Research on Rumen Methanogenesis: Lessons Learned and Future Challenges for Mitigation. Animal 2020, 14, S2–S16. [Google Scholar] [CrossRef]
- Arndt, C.; Hristov, A.N.; Price, W.J.; McClelland, S.C.; Pelaez, A.M.; Cueva, S.F.; Oh, J.; Dijkstra, J.; Bannink, A.; Bayat, A.R.; et al. Full Adoption of the Most Effective Strategies to Mitigate Methane Emissions by Ruminants Can Help Meet the 1.5 °C Target by 2030 but Not 2050. Proc. Natl. Acad. Sci. USA 2022, 119, e2111294119. Available online: https://www.pnas.org/doi/10.1073/pnas.2111294119 (accessed on 20 October 2024). [CrossRef] [PubMed]
- Herrero, M.; Henderson, B.; Havlík, P.; Thornton, P.K.; Conant, R.T.; Smith, P.; Wirsenius, S.; Hristov, A.N.; Gerber, P.; Gill, M.; et al. Greenhouse Gas Mitigation Potentials in the Livestock Sector. Nat. Clim. Chang. 2016, 6, 452–461. [Google Scholar] [CrossRef]
- DOF, G. Seaweed Cultivation. 2020. Available online: https://dof.gov.in/sites/default/files/2020-07/Seaweed_Cultivation.pdf (accessed on 20 October 2024).
- De Bhowmick, G.; Hayes, M. Potential of Seaweeds to Mitigate Production of Greenhouse Gases during Production of Ruminant Proteins. Glob. Chall. 2023, 7, 2200145. [Google Scholar] [CrossRef]
- Mohapatra, A.; Trivedi, S.; Kolte, A.P.; Tejpal, C.S.; Elavarasan, K.; Vaswani, S.; Malik, P.K.; Ravishankar, C.N.; Bhatta, R. Effect of Padina Gymnospora Biowaste Inclusion on In Vitro Methane Production, Feed Fermentation, and Microbial Diversity. Front. Microbiol. 2024, 15, 1431131. [Google Scholar] [CrossRef]
- McGurrin, A.; Maguire, J.; Tiwari, B.K.; Garcia-Vaquero, M. Anti-Methanogenic Potential of Seaweeds and Seaweed-Derived Compounds in Ruminant Feed: Current Perspectives, Risks and Future Prospects. J. Anim. Sci. Biotechnol. 2023, 14, 1–27. [Google Scholar] [CrossRef]
- Liu, Q.; Lei, S.; Zhao, M.; Li, M.; Cong, Y.; Fang, K.; Gao, X.; Zhang, L.; Zhu, C.; Zheng, L.; et al. Potential to Reduce Methane Production of Using Cultivated Seaweeds Supplementation to Reshape the Community Structure of Rumen Microorganisms. Environ. Res. 2024, 259, 119458. [Google Scholar] [CrossRef]
- Wasson, D.E.; Yarish, C.; Hristov, A.N. Enteric Methane Mitigation through Asparagopsis Taxiformis Supplementation and Potential Algal Alternatives. Front. Anim. Sci. 2022, 3, 999338. [Google Scholar] [CrossRef]
- Machado, L.; Magnusson, M.; Paul, N.A.; Kinley, R.; de Nys, R.; Tomkins, N. Identification of Bioactives from the Red Seaweed Asparagopsis Taxiformis That Promote Antimethanogenic Activity In Vitro. J. Appl. Phycol. 2016, 28, 3117–3126. [Google Scholar] [CrossRef]
- Maia, M.R.G.; Fonseca, A.J.M.; Oliveira, H.M.; Mendonça, C.; Cabrita, A.R.J. The Potential Role of Seaweeds in the Natural Manipulation of Rumen Fermentation and Methane Production. Sci. Rep. 2016, 6, 32321. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, S.J.; Kim, H.S.; Eom, J.S.; Jo, S.U.; Guan, L.L.; Park, T.; Seo, J.; Lee, Y.; Bae, D.; et al. Red Seaweed Extracts Reduce Methane Production by Altering Rumen Fermentation and Microbial Composition In Vitro. Front. Vet. Sci. 2022, 9, 985824. [Google Scholar] [CrossRef]
- Association of Officiating Analytical Chemists. AOAC Official Method of Analysis; Association of Officiating Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- ICAR Nutrient Requirements of Cattle and Buffalo. Nutrient Requirements of Animals; Indian Council of Agricultural Research: New Delhi, India, 2013. [Google Scholar]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The Estimation of the Digestibility and Metabolizable Energy Content of Ruminant Feeding Stuffs from the Gas Production When They Are Incubated with Rumen Liquor In Vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Malik, P.K.; Uyeno, Y.; Kolte, A.P.; Kumar, R.; Trivedi, S.; Bhatta, R. Screening of Phyto-Sources from Foothill of Himalayan Mountain for Livestock Methane Reduction. SN Appl. Sci. 2019, 1, 232. [Google Scholar] [CrossRef]
