Unveiling the Mutations and Conservation of InlA in Listeria monocytogenes
Abstract
1. Introduction
2. Materials and Methods
2.1. Genomes of Listeria monocytogenes
2.2. MLST Analysis
2.3. Alignment and Analysis of InlA Sequence
2.4. Statistical Analysis
3. Results
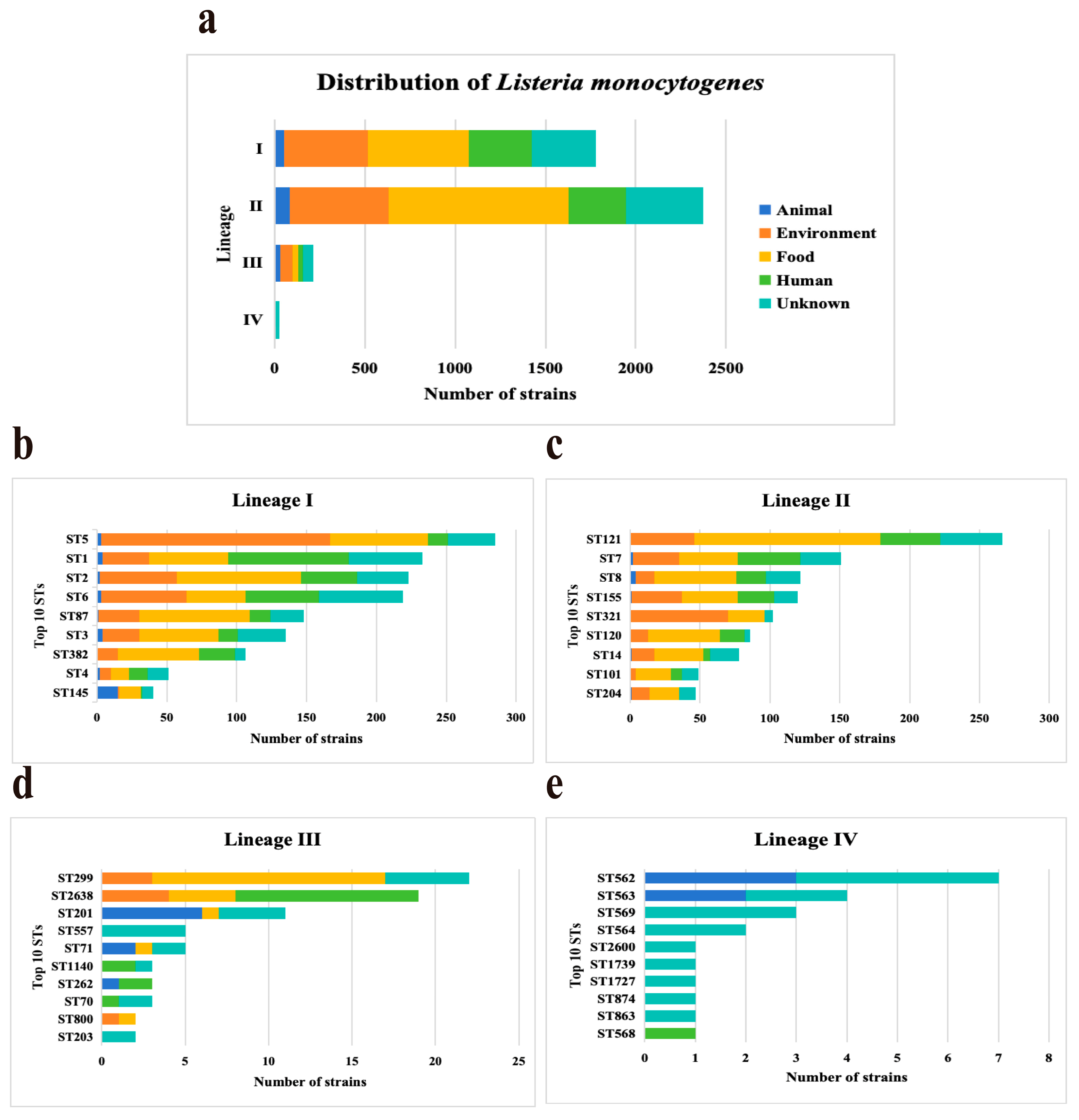
3.1. Distribution Characteristics and MLST of L. monocytogenes Genomes
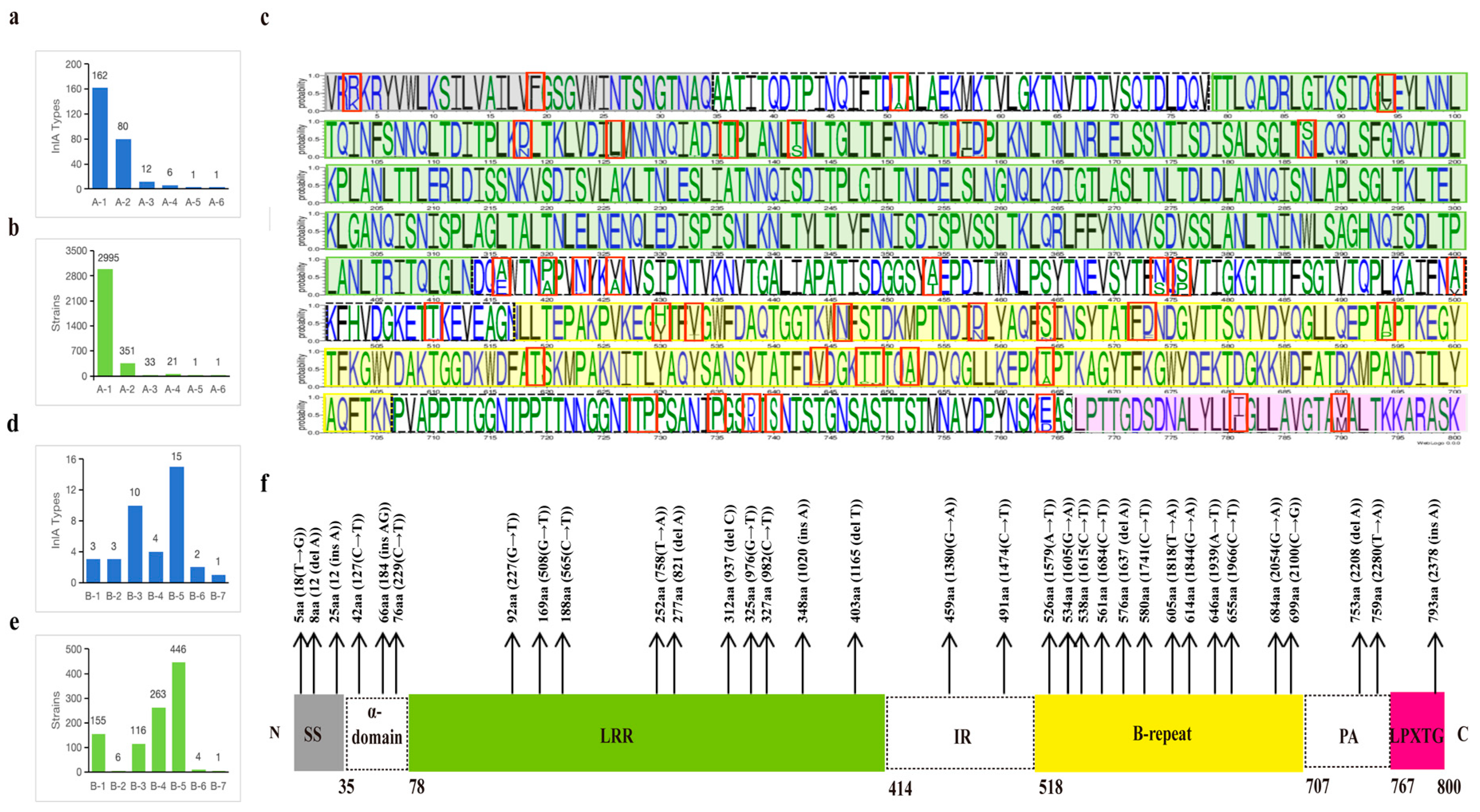
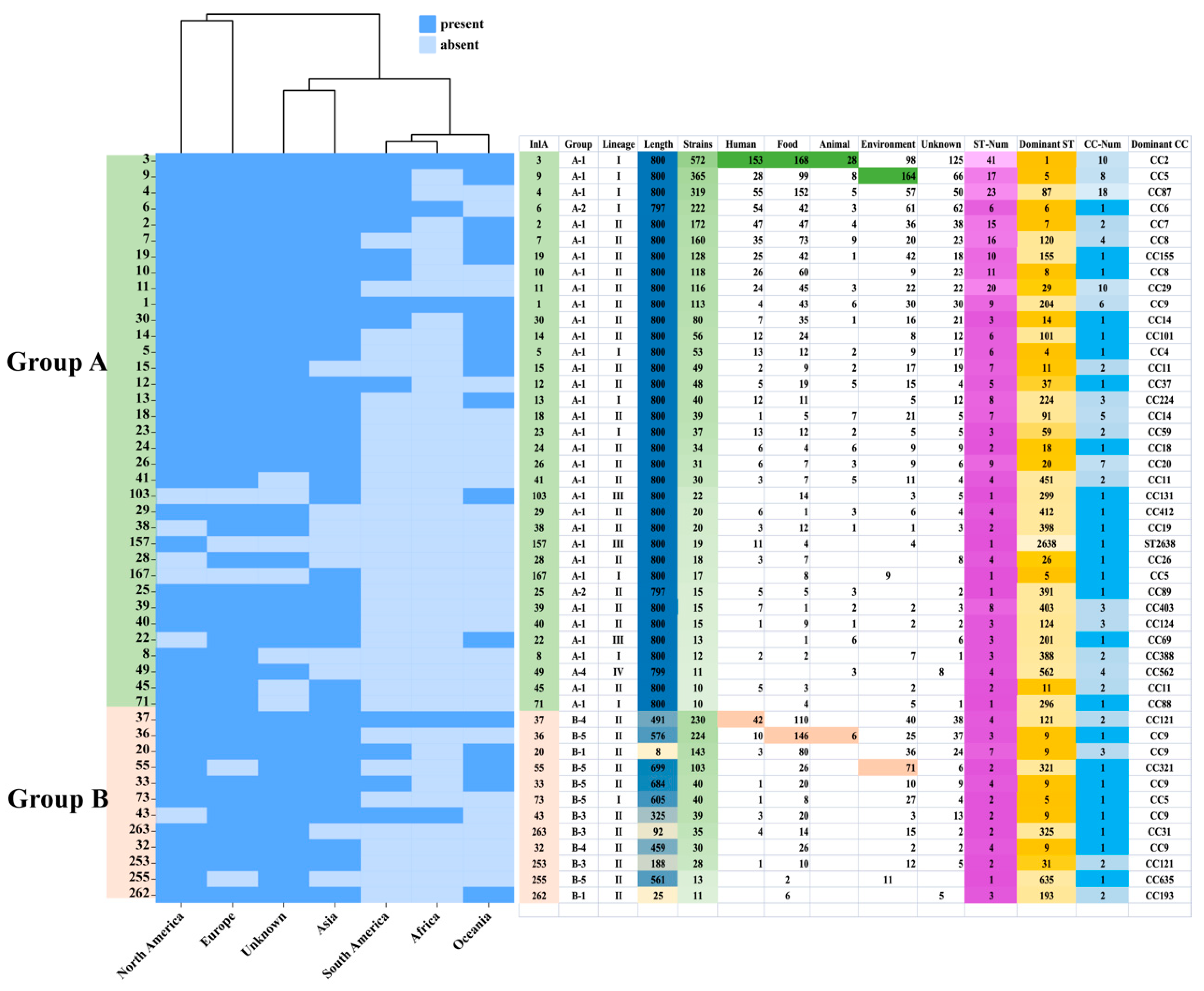
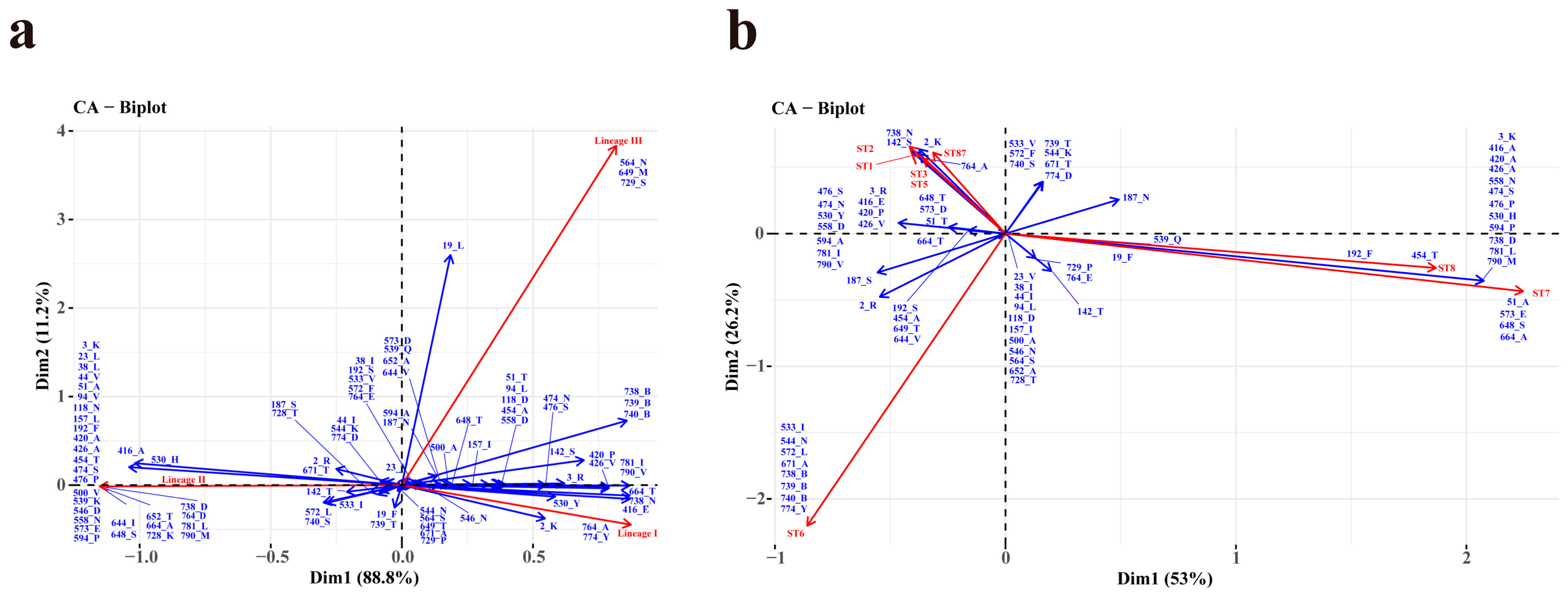
3.2. The Classification and Analysis of InlA Sequence Types
3.3. The Classification and Analysis of InlA Sequence Types
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, X.; Cao, G.; Zhang, J.; Pan, H.; Zhang, D.; Kuang, D.; Yang, X.; Xu, X.; Shi, X.; Meng, J. Characterization of internalin genes in Listeria monocytogenes from food and humans, and their association with the invasion of Caco-2 cells. Gut Pathog. 2019, 11, 30. [Google Scholar] [CrossRef]
- Wagner, E.; Zaiser, A.; Leitner, R.; Quijada, N.; Pracser, N.; