Abstract
Terpenes are diverse specialized metabolites naturally found within plants and have important roles in inter-species communication, adaptation and interaction with the environment. Their industrial applications span a broad range, including fragrances, flavors, cosmetics, natural colorants to agrochemicals and therapeutics, yet formal chemical synthesis is economically challenging due to structural complexities. Engineering terpene biosynthesis could represent an alternative in microbial biotechnological workhorses, such as Saccharomyces cerevisiae or Escherichi coli, utilizing sugars or complex media as feedstocks. Host species that metabolize renewable and affordable carbon sources may offer unique sustainable biotechnological alternatives. Methylotrophs are bacteria with the capacity to utilize one-carbon feedstocks, such as methanol or formate. They colonize the phyllosphere (above-ground area) of plants, and many accumulate abundant carotenoid pigments. Methylotrophs have the capacity to take up and use a subset of the rare earth elements known as lanthanides. These metals can enhance one-carbon (methylotrophic) metabolism. Here, we investigated whether manipulating the metabolism enables and enhances terpene production. A carotenoid-deficient mutant potentially liberates carbon, which may contribute to bioproduct accumulation. To test this hypothesis, terpene-producing bacterial strains regulated by two distinct promoters were generated. Wildtype Methylobacterium extorquens, ∆Meta1_3665, a methylotrophic mutant lacking the carotenoid pathway, and an E. coli strain were transformed with an exogenous terpene pathway and grown both in the presence and absence of lanthanides. The extraction, and the comparison of analytical profiles, provided evidence that engineered cultured M. extorquens under control of a native, inducible methylotrophic promoter can yield the sesquiterpene patchoulol when supplemented with lanthanide. In contrast, using a moderate-strength constitutive promoter failed to give production. We demonstrated colonization of the phyllosphere with the engineered strains, supporting the future engineering of selected species of the plant microbiome and with promising implications for the synthetic biology of small molecules.
Keywords:
metabolism; terpenes; patchoulol; casbene; methylotrophs; inducible expression; lanthanide 1. Introduction
Plants produce specialized metabolites including a broad spectrum of terpenoids that are significant in defense mechanisms, communication and adaptation [1]. Terpenoids also represent industrially relevant bioproducts with a wide range of existing and potentially future renewable applications, including perfumes, pharmaceuticals and food supplements [2]. Typical terpene biosynthetic pathways proceed via the universal 5-carbon precursors isopentenyl diphosphate (IDP) and dimethylallyl diphosphate (DMADP), formed in plants by the cytosolic mevalonate (MVA) and plastidial methylerythritol phosphate (MEP) pathways, with the latter route also found in bacteria. IDP and DMADP are condensed into acyclic isoprene diphosphates of varying lengths in multiples of 5-carbons. Subsequently, terpene synthases can cyclize the intermediates into complex scaffolds with the characteristic monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20) or carotenes/tetraterpenes (C40), as examples. The extraction of terpenes from their native species for industrial applications poses environmental and economic issues with harvesting, limitations in the amount and purification from mixtures of related but unwanted products [3]. Economically viable chemical synthesis is challenging due to its structural complexity [4]. For these reasons, the bioengineering of terpene production through biotechnology is rising in interest for its sustainable production [5,6,7].
The early intermediate pathways are crucial for terpene synthesis and may enable the rewiring of non-plant organisms to host exogenous terpene production. In microbial hosts, the levels of intermediates have been increased through the installation of strong native pathways and engineered alternative routes, as well as the removal or suppression of competing pathways, including sterols/triterpenes (C30) or carotenoids [8,9,10,11]. However, these manipulations may require multiple plasmids to supply the terpene synthase and upregulate the precursors and can represent a burden on the system [12]. These challenges, coupled with the need for glucose or complex feedstocks, raise an opportunity for terpene production in genetically tractable organisms with unique carbon metabolism abilities, such as Methylobacterium extorquens AM1 [13].
Methylotrophs are bacteria unique in their ability to utilize one-carbon compounds including low-cost feedstocks such as methanol or formate. They colonize a spectrum of environments but are exceptionally abundant in the phyllosphere (above-ground area of plants), where they utilize methanol released by cell wall catabolism [14]. Many methylotrophs accumulate carotenoids, giving the microbes a visibly pink hue. Methylotrophs are unique in their uptake and use of rare earth elements known as lanthanides. These metals can alter and enhance one-carbon (methylotrophic) metabolism. M. extorquens AM1 modulates the production of different methanol dehydrogenase (MDH) enzymes (XoxF- and ExaF) depending on the concentration of lanthanides present in the media, allowing a robust metabolic flexibility with the changing of substrate concentrations [15]. Additionally, lanthanides visually intensify the color of cultured isolates and have proven to be essential for growth with some species [16]. Using the MEP pathway, methylotrophs generate abundant terpene precursors for carotenoid synthesis. A previous study isolated the carotenoid-deficient strain CM502, which lacked proposed diapolycopene oxidase activity (META1_3665, GenBank accession AY331188.1) [17]. It is possible that in CM502, the pool of precursors may be liberated and accessible by exogenous pathways. A methylotrophic strain with intact native carotenoid biosynthesis has also been engineered with an exogenous terpene pathway, yet with low resulting yields [13]. The impact of lanthanides on the carbon flow through the carotenoid pathway has not been investigated and presents an additional opportunity to test for enhanced terpene accumulation.