- Malik, P.K.; Trivedi, S.; Mohapatra, A.; Kolte, A.P.; Mech, A.; Victor, T.; Ahasic, E.; Bhatta, R. Oat Brewery Waste Decreased Methane Production and Alters Rumen Fermentation, Microbiota Composition, and CAZymes Profiles. Microorganisms 2024, 12, 1475. [Google Scholar] [CrossRef]
- Malik, P.K.; Trivedi, S.; Kolte, A.P.; Mohapatra, A.; Bhatta, R.; Rahman, H. Effect of an Anti-Methanogenic Supplement on Enteric Methane Emission, Fermentation, and Whole Rumen Metagenome in Sheep. Front. Microbiol. 2022, 13, 1048288. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.K.; Trivedi, S.; Kolte, A.P.; Mohapatra, A.; Biswas, S.; Bhattar, A.V.K.; Bhatta, R.; Rahman, H. Comparative Analysis of Rumen Metagenome, Metatranscriptome, Fermentation and Methane Yield in Cattle and Buffaloes Fed on the Same Diet. Front. Microbiol. 2023, 14, 1266025. [Google Scholar] [CrossRef] [PubMed]
- Filípek, J.; Dvořák, R. Determination of the Volatile Fatty Acid Content in the Rumen Liquid: Comparison of Gas Chromatography and Capillary Isotachophoresis. Acta Vet. Brno 2009, 78, 627–633. [Google Scholar] [CrossRef]
- Conway, E.J. Microdiffusion Analysis and Volumetric Error, 4th ed.; Crosby Lockwood and Son Ltd.: London, UK, 1957. [Google Scholar]
- Malik, P.K.; Trivedi, S.; Mohapatra, A.; Kolte, A.P.; Sejian, V.; Bhatta, R.; Rahman, H. Comparison of Enteric Methane Yield and Diversity of Ruminal Methanogens in Cattle and Buffaloes Fed on the Same Diet. PLoS ONE 2021, 16, e0256048. [Google Scholar] [CrossRef]
- Malik, P.K.; Trivedi, S.; Kolte, A.P.; Sejian, V.; Bhatta, R.; Rahman, H. Diversity of Rumen Microbiota Using Metagenome Sequencing and Methane Yield in Indian Sheep Fed on Straw and Concentrate Diet. Saudi J. Biol. Sci. 2022, 29, 103345. [Google Scholar] [CrossRef]
- Hungate, R.E. The Rumen and Its Microbes; Academic Press Inc.: New York, NY, USA, 1966. [Google Scholar]
- Kamra, D.N.; Agarwal, N. Rumen Protozoa. In Techniques in Rumen Microbiology; Centre of Advance Studies in Animal Nutrition, Indian Veterinary Research Institute, Izatnagar: Bareilly, India, 2003; pp. 35–45. [Google Scholar]
- Yu, Z.; Morrison, M. Improved Extraction of PCR-Quality Community DNA from Digesta and Fecal Samples. Biotechniques 2004, 36, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 8 November 2023).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Olson, R.D.; Assaf, R.; Brettin, T.; Conrad, N.; Cucinell, C.; Davis, J.J.; Dempsey, D.M.; Dickerman, A.; Dietrich, E.M.; Kenyon, R.W.; et al. Introducing the Bacterial and Viral Bioinformatics Resource Center (BV-BRC): A Resource Combining PATRIC, IRD and ViPR. Nucleic Acids Res. 2023, 51, D678–D689. [Google Scholar] [CrossRef]
- Wood, D.E.; Lu, J.; Langmead, B. Improved Metagenomic Analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef]
- Breitwieser, F.P.; Salzberg, S.L. Pavian: Interactive Analysis of Metagenomics Data for Microbiome Studies and Pathogen Identification. Bioinformatics 2020, 36, 1303–1304. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for Comprehensive Statistical, Functional, and Meta-Analysis of Microbiome Data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef]
- Paulson, J.N.; Colin Stine, O.; Bravo, H.C.; Pop, M. Differential Abundance Analysis for Microbial Marker-Gene Surveys. Nat. Methods 2013, 10, 1200–1202. [Google Scholar] [CrossRef] [PubMed]
- Morais, T.; Inácio, A.; Coutinho, T.; Ministro, M.; Cotas, J.; Pereira, L.; Bahcevandziev, K. Seaweed Potential in the Animal Feed: A Review. J. Mar. Sci. Eng. 2020, 8, 559. [Google Scholar] [CrossRef]
- Patel, N.; Banafarr, P.; Ramachandran, P.; Ghosh, A.; Johnson, B.; Dharani, G. Strategy for the Development of Seaweed Value Chain:Fostering Diversified Livelihoods; Government of India: New Delhi, India, 2024.
