Effects of Six Natural Compounds and Their Derivatives on the Control of Coccidiosis in Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animals
2.3. Parasites
2.4. Compounds
2.5. Experimental Design
2.6. Cecum Microbiota Amplification and Sequencing
2.7. Bioinformatics
2.8. Statistical Analysis
3. Results
3.1. Effect of Natural Compounds and Their Derivatives on the Growth Performance of Chickens Infected with E. tenella
3.2. Anticoccidial Effects of Natural Compounds and Their Derivatives
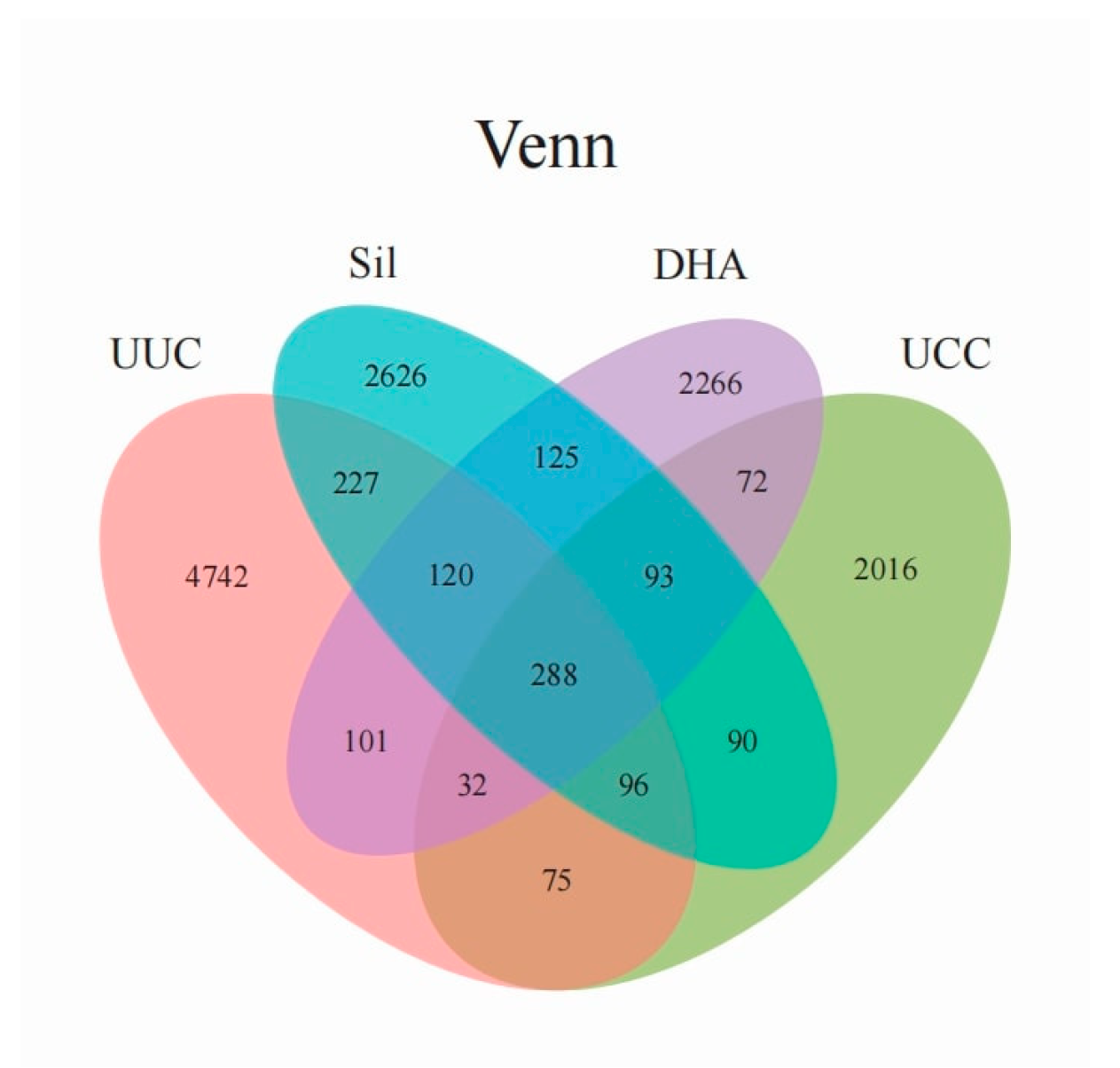
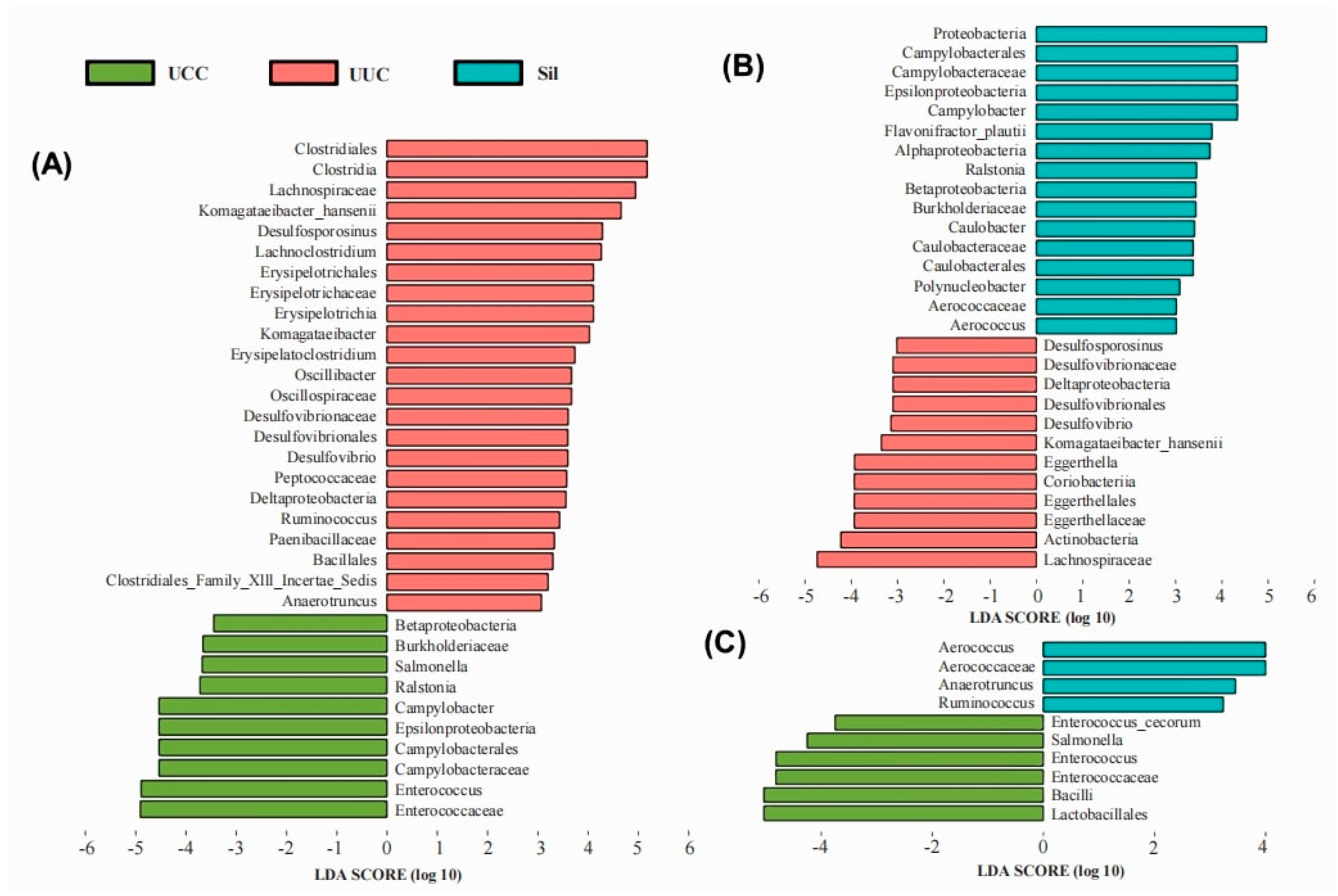
3.3. Cecum Microbiota
3.3.1. Alpha Diversity
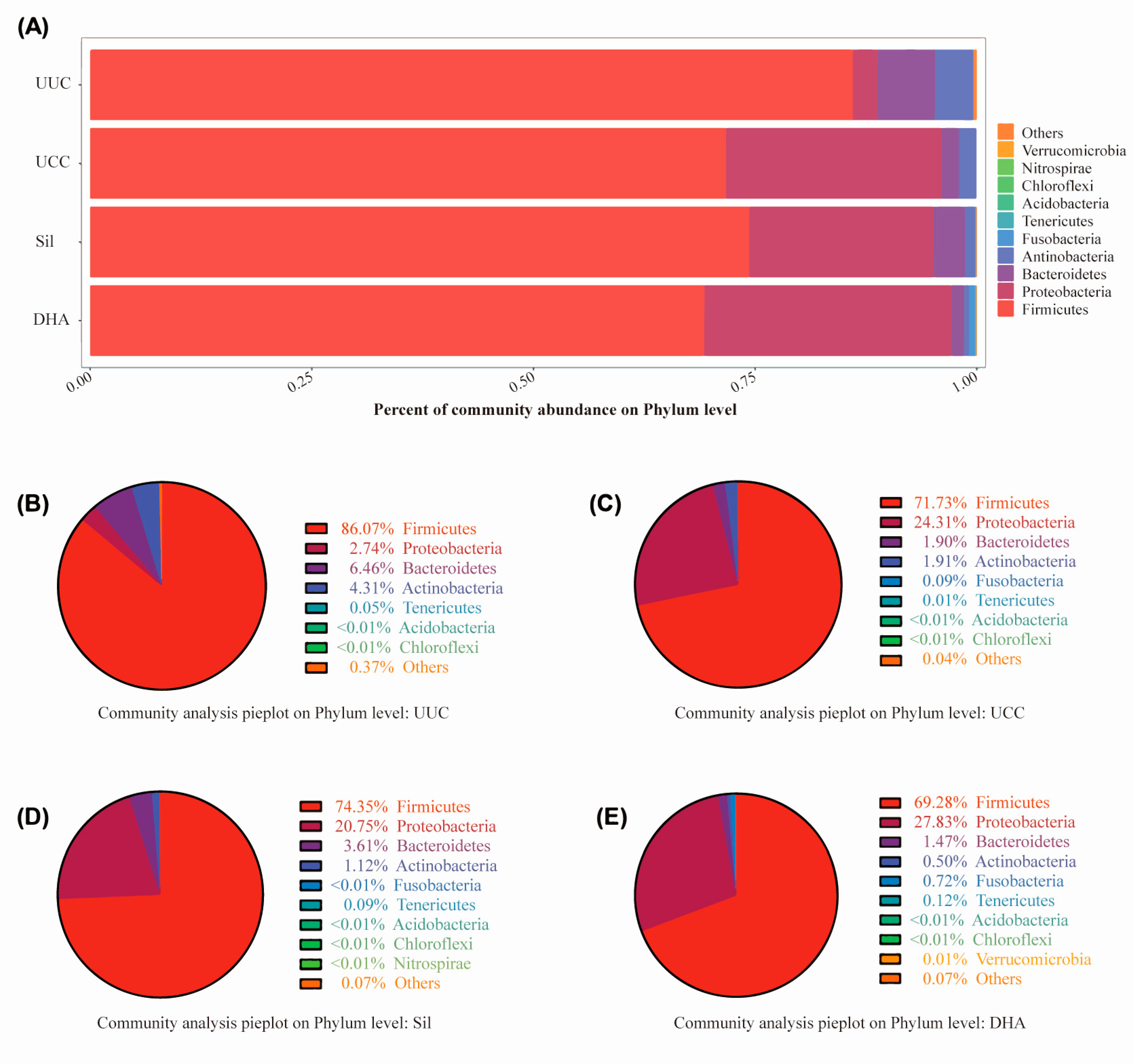
3.3.2. Effects of Treatments on Bacterial Abundance at the Phylum Level
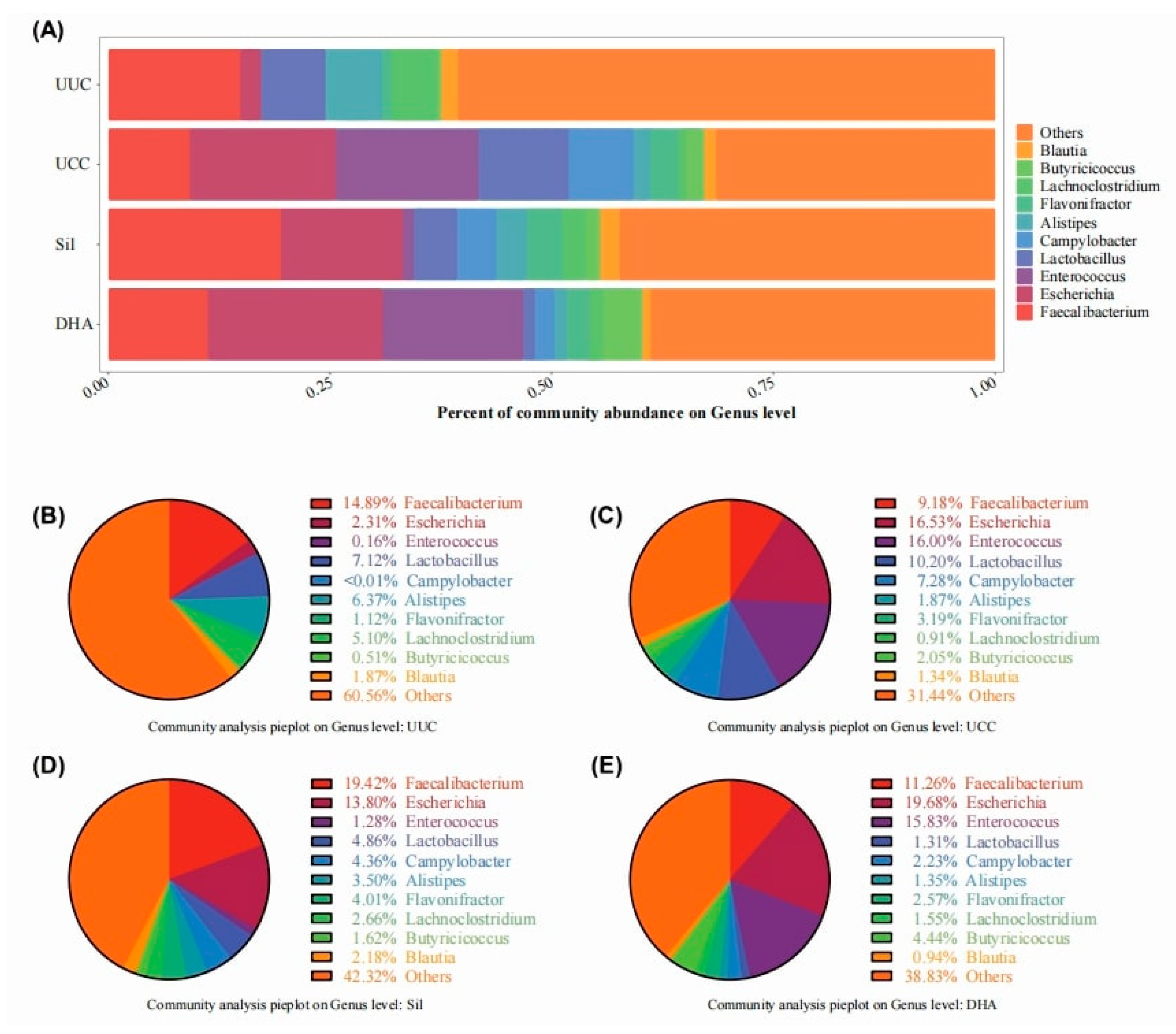
3.3.3. Effects of Treatments on Bacterial Abundance at the Genus Level
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blake, D.P.; Tomley, F.M. Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol. 2014, 30, 12–19. [Google Scholar] [CrossRef]
- Pop, L.; Györke, A.; Tǎbǎran, A.F.; Dumitrache, M.O.; Kalmár, Z.; Magdaş, C.; Mircean, V.; Zagon, D.; Balea, A.; Cozma, V. Effects of artemisinin in broiler chickens challenged with Eimeria acervulina, E. maxima and E. tenella in battery trials. Vet. Parasitol. 2015, 214, 264–271. [Google Scholar] [CrossRef]
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 2020, 51, 115. [Google Scholar] [CrossRef]
- Soutter, F.; Werling, D.; Tomley, F.M.; Blake, D.P. Poultry coccidiosis: Design and interpretation of vaccine studies. Front. Vet. Sci. 2020, 7, 101. [Google Scholar] [CrossRef]
- Chapman, H. Biochemical, genetic and applied aspects of drug resistance in Eimeria parasites of the fowl. Avian Pathol. 1997, 26, 221–244. [Google Scholar] [CrossRef]
- Shirley, M.W.; Smith, A.L.; Blake, D.P. Challenges in the successful control of the avian coccidia. Vaccine 2007, 25, 5540–5547. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-Castañeda, R.E.; Dantán-González, E. Control of avian coccidiosis: Future and present natural alternatives. BioMed Res. Int. 2015, 2015, 430610. [Google Scholar] [CrossRef]
- Abbas, R.; Colwell, D.; Gilleard, J. Botanicals: An alternative approach for the control of avian coccidiosis. World’s Poult. Sci. J. 2012, 68, 203–215. [Google Scholar] [CrossRef]
- Muthamilselvan, T.; Kuo, T.F.; Wu, Y.C.; Yang, W.C. Herbal remedies for coccidiosis control: A review of plants, compounds, and anticoccidial actions. Evid.-Based Complement. Altern. Med. Ecam 2016, 2016, 657981. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, B.; Yue, Y.; Zhou, Q.; Du, A. Anticoccidial activity of traditional Chinese herbal Dichroa febrifuga Lour. extract against Eimeria tenella infection in chickens. Parasitol. Res. 2012, 111, 2229–2233. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Surai, A.; Earle-Payne, K. Silymarin and Inflammation: Food for Thoughts. Antioxidants 2024, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Bahari, H.; Shahraki Jazinaki, M.; Rashidmayvan, M.; Taheri, S.; Amini, M.R.; Malekahmadi, M. The effects of silymarin consumption on inflammation and oxidative stress in adults: A systematic review and meta-analysis. In Inflammopharmacology; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–15. [Google Scholar]
- Soleimani, V.; Delghandi, P.S.; Moallem, S.A.; Karimi, G. Safety and toxicity of silymarin, the major constituent of milk thistle extract: An updated review. Phytother. Res. 2019, 33, 1627–1638. [Google Scholar] [CrossRef]