Here, we focused on two terpene products, patchoulol (C15 sesquiterpene) and casbene (C20 diterpene). Patchoulol is a sesquiterpene alcohol relevant in the perfume and cosmetic industry and known for its earthy aroma. It is naturally formed by cyclization of farnesyl diphosphate (FDP) catalyzed by a sesquiterpene synthase and is the main component (30–40%) of patchouli oil extracted from the Pogostemon cablin plant [1,18,19]. Industrialization of the compound has increased its demand, with prices ranging from $30–$200 per kg [2]. The range in price can be accounted for by the limitations and biological variation in cultured plants. Casbene is a diterpene with antimicrobial effects, formed through a one-step cyclization of geranylgeranyl diphosphate (GGDP) [20]. Casbene synthase, DgTPS1, is natively found in the Daphne genkwa plant [21]. The scaffold can be further modified to afford important precursors in drug discovery [22].
The CM502 mutant arrests the carotenoid route, potentially liberating carbon for the biosynthesis of novel products. It was reported, after metabolic modifications, that CM502 synthesized more of the sesquiterpenoid target α-humulene than the wildtype strain [13]. The introduction of the patchoulol synthase under a native promoter may take advantage of the FDP pool. However, there may be an increase of the products that follow, such as GGDP, allowing for the generation of the diterpene casbene. Given there are two potential metabolite branch points, methylotrophic expression may have the opportunity to increase production, lower cost, and reduce the environmental impacts of terpene synthesis.
This study aims to increase the knowledge of terpene engineering specifically within the native and mutant background of M. extorquens AM1. To test if lanthanides can be used to increase terpene synthesis in methylotrophs, we supplemented engineered and cultured strains with the rare earth metal LaCl3. To investigate if the engineered strains retain the capacity to colonize plants and persist in the phyllosphere, we recovered M. extorquens from leaves four days after inoculation.
2. Materials and Methods
2.1. Strains, Media, and Growth Conditions
MP minimal salts, CoCl2, succinic acid, methanol, and chloramphenicol were purchased from Sigma (Sigma Aldrich, St. Louis, MO, USA), Streptomycin, LB, and TB were purchased from VWR (VWR International, LLC, Radnor, PA, USA). M. extorquens AM1 wildtype and mutant CM502 were used in the study [17] and grown on MP minimal salts agar plates (1.5% w/v) with a CoCl2 concentration of 2.0 μM and 30 mM succinic acid as a carbon source [23]. For liquid growth, MP minimal salts media with 2.0 μM CoCl2 and 125 mM methanol as a carbon source was used [23]. Cultures were incubated at 28.5 °C with the shaking of liquid cultures at 175 rpm. For methylotrophic engineered strains, 12.5 μM CoCl2 was used in MP media with the antibiotic tetracycline hydrochloride (Sigma Aldrich, St. Louis, MO, USA) for selection in a final concentration of 10 μg/mL. The E. coli expression system required tetracycline and streptomycin with a final concentration of 50 μg/mL and chloramphenicol, with a final concentration of 34 μg/mL in the two-plasmid system and 17 μg/mL in the three-plasmid system. E. coli strains were grown on LB plates (1.5% w/v) for solid media. Liquid cultures were in LB or TB media with appropriate antibiotics. All cultures were wrapped in tin foil or kept in the dark to prevent tetracycline degradation.
2.2. Plasmid Generation and Electroporation
Plasmids were generated through PCR amplification and In-Fusion® (Takara Bio USA, Inc., Ann Arbor, MI, USA) cloning. All primers and plasmids used in this study can be found in Table 1 and Table 2, respectively. A modified Tac promoter mTac [24,25] was chosen for expression in both E. coli and methylotrophs to enable a comparison of the impact of lanthanides on carotenoid production. pAP5 [26] was used as a backbone and amplified with primers 1 and 2 to generate a vector for gene and promoter insertion. Patchoulol synthase PcPatS (accession number: AY508730) [27] was amplified using primers 3 and 4. The mTac promoter was synthesized and amplified with primers 5 and 6. When appropriate, overhangs complementary to the gene or backbone next to the insertion site were generated on primers for In-Fusion® HD Cloning Plus (Takara Bio) cloning, and plasmids were transformed into Stellar Competent Cells (Takara Bio). In the pAP5 backbone, the PcPatS gene was inserted with the mTac promoter, resulting in the pAH1 vector (Table 2). Appropriate constructs were selected on LB plus tetracycline hydrochloride plates and confirmed by PCR and through Sanger sequencing (Psomagen, Rockville, MD, USA).

Table 1.
Oligonucleotides used in the study.

Table 2.
Plasmids and strains used in the study.
To investigate the production by a native, lanthanide-inducible promoter, the gene encoding the patchoulol synthase was inserted into a pES503, a plasmid containing the xox1 methylotrophic promoter [26], to generate pAH2 (Table 2). Analogously, the gene encoding casbene synthase DgTPS1 (accession number: MZ485349.1) [21] was amplified using the 9 and 10 primers, resulting in pAH3 (Table 2). A construct containing instead the mTac promoter was generated by using primers 11 and 10, resulting in pAH4 with casbene synthase (Table 2). Constructs were verified by Sanger sequencing. For the E. coli expression system, previously established plasmids for increasing the precursors needed, pIRS and pGG, were utilized [21,28]. An overview of the MEP pathway and vectors is given in Figure 1.
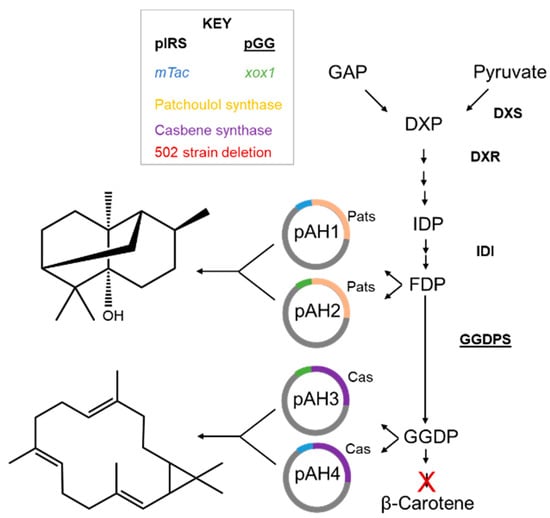
Figure 1.
Scheme of vector insertion into MEP pathway. The four plasmids generated in the study (pAH1, pAH2, pAH3 and pAH4), as well as the previously established E. coli plasmids (pIRS and pGG), are shown. Pats, patchoulol synthase; Cas, casbene synthase.
Competent cells of both AM1 and CM502 were generated using a previously reported method [29]. Overnight cultures of both strains were started from plates and used to inoculate 50 mL flasks of MP media with methanol. The following day, cultures were transferred into sterile 50 mL falcon tubes and centrifuged at 4000× g for 15 min at 4 °C. The media was discarded and chilled sterile ddH2O was used to resuspend the pellet. Another round of centrifugation and wash was performed. After the final spin, the pellet was resuspended in 1 mL 10% glycerol, aliquoted to 50 μL, flash-frozen in liquid nitrogen and stored at −80 °C. E. coli electrocompetent cells were generated using a previously published method [30].
Electroporation was adapted from [29]. Briefly, competent cells in 50 μL aliquots were thawed on ice and 1–2 μL plasmid was added to the suspension and transferred to a chilled cuvette. The mixture was left on ice for 10 min before electroporation in an Eppendorf Electroporator 2510 (USA Scientific, Inc.—BioCT, Ocala, FL, USA) with a set voltage of 2500 V for methylotrophic cells and 1800 V for E. coli cells. Immediately following electroporation, 1 mL of NB media (methylotrophs) or 1 mL SOC media (E. coli) was added to the cuvette. Cultures were transferred to a microcentrifuge tube and then placed in a 28.5 °C incubator at 175 rpm for 20–24 h for methylotrophs. For E. coli strains, the tubes were placed in a 37 °C, 500 rpm shaker for 1–2 h. Following incubation, cells were added to their respective plates with appropriate antibiotics. Colonies were confirmed through PCR with the primers specific to the insert. All strains generated throughout the study are detailed in Table 2.
2.3. Terpene Production and Extraction
A total of 4–5 colonies were inoculated in 3 mL MP with methanol (125 mM) and grown at 28.5 °C with shaking (175 rpm). After 3–4 days, the starter culture was used to inoculate a 50 mL culture in a 250 mL Erlenmeyer flask. Cultures were left to grow for 16 h or until an OD600 of 0.3–0.6 was reached. For the pAH1- and pAH4-containing strains, half of the cultures were given LaCl3 (2 μM) and all were returned to the shaker for 48 h. For strains possessing pAH2 and pAH3, the 50 mL cultures were induced by adding LaCl3 and returned to the shaker for 48 h. Overnight cultures of the E. coli strains were started from frozen glycerol stocks in 5 mL LB plus antibiotics and grown in a 37 °C, 200 rpm shaker. The next morning, 50 mL of TB plus antibiotics were inoculated with 1 mL of culture and returned to the shaker for 4–6 h. Once cultures reached an OD600 of 0.5–0.6, induction was performed by adding 40 mM sodium pyruvate, 2 mM MgCl2 and 1 mM IPTG. Cultures were incubated in a 20 °C, 200 rpm shaker for 48 h.
Cultures were collected and ~20 mL hexane with 1 ng/μL Eicosene as internal standard was added. Cells were then sonicated using the bulk tip on a Misonix S4000 Ultrasonic Liquid Processor (Misonix, Inc., Farmingdale, NY, USA) with the following parameters: 60 amps, 100 s run, 10 s on and 5 s off. After sonication, 5 mL of 100% EtOH was added, and the flasks were then placed on a slow shaker at room temperature for 2 h. The top hexane layer was transferred to a glass tube and samples were reduced under a nitrogen stream to roughly 1.5 mL and transferred to a mass spectrometry glass vial.
The lanthanide-dependent patchoulol formation was determined in triplicate, comparing growth with and without lanthanides and by using 100 mL cultures of M. extorquens strains AM1/pAH2 or CM502/pAH2 harboring PcPatS that were grown to an OD600 of 0.3–0.6 at 175 rpm shaking speed. Lanthanide (2 µM LaCl3) was provided for the plus lanthanide condition and a 20 mL dodecane overlay was added to all cultures, with further incubation at 30 °C for 3 days. The dodecane layer was removed and measured directly on gas chromatography with mass spectrometry (GC-MS) to detect patchoulol peaks.
2.4. GC-FID/GC-MS Analysis
GC-FID analysis was performed using an Agilent 7890A system (Agilent Technologies, Santa Clara, CA, USA) equipped with a 19091S-433 (30 m × 250 μm × 0.25 μm) column and chromatography with the following parameters: helium carrier, rates: 40 °C for 1 min, 40 °C/min hold for 2 min at 200 °C, 20 °C/min to 250 °C, 15 °C/min to 280 °C, 40 °C/min hold at 320 °C for 3 min, splitless: 250 °C, with an injection volume of 1 μL. The patchoulol synthase hexane samples were compared to a patchouli oil standard and the E. coli/pAH1 samples. Comparison of the controls and standard showed the retention time of patchoulol at 7.73 min. For the casbene samples, a previously collected plant extract and the E. coli samples were used as controls. The retention time for casbene was determined at 9.35 min. To further confirm the patchoulol and casbene peaks, representative samples were run on an Agilent A GC-MS instrument with a 30 m VF-5 column and the following parameters: helium carrier, rates: 40 °C hold for 1 min, 40 °C/min hold 200 °C for 4.5 min, 20 °C/min to 240 °C, 10 °C/min to 280 °C, 40 °C/min hold 320 °C for 3 min, splitless: 275 °C and injection volume of 1 μL. Statistical analysis of the GC-FID data was conducted through a one-way ANOVA test of the product to internal standard peak ratios.
2.5. Competition Experiments
Nicotiana benthamiana plants were grown for four weeks in Sure-mix soil (Michigan Grower Products, Inc., Galesburg, MI, USA) in a growth room under controlled conditions with a 16-hour day cycle at 24 °C and an 8-hour night cycle at 17 °C. Methylotrophic cultures of CM502, AM1/pES503, CM502/pAH1, CM502/pAH2, CM502/pAH3, CM502/pAH4 were started in 3 mL MP media with methanol and tetracycline when appropriate. After 4 days, 500 mL of MP media with methanol (125 mM), 2 µM LaCl3 and tetracycline were inoculated with 1.5–2 mL of the starter cultures and were returned to the shaker for 2 days. Cultures were combined and pelleted by centrifugation at 4000× g for 15 min at 4 °C. After two washing steps, the final pellet was resuspended in ddH2O at half the initial volume, and the OD600 of each sample was determined. For coculture inoculation, resuspended cells were mixed in a 1:1 ratio at an OD600 of 1 each. Plants in replicates of three were either left unmanipulated or inoculated with CM502 alone, AM1/pES503 alone, AM1/pES503:CM502/pAH1, AM1/pES503:CM502/pAH2, AM1/pES503:CM502/pAH3 or AM1/pES503:CM502/pAH4. For this, plants were inverted, and the leaves were dipped into the bacterial cultures until all were fully coated. After inoculation, plants were returned to the growth room for 4 days. Bacteria were re-isolated using two medium-sized leaves from each plant. Three 12 mm leaf disks were placed in a sterile 50 mL falcon tube, and 50 mL 100 mM phosphate buffer was added. Tubes were shaken at 22 °C at 120 rpm for 10 min. A total of 100 μL of buffer for the single strain inoculations and 60 μL of buffer for the double strain inoculation plants was plated on MP agar with methanol and LaCl3 and MP agar with methanol, LaCl3 and tetracycline. Plates were covered in tin foil and allowed to grow in the incubator for 5 days before counting CFUs for both pink and white colonies in the presence and absence of tetracycline.
3. Results and Discussion
- Patchoulol synthase under a constitutive promoter fails to produce patchoulol
The pAH1 vector (encoding PcPatS under the constitutive promoter mTac) was electroporated into strain AM1, CM502, and grown until the exponential phase. At this point, LaCl3 was added to half of the methylotrophic cultures. After 48 h of expression, the cultures were harvested. For the E. coli controls, the patchoulol synthase was cloned in pET-28b(+) (EMD Millipore, Burlington, MA, USA) and transformed into chemically competent OverExpressTM C41(DE3) cells (Lucigen, Middleton, WI, USA). Expression was performed as described previously [31]. The extractions of patchoulol with hexane were obtained from each culture and run on a GC-FID instrument. No patchoulol was detected in any sample. It is possible that the strength of the mTac promoter is insufficient for an efficient production of patchoulol that is above the detection limit of our instrument. This is consistent with mTac being only a moderate-strength promoter in both methylotrophic and E. coli systems [32]. It was recently suggested that the engineering of microbial terpenoid biosynthesis requires strong promoters to induce a metabolic pull toward the target product, regardless of the use of native, synthetic or inducible promoters [33]. The same study indicates an inherent benefit from overexpression of potential rate-limiting enzymes to increase the supply of the five-carbon precursors or the isoprenyl diphosphate synthase affording the direct precursor farnesyl diphosphate with synergistic effects of deploying multiple genes [33].
- Native methylotrophic promoter successfully produces patchoulol
To test an alternative promoter, a second construct with a native, lanthanide-inducible methylotrophic promoter was generated, pAH2. The same experimental procedures were conducted, except all the cultures were given LaCl3 to induce the promoter. Analysis of the extracts showed the system was producing patchoulol. In comparison to baseline methylotroph samples and standard control, the patchoulol peak at retention time 7.73 min was found in all the AM1/pAH2 and CM502/pAH2 samples (Figure 2). This demonstrates that the system can produce patchoulol and is further evidence M. extorquens is capable of sesquiterpene synthesis. The analysis of the product and the internal standard peak in each sample were conducted and used for calculating the relative yield (Figure 3). This analysis showed, under these conditions, no significant difference between the relative product collected from the AM1/pAH2 and CM502/pAH2 samples. The lack of difference between the pink and colorless cells may be attributed to the low levels of product and may indicate a bottleneck in the pathway. Additionally, it indicates the natural MEP precursors within the CM502 mutant are not generating an increased pool. As discussed above, overexpression of multiple genes upstream of the terpene synthase may provide a future strategy to increase the carbon flux toward patchoulol [33].
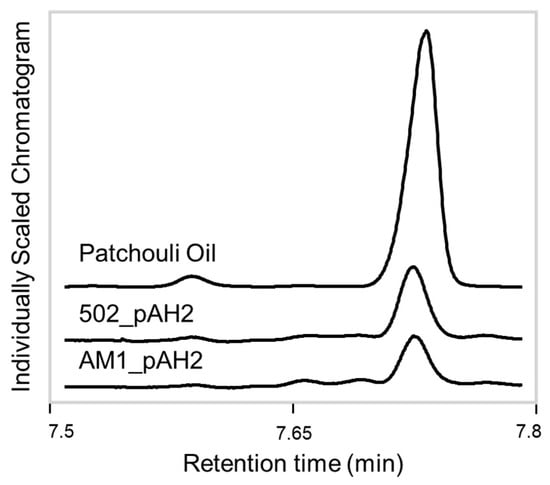
Figure 2.
GC-FID chromatogram of patchoulol yields. Total ion chromatograms focused on the patchoulol peak from the patchouli oil standard, CM502_pAH2 and AM1_pAH2 samples. The peak retention time of patchoulol was determined at 7.73 min and is present in all three samples at varying intensities.
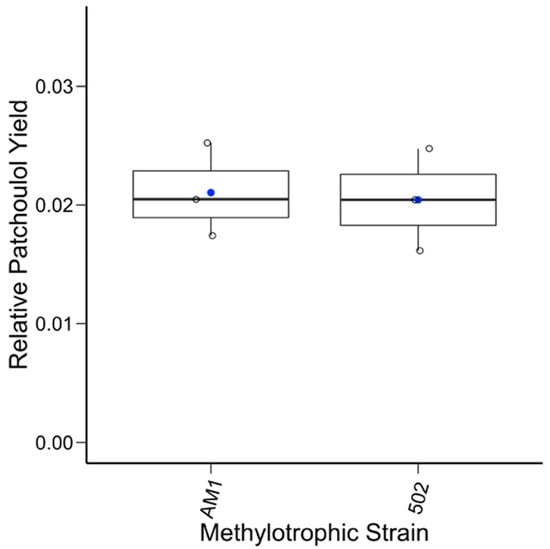
Figure 3.
Relative patchoulol yields from the native promoter. The relative yield of patchoulol for each sample (open circle) from the two methylotrophic strains is plotted along with the mean (blue circle). A one-way ANOVA test was conducted, and no statistical significance between the two sample sets was found.
- Impact of the lanthanide switch on production of patchoulol
Inducible systems are highly attractive in metabolic engineering. To further characterize the use of the xox1 promoter as a lanthanide switch, we tested whether patchoulol biosynthesis in M. extorquens is induced by the presence of LaCl3. Both AM1/pAH2 and CM502/pAH2 were tested for patchoulol production with or without the addition of LaCl3 (2 µM) (Figure 4). In both strains, patchoulol is only seen in the presence of LaCl3, indicating induction of PcPatS gene expression by the xox1 promoter. This supports the xox1 promoter acting as a lanthanide switch that enables inducible expression of biosynthetic pathways. Future studies could further characterize this lanthanide switch, investigating the sensitivity to different LaCl3 concentrations. Similarly, future investigation into the xox1 switch in both AM1 and CM502 may quantitatively elucidate the terpenoid biosynthetic capacity of each strain. In the experiments presented here, both strains may still be limited by PcPatS production, and future improvements to PcPatS expression with xox1 (for example, higher LaCl3 concentrations) may enable greater patchoulol production. Here, it is clear that patchoulol production in M. extorquens is induced by the addition of LaCl3 when using the xox1 promoter, and there is an opportunity to further improve this system in the future.
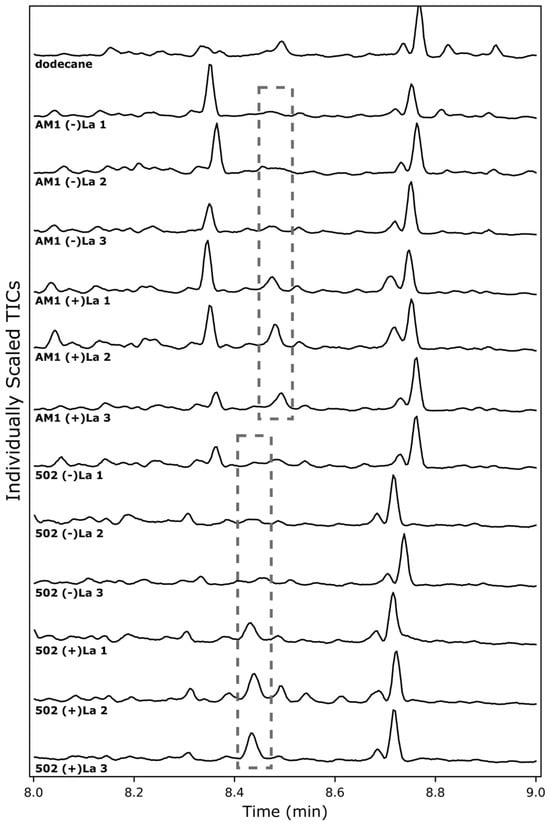
Figure 4.
Chromatograms of M. extorquens pAH2 cultures with or without induction by LaCL3. Dashed boxes indicate elution of patchoulol from GC-MS analysis. Triplicate samples are individually displayed and numbered 1, 2 or 3, with the lack of (-) or presence of (+) LaCl3.