- Olsson, J.; Toth, G.B.; Albers, E. Biochemical Composition of Red, Green and Brown Seaweeds on the Swedish West Coast. J. Appl. Phycol. 2020, 32, 3305–3317. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Cervantes, J.; López-Hernández, J.; Paseiro-Losada, P. Fatty Acids, Total Lipid, Protein and Ash Contents of Processed Edible Seaweeds. Food Chem. 2004, 85, 439–444. [Google Scholar] [CrossRef]
- Hurd, C.L.; Harrison, P.J.; Bischof, K.; Lobban, C.S. Seaweed Ecology and Physiology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Widowati, L.L.; Budi Prayitno, S.; Rejeki, S.; Elfitasari, T.; Purnomo, P.W.; Ariyati, R.W.; Bosma, R.H.; Aubin, J. Organic Matter Reduction Using Four Densities of Seaweed (Gracilaria Verucosa) and Green Mussel (Perna Viridis) to Improve Water Quality for Aquaculture in Java, Indonesia. Aquat. Living Resour. 2021, 34, 5. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Hurd, C.L. Seaweed Nutrient Physiology: Application of Concepts to Aquaculture and Bioremediation. Phycologia 2019, 58, 552–562. [Google Scholar] [CrossRef]
- Kustantinah; Hidayah, N.; Noviandi, C.T.; Astuti, A.; Paradhipta, D.H.V. Nutrients Content of Four Tropical Seaweed Species from Kelapa Beach, Tuban, Indonesia and Their Potential as Ruminant Feed. Biodiversitas 2022, 23, 6191–6197. [Google Scholar] [CrossRef]
- Applegate, R.D.; Gray, P.B. Nutritional Value of Seaweed to Ruminants. Rangifer 1995, 15, 15–18. [Google Scholar] [CrossRef][Green Version]
- Jayasinghe, G.D.T.M.; Jinadasa, B.K.K.K.; Chinthaka, S.D.M. Nutritional Composition and Heavy Metal Content of Five Tropical Seaweeds. Open Sci. J. Anal. Chem. 2018, 3, 17–22. [Google Scholar]
- Fleurence, J.; Morançais, M.; Dumay, J. Seaweed Proteins. In Proteins in Food Processing; Yada, R.Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 245–262. [Google Scholar]
- Fleurence, J.L. Seaweed Proteins: Biochemical, Nutritional Aspects and Potential Uses. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- GOI. 20th Livestock Census: Provisional Key Results; Department of Animal Husbandry and Dairying, Ministry of Fisheries, Animal Husbandry & Dairying, Govt of India: New Delhi, India, 2019.