- Carpio, M.B.; Valdes-Pena, M.A.; Molina, D.A.; Cabello, S.E.E.; Guerrero, C.A.S.; Cribillero, G.; Coca, K.F.V.; Icochea, E. Evaluation of commercial doses of a feed additive and silymarin on broiler performance with and without CCl4-induced liver damage. Poult. Sci. 2024, 103, 103567. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, A.; Samadi, F.; Ramezanpour, S.; Yousefdoust, S. Hepatoprotective effects of silymarin on CCl4-induced hepatic damage in broiler chickens model. Toxicol. Rep. 2019, 6, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Gazak, R.; Walterova, D.; Kren, V. Silybin and silymarin-new and emerging applications in medicine. Curr. Med. Chem. 2007, 14, 315–338. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.R.; Nour, A.A.; Dakrory, A.E. Amelioration of silymarin against cadmium-induced toxicity in pregnant rats and their fetuses. Birth Defects Res. 2023, 115, 1192–1207. [Google Scholar] [CrossRef]
- Jiao, J.; Yang, Y.; Liu, M.; Li, J.; Cui, Y.; Yin, S.; Tao, J. Artemisinin and Artemisia annua leaves alleviate Eimeria tenella infection by facilitating apoptosis of host cells and suppressing inflammatory response. Vet. Parasitol. 2018, 254, 172–177. [Google Scholar] [CrossRef]
- Wiedosari, E.; Wardhana, A.H. Anticoccidial activity of Artemisinin and Extract of Artemesia annua leaves in chicken infected by Eimeria tenella. J. Ilmu Ternak dan Veter 2018, 22, 196–204. [Google Scholar] [CrossRef]
- Guo, Z. Artemisinin anti-malarial drugs in China. Acta Pharm. Sin. B 2016, 6, 115–124. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, X.; Chen, W.; Chen, Y.; Zhang, Q.; Mo, S.; Lu, J. Dihydroartemisinin: A potential natural anticancer drug. Int. J. Biol. Sci. 2021, 17, 603. [Google Scholar] [CrossRef]