- Assessing the methylotrophic engineering of diterpenes
A diterpene pathway using the mTac promoter was generated in the AM1 strain to test the availability of the later C20 terpene precursor, GGDP. To our understanding, there is no report of diterpene production within M. extorquens AM1. The gene encoding casbene synthase was cloned under the xox1 promoter, and no casbene was detected in the methylotrophic samples (2 µM LaCl3). The lack of product is potentially influenced by the availability of the GGDP precursor. Insufficient carbon flow through the pathway to GGDP or enzyme activity of GGDP synthase may be contributing factors.
Methylotrophic and E. coli strains harboring the pAH4 constructs with the mTac promoter were generated to test diterpene synthase expression. The resulting chromatograms from the E. coli and standard hexane samples (Supplemental Figure S1) show the E. coli control is producing both the terpene precursor at retention time 9.035 min (1) and the final diterpene product, casbene, at retention time 9.35 min (2). The methylotrophic samples showed no detectable casbene, possibly due to moderate expression by the constitutive promoter.
- Methylotrophic engineered strains can compete for colonization in the phyllosphere
M. extorquens is a known colonizer of the phyllosphere and metabolizes the methanol released from plant catabolism of pectin. The success of engineering a methylotrophic sesquiterpene system provides the opportunity for creating a production platform on plant leaf surfaces. To determine if the engineered strain can compete with other methylotrophic strains for colonization, a competition experiment in the phyllosphere was conducted. The engineered strain was mixed with a control strain and used to inoculate plant leaves. Strains were re-isolated by selecting with methanol and LaCl3. The resulting plates of the recovered strains show a difference between the CM502/pAH2 and CM502/pAH3 conditions (Figure 5). Specifically, the CM502/pAH2 strain was recovered in low numbers compared to the control AM1/pES503 strain. In contrast, for the CM502/pAH3 strain, the recovered bacteria show a far higher proportion of white colonies, indicating it out-competed the AM1/pES503 control strain. The differences in recovery are possibly associated with the burden of terpene production. However, all engineered strains were recovered. A current established system for terpene expression within plant leaves uses transient infection through agrobacterium [31]. A combination of this expression system and the methylotrophic expression on the leaf surface holds the potential for increasing terpene products generated from one individual plant.
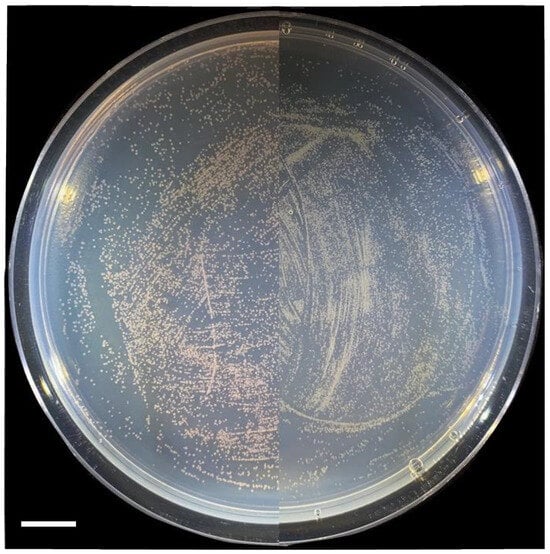
Figure 5.
Recovered bacteria exhibit differences in phenotype. The phyllosphere competition produces a clear color difference in the bacterial cells recovered from the leaf surface. The CM502/pAH2 strain is found in low numbers (left). The CM502/pAH3 strain dominates the pink control strain AM1/pES503 (right). Scale bar, 1 cm.
4. Conclusions
This study adds further evidence that the methylotrophic strain of M. extorquens AM1 has a native, albeit low, capability for production of the C15 sesquiterpene patchoulol. In contrast, the C20 diterpene product casbene failed to accumulate at detectable levels, which could indicate limiting availability of the precursor, geranylgeranyl diphosphate. Here, we emphasize engineering of M. extorquens using a lanthanide-dependent native promoter. This promoter is a unique addition to the growing number of highly specific inducible systems, critical for synthetic biology applications. Comparison of a carotene-free strain with the wildtype strain did not result in a higher yield, indicating that the limitation may reside either in the recombinant heterologous activity of the terpene synthase or the endogenous precursor pathway and lack of C5 precursor building block availability. Lastly, the strains generated were inoculated in the phyllosphere and shown to colonize the model plant Nicotiana benthamiana in sufficient capacity to be recovered from leaves under competitive conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms12030500/s1, Supplemental Figure S1: GC-FID confirmation of casbene production. Extracts from E. coli and M. extorquens casbene sample chromatograms are compared to a positive sample. The E. coli samples from the constitutive promoter when compared to a casbene standard (2) show success of the system at retention time of 9.35 minutes. (1) Geranylgeraniol, product of unspecific phosphatase activity from geranylgeranyl diphosphate.