- Bhatta, R.; Uyeno, Y.; Tajima, K.; Takenaka, A.; Yabumoto, Y.; Nonaka, I.; Enishi, O.; Kurihara, M. Difference in the Nature of Tannins on In Vitro Ruminal Methane and Volatile Fatty Acid Production and on Methanogenic Archaea and Protozoal Populations. J. Dairy Sci. 2009, 92, 5512–5522. [Google Scholar] [CrossRef]
- Gemeda, B.S.; Hassen, A. Effect of Tannin and Species Variation on In Vitro Digestibility, Gas, and Methane Production of Tropical Browse Plants. Asian-Australas J. Anim. Sci. 2015, 28, 188–199. [Google Scholar] [CrossRef]
- Tan, H.Y.; Sieo, C.C.; Abdullah, N.; Liang, J.B.; Huang, X.D.; Ho, Y.W. Effects of Condensed Tannins from Leucaena on Methane Production, Rumen Fermentation and Populations of Methanogens and Protozoa In Vitro. Anim. Feed. Sci. Technol. 2011, 169, 185–193. [Google Scholar] [CrossRef]
- Malik, P.K.; Kolte, A.P.; Baruah, L.; Saravanan, M.; Bakshi, B.; Bhatta, R. Enteric Methane Mitigation in Sheep through Leaves of Selected Tanniniferous Tropical Tree Species. Livest. Sci. 2017, 200, 29–34. [Google Scholar] [CrossRef]
- Malik, P.K.; Kolte, A.P.; Bakshi, B.; Baruah, L.; Dhali, A.; Bhatta, R. Effect of Tamarind Seed Husk Supplementation on Ruminal Methanogenesis, Methanogen Diversity and Fermentation Characteristics. Carbon Manag. 2017, 8, 319–329. [Google Scholar] [CrossRef]
- Malik, P.K.; Singhal, K.K. Influence of Lucerne Fodder Supplementation on Enteric Methane Emission in Crossbred Calves. Indian J. Anim. Sci. 2008, 78, 293. [Google Scholar]
- Malik, P.K.; Singhal, K.K.; Deshpande, S.B. Effect of Saponin Rich Lucerne Fodder Supplementation on Rumen Fermentation, Bacterial and Protozoal Population in Buffalo Bulls. Indian J. Anim. Sci. 2009, 79, 912–916. Available online: https://epubs.icar.org.in/index.php/IJAnS/article/view/2686/800 (accessed on 20 October 2024).
- Malik, P.K.; Singhal, K.K.; Ahlawat, A.; Deshpande, S.B. Effect of Berseem Fodder Supplementation to Wheat Straw Based Diet on In Vitro Total Gas and Methane Production and Fermentation Pattern. Indian J. Anim. Sci. 2010, 80, 551. [Google Scholar]
- Tseten, T.; Sanjorjo, R.A.; Kwon, M.; Kim, S.W. Strategies to Mitigate Enteric Methane Emissions from Ruminant Animals. J. Microbiol. Biotechnol. 2022, 32, 269–277. [Google Scholar] [CrossRef]
- Maweu, A.N.; Bebe, B.O.; Kuria, S.G.; Kashongwe, O.B. In-Vitro Digestibility and Methane Gas Emission of Indigenous and Introduced Grasses in the Rangeland Ecosystems of South Eastern Kenya. Reg. Environ. Chang. 2024, 24, 1–9. [Google Scholar] [CrossRef]
- Chiquettei, J.; Cheng, K.J.; Costerton, J.W.; Milligan, L.P. Effect of Tannins on the Digestibility of Two Isosynthetic Strains of Birdsfoot Trefoil (Lotus corniculatus L.) Using In Vitro and in Sacco Techniques. Cannadian J. Anim. Sci. 1988, 68, 751–760. [Google Scholar] [CrossRef]
- Carulla, J.E.; Kreuzer, M.; Machmüller, A.; Hess, H.D. Supplementation of Acacia Mearnsii Tannins Decreases Methanogenesis and Urinary Nitrogen in Forage-Fed Sheep. Aust. J. Agric. Res. 2005, 56, 961–970. [Google Scholar] [CrossRef]
- Tavendale, M.H.; Meagher, L.P.; Pacheco, D.; Walker, N.; Attwood, G.T.; Sivakumaran, S. Methane Production from In Vitro Rumen Incubations with Lotus Pedunculatus and Medicago Sativa, and Effects of Extractable Condensed Tannin Fractions on Methanogenesis. Anim. Feed. Sci. Technol. 2005, 123–124, 403–419. [Google Scholar] [CrossRef]
- Newbold, C.J.; De la Fuente, G.; Belanche, A.; Ramos-Morales, E.; McEwan, N.R. The Role of Ciliate Protozoa in the Rumen. Front. Microbiol. 2015, 6, 1313. [Google Scholar] [CrossRef]
- Williams, A.G.; Coleman, G.S. Role of Protozoa in the Rumen. In The Rumen Protozoa; Springer: New York, NY, USA, 1992; pp. 317–347. ISBN 978-1-4612-7664-7. [Google Scholar]
- Park, T.; Mao, H.; Yu, Z. Inhibition of Rumen Protozoa by Specific Inhibitors of Lysozyme and Peptidases In Vitro. Front. Microbiol. 2019, 10, 2822. [Google Scholar] [CrossRef]
- Belanche, A.; Abecia, L.; Holtrop, G.; Guada, J.A.; Castrillo, C.; De La Fuente, G.; Balcells, J. Study of the Effect of Presence or Absence of Protozoa on Rumen Fermentation and Microbial Protein Contribution to the Chyme. J. Anim. Sci. 2011, 89, 4163–4174. [Google Scholar] [CrossRef][Green Version]
- Baruah, L.; Malik, P.K.; Kolte, A.P.; Goyal, P.; Dhali, A.; Bhatta, R. Rumen Methane Amelioration in Sheep Using Two Selected Tanniferous Phyto-Leaves. Carbon Manag. 2019, 10, 299–308. [Google Scholar] [CrossRef]
- Poornachandra, K.T.; Malik, P.K.; Dhali, A.; Kolte, A.P.; Bhatta, R. Effect of Combined Supplementation of Tamarind Seed Husk and Soapnut on Enteric Methane Emission in Crossbred Cattle. Carbon Manag. 2019, 10, 465–475. [Google Scholar] [CrossRef]
- Belanche, A.; De la Fuente, G.; Moorby, J.M.; Newbold, C.J. Bacterial Protein Degradation by Different Rumen Protozoal Groups. J. Anim. Sci. 2012, 90, 4495–4504. [Google Scholar] [CrossRef] [PubMed]
- Michaiowski, T. Rumen Protozoa in the Growing Domestic Ruminant. In Biology of Growing Animals; Holzapfel, W.H., Naughton, P.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 2, pp. 54–74. [Google Scholar]
- Kumar, N.J.; Kumar, R.; Patel, K.; Viyol, S.; Bhoi, R. Nutrient Composition and Calorific Value of Some Seaweeds from Bet Dwarka, West Coast of Gujarat, India. Our Nat. 2009, 7, 18–25. [Google Scholar]
- Yang, Y.; Zhang, M.; Alalawy, A.I.; Almutairi, F.M.; Al-Duais, M.A.; Wang, J.; Salama, E.S. Identification and Characterization of Marine Seaweeds for Biocompounds Production. Environ. Technol. Innov. 2021, 24, 101848. [Google Scholar] [CrossRef]
- van Zijderveld, S.M.; Gerrits, W.J.J.; Apajalahti, J.A.; Newbold, J.R.; Dijkstra, J.; Leng, R.A.; Perdok, H.B. Nitrate and Sulfate: Effective Alternative Hydrogen Sinks for Mitigation of Ruminal Methane Production in Sheep. J. Dairy Sci. 2010, 93, 5856–5866. [Google Scholar] [CrossRef] [PubMed]
- National Academy of Sciences Engineering and Medicine. Nutrient Requirement of Beef Cattle, 8th ed.; National Academy Press: Washington, DC, USA, 2016. [Google Scholar]
