Effect of Two Selected Levels of Padina gymnospora Biowaste and Enteric Methane Emission, Nutrient Digestibility, and Rumen Metagenome in Growing Sheep
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Seaweed and Biowaste
2.2. Ethical Approval, Animals, Feeding, and Management
2.3. Chemical Composition
2.4. CH4 Measurement
2.5. Nutrient Intake and Digestibility
2.6. Rumen Fermentation and Protozoa
2.7. Growth
2.8. Microbial Diversity
2.9. Statistical Analysis
3. Results
3.1. Chemical Composition
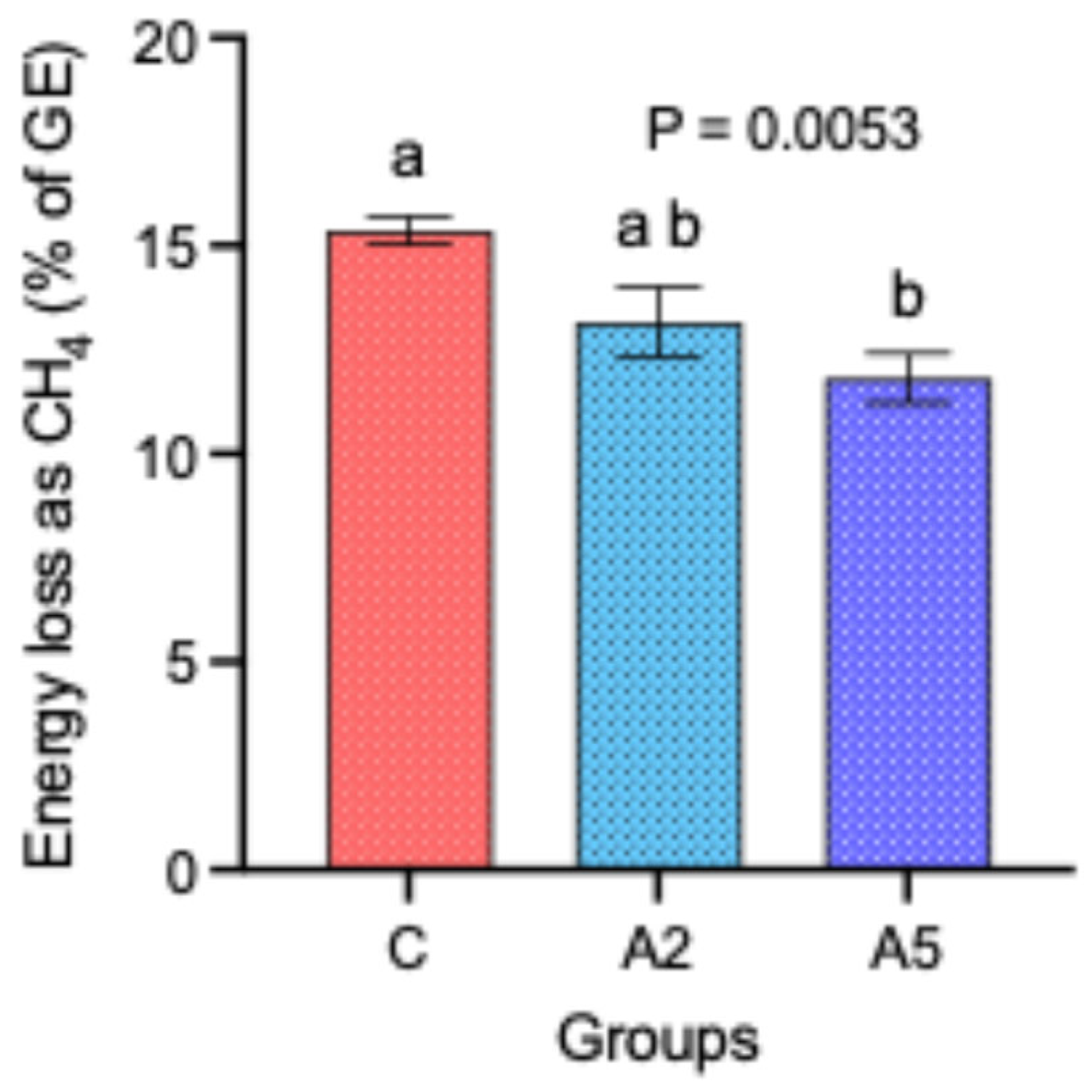
3.2. CH4 Emissions
3.3. Nutrient Intake and Digestibility
3.4. Rumen Fermentation and Protozoa
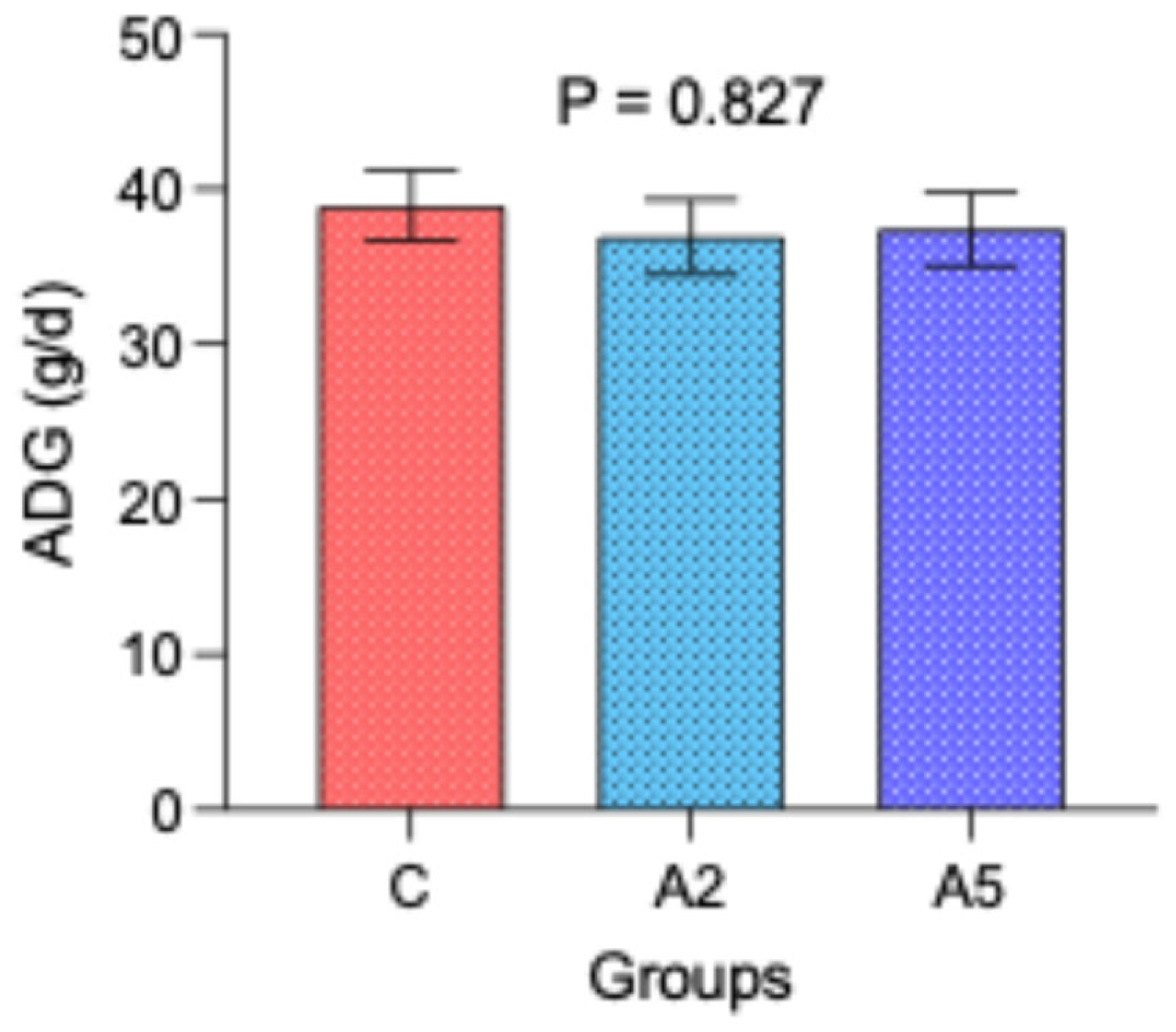
3.5. Growth
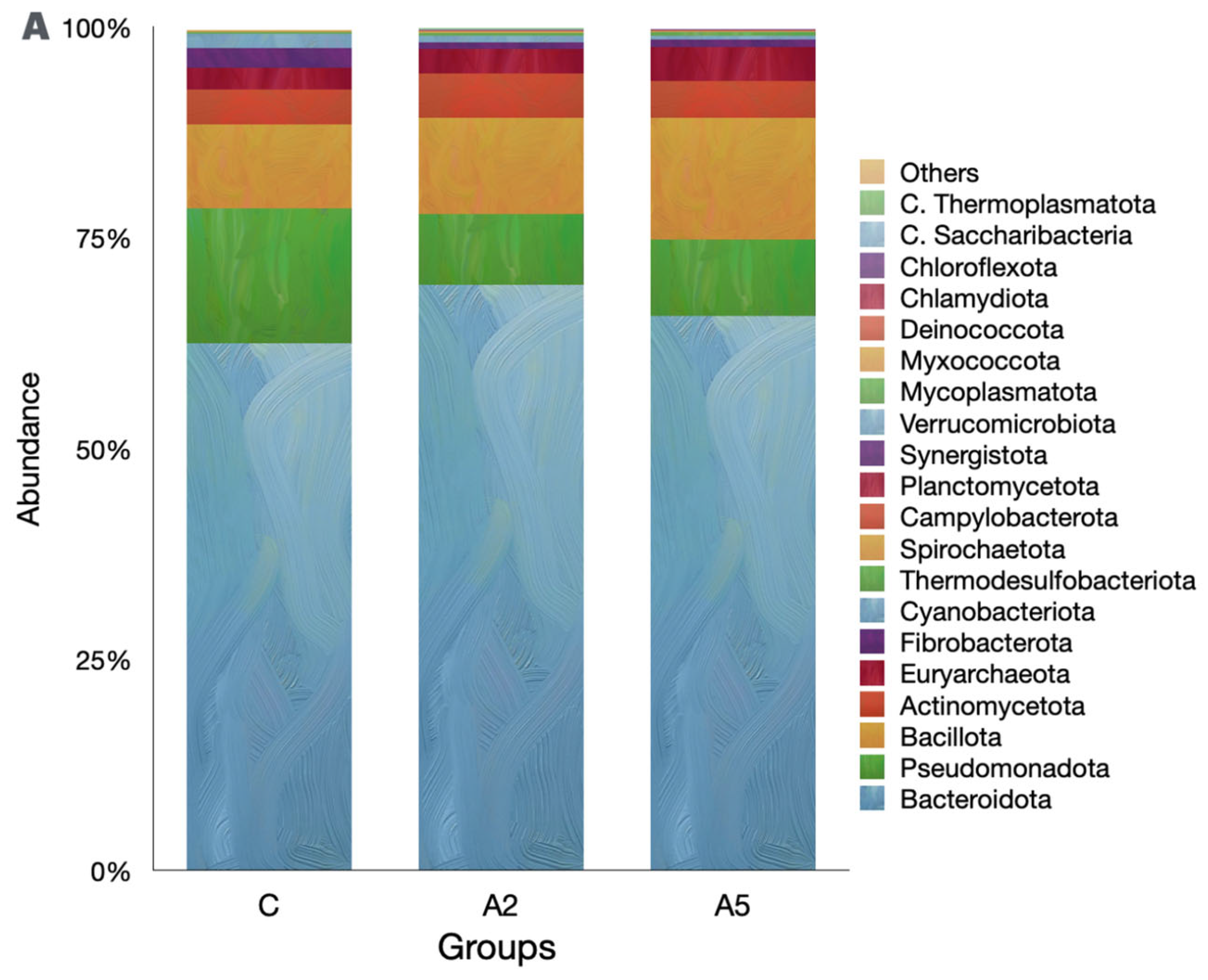
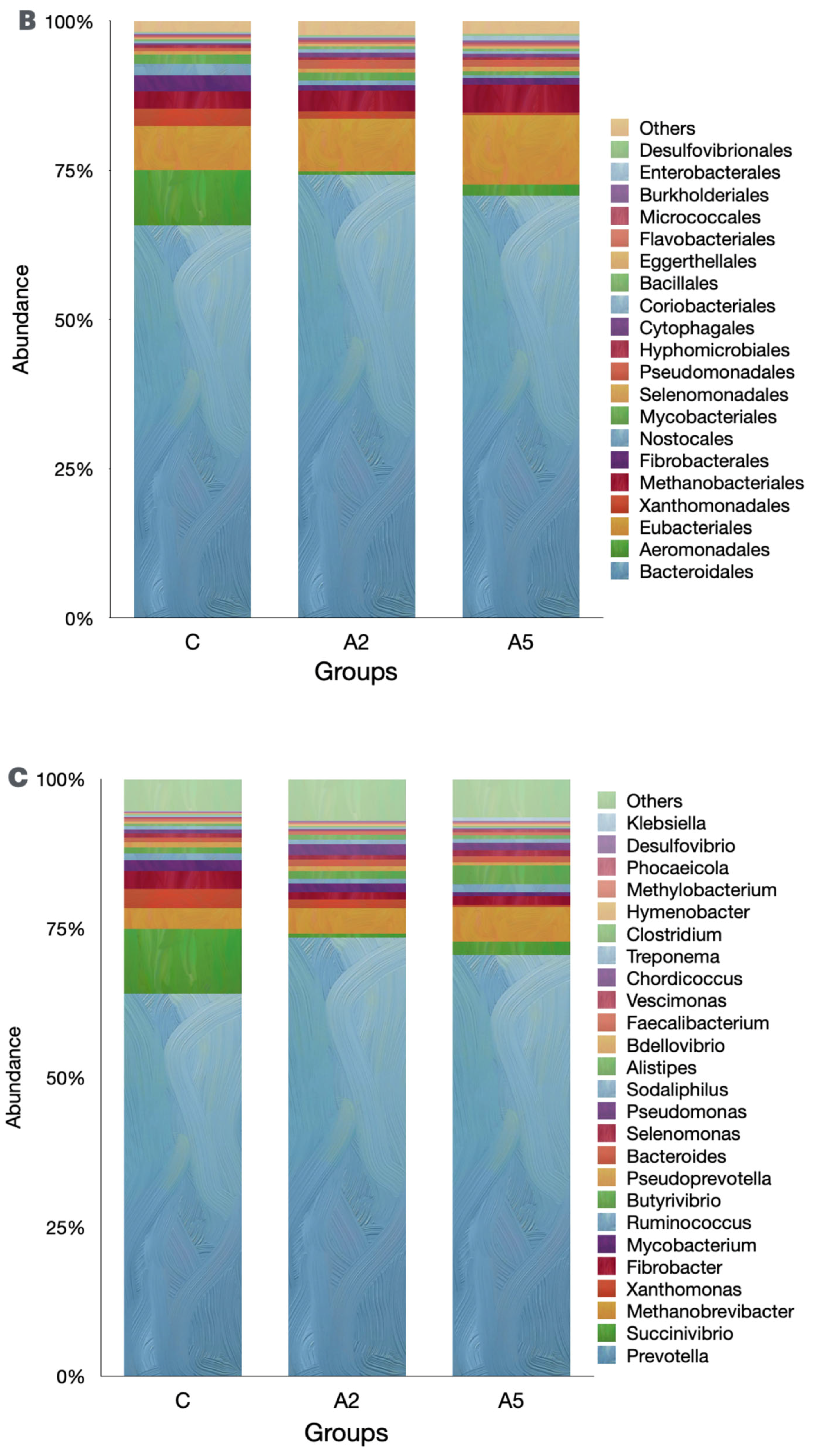
3.6. Microbial Diversity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NOAA. Increase in Atmospheric Methane Set Another Record During 2021. Available online: https://www.noaa.gov/news-release/increase-in-atmospheric-methane-set-another-record-during-2021 (accessed on 19 September 2023).
- IEA. Global Methane Tracker 2024; IEA: Paris, France, 2024. [Google Scholar]
- Jackson, R.B.; Saunois, M.; Bousquet, P.; Canadell, J.G.; Poulter, B.; Stavert, A.R.; Bergamaschi, P.; Niwa, Y.; Segers, A.; Tsuruta, A. Increasing Anthropogenic Methane Emissions Arise Equally from Agricultural and Fossil Fuel Sources. Environ. Res. Lett. 2020, 15, 071002. [Google Scholar]
- Saunois, M.; Stavert, A.R.; Poulter, B.; Bousquet, P.; Canadell, J.G.; Jackson, R.B.; Raymond, P.A.; Dlugokencky, E.J.; Houweling, S.; Patra, P.K.; et al. The Global Methane Budget 2000–2017. Earth Syst. Sci. Data 2020, 12, 1561–1623. [Google Scholar] [CrossRef]
- Chang, J.; Peng, S.; Ciais, P.; Saunois, M.; Dangal, S.R.S.; Herrero, M.; Havlík, P.; Tian, H.; Bousquet, P. Revisiting Enteric Methane Emissions from Domestic Ruminants and Their Δ13CCH4 Source Signature. Nat. Commun. 2019, 10, 3420. [Google Scholar] [CrossRef] [PubMed]
- van Lingen, H.J.; Niu, M.; Kebreab, E.; Valadares Filho, S.C.; Rooke, J.A.; Duthie, C.A.; Schwarm, A.; Kreuzer, M.; Hynd, P.I.; Caetano, M.; et al. Prediction of Enteric Methane Production, Yield and Intensity of Beef Cattle Using an Intercontinental Database. Agric. Ecosyst. Environ. 2019, 283, 106575. [Google Scholar] [CrossRef]
- Arndt, C.; Hristov, A.N.; Price, W.J.; McClelland, S.C.; Pelaez, A.M.; Cueva, S.F.; Oh, J.; Dijkstra, J.; Bannink, A.; Bayat, A.R.; et al. Full Adoption of the Most Effective Strategies to Mitigate Methane Emissions by Ruminants Can Help Meet the 1.5 °C Target by 2030 but Not 2050. Proc. Natl. Acad. Sci. USA 2022, 119, e2111294119. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Kreuzer, M.; O’Mara, F.; McAllister, T.A. Nutritional Management for Enteric Methane Abatement: A Review. Aust. J. Exp. Agric. 2008, 48, 21–27. [Google Scholar] [CrossRef]
- Duffield, T.F.; Rabiee, A.R.; Lean, I.J. A Meta-Analysis of the Impact of Monensin in Lactating Dairy Cattle. Part 2. Production Effects. J. Dairy Sci. 2008, 91, 1347–1360. [Google Scholar] [CrossRef]
- Bhatta, R.; Uyeno, Y.; Tajima, K.; Takenaka, A.; Yabumoto, Y.; Nonaka, I.; Enishi, O.; Kurihara, M. Difference in the Nature of Tannins on in Vitro Ruminal Methane and Volatile Fatty Acid Production and on Methanogenic Archaea and Protozoal Populations. J. Dairy Sci. 2009, 92, 5512–5522. [Google Scholar] [CrossRef]
- Malik, P.K.; Uyeno, Y.; Kolte, A.P.; Kumar, R.; Trivedi, S.; Bhatta, R. Screening of Phyto-Sources from Foothill of Himalayan Mountain for Livestock Methane Reduction. SN Appl. Sci. 2019, 1, 232. [Google Scholar] [CrossRef]
- Jayanegara, A.; Wina, E.; Takahashi, J. Meta-Analysis on Methane Mitigating Properties of Saponin-Rich Sources in the Rumen: Influence of Addition Levels and Plant Sources. Asian-Australas. J. Anim. Sci. 2014, 27, 1426–1435. [Google Scholar] [CrossRef]
- Malik, P.K.; Singhal, K.K. Saponin Content of Lucerne Fodder and Its Effect on Rumen Fermentation and Microbial Population in Crossbred Bulls. Indian J. Anim. Sci. 2008, 78, 298–301. [Google Scholar]
- Lomartire, S.; Marques, J.C.; Gonçalves, A.M.M. An Overview of the Alternative Use of Seaweeds to Produce Safe and Sustainable Bio-Packaging. Appl. Sci. 2022, 12, 3123. [Google Scholar] [CrossRef]
- Patel, N.; Banafarr, P.; Ramachandran, P.; Ghosh, A.; Johnson, B.; Dharani, G. Strategy for the Development of Seaweed Value Chain: Fostering Diversified Livelihoods; Niti Aayog, Government of India: New Delhi, India, 2024. [Google Scholar]
- Ravi, P.; Subramanian, G. Biochemical Studies on Marine Algal Species of Padina (Phaeophyceae) from Mandapam Coastline, Tamil Nadu, India. World J. Pharm. Res. 2017, 6, 44–52. [Google Scholar]
- Tůma, S.; Izaguirre, J.K.; Bondar, M.; Marques, M.M.; Fernandes, P.; da Fonseca, M.M.R.; Cesário, M.T. Upgrading End-of-Line Residues of the Red Seaweed Gelidium Sesquipedale to Polyhydroxyalkanoates Using Halomonas Boliviensis. Biotechnol. Rep. 2020, 27, e00491. [Google Scholar] [CrossRef]
- Cebrián-Lloret, V.; Metz, M.; Martínez-Abad, A.; Knutsen, S.H.; Ballance, S.; López-Rubio, A.; Martínez-Sanz, M. Valorization of Alginate-Extracted Seaweed Biomass for the Development of Cellulose-Based Packaging Films. Algal Res. 2022, 61, 102576. [Google Scholar] [CrossRef]
- Mohapatra, A.; Trivedi, S.; Kolte, A.P.; Tejpal, C.S.; Elavarasan, K.; Vaswani, S.; Malik, P.K.; Ravishankar, C.N.; Bhatta, R. Effect of Padina Gymnospora Biowaste Inclusion on in Vitro Methane Production, Feed Fermentation, and Microbial Diversity. Front. Microbiol. 2024, 15, 1431131. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Berndt, A.; Boland, T.M.; Deighton, M.H.; Gere, J.I.; Grainger, C.; Hegarty, R.S.; Iwaasa, A.D.; Koolaard, J.P.; Lassey, K.R.; Luo, D.; et al. Guidelines for Use of Sulphur Hexafluoride (SF6) Tracer Technique to Measure Enteric Methane Emissions from Ruminants; Lambert, M., Ed.; New Zealand Agricultural Greenhouse Gas Research Centre: Wellington, New Zealand, 2014; ISBN 9780478432107. [Google Scholar]
- Williams, S.R.O.; Moate, P.J.; Deighton, M.H. Sampling Background Air. In Guidelines for Use of Sulphur Hexaflouride (SF6) Tracer Technique to Measure Enteric Methane Emissions from Ruminants; Lambert, M.G., Ed.; New Zealand Agricultural Greenhouse Gas Research Centre: Wellington, New Zealand, 2014; pp. 81–88. ISBN 978-0-478-43211-4. [Google Scholar]
- Malik, P.K.; Trivedi, S.; Mohapatra, A.; Kolte, A.P.; Sejian, V.; Bhatta, R.; Rahman, H. Comparison of Enteric Methane Yield and Diversity of Ruminal Methanogens in Cattle and Buffaloes Fed on the Same Diet. PLoS ONE 2021, 16, e0256048. [Google Scholar] [CrossRef]
- Moate, P.J.; Williams, S.R.O.; Deighton, M.H.; Pinares-Patiño, C.; Lassey, K.R. Estimating Methane Emission Rates and Methane Yield Using the SF6 Technique. In Guidelines for Use of Sulphur Hexaflouride (SF6) Tracer Technique to Measure Enteric Methane Emissions from Ruminants; Lambert, M.G., Ed.; New Zealand Agricultural Greenhouse Gas Research Centre: New Zealand, 2014; pp. 126–133. ISBN 978-0-478-43211-4. [Google Scholar]
- Pinares-Patiño, C.S.; Clark, H. Reliability of the Sulfur Hexafluoride Tracer Technique for Methane Emission Measurement from Individual Animals: An Overview. Aust. J. Exp. Agric. 2008, 48, 223–229. [Google Scholar] [CrossRef]
- Malik, P.K.; Trivedi, S.; Kolte, A.P.; Mohapatra, A.; Biswas, S.; Bhattar, A.V.K.; Bhatta, R.; Rahman, H. Comparative Rumen Metagenome and CAZyme Profiles in Cattle and Buffaloes: Implications for Methane Yield and Rumen Fermentation on a Common Diet. Microorganisms 2023, 12, 47. [Google Scholar] [CrossRef]
- Malik, P.K.; Soren, N.M.; Thulasi, A.; Prasad, C.S. Simple Method for Rumen Content Collection from 2 Days Old Lambs. Indian Vet. J. 2015, 92, 46–48. [Google Scholar]
- Filípek, J.; Dvořák, R. Determination of the Volatile Fatty Acid Content in the Rumen Liquid: Comparison of Gas Chromatography and Capillary Isotachophoresis. Acta Vet. Brno 2009, 78, 627–633. [Google Scholar] [CrossRef]
- Malik, P.K.; Trivedi, S.; Kolte, A.P.; Mohapatra, A.; Biswas, S.; Bhattar, A.V.K.; Bhatta, R.; Rahman, H. Comparative Analysis of Rumen Metagenome, Metatranscriptome, Fermentation and Methane Yield in Cattle and Buffaloes Fed on the Same Diet. Front. Microbiol. 2023, 14, 1266025. [Google Scholar] [CrossRef]
- Malik, P.K.; Trivedi, S.; Mohapatra, A.; Kolte, A.P.; Mech, A.; Victor, T.; Ahasic, E.; Bhatta, R. Oat Brewery Waste Decreased Methane Production and Alters Rumen Fermentation, Microbiota Composition, and CAZymes Profiles. Microorganisms 2024, 12, 1475. [Google Scholar] [CrossRef]
- Conway, E.J. Microdiffusion Analysis and Volumetric Error, 4th ed.; Crosby Lockwood and Son Ltd.: London, UK, 1957. [Google Scholar]
- Hungate, R.E. The Rumen and Its Microbes; Academic Press Inc: New York, NY, USA, 1966. [Google Scholar]
- Kamra, D.N.; Agarwal, N. Techniques in Rumen Microbiology; Indian Veterinary Research Institute: Bareilly, India, 2003; Volume 243. [Google Scholar]
- Yu, Z.; Morrison, M. Improved Extraction of PCR-Quality Community DNA from Digesta and Fecal Samples. Biotechniques 2004, 36, 808–812. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Olson, R.D.; Assaf, R.; Brettin, T.; Conrad, N.; Cucinell, C.; Davis, J.J.; Dempsey, D.M.; Dickerman, A.; Dietrich, E.M.; Kenyon, R.W.; et al. Introducing the Bacterial and Viral Bioinformatics Resource Center (BV-BRC): A Resource Combining PATRIC, IRD and ViPR. Nucleic Acids Res. 2023, 51, D678–D689. [Google Scholar] [CrossRef]
- Wood, D.E.; Lu, J.; Langmead, B. Improved Metagenomic Analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef]
