Streptococcus thermophilus iHA318 Improves Dry Eye Symptoms by Mitigating Ocular Surface Damage in a Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Activation and Culture
2.2. Bacterial Identification
2.3. Cell Culture and Treatment
2.4. NO Production Assay for Assessment of Inflammatory Response
2.5. Osmolality Damage Test
2.6. MTT Assay for Cell Viability
2.7. ROS Production Analysis
2.8. Quantification of Hyaluronic Acid
2.9. Quantification of Sialic Acid
2.10. UVB-Induced Mouse Dry Eye Model and Treatment
2.11. Tear Volume (Schirmer’s Test) and Tear Film Breakup Time (TBUT) Measurements
2.12. Ocular Surface Photography
2.13. Statistics
3. Results
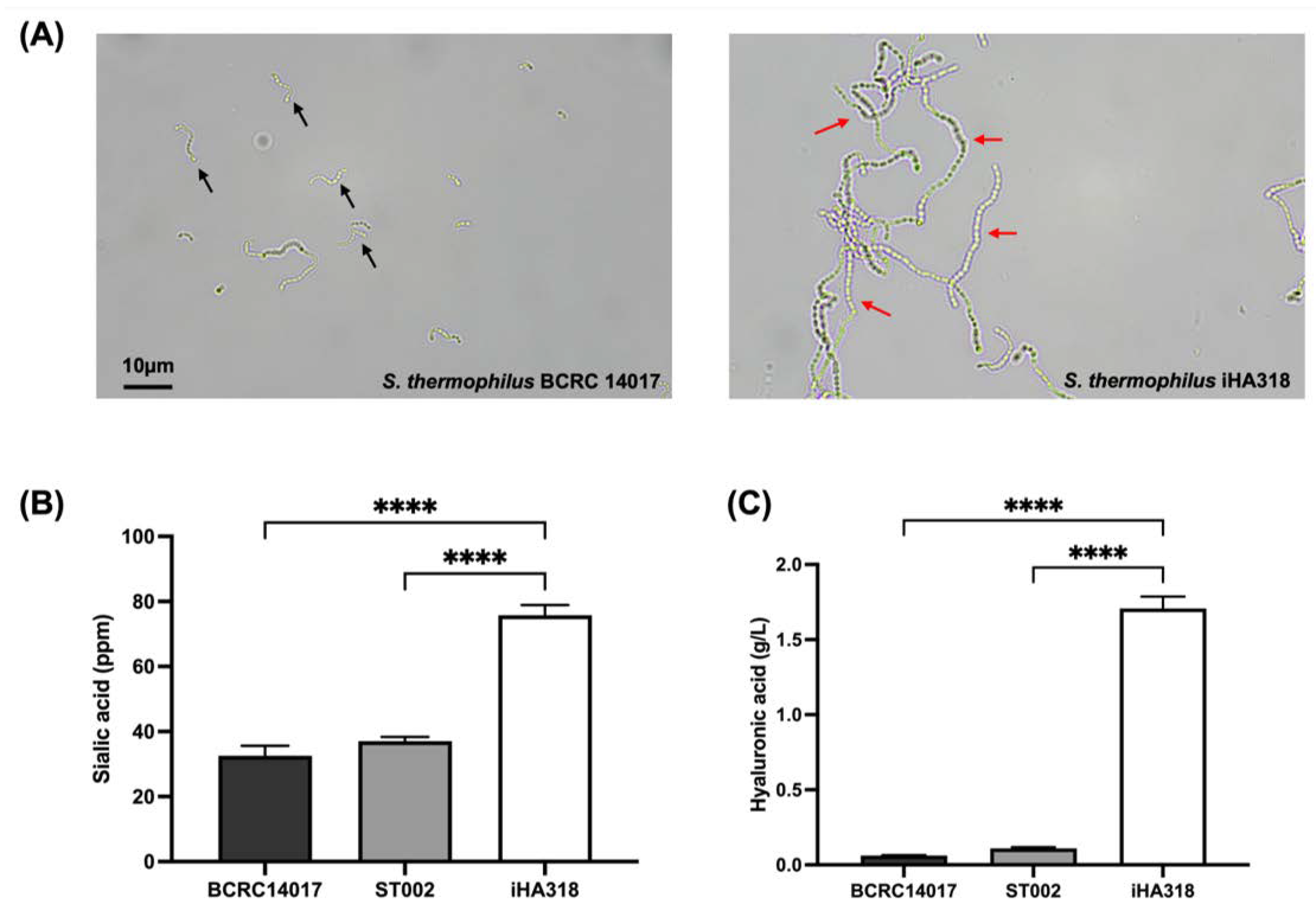
3.1. Colony Characteristics and Bacterial Morphology
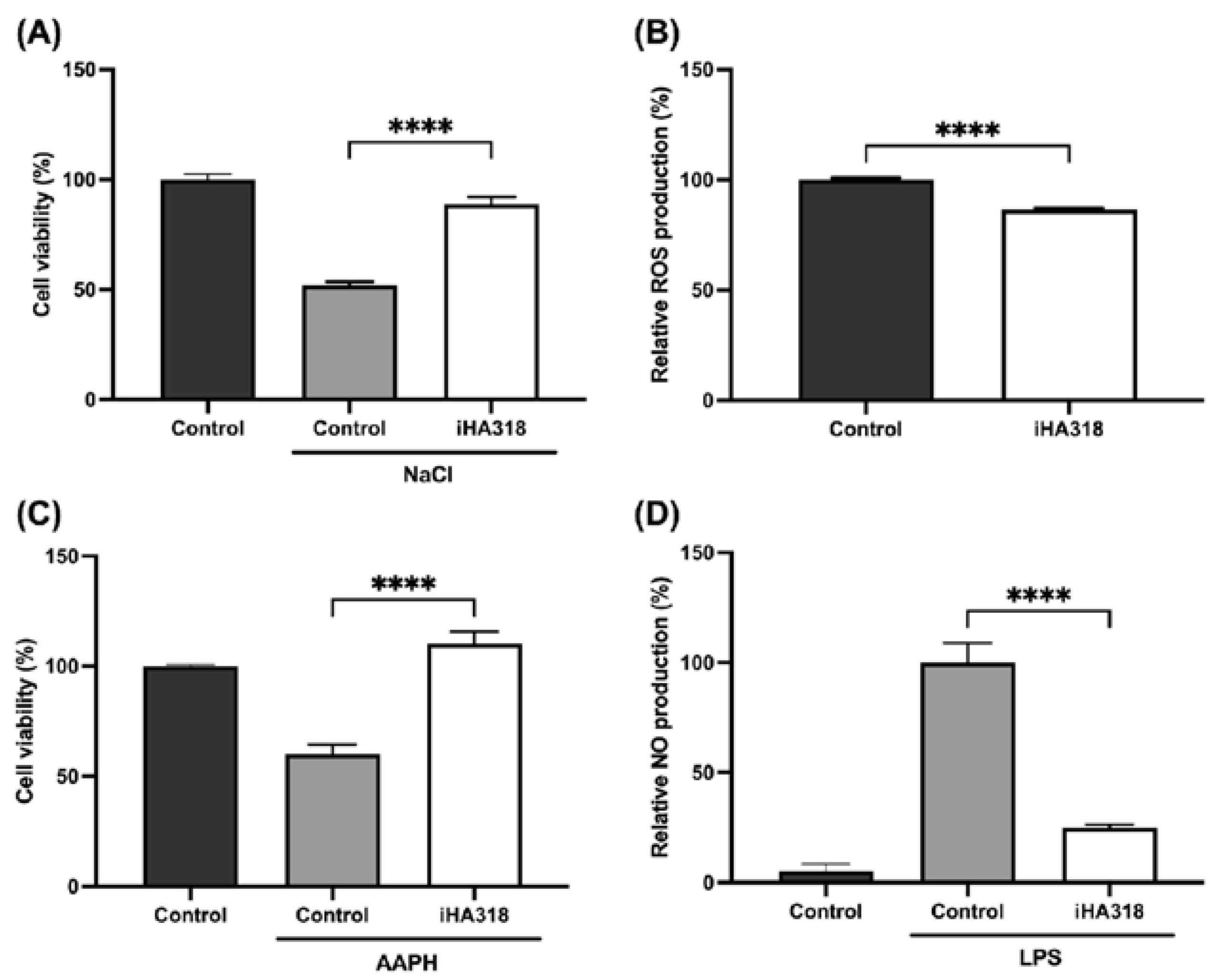
3.2. Protective Effects of S. thermophilus iHA318 on Human Retinal Pigment Epithelial Cells
3.3. Immune Modulatory Effects of S. thermophilus iHA318
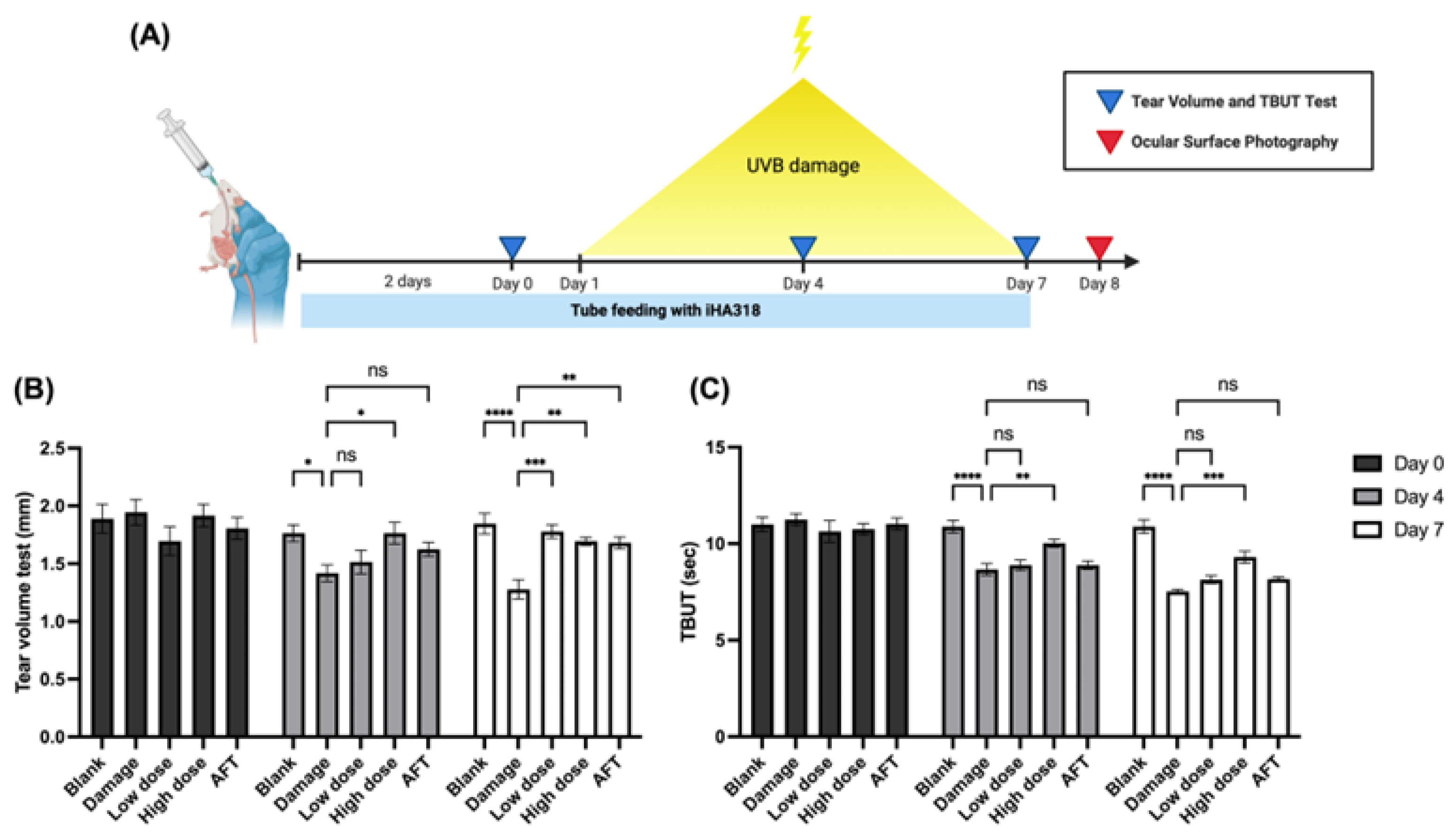
3.4. In Vivo Effects of Probiotics on Tear Production
3.5. Tear Film Break-Up Time (TBUT) Assessment
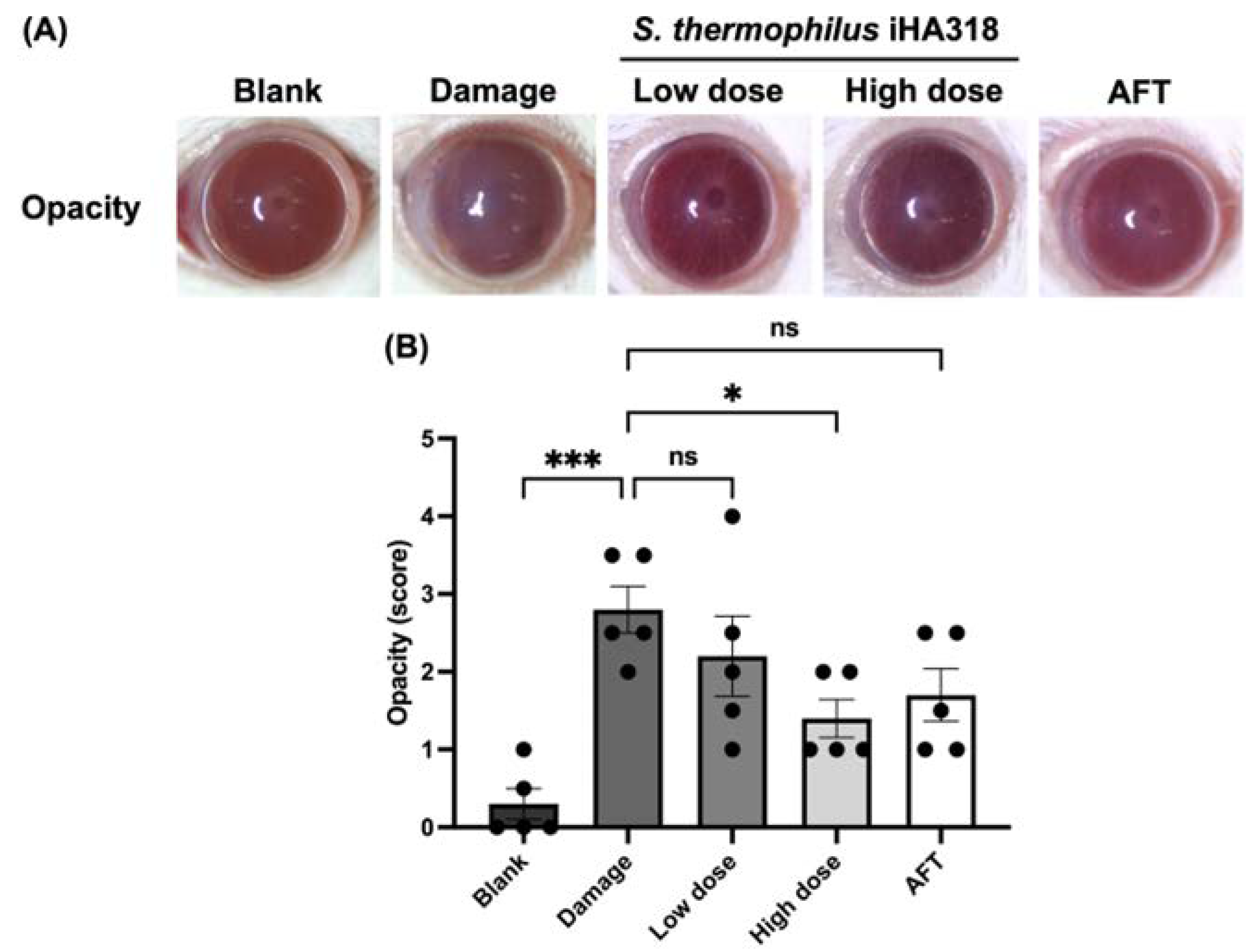
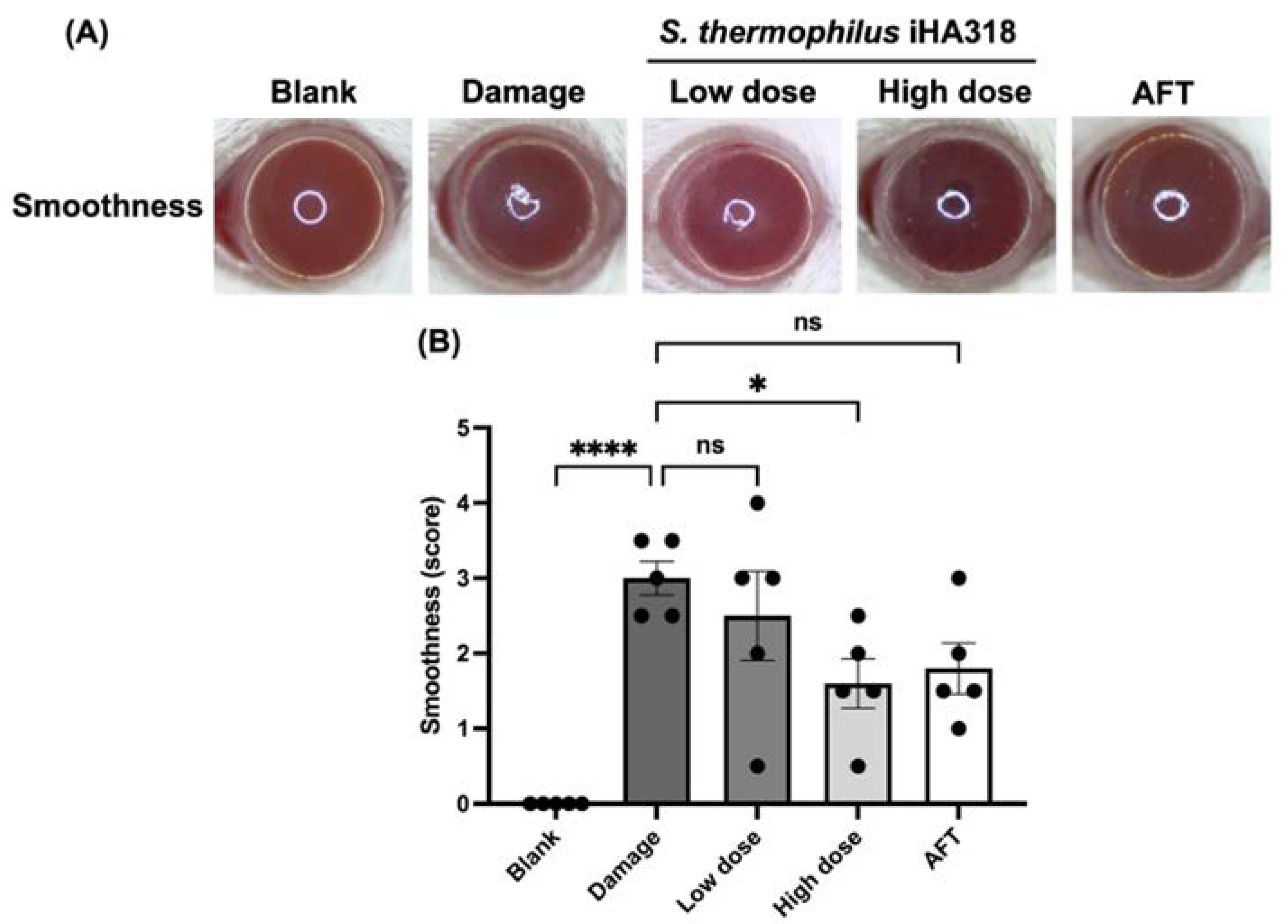
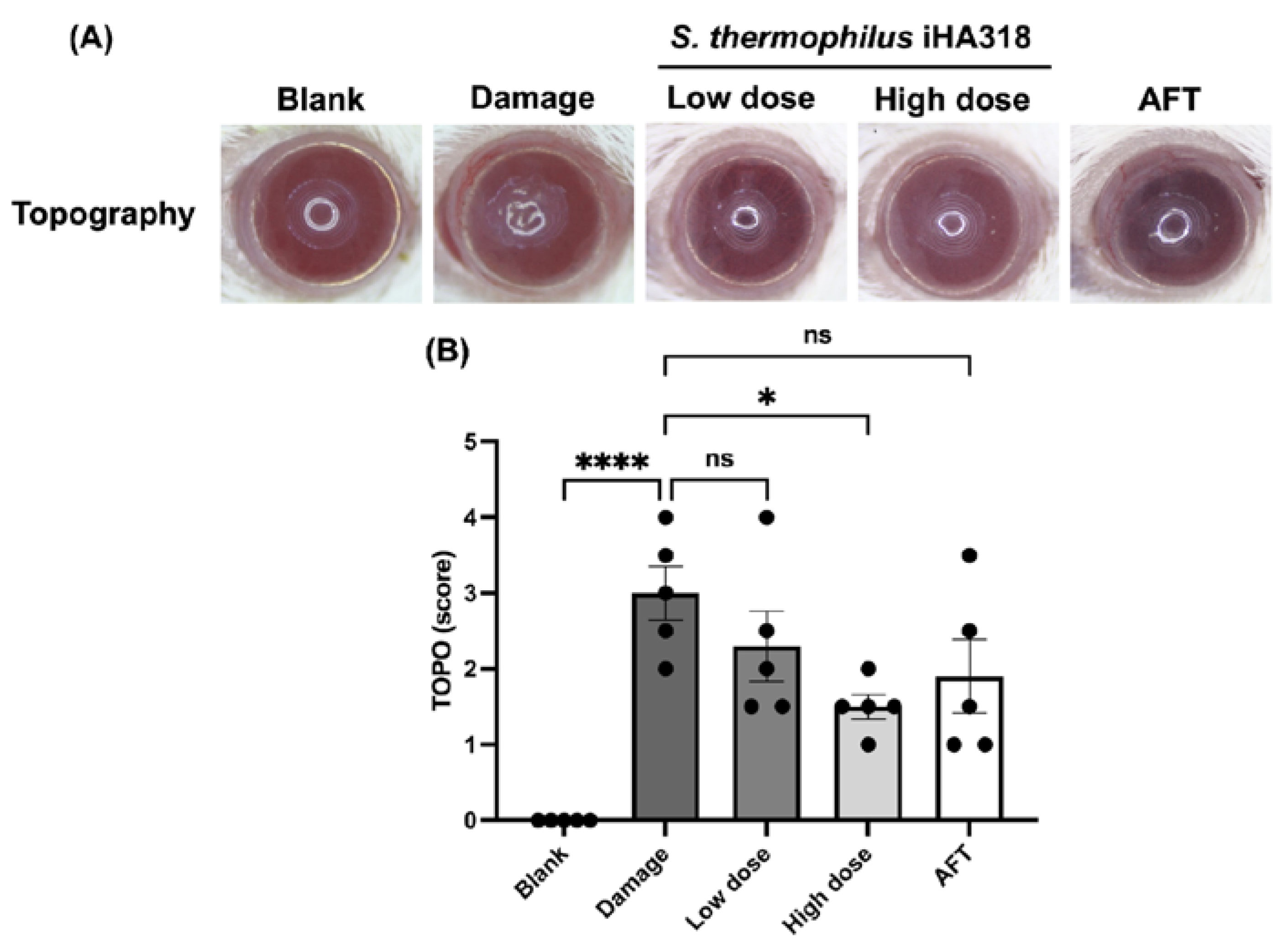
3.6. Ocular Surface Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Bu, J.; Liu, Y.; Zhang, R.; Lin, S.; Zhuang, J.; Sun, L.; Zhang, L.; He, H.; Zong, R.; Wu, Y.; et al. Potential New Target for Dry Eye Disease—Oxidative Stress. Antioxidants 2024, 13, 422. [Google Scholar] [CrossRef] [PubMed]
- Dogru, M.; Kojima, T.; Simsek, C.; Tsubota, K. Potential Role of Oxidative Stress in Ocular Surface Inflammation and Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES163–DES168. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cai, S.; Wu, H.; Pan, J.; Su, M.; Wei, X.; Ye, J.; Ke, L.; Liu, G.; Chu, C. Revolutionizing eye care: The game-changing applications of nano-antioxidants in ophthalmology. Nanoscale 2024, 16, 7307–7322. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Rigas, Y.; Kantor, N.B.; Cohen, N.K.; Tomlinson, A.; Leger, A.J.S.; Galor, A. Living with your biome: How the bacterial microbiome impacts ocular surface health and disease. Expert. Rev. Ophthalmol. 2024, 19, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Kunst, C.; Schmid, S.; Michalski, M.; Tümen, D.; Buttenschön, J.; Müller, M.; Gülow, K. The Influence of Gut Microbiota on Oxidative Stress and the Im mune System. Biomedicines 2023, 11, 1388. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Mousa, W.K.; Chehadeh, F.; Husband, S. Microbial dysbiosis in the gut drives systemic autoimmune diseases. Front. Immunol. 2022, 13, 906258. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Zogg, H.; Wei, L.; Bartlett, A.; Ghoshal, U.C.; Rajender, S.; Ro, S. Gut Microbial Dysbiosis in the Pathogenesis of Gastrointestinal Dysmotility and Metabolic Disorders. J. Neurogastroenterol. Motil. 2021, 27, 19–34. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Bene ficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kim, S.W.; Verma, R.; Noh, J.; Park, J.C.; Park, S.; Lee, H.; Park, H.E.; Kim, C.J.; Byun, S.; et al. Probiotic Consortium Confers Synergistic Anti-Inflammatory Effects in Inflammatory Disorders. Nutrients 2024, 16, 790. [Google Scholar] [CrossRef]