- Uniyal, S.; Chaudhary, L.C.; Kala, A.; Agarwal, N.; Kamra, D.N. Effect of Sulphur Supplementation on Methane Emission, Energy Partitioning and Nutrient Utilization in Goats. Anim. Nutr. Feed. Technol. 2020, 20, 111–119. [Google Scholar] [CrossRef]
- Li, L.; Silveira, C.I.; Nolan, J.V.; Godwin, I.R.; Leng, R.A.; Hegarty, R.S. Effect of Added Dietary Nitrate and Elemental Sulfur on Wool Growth and Methane Emission of Merino Lambs. Anim. Prod. Sci. 2013, 53, 1195–1201. [Google Scholar] [CrossRef]
- Silivong, P.; Preston, T.R.; Leng, R.A. Effect of Sulphur and Calcium Nitrate on Methane Production by Goats Fed a Basal Diet of Molasses Supplemented with Mimosa (Mimosa Pigra) Foliage. Livest. Res. Rural. Dev. 2011, 23, 2011. [Google Scholar]
- Ungerfeld, E.M. Shifts in Metabolic Hydrogen Sinks in the Methanogenesis-Inhibited Ruminal Fermentation: A Meta-Analysis. Front. Microbiol. 2015, 6, 1–17. [Google Scholar] [CrossRef]
- Kohn, R.A. Thermodynamic and Kinetic Control of Methane Emission from Ruminants. In Livestock Production and Climate Change; Malik, P.K., Bhatta, R., Takahasi, J., Kohn, R.A., Prasad, C.S., Eds.; CABI: Wallingford, UK, 2015; pp. 245–262. ISBN 978-1-78064-432-5. [Google Scholar]
- Paul, S.S.; Deb, S.M.; Singh, D. Isolation and Characterization of Novel Sulphate-Reducing Fusobacterium Sp. and Their Effects on In Vitro Methane Emission and Digestion of Wheat Straw by Rumen Fluid from Indian Riverine Buffaloes. Anim. Feed. Sci. Technol. 2011, 166–167, 132–140. [Google Scholar] [CrossRef]
- Tanji, Y.; Toyama, K.; Hasegawa, R.; Miyanaga, K. Biological Souring of Crude Oil under Anaerobic Conditions. Biochem. Eng. J. 2014, 90, 114–120. [Google Scholar] [CrossRef]
- Liamleam, W.; Annachhatre, A.P. Electron Donors for Biological Sulfate Reduction. Biotechnol. Adv. 2007, 25, 452–463. [Google Scholar] [CrossRef]
- Jami, E.; Mizrahi, I. Composition and Similarity of Bovine Rumen Microbiota across Individual Animals. PLoS ONE 2012, 7, e33306. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for Livestock Diets: A Review. Anim. Feed. Sci. Technol. 2016, 212, 1–17. [Google Scholar] [CrossRef]

| Seaweed | Composition (% DM) | |||||||
|---|---|---|---|---|---|---|---|---|
| OM | CP | CF | EE | Ash | GE * | Tannins | Saponins | |
| KA | 60.4 | 3.25 | 4.68 | 0.26 | 39.6 | 8.56 | 8.58 | 8.04 |
| SF | 54.9 | 12.7 | 4.76 | 0.73 | 45.1 | 11.6 | 3.47 | 6.41 |
| AS | 56.0 | 15.3 | 5.47 | 0.28 | 44.0 | 8.40 | 9.46 | 4.92 |
| SW | 69.8 | 6.91 | 8.97 | 0.35 | 30.2 | 9.54 | 7.91 | 5.36 |
| PG | 56.3 | 6.40 | 6.49 | 0.07 | 43.7 | 6.96 | 6.20 | 7.14 |
| Attributes | Seaweeds | ||||
|---|---|---|---|---|---|
| KA | SF | As | SW | PG | |
| Minerals | |||||
| Ca (%) | 0.340 | 1.98 | 0.850 | 1.55 | 4.81 |
| P (%) | 0.021 | 0.062 | 0.086 | 0.050 | 0.052 |
| Mg (%) | 0.210 | 0.860 | 0.930 | 0.780 | 2.50 |
| Fe (%) | 0.013 | 0.003 | 0.022 | 0.011 | 0.052 |
| Zn (mg/kg) | 15.6 | 31.9 | 10.2 | 4.92 | 7.25 |
| Cu (mg/kg) | 3.54 | 1.77 | 385 | 3.88 | 1.85 |
| I (mg/kg) | 51.9 | 137 | 24.9 | 279 | 38.9 |
| Treatments | Composition (% DM) | ||||