- Breitwieser, F.P.; Salzberg, S.L. Pavian: Interactive Analysis of Metagenomics Data for Microbiome Studies and Pathogen Identification. Bioinformatics 2020, 36, 1303–1304. [Google Scholar] [CrossRef]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for Comprehensive Statistical, Functional, and Meta-Analysis of Microbiome Data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef] [PubMed]
- Paulson, J.N.; Colin Stine, O.; Bravo, H.C.; Pop, M. Differential Abundance Analysis for Microbial Marker-Gene Surveys. Nat. Methods 2013, 10, 1200–1202. [Google Scholar] [CrossRef] [PubMed]
- Doreau, M.; Benhissi, H.; Thior, Y.E.; Bois, B.; Leydet, C.; Genestoux, L.; Lecomte, P.; Morgavi, D.P.; Ickowicz, A. Methanogenic Potential of Forages Consumed throughout the Year by Cattle in a Sahelian Pastoral Area. Anim. Prod. Sci. 2016, 56, 613–618. [Google Scholar] [CrossRef]
- Jiyana, S.T.; Ratsaka, M.M.; Leeuw, K.J.; Mbatha, K.R. Impacts of Graded Dietary Fiber Levels on Feed Efficiency and Carbon Footprint of Two Beef Breeds. Livest. Sci. 2022, 258, 104867. [Google Scholar] [CrossRef]
- McSweeney, C.S.; Palmer, B.; McNeill, D.M.; Krause, D.O. Microbial Interactions with Tannins: Nutritional Consequences for Ruminants. Anim. Feed. Sci. Technol. 2001, 91, 83–93. [Google Scholar] [CrossRef]
- Haque, M.N.; Hansen, H.H.; Storm, I.M.L.D.; Madsen, J. Comparative Methane Estimation from Cattle Based on Total CO2 Production Using Different Techniques. Anim. Nutr. 2017, 3, 175–179. [Google Scholar] [CrossRef]
- Van Lingen, H.J.; Plugge, C.M.; Fadel, J.G.; Kebreab, E.; Bannink, A.; Dijkstra, J. Thermodynamic Driving Force of Hydrogen on Rumen Microbial Metabolism: A Theoretical Investigation. PLoS ONE 2016, 11, e0161362. [Google Scholar] [CrossRef]
- Laanbroek, H.J.; Abee, T.; Voogd, I.L. Alcohol Conversion by Desulfobulbus Propionicus Lindhorst in the Presence and Absence of Sulfate and Hydrogen. Arch. Microbiol. 1982, 133, 178–184. [Google Scholar] [CrossRef]
- Denman, S.E.; Tomkins, N.W.; McSweeney, C.S. Quantitation and Diversity Analysis of Ruminal Methanogenic Populations in Response to the Antimethanogenic Compound Bromochloromethane. FEMS Microbiol. Ecol. 2007, 62, 313–322. [Google Scholar] [CrossRef]
- Mcsweeney, C.; Kang, S.; Gagen, E.; Davis, C.; Morrison, M.; Denman, S. Recent Developments in Nucleic Acid Based Techniques for Use in Rumen Manipulation. Rev. Bras. De Zootec. 2009, 38, 341–351. [Google Scholar] [CrossRef]
- Altermann, E.; Schofield, L.R.; Ronimus, R.S.; Beatty, A.K.; Reilly, K. Inhibition of Rumen Methanogens by a Novel Archaeal Lytic Enzyme Displayed on Tailored Bionanoparticles. Front. Microbiol. 2018, 9, 2378. [Google Scholar] [CrossRef]
- Wright, A.D.G.; Williams, A.J.; Winder, B.; Christophersen, C.T.; Rodgers, S.L.; Smith, K.D. Molecular Diversity of Rumen Methanogens from Sheep in Western Australia. Appl. Environ. Microbiol. 2004, 70, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.; Toovey, A.; Pimm, C. Molecular Identification of Methanogenic Archaea from Sheep in Queensland, Australia Reveal More Uncultured Novel Archaea. Anaerobe 2006, 12, 134–139. [Google Scholar] [PubMed]
- Janssen, P.H.; Kirs, M. Structure of the Archaeal Community of the Rumen. Appl. Environ. Microbiol. 2008, 74, 3619–3625. [Google Scholar] [CrossRef]
- Snelling, T.J.; Genç, B.; McKain, N.; Watson, M.; Waters, S.M.; Creevey, C.J.; Wallace, R.J. Diversity and Community Composition of Methanogenic Archaea in the Rumen of Scottish Upland Sheep Assessed by Different Methods. PLoS ONE 2014, 9, e106491. [Google Scholar] [CrossRef]
- Pitta, D.W.; Indugu, N.; Melgar, A.; Hristov, A.; Challa, K.; Vecchiarelli, B.; Hennessy, M.; Narayan, K.; Duval, S.; Kindermann, M.; et al. The Effect of 3-Nitrooxypropanol, a Potent Methane Inhibitor, on Ruminal Microbial Gene Expression Profiles in Dairy Cows. Microbiome 2022, 10, 146. [Google Scholar] [CrossRef]
- Malik, P.K.; Trivedi, S.; Kolte, A.P.; Mohapatra, A.; Bhatta, R.; Rahman, H. Effect of an Anti-Methanogenic Supplement on Enteric Methane Emission, Fermentation, and Whole Rumen Metagenome in Sheep. Front. Microbiol. 2022, 13, 1048288. [Google Scholar] [CrossRef]