- Yang, C.; Luo, P.; Chen, S.-J.; Deng, Z.-C.; Fu, X.-L.; Xu, D.-N.; Tian, Y.-B.; Huang, Y.-M.; Liu, W.-J. Resveratrol sustains intestinal barrier integrity, improves antioxidant capacity, and alleviates inflammation in the jejunum of ducks exposed to acute heat stress. Poult. Sci. 2021, 100, 101459. [Google Scholar] [CrossRef] [PubMed]
- Bastaki, S.M.; Amir, N.; Adeghate, E.; Ojha, S. Nerolidol, a sesquiterpene, attenuates oxidative stress and inflammation in acetic acid-induced colitis in rats. Mol. Cell. Biochem. 2021, 476, 3497–3512. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.M.; Ma, Y.F.; Takvorian, P.M.; Tanowitz, H.B.; Wittner, M. Bradyzoite development in Toxoplasma gondii and the hsp70 stress response. Infect. Immun. 1998, 66, 3295–3302. [Google Scholar] [CrossRef]
- Rodrigues Goulart, H.; Kimura, E.A.; Peres, V.J.; Couto, A.S.; Aquino Duarte, F.A.; Katzin, A.M. Terpenes arrest parasite development and inhibit biosynthesis of isoprenoids in Plasmodium falciparum. Antimicrob. Agents Chemother. 2004, 48, 2502–2509. [Google Scholar] [CrossRef]
- Chen, Q.W.; Dong, K.; Qin, H.X.; Yang, Y.K.; He, J.L.; Li, J.; Zheng, Z.W.; Chen, D.L.; Chen, J.P. Direct and indirect inhibition effects of resveratrol against Toxoplasma gondii tachyzoites in vitro. Antimicrob. Agents Chemother. 2019, 63, e01233-01218. [Google Scholar] [CrossRef]
- Seo, S.H.; Kim, S.G.; Shin, J.H.; Ham, D.W.; Shin, E.H. Toxoplasma gra16 inhibits nf-κb activation through pp2a-b55 upregulation in non-small-cell lung carcinoma cells. Int. J. Mol. Sci. 2020, 21, 6642. [Google Scholar] [CrossRef] [PubMed]
- Eckert, J.; Braun, R.; Shirley, M.; Coudert, P. Biotechnology: Guidelines on Techniques in Coccidiosis Research; OPOCE: Luxembourg, 1995. [Google Scholar]
- Yin, W.; Gao, Q.; Liu, X.; Tang, H. In vivo Effect of Dihydroartemisinin and Azithromycin on the Ultrastructure of Toxoplasma gondii Tachyzoites. Chin. J. Parasitol. Parasit. 2009, 27, 325–327. [Google Scholar]
- Armanini, E.H.; Boiago, M.M.; Cécere, B.G.d.O.; Oliveira, P.V.; Teixeira, C.J.; Strapazzon, J.V.; Bottari, N.B.; Silva, A.D.; Fracasso, M.; Vendruscolo, R.G. Protective effects of silymarin in broiler feed contaminated by mycotoxins: Growth performance, meat antioxidant status, and fatty acid profiles. Trop. Anim. Health Prod. 2021, 53, 442. [Google Scholar] [CrossRef]
- Lv, Z.; Fan, H.; Zhang, B.; Xing, K.; Guo, Y. Dietary genistein supplementation for breeders and their offspring improves the growth performance and immune function of broilers. Sci. Rep. 2018, 8, 5161. [Google Scholar] [CrossRef]
- Zhou, N.; Tian, Y.; Liu, W.; Tu, B.; Xu, W.; Gu, T.; Zou, K.; Lu, L. Protective effects of resveratrol and apigenin dietary supplementation on serum antioxidative parameters and mRNAs expression in the small intestines of diquat-challenged pullets. Front. Vet. Sci. 2022, 9, 850769. [Google Scholar] [CrossRef]
- Sun, L.; Guo, L.; Xu, G.; Li, Z.; Appiah, M.O.; Yang, L.; Lu, W. Quercetin reduces inflammation and protects gut microbiota in broilers. Molecules 2022, 27, 3269. [Google Scholar] [CrossRef]
- Klopell, F.C.; Lemos, M.; Sousa, J.P.B.; Comunello, E.; Maistro, E.L.; Bastos, J.K.; Andrade, S.F.d. Nerolidol, an antiulcer constituent from the essential oil of Baccharis dracunculifolia DC (Asteraceae). Z. Naturforsch. C 2007, 62, 537–542. [Google Scholar] [CrossRef]
- Johnson, J.; Reid, W.M. Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970, 28, 30–36. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Fraschini, F.; Demartini, G.; Esposti, D. Pharmacology of silymarin. Clin. Drug Investig. 2002, 22, 51–65. [Google Scholar] [CrossRef]