|---|---|---|---|---|---|
| OM | CP | CF | EE | Ash | |
| Control (C) | 92.3 | 11.5 | 18.3 | 0.979 | 7.74 |
| KA-based | |||||
| A1 | 91.5 | 11.8 | 18.7 | 0.877 | 8.47 |
| A2 | 91.2 | 12.8 | 19.3 | 0.956 | 8.82 |
| A3 | 90.9 | 12.9 | 19.9 | 0.969 | 9.10 |
| A4 | 90.5 | 13.6 | 21.0 | 1.09 | 9.52 |
| A5 | 89.8 | 13.7 | 21.2 | 1.37 | 10.2 |
| SW-based | |||||
| A1 | 91.4 | 14.2 | 18.5 | 1.14 | 8.61 |
| A2 | 91.3 | 14.8 | 18.7 | 1.16 | 8.67 |
| A3 | 91.0 | 15.3 | 19.7 | 1.19 | 8.97 |
| A4 | 90.7 | 16.2 | 20.2 | 1.84 | 9.30 |
| A5 | 90.5 | 16.4 | 21.9 | 1.90 | 9.49 |
| Source/Attributes | Treatments | SEM | p | |||||
|---|---|---|---|---|---|---|---|---|
| C | A1 | A2 | A3 | A4 | A5 | |||
| KA | ||||||||
| TG (mL/200 mg DM) | 45.2 a | 43.9 a | 43.3 a | 42.5 a | 41.1 a | 35.7 b | 1.37 | <0.0001 |
| CH4 (mL/200 mg DM) | 8.58 a | 7.30 a | 6.78 a | 5.93 b | 4.96 b | 5.05 b | 0.572 | 0.0008 |
| IVDMD (%) | 55.2 | 53.4 | 53.6 | 54.6 | 54.5 | 54.5 | 0.276 | 0.8990 |
| IVOMD (%) | 55.4 | 53.1 | 53.3 | 54.1 | 53.9 | 53.5 | 0.339 | 0.4893 |
| CH4 (mL/g dig. DM) | 77.7 a | 68.9 ab | 63.4 ab | 54.1 ab | 45.6 b | 46.0 b | 5.28 | 0.0012 |
| CH4 (mL/g dig. OM) | 83.9 a | 75.7 ab | 70.0 ab | 60.1 ab | 51.0 b | 52.6 b | 5.38 | 0.0033 |
| Ammonia-N (mg/dL) | 14.2 | 13.5 | 13.3 | 12.4 | 15.1 | 14.5 | 0.393 | 0.2281 |
| SW | ||||||||
| TG (mL/200 mg DM) | 40.8 a | 42.5 a | 40.4 a | 38.7 a | 37.3 ab | 31.4 b | 1.60 | 0.0001 |
| CH4 (mL/200 mg DM) | 7.20 a | 6.40 ab | 5.86 ab | 5.10 ab | 4.61 bc | 3.76 c | 0.510 | 0.0038 |
| IVDMD (%) | 53.5 | 53.4 | 51.9 | 52.1 | 52.5 | 52.3 | 0.276 | 0.3140 |
| IVOMD (%) | 53.6 | 53.1 | 51.5 | 51.6 | 52.0 | 51.5 | 0.372 | 0.6828 |
| CH4 (mL/g dig. DM) | 67.3 a | 60.1 ab | 56.3 ab | 48.8 ab | 43.7 ab | 36.2 b | 4.64 | 0.0056 |
| CH4 (mL/g dig. OM) | 72.8 a | 66.0 ab | 62.2 ab | 54.0 ab | 48.6 ab | 40.5 b | 4.86 | 0.0107 |
| Ammonia-N (mg/dL) | 16.3 | 14.7 | 13.7 | 14.9 | 15.4 | 14.9 | 0.349 | 0.0692 |
| TG (mL/200 mg) source x levels | Source p < 0.0001 | Levels p < 0.0001 | Interaction p = 0.767 | |||||
| CH4 (mL/200 mg) source x levels | Source p = 0.196 | Levels p = 0.243 | Interaction p = 0.022 | |||||
| Source/Attributes | Treatments | SEM | p | |||||
|---|---|---|---|---|---|---|---|---|
| C | A1 | A2 | A3 | A4 | A5 | |||
| KA | ||||||||
| TVFA | 35.7 a | 35.4 a | 38.7 ab | 42.1 c | 42.2 c | 39.6 bc | 1.21 | <0.0001 |
| Acetate | 14.5 a | 15.3 a | 16.8 b | 18.2 c | 18.3 c | 17.6 bc | 0.643 | <0.0001 |
| Propionate | 10.4 b | 8.72 a | 9.56 c | 10.3 b | 9.37 bc | 9.56 c | 0.255 | <0.0001 |
| Butyrate | 7.75 a | 8.38 ab | 9.06 bc | 10.0 c | 10.1 c | 9.33 bc | 0.374 | <0.0001 |
| Iso-butyrate | 0.814 ab | 0.605 a | 0.885 b | 1.17 c | 1.11 c | 0.900 b | 0.084 | <0.0001 |
| Valerate | 1.72 | 1.82 | 1.83 | 1.86 | 1.84 | 1.79 | 0.020 | 0.0670 |
| Isovalerate | 0.421 | 0.511 | 0.557 | 0.514 | 0.637 | 0.557 | 0.029 | 0.0097 |
| SW | ||||||||
| TVFA | 29.1 b | 29.6 b | 28.8 ab | 26.0 a | 27.1 ab | 28.1 ab | 0.552 | 0.0058 |
