Effects of Fungal Solid-State Fermentation on the Profile of Phenolic Compounds and on the Nutritional Properties of Grape Pomace
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Substrate and Microorganism
2.3. Biological Treatment of Grape Pomace by Trametes Versicolor
2.3.1. Laboratory Jars
2.3.2. Tray Bioreactor
2.4. Enzyme Activity Measurement
2.5. Determination of Biomass Concentration
2.6. Analysis of Chemical Composition of Grape Pomace
2.6.1. UHPLC Analysis of Individual Phenolic Compounds
2.6.2. Analyses of Total Phenolic Compounds, Total Flavonoids, and Total Extractable Proanthocyanidins
2.6.3. Determination of Antioxidant Activity by DPPH, FRAP, and ABTS Methods
2.6.4. Measurements of Total Organic Carbon and Total Nitrogen
2.6.5. Measurements of Reducing and Individual Sugars Concentrations
2.6.6. Determination of Dry Matter, Ash, Crude Protein, and Free Fats
2.6.7. Crude Fiber Content Determination
2.7. Statistical Analysis
3. Results and Discussion
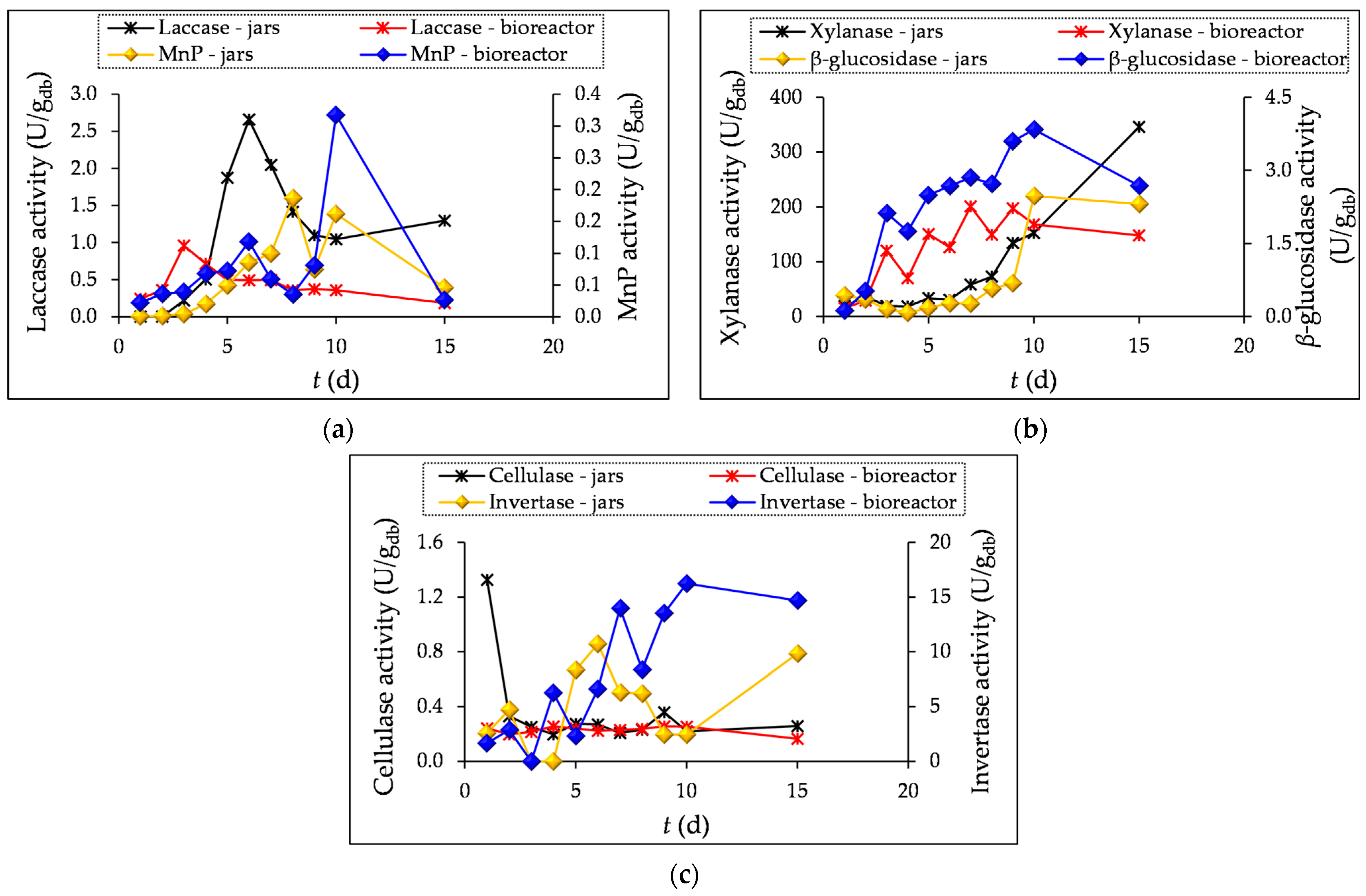
3.1. Enzyme Activity Measurement
3.2. Determination of Biomass Concentration and pH Measurement
3.3. Chemical Composition of Grape Pomace
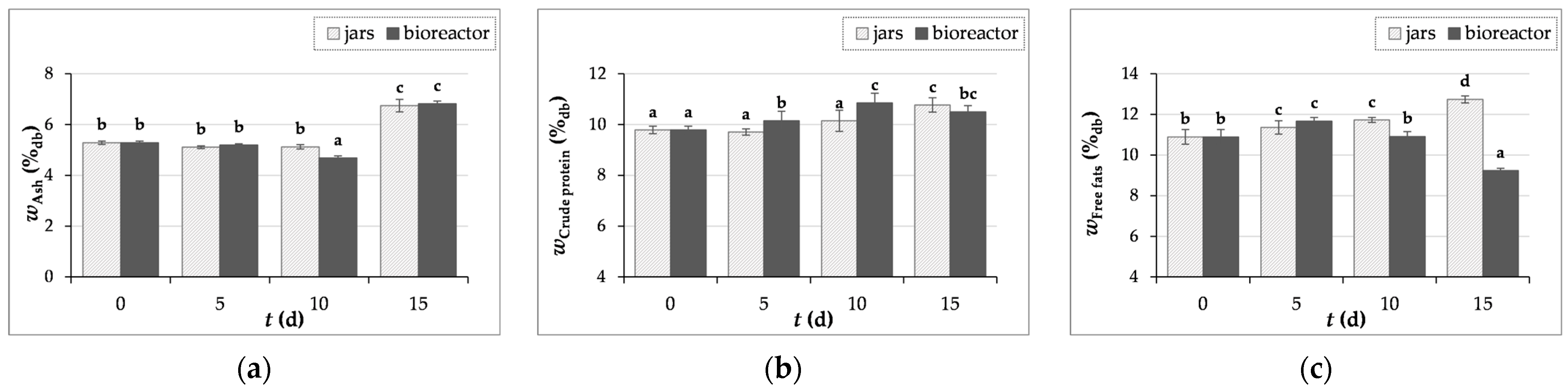
3.3.1. Determination of Ash, Crude Proteins, and Free Fats Content
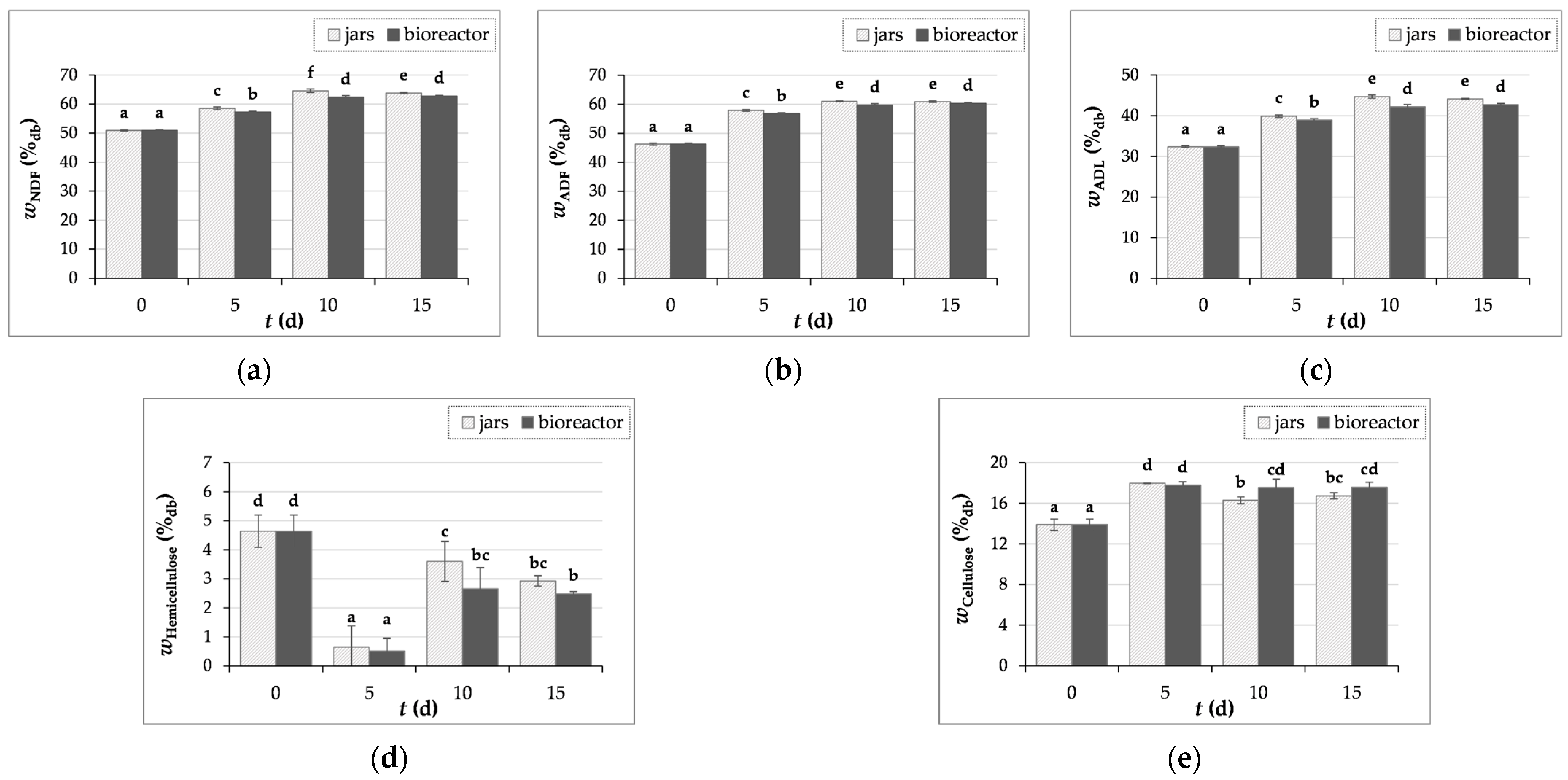
3.3.2. Crude Fiber Content Determination
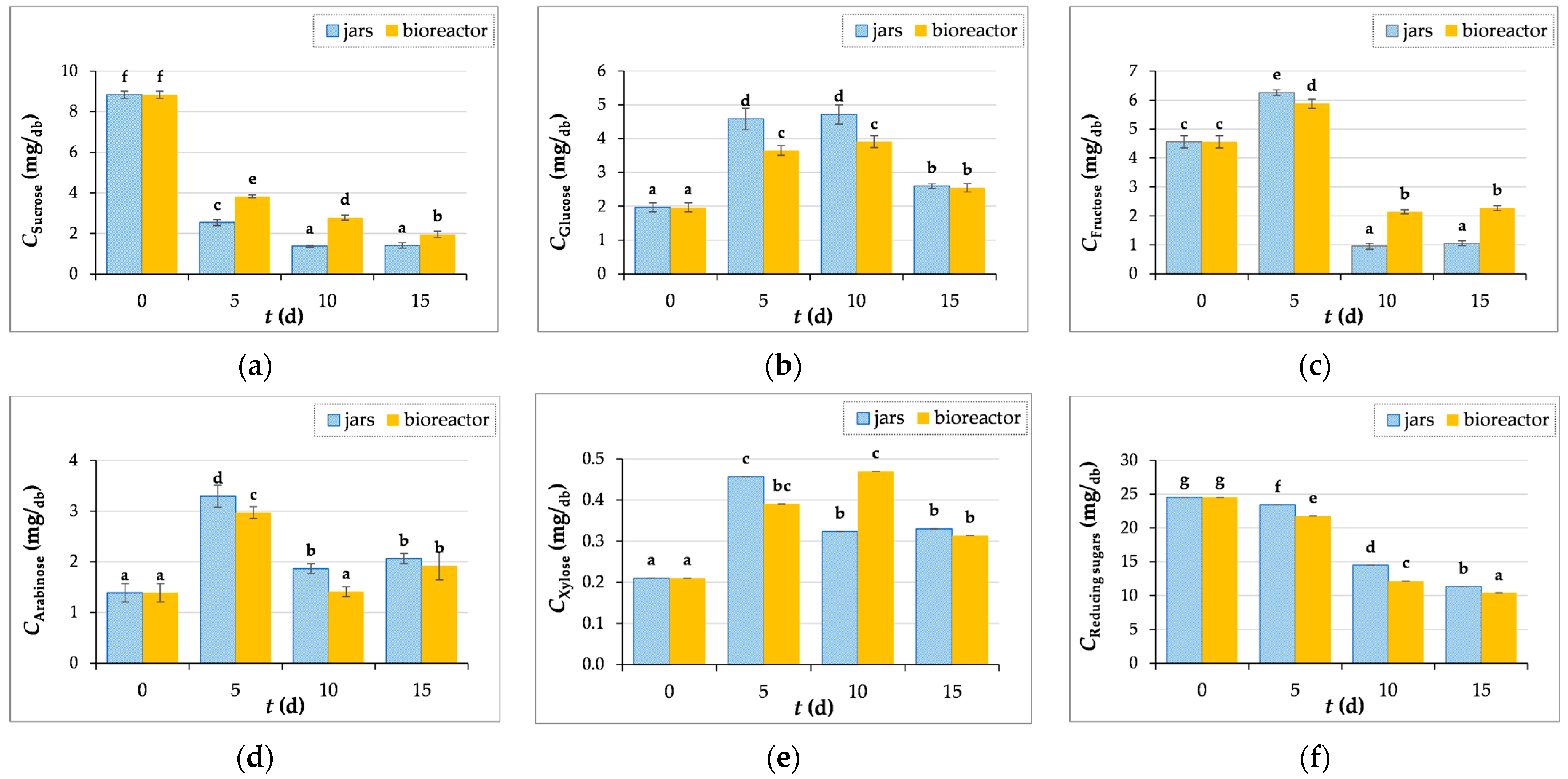
3.3.3. Measurements of Reducing and Individual Sugars Concentrations
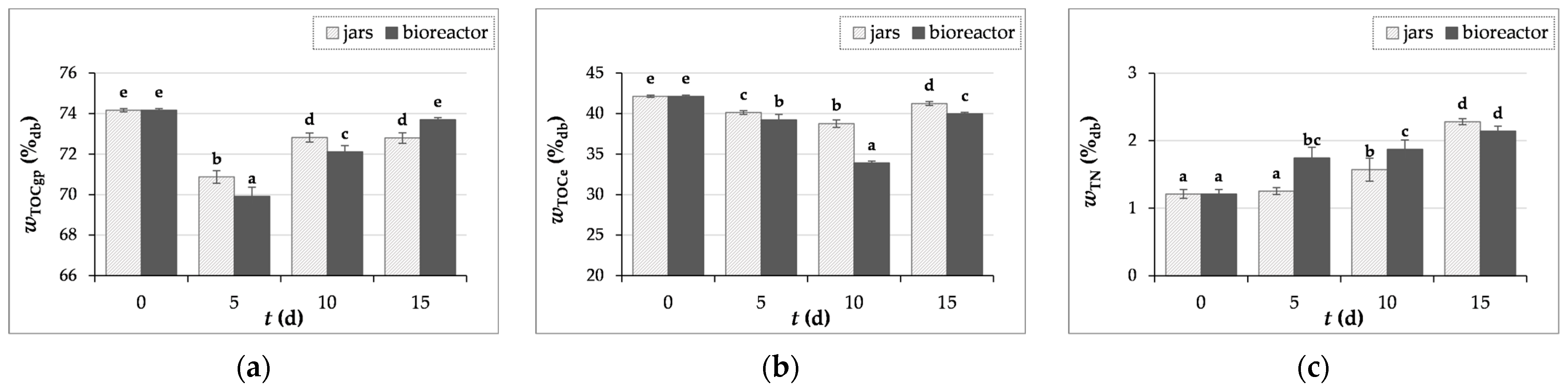
3.3.4. Measurements of Carbon and Nitrogen Content
3.4. Phenolic Compound and Antioxidant Activity Measurements
3.4.1. Determination of Total Phenolic Compound and Antioxidant Activity
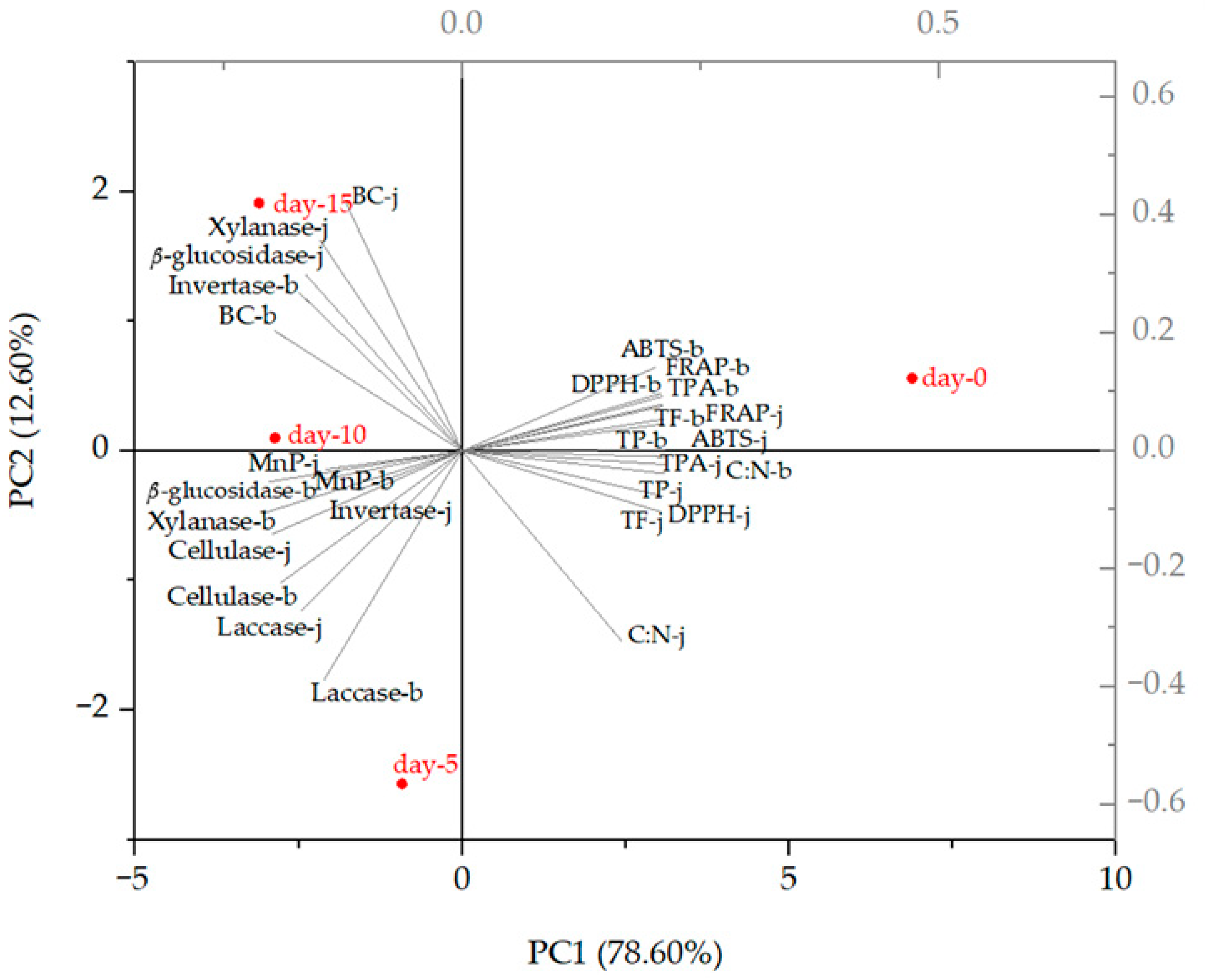
3.4.2. Principal Components Analysis
3.4.3. Determination of Individual Phenolic Compound Content
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 30 May 2022).
- Cui, W.; Wang, Y.; Sun, Z.; Cui, C.; Li, H.; Luo, K.; Cheng, A. Effects of Steam Explosion on Phenolic Compounds and Dietary Fiber of Grape Pomace. LWT 2023, 173, 114350. [Google Scholar] [CrossRef]
- Bucić-Kojić, A.; Tišma, M.; Šelo, G.; Grgić, J.; Perković, G.; Planinić, M. Winery Production Residues as Feedstocks within the Biorefinery Concept. Eng. Power 2022, 17, 11–17. [Google Scholar]
- Siller-Sánchez, A.; Luna-Sánchez, K.A.; Bautista-Hernández, I.; Chávez-González, M.L. Use of Grape Pomace from the Wine Industry for the Extraction of Valuable Compounds with Potential Use in the Food Industry. Curr. Food Sci. Technol. Rep. 2024, 2, 7–16. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Nobre, C.; Rodrigues, R.M.; Genisheva, Z.; Botelho, C.; Teixeira, J.A. Extraction of Phenolic Compounds from Grape Pomace Using Ohmic Heating: Chemical Composition, Bioactivity and Bioaccessibility. Food Chem. 2024, 436, 137780. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, D.; Zhang, J.; Shen, J.; Cao, J.; Gu, H.; Cui, M.; He, L.; Chen, G.; Liu, S.; et al. Improving Soluble Phenolic Profile and Antioxidant Activity of Grape Pomace Seeds through Fungal Solid-State Fermentation. Foods 2024, 13, 1158. [Google Scholar] [CrossRef]
- Silva, A.; Silva, V.; Igrejas, G.; Aires, A.; Falco, V.; Valentão, P.; Poeta, P. Phenolic Compounds Classification and Their Distribution in Winemaking By-Products. Eur. Food Res. Technol. 2023, 249, 207–239. [Google Scholar] [CrossRef]
- Martinović, J.; Lukinac, J.; Jukić, M.; Ambrus, R.; Planinić, M.; Šelo, G.; Klarić, A.-M.; Perković, G.; Bucić-Kojić, A. Physicochemical Characterization and Evaluation of Gastrointestinal In Vitro Behavior of Alginate-Based Microbeads with Encapsulated Grape Pomace Extracts. Pharmaceutics 2023, 15, 980. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Martinović, J.; Perković, G.; Bucić-Kojić, A. Bioconversion of Grape Pomace with Rhizopus oryzae under Solid-State Conditions: Changes in the Chemical Composition and Profile of Phenolic Compounds. Microorganisms 2023, 11, 956. [Google Scholar] [CrossRef]
- Caponio, G.R.; Minervini, F.; Tamma, G.; Gambacorta, G.; De Angelis, M. Promising Application of Grape Pomace and Its Agri-Food Valorization: Source of Bioactive Molecules with Beneficial Effects. Sustainability 2023, 15, 9075. [Google Scholar] [CrossRef]
- Abid, K.; Boudagga, S.; Abid, O.; Najar, T.; Jaouani, A. Bioconversion of Grape Pomace Waste into Suitable Alternative Feed for Ruminants with Pleurotus cornucopiae and Ganoderma resinaceum via Solid-State Fermentation Bioprocess. Biomass Conv. Bioref. 2023, 1–10, EarlyAccess. [Google Scholar] [CrossRef]
- Gerardi, C.; D’Amico, L.; Durante, M.; Tufariello, M.; Giovinazzo, G. Whole Grape Pomace Flour as Nutritive Ingredient for Enriched Durum Wheat Pasta with Bioactive Potential. Foods 2023, 12, 2593. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Tomas, S.; Koceva Komlenić, D.; Bucić-Kojić, A. A Comprehensive Review on Valorization of Agro-Food Industrial Residues by Solid-State Fermentation. Foods 2021, 10, 927. [Google Scholar] [CrossRef]
- Cerda-Cejudo, N.D.; Buenrostro-Figueroa, J.J.; Sepúlveda-Torre, L.; Torres-León, C.; Chávez-González, M.L.; Ascacio-Valdés, J.A.; Aguilar, C.N. Solid-State Fermentation for the Recovery of Phenolic Compounds from Agro-Wastes. Resources 2023, 12, 36. [Google Scholar] [CrossRef]
- Tišma, M.; Žnidaršič-Plazl, P.; Šelo, G.; Tolj, I.; Šperanda, M.; Bucić-Kojić, A.; Planinić, M. Trametes versicolor in Lignocellulose-Based Bioeconomy: State of the Art, Challenges and Opportunities. Bioresour. Technol. 2021, 330, 124997. [Google Scholar] [CrossRef]
- Torreggiani, A.; Beccaccioli, M.; Verni, M.; Cecchetti, V.; Minisci, A.; Reverberi, M.; Pontonio, E.; Rizzello, C.G. Combined Use of Trametes versicolor Extract and Sourdough Fermentation to Extend the Microbiological Shelf-Life of Baked Goods. LWT 2023, 189, 115467. [Google Scholar] [CrossRef]
- Planinić, M.; Zelić, B.; Čubel, I.; Bucić-Kojić, A.; Tišma, M. Corn Forage Biological Pretreatment by Trametes Versicolor in a Tray Bioreactor. Waste Manag. Res. 2016, 34, 802–809. [Google Scholar] [CrossRef]
- Bailey, M.J.; Biely, P.; Poutanen, K. Interlaboratory Testing of Methods for Assay of Xylanase Activity. J. Biotechnol. 1992, 23, 257–270. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of Cellulase Activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Karpe, A.V.; Dhamale, V.V.; Morrison, P.D.; Beale, D.J.; Harding, I.H.; Palombo, E.A. Winery Biomass Waste Degradation by Sequential Sonication and Mixed Fungal Enzyme Treatments. Fungal Genet. Biol. 2017, 102, 22–30. [Google Scholar] [CrossRef]
- Margetić, A.; Vujčić, Z. Comparative Study of Stability of Soluble and Cell Wall Invertase from Saccharomyces cerevisiae. Prep. Biochem. Biotechnol. 2017, 47, 305–311. [Google Scholar] [CrossRef]
- Lueangjaroenkit, P.; Kunitake, E.; Sakka, M.; Kimura, T.; Teerapatsakul, C.; Sakka, K.; Chitradon, L. Light Regulation of Two New Manganese Peroxidase-Encoding Genes in Trametes Polyzona KU-RNW027. Microorganisms 2020, 8, 852. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Development of a Novel Methodology for the Analysis of Ergosterol in Mushrooms. Food Anal. Methods 2014, 7, 217–223. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Grgić, J.; Perković, G.; Koceva Komlenić, D.; Bucić-Kojić, A. A Comparative Study of the Influence of Various Fungal-Based Pretreatments of Grape Pomace on Phenolic Compounds Recovery. Foods 2022, 11, 1665. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of Total Phenolics. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Ed.; John Wiley & Sons Inc.: New York, NY, USA, 2001; pp. I1.1.1–I1.1.8. [Google Scholar]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, Proanthocyanidins, Flavones and Flavonols in Some Plant Materials and Their Antioxidant Activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Bucić-Kojić, A.; Planinić, M.; Tomas, S.; Jakobek, L.; Šeruga, M. Influence of Solvent and Temperature on Extraction of Phenolic Compounds from Grape Seed, Antioxidant Activity and Colour of Extract. Int. J. Food Sci. Technol. 2009, 44, 2394–2401. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Alarcón, E.; Hernández, C.; García, G.; Ziarelli, F.; Gutiérrez-Rivera, B.; Musule, R.; Vázquez-Marrufo, G.; Gardner, T.G. Changes in Chemical and Structural Composition of Sugarcane Bagasse Caused by Alkaline Pretreatments [Ca(OH)2 and NaOH] Modify the Amount of Endoglucanase and β-Glucosidase Produced by Aspergillus niger in Solid-State Fermentation. Chem. Eng. Commun. 2021, 209, 594–606. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Method 942.05. In Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Arlington, VA, USA, 1995. [Google Scholar]
- AOAC. AOAC Official Method 2001.11. In Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC. AOAC Official Method 945.16. In Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses (Apparatus, Reagents, Procedures, and Some Applications). In Agriculture Handbook; No. 379; Agricultural Research Service, U.S. Department of Agriculture: Washington, DC, USA, 1970; pp. 1–24. [Google Scholar]
- Kumar, D. Fungal Lipase Production by Solid State Fermentation—An Overview. J. Anal. Bioanal. Tech. 2015, 6, 1000230. [Google Scholar] [CrossRef]
- Mostafa, Y.S.; Širić, I.; Alamri, S.A.M.; Alrumman, S.A.; Kumar, P.; Abou Fayssal, S.; Zjalić, S.; Singh, R.; Eid, E.M. Assessment of Metal Elements and Biochemical Constituents of Wild Turkey Tail (Trametes versicolor) Mushrooms Collected from the Shivalik Foothills of the Himalayas, India. Forests 2023, 14, 2247. [Google Scholar] [CrossRef]
- Fabros, J.A.; Lazo, M.K.M.; Magpantay, J.E.S.; Abon, M.D.; Dulay, R.M.R.; Kalaw, S.P.; Reyes, R.G. The Effect of Nutritional and Physical Factors on the Growth of Trametes versicolor (L.) Lloyd and Its Mycochemical and Cytotoxic Properties. Stud. Fungi 2023, 8, 18. [Google Scholar] [CrossRef]
- Athanasiou, P.E.; Gkountela, C.I.; Patila, M.; Fotiadou, R.; Chatzikonstantinou, A.V.; Vouyiouka, S.N.; Stamatis, H. Laccase-Mediated Oxidation of Phenolic Compounds from Wine Lees Extract towards the Synthesis of Polymers with Potential Applications in Food Packaging. Biomolecules 2024, 14, 323. [Google Scholar] [CrossRef]
- Erskine, E.; Ozkan, G.; Lu, B.; Capanoglu, E. Effects of Fermentation Process on the Antioxidant Capacity of Fruit Byproducts. ACS Omega 2023, 8, 4543–4553. [Google Scholar] [CrossRef]
- Li, X.; Dilokpimol, A.; Kabel, M.A.; de Vries, R.P. Fungal Xylanolytic Enzymes: Diversity and Applications. Bioresour. Technol. 2022, 344, 126290. [Google Scholar] [CrossRef]
- Manoochehri, H.; Hosseini, N.F.; Saidijam, M.; Taheri, M.; Rezaee, H.; Nouri, F. A Review on Invertase: Its Potentials and Applications. Biocatal. Agric. Biotechnol. 2020, 25, 101599. [Google Scholar] [CrossRef]
- Botella, C.; Diaz, A.; de Ory, I.; Webb, C.; Blandino, A. Xylanase and Pectinase Production by Aspergillus awamori on Grape Pomace in Solid State Fermentation. Process Biochem. 2007, 42, 98–101. [Google Scholar] [CrossRef]
- De Bellis, P.; Maggiolino, A.; Albano, C.; De Palo, P.; Blando, F. Ensiling Grape Pomace With and Without Addition of a Lactiplantibacillus Plantarum Strain: Effect on Polyphenols and Microbiological Characteristics, in Vitro Nutrient Apparent Digestibility, and Gas Emission. Front. Vet. Sci. 2022, 9, 808293. [Google Scholar] [CrossRef]
- Mohamed Ahmed, I.A.; Özcan, M.M.; Al Juhaimi, F.; Babiker, E.F.E.; Ghafoor, K.; Banjanin, T.; Osman, M.A.; Gassem, M.A.; Alqah, H.A.S. Chemical Composition, Bioactive Compounds, Mineral Contents, and Fatty Acid Composition of Pomace Powder of Different Grape Varieties. J. Food Process. Preserv. 2020, 44, e14539. [Google Scholar] [CrossRef]
- Altop, A.; Güngör, E.; Erener, G. Aspergillus niger May Improve Nutritional Quality of Grape Seed and Its Usability in Animal Nutrition through Solid-State Fermentation. Int. Adv. Res. Eng. J. 2018, 2, 273–277. [Google Scholar]
- Gungor, E.; Altop, A.; Erener, G. Effect of Raw and Fermented Grape Pomace on the Growth Performance, Antioxidant Status, Intestinal Morphology, and Selected Bacterial Species in Broiler Chicks. Animals 2021, 11, 364. [Google Scholar] [CrossRef]
- Ruiz-Herrera, J. Fungal Cell Wall: Structure, Synthesis, and Assembly, 1st ed.; CRC Press: Boca Raton, FL, USA, 1991; ISBN 978-0-8493-6672-7. [Google Scholar]
- Sukma, A.; Jos, B.; Sumardiono, S. Kinetic of Biomass Growth and Protein Formation on Rice Bran Fermentation Using Rhizopus oryzae. MATEC Web. Conf. 2018, 156, 01023. [Google Scholar] [CrossRef][Green Version]
- Machado, A.R.; Voss, G.B.; Machado, M.; Paiva, J.A.; Nunes, J.; Pintado, M. Chemical Characterization of the Cultivar ‘Vinhão’ (Vitis vinifera L.) Grape Pomace Towards Its Circular Valorisation. Meas. Food 2024, 15, 100175. [Google Scholar] [CrossRef]
- Zheng, Y.; Lee, C.; Yu, C.; Cheng, Y.-S.; Simmons, C.W.; Zhang, R.; Jenkins, B.M.; VanderGheynst, J.S. Ensilage and Bioconversion of Grape Pomace into Fuel Ethanol. J. Agric. Food Chem. 2012, 60, 11128–11134. [Google Scholar] [CrossRef]
- Manara, P.; Zabaniotou, A.; Vanderghem, C.; Richel, A. Lignin Extraction from Mediterranean Agro-Wastes: Impact of Pretreatment Conditions on Lignin Chemical Structure and Thermal Degradation Behavior. Catal. Today 2014, 223, 25–34. [Google Scholar] [CrossRef]
- Teles, A.S.C.; Chávez, D.W.H.; Oliveira, R.A.; Bon, E.P.S.; Terzi, S.C.; Souza, E.F.; Gottschalk, L.M.F.; Tonon, R.V. Use of Grape Pomace for the Production of Hydrolytic Enzymes by Solid-State Fermentation and Recovery of Its Bioactive Compounds. Food Res. Int. 2019, 120, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Zamora Zamora, H.D.; Silva, T.A.L.; Varão, L.H.R.; Baffi, M.A.; Pasquini, D. Simultaneous Production of Cellulases, Hemicellulases, and Reducing Sugars by Pleurotus Ostreatus Growth in One-Pot Solid State Fermentation Using Alstroemeria Sp. Waste. Biomass Conv. Bioref. 2021, 13, 4879–4982. [Google Scholar] [CrossRef]
- Yu, H.; Xie, B.; Khan, R.; Dong, J.; Shen, G. The Changes in Macronutrients and Microbial Community Structure during the Co-Composting of White Wine Distillers’ Grains and Potassium Silicate. J. Clean. Prod. 2021, 319, 128681. [Google Scholar] [CrossRef]
- Zambrano, C.; Kotogán, A.; Bencsik, O.; Papp, T.; Vágvölgyi, C.; Mondal, K.C.; Krisch, J.; Takó, M. Mobilization of Phenolic Antioxidants from Grape, Apple and Pitahaya Residues via Solid State Fungal Fermentation and Carbohydrase Treatment. LWT Food Sci. Technol. 2018, 89, 457–465. [Google Scholar] [CrossRef]
- Chen, Q.; Su, J.; Zhang, Y.; Li, C.; Zhu, S. Phytochemical Profile and Bioactivity of Bound Polyphenols Released from Rosa Roxburghii Fruit Pomace Dietary Fiber by Solid-State Fermentation with Aspergillus niger. Molecules 2024, 29, 1689. [Google Scholar] [CrossRef]
- Tian, Z.-X.; Li, Y.-F.; Long, M.-X.; Liang, Q.; Chen, X.; Huang, D.-M.; Ran, Y.-Q. Effects of Six Different Microbial Strains on Polyphenol Profiles, Antioxidant Activity, and Bioaccessibility of Blueberry Pomace with Solid-State Fermentation. Front. Nutr. 2023, 10, 1282438. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, M.; Çoklar, H.; Bulut, A.N.; Hosseini, S.R. Evaluation of Black Grape Pomace, a Fruit Juice by-Product, in Shalgam Juice Production: Effect on Phenolic Compounds, Anthocyanins, Resveratrol, Tannin, and in Vitro Antioxidant Activity. Food Sci. Nutr. 2024, 12, 4372–4384. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, G.; Boll, M.; Heider, J. Microbial Degradation of Aromatic Compounds—From One Strategy to Four. Nat. Rev. Microbiol. 2011, 9, 803–816. [Google Scholar] [CrossRef] [PubMed]

| Lignolytic Enzymes | Buffer Solution | pH |
|---|---|---|
| Laccase | 50 mM sodium malonate buffer | 4.5 |
| Manganese peroxidase (MnP) | 50 mM sodium malonate buffer | 4.5 |
| Hydrolytic enzymes | Buffer solution | pH |
| Xylanase | 50 mM sodium citrate buffer | 5.3 |
| Cellulase | 50 mM sodium citrate buffer | 4.8 |
| β-glucosidase | 100 mM sodium acetate buffer | 5.0 |
| Invertase | 100 mM sodium acetate buffer | 4.5 |
| Liquid Extract Preparation | Analysis |
|---|---|
|
|
|
|
|
|
| Solid samples of GP | Analysis |
|
|
| Day “0” | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 10 | Day 15 | |
|---|---|---|---|---|---|---|---|---|
| Weight loss—jars (%db) | - | 9.0 | 11.7 | 14.8 | 15.6 | 16.4 | 25.2 | 38.5 |
| Moisture content—jars (%db) | 71.8 | 71.7 | 70.5 | 68.5 | 65.9 | 68.4 | 60.6 | 51.1 |
| Moisture content—bioreactor (%db) | 66.9 | 58.7 | 57.1 | 57.2 | 57.1 | 49.4 | 45.4 | 29.7 |
| Tbioreactor (°C) | - | 27.3 | 27.0 | 27.5 | 27.5 | 27.6 | 27.2 | 27.5 |
| TGP in bioreactor (°C) | - | 26.7 | 26.6 | 27.6 | 27.7 | 27.6 | 27.4 | 27.3 |
| Laboratory Jars | ||||||
|---|---|---|---|---|---|---|
| (mg/gdb) | (mg/gdb) | (mg/gdb) | (mgT/gdb) | (mgT/gdb) | (mgT/gdb) | |
| Day of SSF | TP | TF | TPA | DPPH | ABTS | FRAP |
| day “0” | 50.08 ± 0.08 m | 25.14 ± 0.06 n | 8.55 ± 0.04 n | 57.50 ± 0.00 g | 314.00 ± 0.00 k | 212.50 ± 0.00 h |
| day 1 | 48.95 ± 0.41 l | 26.61 ± 0.07 o | 7.57 ± 0.06 m | 57.00 ± 0.01 fg | 206.50 ± 0.00 i | 138.50 ± 0.00 g |
| day 2 | 41.54 ± 0.01 k | 22.21 ± 0.07 m | 6.37 ± 0.05 l | 53.00 ± 0.00 f | 264.00 ± 0.00 j | 119.50 ± 0.00 f |
| day 3 | 37.47 ± 0.18 j | 20.21 ± 0.01 l | 5.74 ± 0.04 k | 47.00 ± 0.00 e | 185.00 ± 0.00 h | 114.00 ± 0.00 f |
| day 4 | 31.85 ± 0.03 i | 16.53 ± 0.04 k | 4.45 ± 0.06 j | 42.00 ± 0.00 d | 167.50 ± 0.01 g | 83.50 ± 0.00 e |
| day 5 | 23.68 ± 0.11 h | 13.91 ± 0.03 j | 3.32 ± 0.04 i | 27.50 ± 0.00 c | 114.00 ± 0.01 f | 67.50 ± 0.01 d |
| day 10 | 14.79 ± 0.28 e | 8.23 ± 0.06 g | 1.80 ± 0.03 f | 18.50 ± 0.00 b | 85.50 ± 0.00 d | 58.50 ± 0.01 c |
| day 15 | 12.43 ± 0.07 b | 5.99 ± 0.03 d | 1.44 ± 0.03 d | 10.50 ± 0.00 a | 57.50 ± 0.00 a | 48.50 ± 0.00 b |
| Tray bioreactor | ||||||
| (mg/gdb) | (mg/gdb) | (mg/gdb) | (mgT/gdb) | (mgT/gdb) | (mgT/gdb) | |
| TP | TF | TPA | DPPH | ABTS | FRAP | |
| day “0” | 50.08 ± 0.08 m | 25.14 ± 0.06 n | 8.55 ± 0.04 n | 57.50 ± 0.00 g | 314.00 ± 0.00 k | 212.50 ± 0.00 h |
| day 1 | 22.62 ± 0.11 g | 12.63 ± 0.04 i | 2.84 ± 0.04 h | 56.50 ± 0.00 fg | 118.00 ± 0.00 f | 67.50 ± 0.00 d |
| day 2 | 20.92 ± 0.04 f | 11.32 ± 0.04 h | 2.57 ± 0.06 g | 55.50 ± 0.00 fg | 105.00 ± 0.00 e | 57.50 ± 0.00 c |
| day 3 | 13.30 ± 0.14 c | 5.79 ± 0.04 c | 1.22 ± 0.04 b | 10.50 ± 0.00 a | 59.50 ± 0.00 a | 34.00 ± 0.01 a |
| day 4 | 13.19 ± 0.02 c | 6.17 ± 0.06 e | 1.34 ± 0.04 c | 10.50 ± 0.00 a | 78.00 ± 0.00 c | 32.00 ± 0.00 a |
| day 5 | 14.31 ± 0.04 d | 6.91 ± 0.04 f | 1.55 ± 0.03 e | 11.50 ± 0.00 a | 72.00 ± 0.00 b | 37.50 ± 0.00 a |
| day 10 | 12.64 ± 0.06 b | 4.52 ± 0.03 b | 1.32 ± 0.02 c | 10.50 ± 0.00 a | 102.50 ± 0.00 e | 35.00 ± 0.00 a |
| day 15 | 11.62 ± 0.07 a | 4.22 ± 0.07 a | 1.09 ± 0.04 a | 10.0 ± 0.00 a | 88.50 ± 0.00 d | 34.00 ± 0.00 a |
| Phenolic Compound | Day “0” | SSF in Laboratory Jars | SSF in Tray Bioreactor | ||||
|---|---|---|---|---|---|---|---|
| Co (µg/gdb) * | Ci,max. (µg/gdb) * | p ** | tSSF (d) | Ci,max. (µg/gdb) * | p ** | tSSF (d) | |
| Phenolic acids (hydroxybenzoic acids) | |||||||
| GA | 267.77 ± 11.78 | 275.59 ± 11.90 | 0.6249 | 1. | 248.40 ± 4.41 | 0.0450 | 1. |
| EA | 34.65 ± 3.66 | 129.11 ± 14.82 | 0.0125 | 1. | 136.02 ± 3.84 | 0.0000 | 1. |
| p-HBA | 5.05 ± 2.15 | 9.33 ± 0.02 | 0.0007 | 10. | 10.96 ± 0.43 | 0.0040 | 15. |
| SA | 86.37 ± 2.15 | 95.79 ± 3.22 | 0.0933 | 10. | 86.64 ± 2.86 | 0.5661 | 3. |
| 3,4-DHBA | 138.61 ± 9.87 | 237.46 ± 5.73 | 0.0082 | 10. | 338.03 ± 2.19 | 0.0005 | 3. |
| Phenolic acid (hydroxycinnamic acid) | |||||||
| o-CoA | 4.43 ± 0.11 | 7.74 ± 0.33 | 0.0082 | 10. | 6.83 ± 0.58 | 0.0263 | 4. |
| Flavan-3-ols | |||||||
| EPG | 166.69 ± 8.42 | 246.27 ± 7.32 | 0.0128 | 2. | 373.05 ± 4.25 | 0.0001 | 2. |
| GCG | 291.57 ± 2.35 | 408.79 ± 15.58 | 0.0042 | 2. | 480.89 ± 4.18 | 0.0000 | 2. |
| Flavonols | |||||||
| QU | 173.32 ± 16.54 | 504.31 ± 16.98 | 0.0034 | 1. | 507.29 ± 31.17 | 0.0067 | 1. |
| KA | 10.22 ± 1.06 | 33.78 ± 0.65 | 0.0017 | 1. | 35.75 ± 1.09 | 0.0024 | 1. |
| Procyanidin | |||||||
| PB1 | 304.27 ± 0.37 | 460.39 ± 12.31 | 0.0019 | 2. | 510.34 ± 18.72 | 0.0028 | 1. |
| Stilbenes | |||||||
| RES | 46.07 ± 3.48 | 56.65 ± 3.54 | 0.1204 | 1. | 54.46 ± 0.67 | 0.0353 | 2. |
| VIN | 17.52 ± 1.64 | 44.53 ± 1.12 | 0.0035 | 1. | 46.55 ± 1.30 | 0.0034 | 1. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šelo, G.; Planinić, M.; Tišma, M.; Klarić, A.-M.; Bucić-Kojić, A. Effects of Fungal Solid-State Fermentation on the Profile of Phenolic Compounds and on the Nutritional Properties of Grape Pomace. Microorganisms 2024, 12, 1310. https://doi.org/10.3390/microorganisms12071310
Šelo G, Planinić M, Tišma M, Klarić A-M, Bucić-Kojić A. Effects of Fungal Solid-State Fermentation on the Profile of Phenolic Compounds and on the Nutritional Properties of Grape Pomace. Microorganisms. 2024; 12(7):1310. https://doi.org/10.3390/microorganisms12071310
Chicago/Turabian StyleŠelo, Gordana, Mirela Planinić, Marina Tišma, Ana-Marija Klarić, and Ana Bucić-Kojić. 2024. "Effects of Fungal Solid-State Fermentation on the Profile of Phenolic Compounds and on the Nutritional Properties of Grape Pomace" Microorganisms 12, no. 7: 1310. https://doi.org/10.3390/microorganisms12071310
APA StyleŠelo, G., Planinić, M., Tišma, M., Klarić, A.-M., & Bucić-Kojić, A. (2024). Effects of Fungal Solid-State Fermentation on the Profile of Phenolic Compounds and on the Nutritional Properties of Grape Pomace. Microorganisms, 12(7), 1310. https://doi.org/10.3390/microorganisms12071310