- Janse, C.; Waters, A.; Kos, J.; Lugt, C. Comparison of in vivo and in vitro antimalarial activity of artemisinin, dihydroartemisinin and sodium artesunate in the Plasmodium berghei-rodent model. Int. J. Parasitol. 1994, 24, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Angus, B. Novel anti-malarial combinations and their toxicity. Expert Rev. Clin. Pharmacol. 2014, 7, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Lapczynski, A.; Bhatia, S.; Letizia, C.; Api, A. Fragrance material review on nerolidol (isomer unspecified). Food Chem. Toxicol. 2008, 46, S247–S250. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.M.; Palmeira, C.M.; Oliveira, M.M.; Santos, D.; Simões, A.M.; Rocha, S.M.; Coimbra, M.A.; Peixoto, F. Nerolidol effects on mitochondrial and cellular energetics. Toxicology In Vitro 2012, 26, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Madlala, T.; Okpeku, M.; Adeleke, M.A. Understanding the interactions between Eimeria infection and gut microbiota, towards the control of chicken coccidiosis: A review. Parasite 2021, 28, 48. [Google Scholar] [CrossRef] [PubMed]
- Jung, A.; Chen, L.R.; Suyemoto, M.M.; Barnes, H.J.; Borst, L.B. A review of Enterococcus cecorum infection in poultry. Avian Dis. 2018, 62, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Botin, T.; Ramirez-Chamorro, L.; Vidic, J.; Langella, P.; Martin-Verstraete, I.; Chatel, J.-M.; Auger, S. The Tolerance of Gut Commensal Faecalibacterium to Oxidative Stress Is Strain Dependent and Relies on Detoxifying Enzymes. Appl. Environ. Microbiol. 2023, 89, e00606–e00623. [Google Scholar] [CrossRef]
- Latorre, J.D.; Adhikari, B.; Park, S.H.; Teague, K.D.; Graham, L.E.; Mahaffey, B.D.; Baxter, M.F.; Hernandez-Velasco, X.; Kwon, Y.M.; Ricke, S.C. Evaluation of the epithelial barrier function and ileal microbiome in an established necrotic enteritis challenge model in broiler chickens. Front. Vet. Sci. 2018, 5, 199. [Google Scholar] [CrossRef]
- Zhou, B.-H.; Jia, L.-S.; Wei, S.-S.; Ding, H.-Y.; Yang, J.-Y.; Wang, H.-W. Effects of Eimeria tenella infection on the barrier damage and microbiota diversity of chicken cecum. Poult. Sci. 2020, 99, 1297–1305. [Google Scholar] [CrossRef]
- Singh, K.; Shah, T.; Deshpande, S.; Jakhesara, S.; Koringa, P.; Rank, D.; Joshi, C. High through put 16S rRNA gene-based pyrosequencing analysis of the fecal microbiota of high FCR and low FCR broiler growers. Mol. Biol. Rep. 2012, 39, 10595–10602. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Faecalibacterium prausnitzii: From microbiology to diagnostics and prognostics. ISME J. 2017, 11, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Steck, N.; Hoffmann, M.; Sava, I.G.; Kim, S.C.; Hahne, H.; Tonkonogy, S.L.; Mair, K.; Krueger, D.; Pruteanu, M.; Shanahan, F. Enterococcus faecalis metalloprotease compromises epithelial barrier and contributes to intestinal inflammation. Gastroenterology 2011, 141, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Shogan, B.D.; Belogortseva, N.; Luong, P.M.; Zaborin, A.; Lax, S.; Bethel, C.; Ward, M.; Muldoon, J.P.; Singer, M.; An, G. Collagen degradation and MMP9 activation by Enterococcus faecalis contribute to intestinal anastomotic leak. Sci. Transl. Med. 2015, 7, ra268–ra286. [Google Scholar] [CrossRef] [PubMed]

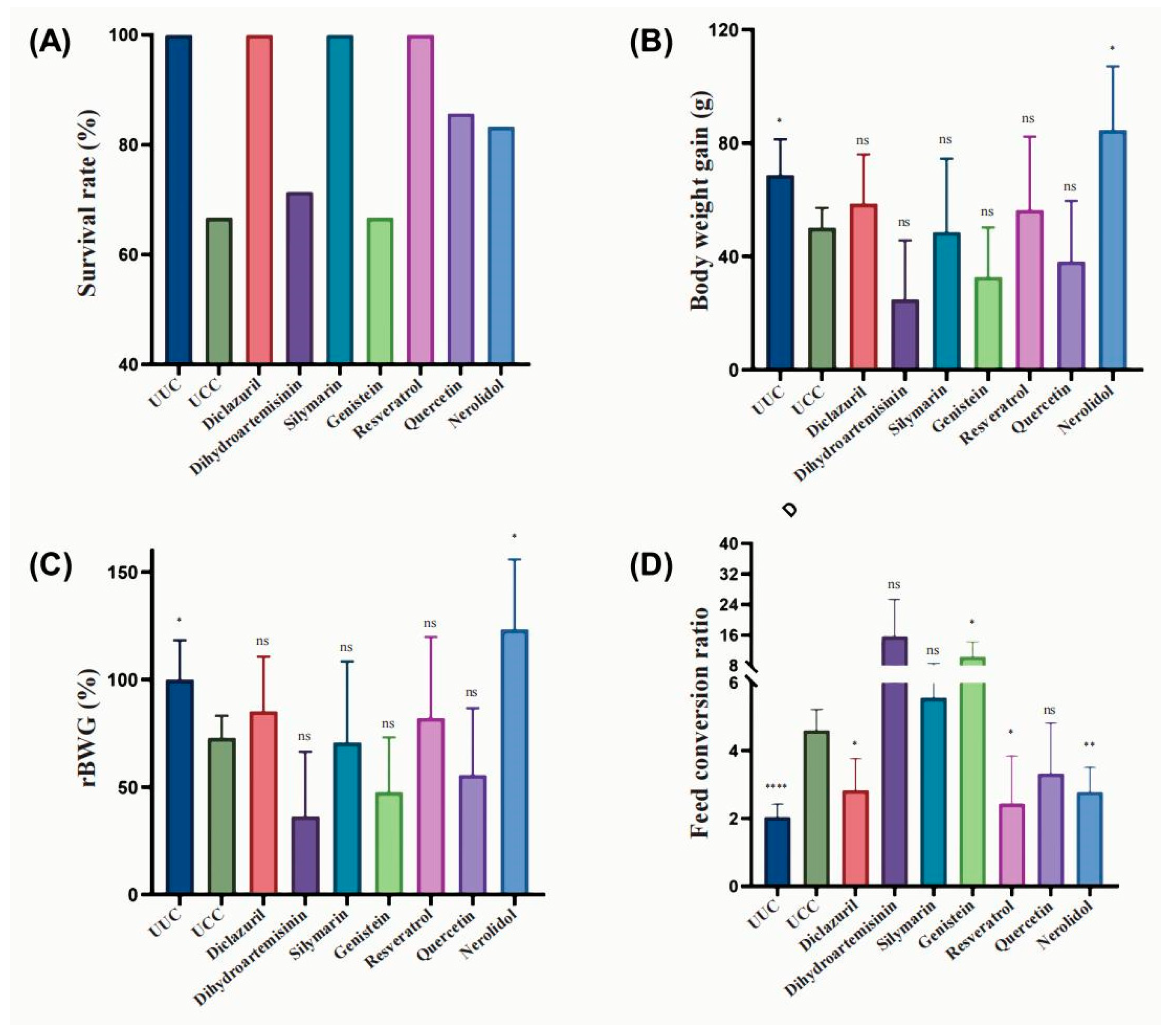
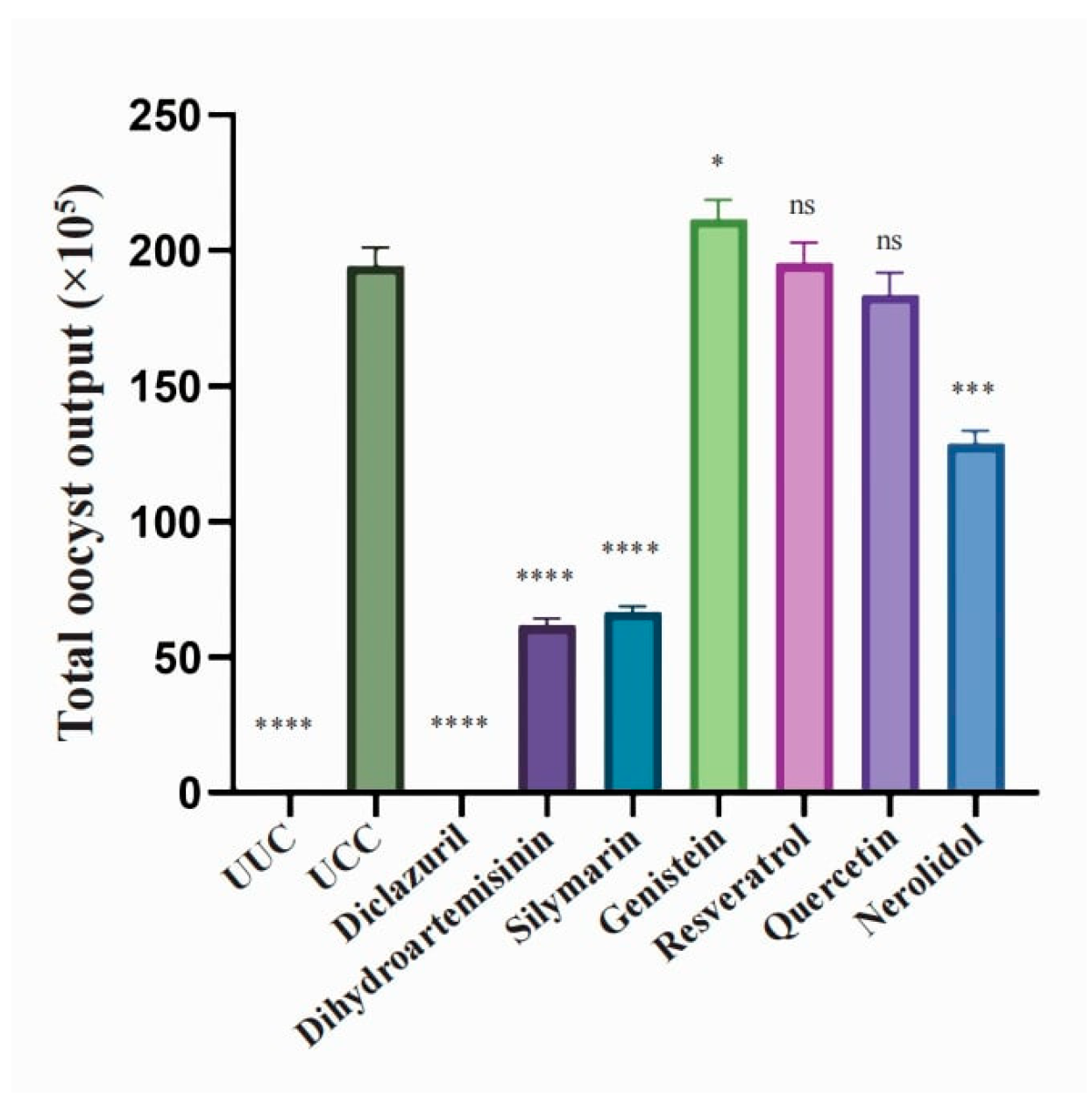
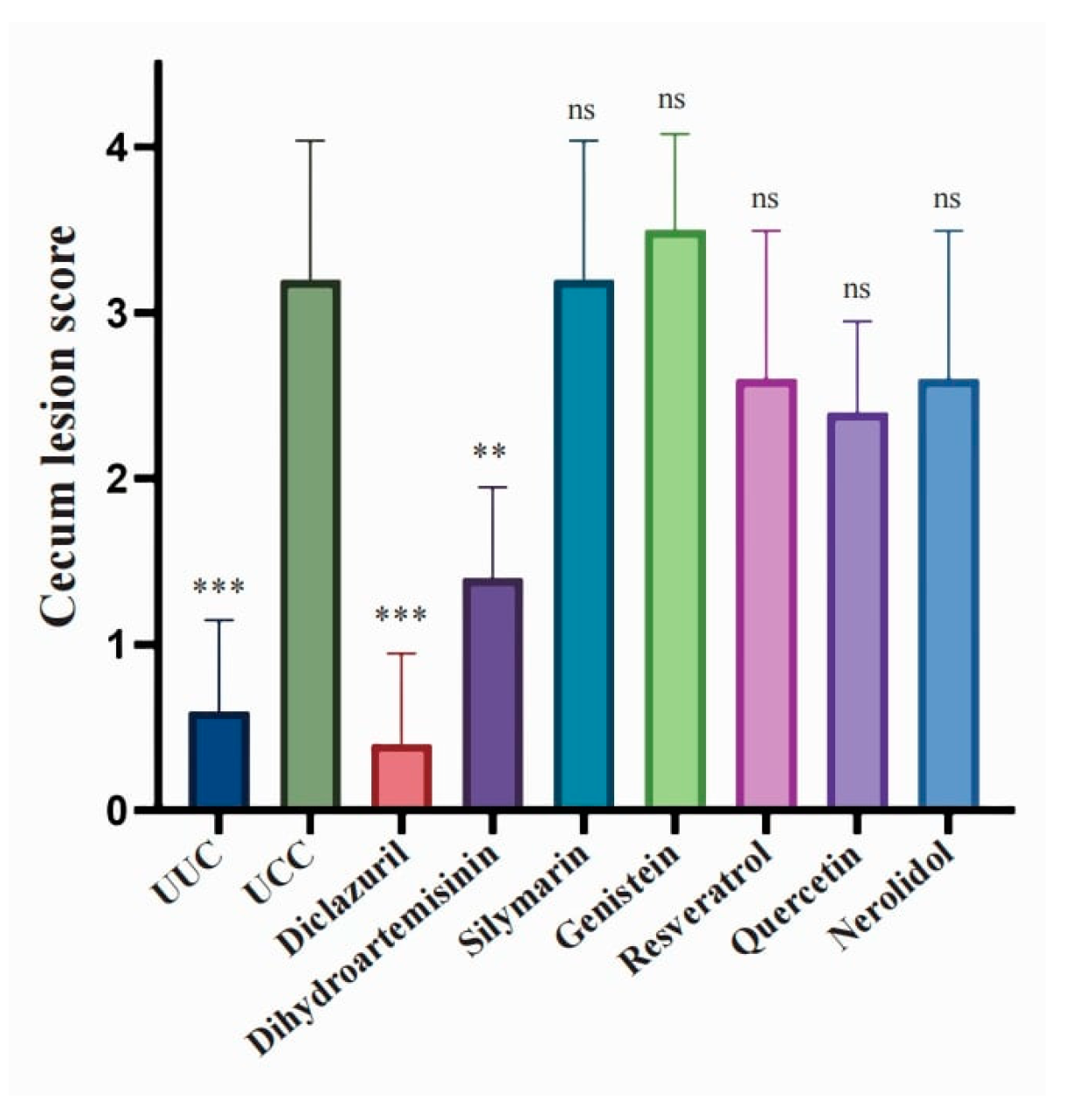
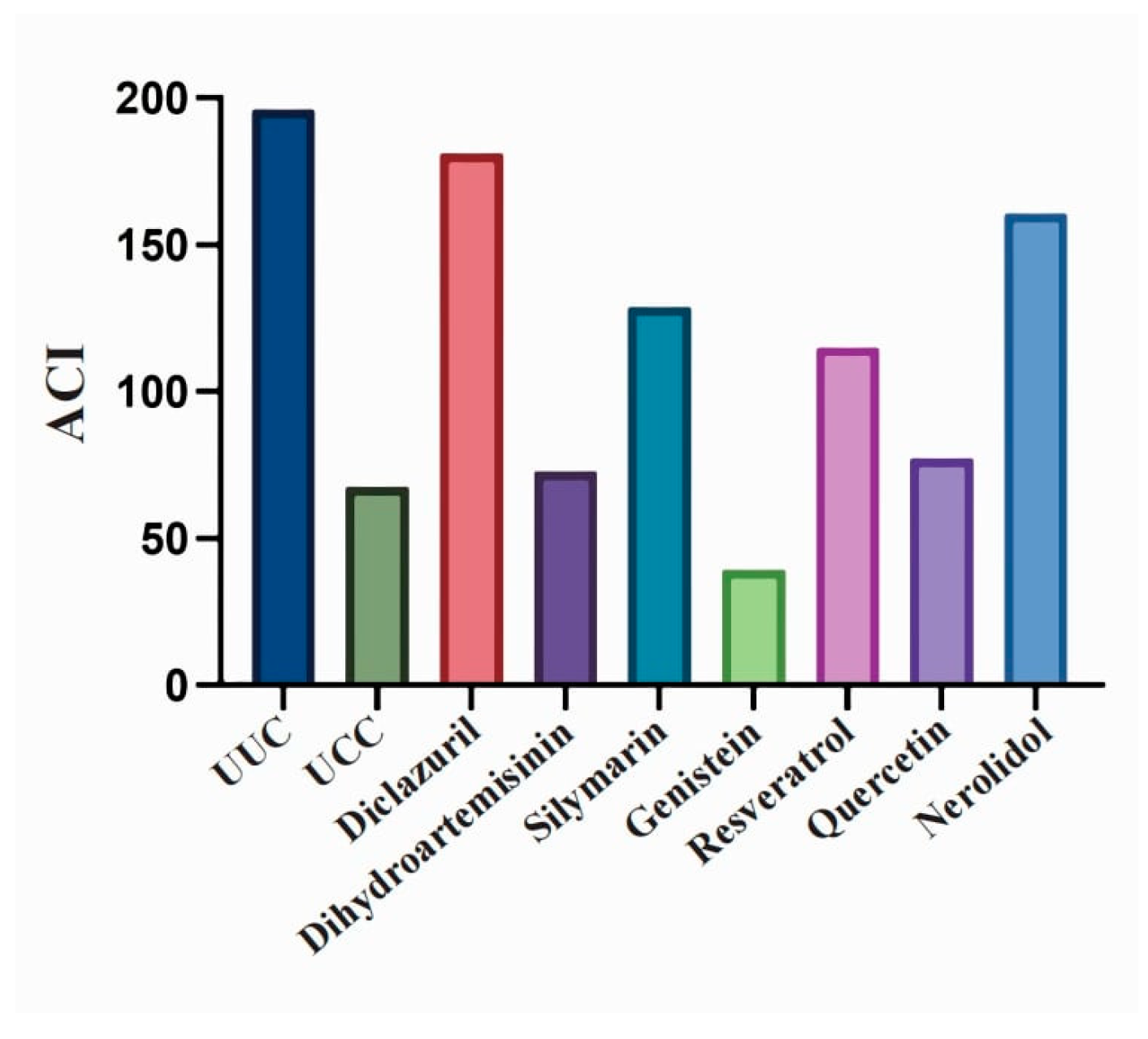

| UUC | UCC | Diclazuril | Dihydroartemisinin | Silymarin | Genistein | Resveratrol | Quercetin | Nerolidol | |
|---|---|---|---|---|---|---|---|---|---|
| Survival rate | 100.0 | 71.42 | 100.0 | 71.42 | 100.0 | 66.67 | 100.0 | 85.71 | 83.33 |
| BWG | 68.73 ± 12.59 * | 50.15 ± 6.99 | 58.60 ± 17.41 | 24.97 ± 20.71 | 48.62 ± 25.93 | 32.78 ± 17.49 | 56.43 ± 25.88 | 38.22 ± 21.39 | 84.71 ± 22.37 * |