- Campagnoli, L.I.M.; Varesi, A.; Barbieri, A.; Marchesi, N.; Pascale, A. Targeting the Gut–Eye Axis: An Emerging Strategy to Face Ocular Diseases. Int. J. Mol. Sci. 2023, 24, 13338. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive oxygen species (ROS) scavenging biomaterials for anti-in flammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef]
- Jurášková, D.; Ribeiro, S.C.; Silva, C.C.G. Exopolysaccharides Produced by Lactic Acid Bacteria: From Biosynthesis to Health-Promoting Properties. Foods 2022, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Salimi, F.; Farrokh, P. Recent advances in the biological activities of microbial exopolysaccharides. World J. Microbiol. Biotechnol. 2023, 39, 213. [Google Scholar] [CrossRef] [PubMed]
- Sudha, P.N.; Rose, M.H. Beneficial effects of hyaluronic acid. Adv. Food Nutr. Res. 2014, 72, 137–176. [Google Scholar]
- Hynnekleiv, L.; Magno, M.; Vernhardsdottir, R.R.; Moschowits, E.; Tønseth, K.A.; Dartt, D.A.; Vehof, J.; Utheim, T.P. Hyaluronic acid in the treatment of dry eye disease. Acta Ophthalmol. 2022, 100, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-J.; Lee, W.-Y.; Kim, Y.-J.; Hong, Y.-P. A Meta-Analysis of the Efficacy of Hyaluronic Acid Eye Drops for the Treatment of Dry Eye Syndrome. Int. J. Environ. Res. Public Health 2021, 18, 2383. [Google Scholar] [CrossRef] [PubMed]
- Böhm, S.; Schwab, I.; Lux, A.; Nimmerjahn, F. The role of sialic acid as a mod ulator of the anti-inflammatory activity of IgG. Semin. Immunopathol. 2012, 34, 443–453. [Google Scholar] [CrossRef]
- Li, D.; Xie, T.; Guo, T.; Hu, Z.; Li, M.; Tang, Y.; Wu, Q.; Luo, F.; Lin, Q.; Wang, H. Sialic acid exerts anti-inflammatory effect through inhibiting MAPK-NF-κB/AP-1 pathway and apoptosis in ulcerative colitis. J. Funct. Foods 2023, 101, 105416. [Google Scholar] [CrossRef]
- Wang, B. Sialic Acid Is an Essential Nutrient for Brain Development and Cognition. Annu. Rev. Nutr. 2009, 29, 177–222. [Google Scholar] [CrossRef]
- Sakellaris, G.; Kolisis, F.N.; Evangelopoulos, A.E. Presence of sialic acids in Lactobacillus plantarum. Biochem. Biophys. Res. Commun. 1988, 155, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, A.; Markoulli, M.; Papas, E.; Flanagan, J. The Impact of Probiotics and Prebiotics on Dry Eye Disease Signs and Symptoms. J. Clin. Med. 2022, 11, 4889. [Google Scholar] [CrossRef]
- Lee, K.; Jeong, J.W.; Shim, J.J.; Hong, H.S.; Kim, J.Y.; Lee, J.L. Lactobacillus fermentum HY7302 Improves Dry Eye Symptoms in a Mouse Model of Benzalkonium Chloride-Induced Eye Dysfunction and Human Conjunctiva Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 10378. [Google Scholar] [CrossRef]
- Chan, K.G.; Atkinson, S.; Mathee, K.; Sam, C.K.; Chhabra, S.R.; Cámara, M.; Koh, C.; Williams, P. Characterization of N-acylhomoserine lactone-degrading bacte ria associated with the Zingiber officinale (ginger) rhizosphere: Co-existence of quorum quenching and quorum sensing in Acinetobacter and Burkholderia. BMC Microbiol. 2011, 11, 51. [Google Scholar] [CrossRef]