Author Contributions
Conceptualization, N.C.M.-G., A.H. and B.H. conceived the study. Methodology, A.H. generated all strains and performed acquisition, analysis and interpretation of the data. Resources, N.C.M.-G. provided the original strains and methodology for their manipulation. B.H. provided funding. A.H. provided the original draft. J.D.B. assisted with the critical final experiments, analysis, interpretation and visualization of the data. All authors contributed to review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by the Great Lakes Bioenergy Research Center, U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research under Award Number DE-SC0018409, the Department of Biochemistry and Molecular Biology and startup funding and support from AgBioResearch (MICL02454).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We would like to thank Garret Miller for generating the initial chromatograms. We collectively acknowledge that Michigan State University occupies the ancestral, traditional and contemporary Lands of the Anishinaabeg—Three Fires Confederacy of Ojibwe, Odawa and Potawatomi peoples. In particular, the University resides on Land ceded in the 1819 Treaty of Saginaw. We recognize, support and advocate for the sovereignty of Michigan’s twelve federally recognized Indian nations, for historic Indigenous communities in Michigan, for Indigenous individuals and communities who live here now and for those who were forcibly removed from their Homelands. By offering this Land Acknowledgement, we affirm Indigenous sovereignty and will work to hold Michigan State University more accountable to the needs of American Indian and Indigenous peoples.
Conflicts of Interest
Author Allison Hurt is currently employed by the company Pfizer. This employment is unrelated to the study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Henke, N.A.; Wichmann, J.; Baier, T.; Frohwitter, J.; Lauersen, K.J.; Risse, J.M.; Peters-Wendisch, P.; Kruse, O.; Wendisch, V.F. Patchoulol production with metabolically engineered Corynebacterium glutamicum. Genes 2018, 9, 219. [Google Scholar] [CrossRef]
- Zhan, X.; Zhang, Y.H.; Chen, D.F.; Simonsen, H.T. Metabolic engineering of the moss Physcomitrella patens to produce the sesquiterpenoids patchoulol and α/β-santalene. Front. Plant Sci. 2014, 5, 536. [Google Scholar] [CrossRef]
- Zhou, F.; Pichersky, E. More is better: The diversity of terpene metabolism in plants. Curr. Opin. Plant Biol. 2020, 55, 1–10. [Google Scholar] [CrossRef]
- Veitch, G.E.; Beckmann, E.; Burke, B.J.; Boyer, A.; Maslen, S.L.; Ley, S.V. Synthesis of azadirachtin: A long but successful journey. Angew. Chem. Int. Ed. 2007, 46, 7629–7632. [Google Scholar] [CrossRef] [PubMed]
- Paddon, C.J.; Keasling, J.D. Semi-synthetic artemisinin: A model for the use of synthetic biology in pharmaceutical development. Nat. Rev. Microbiol. 2014, 12, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Kadkade, P.G.; Prince, C.L.; Roach, B.L.; Antonio, S. Enhanced Production of Taxol and Taxanes by Cell Cultures of Taxus Species. Patent WO/1993/017121, 2013. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiTv6Gvy8-EAxVrnK8BHY-OD5EQFnoECBMQAQ&url=https%3A%2F%2Fdata.epo.org%2Fgpi%2FEP0960944A1.pdf%3Fdownload%3Dtrue&usg=AOvVaw0IeO8sGVHlyWVZ6IiGvLAJ&opi=89978449 (accessed on 11 May 2023).
- Mao, G.; Chaturvedula, P.V.S.; Yu, O. Non-Caloric Sweetener. U.S. Patent 9,765,104B2, 2017. Available online: https://uspto.report/patent/grant/9765104 (accessed on 11 May 2023).
- Leonard, E.; Ajikumar, P.K.; Thayer, K.; Xiao, W.-H.; Mo, J.D.; Tidor, B.; Stephanopoulos, G.; Prather, K.L.J. Combining metabolic and protein engineering of a terpenoid biosynthetic pathway for overproduction and selectivity control. Proc. Natl. Acad. Sci. USA 2010, 107, 13654–13659. [Google Scholar] [CrossRef] [PubMed]
- Frontiers|Microbial Platform for Terpenoid Production: Escherichia coli and Yeast [Internet]. Available online: https://www.frontiersin.org/articles/10.3389/fmicb.2018.02460/full (accessed on 22 January 2024).
- Kirby, J.; Nishimoto, M.; Chow, R.W.N.; Baidoo, E.E.K.; Wang, G.; Martin, J.; Schackwitz, W.; Chan, R.; Fortman, J.L.; Keasling, J.D. Enhancing Terpene Yield from Sugars via Novel Routes to 1-Deoxy-d-Xylulose 5-Phosphate. Appl. Environ. Microbiol. 2015, 81, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, J.; Keeling, C.I. Terpenoid biomaterials. Plant J. 2008, 54, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Morrone, D.; Lowry, L.; Determan, M.K.; Hershey, D.M.; Xu, M.; Peters, R.J. Increasing diterpene yield with a modular metabolic engineering system in E. coli: Comparison of MEV and MEP isoprenoid precursor pathway engineering. Appl. Microbiol. Biotechnol. 2010, 85, 1893–1906. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, F.; Kroner, C.; Lubuta, P.; Peyraud, R.; Horst, A.; Buchhaupt, M.; Schrader, J. Engineering Methylobacterium extorquens for de novo synthesis of the sesquiterpenoid α-humulene from methanol. Metab. Eng. 2015, 32, 82–94. [Google Scholar] [CrossRef]
- Delmotte, N.; Knief, C.; Chaffron, S.; Innerebner, G.; Roschitzki, B.; Schlapbach, R.; von Mering, C.; Vorholt, J.A. Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proc. Natl. Acad. Sci. USA 2009, 106, 16428–16433. [Google Scholar] [CrossRef]
- Good, N.M.; Vu, H.N.; Suriano, C.J.; Subuyuj, G.A.; Skovran, E.; Martinez-Gomez, N.C. Pyrroloquinoline quinone ethanol dehydrogenase in Methylobacterium extorquens AM1 extends lanthanide-dependent metabolism to multicarbon substrates. J. Bacteriol. 2016, 198, 3109–3118. [Google Scholar] [CrossRef]
- Skovran, E.; Martinez-Gomez, N.C. Just add lanthanides. Science 2015, 348, 862–863. [Google Scholar] [CrossRef]
- Van Dien, S.J.; Marx, C.J.; O’Brien, B.N.; Lidstrom, M.E. Genetic Characterization of the Carotenoid Biosynthetic Pathway in Methylobacterium extorquens AM1 and Isolation of a Colorless Mutant. Appl. Environ. Microbiol. 2003, 69, 7563–7566. [Google Scholar] [CrossRef]
- Ma, B.; Liu, M.; Li, Z.H.; Tao, X.; Wei, D.Z.; Wang, F.Q. Significantly Enhanced Production of Patchoulol in Metabolically Engineered Saccharomyces cerevisiae. J. Agric. Food Chem. 2019, 67, 8590–8598. [Google Scholar] [CrossRef]
- Munck, S.L.; Croteau, R. Purification and characterization of the sesquiterpene cyclase patchoulol synthase from Pogostemon cablin. Arch. Biochem. Biophys. 1990, 282, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Dueber, M.; Adolf, W.; West, C. Biosynthesis of the Diterpene Phytoalexin Casbene: Partial Purification and Characterization of Casbene Synthetase from Ricinis communis. Plant Physiol. 1978, 62, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Bibik, J.D.; Weraduwage, S.M.; Banerjee, A.; Robertson, K.; Espinoza-Corral, R.; Sharkey, T.D.; Lundquist, P.K.; Hamberger, B.R. Pathway Engineering, Re-targeting, and Synthetic Scaffolding Improve the Production of Squalene in Plants. ACS Synth. Biol. 2022, 11, 2121–2133. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Callari, R.; Hamberger, B.; Wubshet, S.G.; Nielsen, M.T.; Andersen-Ranberg, J.; Hallström, B.M.; Cozzi, F.; Heider, H.; Møller, B.L.; et al. Oxidation and cyclization of casbene in the biosynthesis of Euphorbia factors from mature seeds of Euphorbia lathyris L. Proc. Natl. Acad. Sci. USA 2016, 113, E5082–E5089. [Google Scholar] [CrossRef] [PubMed]
- Delaney, N.F.; Kaczmarek, M.E.; Ward, L.M.; Swanson, P.K.; Lee, M.C.; Marx, C.J. Development of an Optimized Medium, Strain and High-Throughput Culturing Methods for Methylobacterium extorquens. PLoS ONE 2013, 8, e62957. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Chou, H.H.; Marx, C.J. Asymmetric, bimodal trade-offs during adaptation of methylobacterium to distinct growth substrates. Evolution 2009, 63, 2816–2830. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Metcalf, W.W. Distinct regulators control the expression of methanol methyltransferase isozymes in Methanosarcina acetivorans C2A. Mol. Microbiol. 2008, 67, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Skovran, E.; Palmer, A.D.; Rountree, A.M.; Good, N.M.; Lidstrom, M.E. XoxF is required for expression of methanol dehydrogenase in Methylobacterium extorquens AM1. J. Bacteriol. 2011, 193, 6032–6038. [Google Scholar] [CrossRef] [PubMed]
- Deguerry, F.; Pastore, L.; Wu, S.; Clark, A.; Chappell, J.; Schalk, M. The diverse sesquiterpene profile of patchouli, Pogostemon cablin, is correlated with a limited number of sesquiterpene synthases. Arch. Biochem. Biophys. 2006, 454, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Cyr, A.; Wilderman, P.R.; Determan, M.; Peters, R.J. A modular approach for facile biosynthesis of labdane-related diterpenes. J. Am. Chem. Soc. 2007, 129, 6684–6685. [Google Scholar] [CrossRef] [PubMed]
- Toyama, H.; Anthony, C.; Lidstrom, M.E. Construction of insertion and deletion mxa mutants of Methylobacterium extorquens AM1 by electroporation. FEMS Microbiol. Lett. 1998, 166, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, M.F.; Brooks, T.; Pukatzki, S.U.; Provenzano, D. Rapid protocol for preparation of electrocompetent Escherichia coli and Vibrio cholerae. J. Vis. Exp. 2013, 2013, 50684. [Google Scholar]
- Miller, G.P.; Bhat, W.W.; Lanier, E.R.; Johnson, S.R.; Mathieu, D.T.; Hamberger, B. The biosynthesis of the anti-microbial diterpenoid leubethanol in Leucophyllum frutescens proceeds via an all-cis prenyl intermediate. Plant J. 2020, 104, 693–705. [Google Scholar] [CrossRef]
- Martinez-Gomez, N.C.; Good, N.M.; Lidstrom, M.E. Methenyl-Dephosphotetrahydromethanopterin Is a Regulatory Signal for Acclimation to Changes in Substrate Availability in Methylobacterium extorquens AM1. J. Bacteriol. 2015, 197, 2020–2026. [Google Scholar] [CrossRef]
- Klaus, O.; Hilgers, F.; Nakielski, A.; Hasenklever, D.; Jaeger, K.E.; Axmann, I.M.; Axmann, I.M.; Drepper, T. Engineering phototrophic bacteria for the production of terpenoids. Curr. Opin. Biotechnol. 2022, 77, 102764. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).