| Acetate | 15.5 | 16.2 | 16.2 | 14.3 | 15.1 | 15.8 | 0.298 | 0.1062 |
| Propionate | 7.14 | 7.19 | 7.02 | 6.51 | 7.05 | 7.24 | 0.108 | 0.0794 |
| Butyrate | 2.72 b | 2.54 ab | 2.37 ab | 2.20 a | 2.32 ab | 2.46 ab | 0.074 | 0.0073 |
| Iso-butyrate | 1.89 b | 1.83 ab | 1.53 ab | 1.38 a | 1.55 ab | 1.70 ab | 0.080 | 0.0153 |
| Valerate | 1.60 b | 1.50 ab | 1.40 ab | 1.30 a | 1.37 ab | 1.46 ab | 0.043 | 0.0073 |
| Isovalerate | 0.145 | .0282 | 0.262 | 0.239 | 0.231 | 0.293 | 0.040 | 0.0630 |
| Source/Attributes | Treatments | SEM | p | |||||
|---|---|---|---|---|---|---|---|---|
| C | A1 | A2 | A3 | A4 | A5 | |||
| KA | ||||||||
| Total protozoa (×107 cells/mL) | 8.69 c | 8.37 bc | 8.15 bc | 7.59 ab | 7.70 ab | 6.99 a | 0.2496 | 0.0001 |
| Entodinomorphs (×107 cells/mL) | 8.65 c | 8.33 bc | 8.12 bc | 7.55 ab | 7.66 ab | 6.94 a | 0.2512 | <0.0001 |
| Holotrichs (×106 cells/mL) | 0.312 | 0.350 | 0.390 | 0.382 | 0.435 | 0.375 | 0.0170 | 0.9239 |
| SW | ||||||||
| Total protozoa (×107 cells/mL) | 8.69 a | 7.85 ac | 7.50 c | 7.05 cd | 6.40 bd | 5.53 b | 0.4538 | <0.0001 |
| Entodinomorphs (×107 cells/mL) | 8.65 a | 7.81 ab | 7.64 b | 6.99 bc | 6.36 cd | 5.48 d | 0.4605 | <0.0001 |
| Holotrichs (×106 cells/mL) | 0.312 | 0.365 | 0.375 | 0.620 | 0.422 | 0.490 | 0.0450 | 0.1792 |
| Comparison (source, levels) | ||||||||
| Total protozoa | NS | NS | NS | NS | * | * | Source < 0.0001; levels < 0.0001; interaction 0.008 | |
| Entodinomorphs | NS | NS | NS | NS | * | * | Source < 0.0001; levels < 0.0001; interaction 0.008 | |
| Holotrichs | NS | NS | NS | NS | NS | NS | Source 0.2362; levels 0.2786; interaction 0.5791 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, P.K.; Kolte, A.P.; Trivedi, S.; Tamilmani, G.; Mohapatra, A.; Vaswani, S.; Belevendran, J.; Sahoo, A.; Gopalakrishnan, A.; Bhatta, R. Anti-Methanogenic Potential of Seaweeds and Impact on Feed Fermentation and Rumen Microbiome In Vitro. Microorganisms 2025, 13, 123. https://doi.org/10.3390/microorganisms13010123
Malik PK, Kolte AP, Trivedi S, Tamilmani G, Mohapatra A, Vaswani S, Belevendran J, Sahoo A, Gopalakrishnan A, Bhatta R. Anti-Methanogenic Potential of Seaweeds and Impact on Feed Fermentation and Rumen Microbiome In Vitro. Microorganisms. 2025; 13(1):123. https://doi.org/10.3390/microorganisms13010123
Chicago/Turabian StyleMalik, Pradeep Kumar, Atul Purshottam Kolte, Shraddha Trivedi, Govindan Tamilmani, Archit Mohapatra, Shalini Vaswani, Johnson Belevendran, Artabandhu Sahoo, Achamveetil Gopalakrishnan, and Raghavendra Bhatta. 2025. "Anti-Methanogenic Potential of Seaweeds and Impact on Feed Fermentation and Rumen Microbiome In Vitro" Microorganisms 13, no. 1: 123. https://doi.org/10.3390/microorganisms13010123
APA StyleMalik, P. K., Kolte, A. P., Trivedi, S., Tamilmani, G., Mohapatra, A., Vaswani, S., Belevendran, J., Sahoo, A., Gopalakrishnan, A., & Bhatta, R. (2025). Anti-Methanogenic Potential of Seaweeds and Impact on Feed Fermentation and Rumen Microbiome In Vitro. Microorganisms, 13(1), 123. https://doi.org/10.3390/microorganisms13010123






