Endogenous Microbacteria Can Contribute to Ovarian Carcinogenesis by Reducing Iron Concentration in Cysts: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. DNA Extraction and Bacterial 16S rDNA Sequencing from Cyst Fluid Samples
2.3. Tumor Imaging and Diagnoses
2.4. Statistical Analysis
3. Results
3.1. Patients
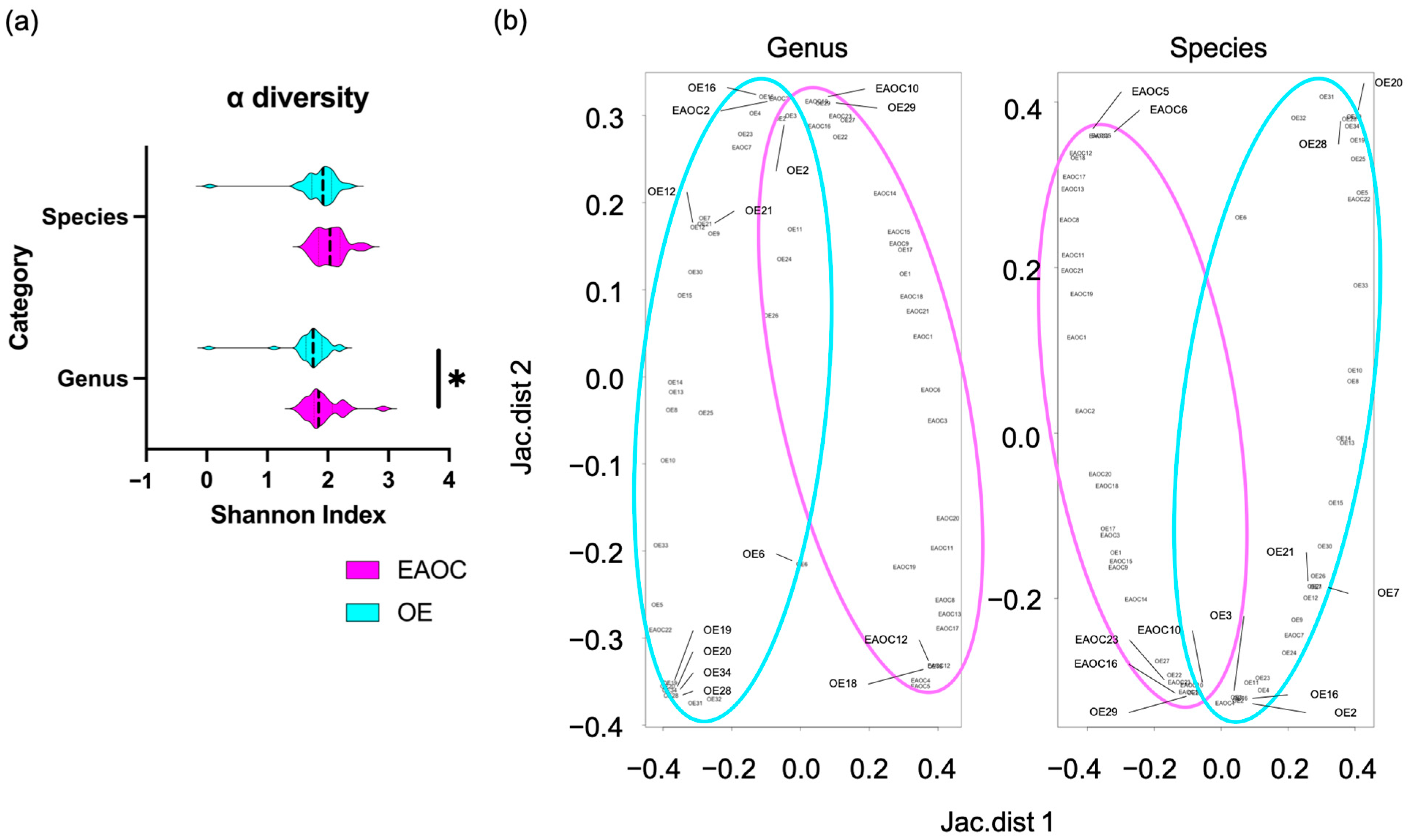
3.2. The Taxonomic Diversity of Cyst Fluid Microbiota
3.3. Isolation of Key Bacteria in Cyst Fluid
3.4. To Elucidate Specific Bacteria That Reduce the Iron Correlation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siegel, L.R.; Miller, D.K.; Wagle, S.N.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Bharwani, N.; Reznek, R.H.; Rockall, A.G. Ovarian Cancer Management: The role of imaging and diagnostic challenges. Eur. J. Radiol. 2011, 78, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Debuquoy, C.; Romeo, C.; Vanacker, H.; Ray-Coquard, I. Rare ovarian tumors: An update on diagnosis and treatment. Int. J. Gynecol. Cancer 2020, 30, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Gilks, C.; Bell, D.; Huntsman, D. Clear cell tumours. In WHO Classification of Tumours of Female Reproductive Organs; Kurman, R.J., Carcangiu, M.L., Herrington, C.S., Young, R.H., Eds.; IARC: Lyon, France, 2014; pp. 33–34. [Google Scholar]
- Kurman, R.J.; Shih, I.e.M. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am. J. Surg. Pathol. 2010, 34, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Sumimoto, K.; Moniwa, N.; Imai, M.; Takakura, K.; Kuromaki, T.; Morioka, E.; Arisawa, K.; Terao, T. Risk of developing ovarian cancer among women with ovarian endometrioma: A cohort study in Shizuoka, Japan. Int. J. Gynecol. Cancer 2007, 17, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H. Ovarian cancer in endometriosis: Epidemiology, natural history, and clinical diagnosis. Int. J. Clin. Oncol. 2009, 14, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H. Potential scenarios leading to ovarian cancer arising from endometriosis. Redox Rep. 2016, 21, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Amano, T.; Chano, T.; Isono, T.; Kimura, F.; Kushima, R.; Murakami, T. Abundance of mitochondrial superoxide dismutase is a negative predictive biomarker for endometriosis-associated ovarian cancers. World J. Surg. Oncol. 2019, 17, 24. [Google Scholar] [CrossRef]