- Smith, N.W.; Shorten, P.R.; Altermann, E.; Roy, N.C.; McNabb, W.C. Competition for Hydrogen Prevents Coexistence of Human Gastrointestinal Hydrogenotrophs in Continuous Culture. Front. Microbiol. 2020, 11, 1073. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, B.; Gao, J.; Zhao, G. Dietary Supplementation with Sodium Sulfate Improves Rumen Fermentation, Fiber Digestibility, and the Plasma Metabolome through Modulation of Rumen Bacterial Communities in Steers. Appl. Environ. Microbiol. 2020, 86, e01412-20. [Google Scholar] [CrossRef]
- Wu, H.; Li, Y.; Meng, Q.; Zhou, Z. Effect of High Sulfur Diet on Rumen Fermentation, Microflora, and Epithelial Barrier Function in Steers. Animals 2021, 11, 2545. [Google Scholar] [CrossRef]
- Antal, V.; Andriana, C.; Nicolae, C.; Kévin, G.; Christine, D. Effects of Sulfur Sources on Ruminal S Bioavailability, Fermentation Activity and Microbial Populations Measured In Vitro. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Anim. Sci. Biotechnol. 2023, 80, 15–26. [Google Scholar] [CrossRef]
- Neville, B.W.; Schauer, C.S.; Karges, K.; Gibson, M.L.; Thompson, M.M.; Kirschten, L.A.; Dyer, N.W.; Berg, P.T.; Lardy, G.P. Effect of Thiamine Concentration on Animal Health, Feedlot Performance, Carcass Characteristics, and Ruminal Hydrogen Sulfide Concentrations in Lambs Fed Diets Based on 60% Distillers Dried Grains plus Solubles. J. Anim. Sci. 2010, 88, 2444–2455. [Google Scholar] [CrossRef] [PubMed]
- Neville, B.W.; Lardy, G.P.; Karges, K.K.; Schauer, C.S. Sulfur Intake, Excretion, and Ruminal Hydrogen Sulfide Concentrations in Lambs Fed Increasing Concentrations of Distillers Dried Grains with Solubles. Goat Res. J. Sheep Goat Res. J. 2011, 26, 13–19. [Google Scholar]
- Gooneratne, S.R.; Christensent, D.A. Review of Copper Deficiency and Metabolism in Ruminants. Can. J. Anim. Sci. 1989, 69, 819–845. [Google Scholar]
- Gould, D.H.; Cummings, B.A.; Hamar, D.W. In Vivo Indicators of Pathologic Ruminal Sulfide Production in Steers with Diet-Induced Polioencephalomalacia. J. Vet. Diagn. Investig. 1997, 9, 72–76. [Google Scholar]
- Delfiol, D.J.Z.; Cunha, P.H.J.D.; Borges, A.S. Determination of Ruminal Hydrogen Sulfide in Sheep. Vet. Zootec. 2011, 18, 625–628. [Google Scholar]
- Lewis, D. The Reduction of Sulphate in the Rumen of the Sheep. Biochem. J. 1954, 56, 391–399. [Google Scholar]
- Morgavi, D.P.; Martin, C.; Jouany, J.P.; Ranilla, M.J. Rumen Protozoa and Methanogenesis: Not a Simple Cause-Effect Relationship. Br. J. Nutr. 2012, 107, 388–397. [Google Scholar] [CrossRef]
- Ushida, K.; Kayouli, C.; De Smet, S.; Jouany, J.P. Effect of Defaunation on Protein and Fibre Digestion in Sheep Fed on Ammonia-Treated Straw-Based Diets with or without Maize. Br. J. Nutr. 1990, 64, 765–775. [Google Scholar] [CrossRef]
- Soetanto, H.; Gordon, G.L.; Hume, I.D.; Leng, R.A. The Role of Protozoa and Fungi in Fibre Digestion in the Rumen of Sheep. In Proceedings of the 3rd AAAP Animal Science Congress, Efficient Animal Production for Asian Welfare, Seoul, Republic of Korea, 6–10 May 1985; pp. 805–807. [Google Scholar]
- Romulo, B.H.; Bird, S.H.; Leng, R.A. The Effects of Defaunation on Digestibility and Rumen Fungi Counts in Sheep Fed High-Fibre Diets. Proc. Aust. Soc. Anim. Prod. 1986, 16, 327–330. [Google Scholar]
- Jouany, J.P. Effect of Rumen Protozoa on Nitrogen Utilization by Ruminants. J. Nutr. 1996, 126, 1335S–1346S. [Google Scholar] [CrossRef] [PubMed]
- Han, C.Y.; Lu, D.X.; Hu, M.; Tan, Z.L. Influence of Controlling Protozoa on the Degradation and Utilization of Dietary Fibre and Protein in the Rumen and Nitrogenous Flow Entering the Duodenum of Sheep. Asian-Aust. J. Anim. Sci. 1999, 12, 1241–1245. [Google Scholar] [CrossRef]
- Morgavi, D.P.; Cantalapiedra-Hijar, G.; Eugène, M.; Martin, C.; Noziere, P.; Popova, M.; Ortigues-Marty, I.; Muñoz-Tamayo, R.; Ungerfeld, E.M. Review: Reducing Enteric Methane Emissions Improves Energy Metabolism in Livestock: Is the Tenet Right? Animal 2023, 17, 100830. [Google Scholar] [CrossRef] [PubMed]
- Roque, B.M.; Salwen, J.K.; Kinley, R.; Kebreab, E. Inclusion of Asparagopsis Armata in Lactating Dairy Cows’ Diet Reduces Enteric Methane Emission by over 50 Percent. J. Clean. Prod. 2019, 234, 132–138. [Google Scholar] [CrossRef]