- Nikkari, S. Broad-range bacterial detection and the analysis of unexplained death and critical illness. Emerg. Infect. Dis. 2002, 8, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Little, S.V.; Hillhouse, A.E.; Lawhon, S.D.; Dunning Hotopp, J.C. Whole-Ge nome Sequences of an Abortive Bacillus safensis Strain Isolated from a Mare’s Uterus. Microbiol. Resour. Announc. 2020, 9, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Skoza, L.; Mohos, S. Stable thiobarbituric acid chromophore with dimethyl sulphoxide. Application to sialic acid assay in analytical de-O-acetylation. Biochem. J. 1976, 159, 457–462. [Google Scholar] [CrossRef]
- Chang, H.H.; Chang, W.J.; Jhou, B.Y.; Kuo, S.Y.; Hsu, J.H.; Chen, Y.L.; Chen, C.; Lin, D.P.-C. Efficacy of Cordyceps cicadae (Ascomycota) Mycelium Sup plementation for Amelioration of Dry Eye Symptoms: A Randomized, Double-Blind Clinical Pilot Study. Int. J. Med. Mushrooms 2022, 24, 57–67. [Google Scholar] [CrossRef]
- Tsubota, K. Short Tear Film Breakup Time–Type Dry Eye. Investig. Ophthal Mology Vis. Sci. 2018, 59, DES64–DES70. [Google Scholar] [CrossRef]
- Chen, B.Y.; Lin, D.P.C.; Chang, L.S.; Huang, T.P.; Liu, H.J.; Luk, C.P.; Lo, Y.-L.; Chang, H.-H. Dietary α-lipoic acid prevents UVB-induced corneal and con junctival degeneration through multiple effects. Investig. Ophthalmol. Vis Sci. 2013, 54, 6757–6766. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cougnard-Gregoire, A.; Merle, B.M.; Aslam, T.; Seddon, J.M.; Aknin, I.; Klaver, C.C.; Garhofer, G.; Layana, A.G.; Minnella, A.M.; Silva, R.; et al. Blue Light Exposure: Ocular Hazards and Preven tion-A Narrative Review. Ophthalmol. Ther. 2023, 12, 755–788. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, X.; Li, J.; Wang, Y.; Chen, Q.; Hou, C.; Garrett, Q. Efficacy of Osmoprotectants on Prevention and Treatment of Mu rine Dry Eye. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6287–6297. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-H.; Lee, C.-H.; Wang, H.-M.; Chang, Y.-W.; Lin, C.-Y.; Chen, C.-Y.; Chen, Y.-H. 6-Dehydrogingerdione restrains lipopolysaccharide-induced inflammatory responses in RAW 264.7 macrophages. J. Agric. Food Chem. 2014, 62, 9171–9179. [Google Scholar] [CrossRef] [PubMed]
- Suriyaprom, S.; Srisai, P.; Intachaisri, V.; Kaewkod, T.; Pekkoh, J.; Desvaux, M.; Tragoolpua, Y. Antioxidant and Anti-Inflammatory Activity on LPS-Stimulated RAW 264.7 Macrophage Cells of White Mulberry (Morus alba L.) Leaf Extracts. Molecules 2023, 28, 4395. [Google Scholar] [CrossRef] [PubMed]
- Ohgami, K.; Shiratori, K.; Kotake, S.; Nishida, T.; Mizuki, N.; Yazawa, K.; Ohno, S. Effects of Astaxanthin on Lipopolysaccharide-Induced Inflammation In Vitro and In Vivo. Investig. Opthalmol. Vis. Sci. 2003, 44, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Golden, M.I.; Meyer, J.J.; Patel, B.C. Dry Eye Syndrome. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Nachtigall, C.; Weber, C.; Rothenburger, S.; Jaros, D.; Rohm, H. Test parameters and cell chain length of Streptococcus ther mophilus affect the microbial adhesion to hydrocarbons assay: A methodical approach. FEMS Microbiol. Lett. 2019, 366, fnz150. [Google Scholar] [CrossRef] [PubMed]
- Paterniti, I.; Scuderi, S.A.; Cambria, L.; Nostro, A.; Esposito, E.; Marino, A. Protective Effect of Probiotics against Pseudomonas aeruginosa Infection of Human Corneal Epithelial Cells. Int. J. Mol. Sci. 2024, 25, 1770. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Miwa, Y.; Jounai, K.; Fujiwara, D.; Kurihara, T.; Kanauchi, O. Lactobacillus paracasei KW3110 Prevents Blue Light-Induced Inflammation and Degeneration in the Retina. Nutrients 2018, 10, 1991. [Google Scholar] [CrossRef]
- Xing, Y.; Liang, S.; Zhang, L.; Ni, H.; Zhang, X.; Wang, J.; Yang, L.; Song, S.; Li, H.H.; Jia, C.; et al. Combination of Lactobacillus fermentum NS9 and aronia anthocy anidin extract alleviates sodium iodate-induced retina degeneration. Sci. Rep. 2023, 13, 8380. [Google Scholar] [CrossRef] [PubMed]
- de Paiva, C.S.; Leger, A.J.S.; Caspi, R.R. Mucosal immunology of the ocular surface. Mucosal Immunol. 2022, 15, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.; Pflugfelder, S.C.; Britton, R.; De Paiva, C.S. Probiotic Limosilacto bacillus reuteri DSM 17938 suppresses corneal barrier dysfunction and conjunctival goblet cell reduction in mice subjected to desiccating stress. Investig. Ophthalmol. Vis. Sci. 2023, 64, 697. [Google Scholar]
- Nagai, N.; Otake, H. Novel drug delivery systems for the management of dry eye. Adv. Drug Deliv. Rev. 2022, 191, 114582. [Google Scholar] [CrossRef]
- Chhadva, P.; Goldhardt, R.; Galor, A. Meibomian Gland Disease: The Role of Gland Dysfunction in Dry Eye Disease. Ophthalmology 2017, 124, S20–S26. [Google Scholar] [CrossRef]
- Yu, K.; Bunya, V.; Maguire, M.; Asbell, P.; Ying, G.-S. Systemic Conditions Associated with Severity of Dry Eye Signs and Symptoms in the Dry Eye Assessment and Management Study. Ophthalmology 2021, 128, 1384–1392. [Google Scholar] [CrossRef]

| Group | Universal Primer | Sequences |
|---|---|---|
| 1 | 27F | AGAGTTTGATCMTGGCTCAG |
| 1525R | AAGGAGGTGWTCCARCC | |
| 2 | 8F2 | TGGAGAGTTTGATCCTGGCTCAG |
| 806R | GGACTACCAGGGTATCTAAT | |
| 3 | fD1 mod | AGAGTTTGATCYTGGYTYAG |
| 16S1RR-B | CTTTACGCCCARTRAWTCCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-W.; Sun, Y.-L.; Chu, E.; Hung, Y.-Y.; Liao, W.-C.; Tsai, S.-M.; Lu, T.-H.; Huang, P.-C.; Yu, C.-H.; Lee, S.-Y.; et al. Streptococcus thermophilus iHA318 Improves Dry Eye Symptoms by Mitigating Ocular Surface Damage in a Mouse Model. Microorganisms 2024, 12, 1306. https://doi.org/10.3390/microorganisms12071306
Chang Y-W, Sun Y-L, Chu E, Hung Y-Y, Liao W-C, Tsai S-M, Lu T-H, Huang P-C, Yu C-H, Lee S-Y, et al. Streptococcus thermophilus iHA318 Improves Dry Eye Symptoms by Mitigating Ocular Surface Damage in a Mouse Model. Microorganisms. 2024; 12(7):1306. https://doi.org/10.3390/microorganisms12071306
Chicago/Turabian StyleChang, Yu-Wei, Yen-Ling Sun, Evelyn Chu, Yi-Yun Hung, Wei-Chieh Liao, Su-Min Tsai, Tsung-Han Lu, Pin-Chao Huang, Chin-Hsiu Yu, Shao-Yu Lee, and et al. 2024. "Streptococcus thermophilus iHA318 Improves Dry Eye Symptoms by Mitigating Ocular Surface Damage in a Mouse Model" Microorganisms 12, no. 7: 1306. https://doi.org/10.3390/microorganisms12071306
APA StyleChang, Y.-W., Sun, Y.-L., Chu, E., Hung, Y.-Y., Liao, W.-C., Tsai, S.-M., Lu, T.-H., Huang, P.-C., Yu, C.-H., Lee, S.-Y., Chang, H.-H., & Lin, D. P.-C. (2024). Streptococcus thermophilus iHA318 Improves Dry Eye Symptoms by Mitigating Ocular Surface Damage in a Mouse Model. Microorganisms, 12(7), 1306. https://doi.org/10.3390/microorganisms12071306