- Itamochi, H.; Kigawa, J.; Terakawa, N. Mechanisms of chemoresistance and poor prognosis in ovarian clear cell carcinoma. Cancer Sci. 2008, 99, 653–658. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Mandai, M.; Toyokuni, S.; Hamanishi, J.; Higuchi, T.; Takakura, K.; Fujii, S. Contents of en-dometriotic cysts, especially the high concentration of free iron, are a possible cause of carcinogenesis in the cysts through the iron-induced persistent oxidative stress. Clin. Cancer Res. 2008, 14, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Agarwal, A.; Krajcir, N.; Alvarez, J.G. Role of oxidative stress in endometriosis. Reprod. Biomed. Online 2006, 13, 126–134. [Google Scholar] [CrossRef]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 789–799. [Google Scholar] [CrossRef]
- Yoshimoto, C.; Iwabuchi, T.; Shigetomi, H.; Kobayashi, H. Cyst fluid iron-related compounds as useful markers to distinguish malignant transformation from benign endometriotic cysts. Cancer Biomark. 2015, 15, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Wood, J.C.; Enriquez, C.; Ghugre, N.; Tyzka, J.M.; Carson, S.; Nelson, M.D.; Coates, T. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood 2005, 106, 1460–1465. [Google Scholar] [CrossRef]
- Pineda, N.; Sharma, P.; Xu, Q.; Hu, X.; Vos, M.; Martin, D.R. Measurement of Hepatic Lipid: High-Speed T2-Corrected Multiecho Acquisition at1H MR Spectroscopy—A Rapid and Accurate Technique. Radiology 2009, 252, 568–576. [Google Scholar] [CrossRef]
- Hasegawa, T.; Inagaki, K.; Haraguchi, H. Multielement correlation analysis of major-to-trace elements in human blood serum for medical diagnosis as studied by ICP-AES and ICP-MS. Anal. Sci. 2001, 17, i979–i982. [Google Scholar]
- Wang, Z.J.; Haselgrove, J.C.; Rn, M.B.M.; Hubbard, A.M.; Li, S.; Loomes, K.; Moore, J.R.; Zhao, H.; Cohen, A.R. Evaluation of iron overload by single voxel MRS measurement of liver T2. J. Magn. Reson. Imaging 2002, 15, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, C.; Takahama, J.; Iwabuchi, T.; Uchikoshi, M.; Shigetomi, H.; Kobayashi, H. Transverse Relaxation Rate of Cyst Fluid Can Predict Malignant Transformation of Ovarian Endometriosis. Magn. Reson. Med. Sci. 2017, 16, 137–145. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yamada, Y.; Kawahara, N.; Ogawa, K.; Yoshimoto, C. Modern approaches to noninvasive diagnosis of malignant transformation of endometriosis. Oncol. Lett. 2019, 17, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.; Villeneuve, N.F.; Sun, Z.; Wong, P.K.; Zhang, D.D. Dual roles of Nrf2 in cancer. Pharmacol. Res. 2008, 58, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Jezierska-Drutel, A.; Rosenzweig, S.A.; Neumann, C.A. Role of oxidative stress and the microenvironment in breast cancer development and progression. Adv. Cancer Res. 2013, 119, 107–125. [Google Scholar]