- Angellotti, M.; Lindberg, M.; Ramin, M.; Krizsan, S.J.; Danielsson, R. Asparagopsis Taxiformis Supplementation to Mitigate Enteric Methane Emissions in Dairy Cows—Effects on Performance and Metabolism. J. Dairy Sci. 2025, 108, 2503–2516. [Google Scholar] [CrossRef]
- Li, X.; Norman, H.C.; Kinley, R.D.; Laurence, M.; Wilmot, M.; Bender, H.; De Nys, R.; Tomkins, N. Asparagopsis Taxiformis Decreases Enteric Methane Production from Sheep. Anim. Prod. Sci. 2018, 58, 681–688. [Google Scholar] [CrossRef]
- Romero, P.; Ungerfeld, E.M.; Popova, M.; Morgavi, D.P.; Yáñez-Ruiz, D.R.; Belanche, A. Exploring the Combination of Asparagopsis Taxiformis and Phloroglucinol to Decrease Rumen Methanogenesis and Redirect Hydrogen Production in Goats. Anim. Feed Sci. Technol. 2024, 316, 116060. [Google Scholar] [CrossRef]


| Attributes | Concentrate | Ragi Straw | Biowaste * | ||
|---|---|---|---|---|---|
| C | A2 | A5 | |||
| OM | 937 | 921 | 906 | 927 | 786 |
| CP | 215 | 219 | 222 | 35.2 | 139 |
| NDF | 378 | 386 | 391 | 733 | 690 |
| ADF | 98.7 | 105 | 124 | 514 | 433 |
| TA | 63.1 | 78.5 | 94.4 | 73.4 | 214 |
| GE (MJ/kg) | 16.99 | 16.96 | 16.95 | 16.48 | 19.0 |
| Attributes | C | A2 | A5 | SEM | p |
|---|---|---|---|---|---|
| CH4 emissions | |||||
| g/100 DM | 4.96 a | 4.11 b | 3.53 b | 0.415 | 0.0019 |
| g/100 g OM | 5.28 b | 4.43 ab | 3.82 a | 0.423 | 0.0028 |
| g/100 g dig DM | 6.67 a | 5.54 b | 4.88 b | 0.522 | 0.0001 |
| g/100 g dig. OM | 7.12 b | 6.14 ab | 5.60 a | 0.444 | 0.0232 |
| Intake (g/d) | |||||
| DM | 493 | 496 | 493 | 1.201 | 0.994 |
| OM | 463 | 459 | 455 | 2.309 | 0.825 |
| CP | 81.3 | 82.2 | 78.4 | 1.146 | 0.577 |
| NDF | 239 | 253 | 251 | 4.371 | 0.648 |
| ADF | 111 | 132 | 123 | 6.082 | 0.07 |
| Digestibility (%) | |||||
| DM | 73.6 | 73.1 | 72.2 | 0.409 | 0.067 |
| OM | 73.3 | 69.7 | 69.8 | 1.183 | 0.124 |
| CP | 76.9 | 75.9 | 75.3 | 0.466 | 0.826 |
| NDF | 62.8 | 57.2 | 62.4 | 1.803 | 0.154 |
| ADF | 49.5 | 46.7 | 50.1 | 1.047 | 0.710 |
| Attributes | C | A2 | A5 | SEM | p |
|---|---|---|---|---|---|
| Ammonia-N (mg/dL) | 14.5 | 11.8 | 10.5 | 1.178 | 0.0993 |
| TVFA (mmol/L) | 81.2 | 83.9 | 81.0 | 0.929 | 0.9227 |
| Acetate (mmol/L) | 54.2 | 57.9 | 56.5 | 1.078 | 0.8034 |
| Propionate (mmol/L) | 16.5 | 18.3 | 17.5 | 0.520 | 0.6624 |
| Butyrate (mmol/L) | 8.21 | 6.28 | 6.10 | 0.677 | 0.1861 |
| Iso-butyrate (mmol/L) | 0.638 b | 0.177 a | 0.021 a | 0.185 | 0.0002 |
| Valerate (mmol/L) | 0.806 b | 0.535 ab | 0.265 a | 0.156 | 0.0182 |
| Isovalerate (mmol/L) | 0.910 | 0.662 | 0.703 | 0.076 | 0.3604 |
| Total protozoa (cells × 107/mL) | 22.3 b | 18.8 b | 11.4 a | 3.219 | 0.0014 |
| Holotrichs (cells × 106/mL) | 1.32 b | 1.27 b | 0.58 a | 0.239 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohapatra, A.; Trivedi, S.; Tejpal, C.S.; Aware, M.J.; Vaswani, S.; Prajapati, V.J.; Kolte, A.P.; Malik, P.K.; Sahoo, A.; Ravishankar, C.N.; et al. Effect of Two Selected Levels of Padina gymnospora Biowaste and Enteric Methane Emission, Nutrient Digestibility, and Rumen Metagenome in Growing Sheep. Microorganisms 2025, 13, 780. https://doi.org/10.3390/microorganisms13040780
Mohapatra A, Trivedi S, Tejpal CS, Aware MJ, Vaswani S, Prajapati VJ, Kolte AP, Malik PK, Sahoo A, Ravishankar CN, et al. Effect of Two Selected Levels of Padina gymnospora Biowaste and Enteric Methane Emission, Nutrient Digestibility, and Rumen Metagenome in Growing Sheep. Microorganisms. 2025; 13(4):780. https://doi.org/10.3390/microorganisms13040780
Chicago/Turabian StyleMohapatra, Archit, Shraddha Trivedi, Chaluvanahalli S. Tejpal, Manojkumar Janardhan Aware, Shalini Vaswani, Vedant Jayeshkumar Prajapati, Atul Purshottam Kolte, Pradeep Kumar Malik, Artabandhu Sahoo, Chandragiri Nagarajarao Ravishankar, and et al. 2025. "Effect of Two Selected Levels of Padina gymnospora Biowaste and Enteric Methane Emission, Nutrient Digestibility, and Rumen Metagenome in Growing Sheep" Microorganisms 13, no. 4: 780. https://doi.org/10.3390/microorganisms13040780
APA StyleMohapatra, A., Trivedi, S., Tejpal, C. S., Aware, M. J., Vaswani, S., Prajapati, V. J., Kolte, A. P., Malik, P. K., Sahoo, A., Ravishankar, C. N., & Bhatta, R. (2025). Effect of Two Selected Levels of Padina gymnospora Biowaste and Enteric Methane Emission, Nutrient Digestibility, and Rumen Metagenome in Growing Sheep. Microorganisms, 13(4), 780. https://doi.org/10.3390/microorganisms13040780