| rBWG | 100.00 | 73.00 ± 10.18 | 85.30 ± 25.35 | 36.34 ± 30.14 | 70.77 ± 37.74 | 47.71 ± 25.46 | 82.14 ± 37.68 | 55.63 ± 31.14 | 123.31 ± 32.56 * |
| FCR | 2.03 ± 0.38 **** | 4.59 ± 0.62 | 2.83 ± 0.92 * | 15.63 ± 9.72 | 5.55 ± 3.06 | 10.30 ± 3.95 * | 2.44 ± 1.40 * | 3.32 ± 1.49 | 2.78 ± 0.72 ** |
| Total oocyst output (*105) | 0 **** | 194.24 ± 6.93 | 0 **** | 61.73 ± 2.55 **** | 66.86 ± 1.82 **** | 211.58 ± 7.07 * | 195.35 ± 7.50 | 183.49 ± 8.38 | 128.69 ± 4.86 *** |
| Cecum lesion score | 0.60 ± 0.54 *** | 3.20 ± 0.84 | 0.40 ± 0.54 *** | 1.40 ± 0.54 ** | 3.20 ± 0.83 | 3.50 ± 0.57 | 2.60 ± 0.89 | 2.40 ± 0.55 | 2.60 ± 0.89 |
| ACI | 196.0 | 67.57 | 181.3 | 72.76 | 128.7 | 39.2 | 115.0 | 77.31 | 160.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.; Han, B.; Lin, Z.; Liu, Q.; Liu, Z.; Si, H.; Hu, D. Effects of Six Natural Compounds and Their Derivatives on the Control of Coccidiosis in Chickens. Microorganisms 2024, 12, 601. https://doi.org/10.3390/microorganisms12030601
Hou Y, Han B, Lin Z, Liu Q, Liu Z, Si H, Hu D. Effects of Six Natural Compounds and Their Derivatives on the Control of Coccidiosis in Chickens. Microorganisms. 2024; 12(3):601. https://doi.org/10.3390/microorganisms12030601
Chicago/Turabian StyleHou, Yue, Bo Han, Zehua Lin, Qizheng Liu, Zhenhe Liu, Hongbin Si, and Dandan Hu. 2024. "Effects of Six Natural Compounds and Their Derivatives on the Control of Coccidiosis in Chickens" Microorganisms 12, no. 3: 601. https://doi.org/10.3390/microorganisms12030601
APA StyleHou, Y., Han, B., Lin, Z., Liu, Q., Liu, Z., Si, H., & Hu, D. (2024). Effects of Six Natural Compounds and Their Derivatives on the Control of Coccidiosis in Chickens. Microorganisms, 12(3), 601. https://doi.org/10.3390/microorganisms12030601