- Smolková, K.; Mikó, E.; Kovács, T.; Leguina-Ruzzi, A.; Sipos, A.; Bai, P. Nuclear Factor Erythroid 2-Related Factor 2 in Regulating Cancer Metabolism. Antioxid. Redox Signal. 2020, 33, 966–997. [Google Scholar] [CrossRef]
- Sipos, A.; Ujlaki, G.; Mikó, E.; Maka, E.; Szabó, J.; Uray, K.; Krasznai, Z.; Bai, P. The role of the microbiome in ovarian cancer: Mechanistic insights into oncobiosis and to bacterial metabolite signaling. Mol. Med. 2021, 27, 33. [Google Scholar] [CrossRef]
- Ellermann, M.; Arthur, J.C. Siderophore-mediated iron acquisition and modulation of host-bacterial interactions. Free Radic. Biol. Med. 2017, 105, 68–78. [Google Scholar] [CrossRef]
- Rockfield, S.; Raffel, J.; Mehta, R.; Rehman, N.; Nanjundan, M. Iron overload and altered iron metabolism in ovarian cancer. Biol. Chem. 2017, 398, 995–1007. [Google Scholar] [CrossRef]
- Tolg, C.; Sabha, N.; Cortese, R.; Panchal, T.; Ahsan, A.; Soliman, A.; Aitken, K.J.; Petronis, A.; Bägli, D.J.; Uropathogenic, E. coli infection provokes epigenetic downregulation of CDKN2A (p16INK4A) in uroepithelial cells. Lab. Investig. 2011, 91, 825–836. [Google Scholar] [CrossRef]
- Huang, Q.; Wei, X.; Li, W.; Ma, Y.; Chen, G.; Zhao, L.; Jiang, Y.; Xie, S.; Chen, Q.; Chen, T. Endogenous Propionibacterium acnes Promotes Ovarian Cancer Progression via Regulating Hedgehog Signalling Pathway. Cancers 2022, 14, 5178. [Google Scholar] [CrossRef] [PubMed]
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus As Potential Probiotics: Status, Concerns, and Future Perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef] [PubMed]
- Kapse, N.G.; Engineer, A.S.; Gowdaman, V.; Wagh, S.; Dhakephalkar, P.K. Functional annotation of the genome unravels probiotic potential of Bacillus coagulans HS243. Genomics 2019, 111, 921–929. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, J.; Cheng, Z.; Wang, T.; Chen, J.; Long, M. Bacillus coagulans TL3 Inhibits LPS-Induced Caecum Damage in Rat by Regulating the TLR4/MyD88/NF-κB and Nrf2 Signal Pathways and Modulating Intestinal Microflora. Oxidative Med. Cell. Longev. 2022, 2022, 5463290. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qiu, X.; Wang, W.; Li, D.; Wu, A.; Hong, Z.; Di, W.; Qiu, L. Human papillomavirus infection and cervical intraepithelial neoplasia progression are associated with increased vaginal microbiome diversity in a Chinese cohort. BMC Infect. Dis. 2020, 20, 629. [Google Scholar] [CrossRef]
- Radej, S.; Szewc, M.; Maciejewski, R. Prostate Infiltration by Treg and Th17 Cells as an Immune Response to Propionibacterium acnes Infection in the Course of Benign Prostatic Hyperplasia and Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 8849. [Google Scholar] [CrossRef]
- Li, Q.; Wu, W.; Gong, D.; Shang, R.; Wang, J.; Yu, H. Propionibacterium acnes overabundance in gastric cancer promote M2 polarization of macrophages via a TLR4/PI3K/Akt signaling. Gastric Cancer 2021, 24, 1242–1253. [Google Scholar] [CrossRef]



| 0E | EAOC | p-Value | |
|---|---|---|---|
| Number | n = 34 | n = 23 | |
| Age (years) | |||
| Median (range) | 39.00 (20–63) | 52.00 (31–74) | |
| Mean ± SD | 37.38 ± 9.50 | 50.57 ± 12.11 | <0.001 |
| BMI | |||
| Median (range) | 20.15 (16.02–26.72) | 23.30 (17.00–28.90) | |
| Mean ± SD | 20.59 ± 2.69 | 22.50 ± 3.79 | 0.104 |
| Parity | |||
| 0 | 20 | 10 | |
| ≥1 | 14 | 13 | 0.193 |
| Tumor volume (cm3) | |||
| Median (range) | 121.78 (5.71–637.94) | 688.84 (44.71–2493.19) | |
| Mean ± SD | 176.38 ± 154.51 | 824.67 ± 709.78 | <0.001 |
| R2 value (s−1) | *1 | *2 | |
| Median (range) | 17.71(5.94–42.14) | 9.12 (5.66–31.22) | |
| Mean ± SD | 17.05 ± 7.27 | 11.58 ± 6.92 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawahara, N.; Yamanaka, S.; Nishikawa, K.; Matsuoka, M.; Maehana, T.; Kawaguchi, R.; Ozu, N.; Fujii, T.; Sugimoto, A.; Yoshizawa, A.; et al. Endogenous Microbacteria Can Contribute to Ovarian Carcinogenesis by Reducing Iron Concentration in Cysts: A Pilot Study. Microorganisms 2024, 12, 538. https://doi.org/10.3390/microorganisms12030538
Kawahara N, Yamanaka S, Nishikawa K, Matsuoka M, Maehana T, Kawaguchi R, Ozu N, Fujii T, Sugimoto A, Yoshizawa A, et al. Endogenous Microbacteria Can Contribute to Ovarian Carcinogenesis by Reducing Iron Concentration in Cysts: A Pilot Study. Microorganisms. 2024; 12(3):538. https://doi.org/10.3390/microorganisms12030538
Chicago/Turabian StyleKawahara, Naoki, Shoichiro Yamanaka, Kyohei Nishikawa, Motoki Matsuoka, Tomoka Maehana, Ryuji Kawaguchi, Naoki Ozu, Tomomi Fujii, Aya Sugimoto, Akihiko Yoshizawa, and et al. 2024. "Endogenous Microbacteria Can Contribute to Ovarian Carcinogenesis by Reducing Iron Concentration in Cysts: A Pilot Study" Microorganisms 12, no. 3: 538. https://doi.org/10.3390/microorganisms12030538
APA StyleKawahara, N., Yamanaka, S., Nishikawa, K., Matsuoka, M., Maehana, T., Kawaguchi, R., Ozu, N., Fujii, T., Sugimoto, A., Yoshizawa, A., & Kimura, F. (2024). Endogenous Microbacteria Can Contribute to Ovarian Carcinogenesis by Reducing Iron Concentration in Cysts: A Pilot Study. Microorganisms, 12(3), 538. https://doi.org/10.3390/microorganisms12030538






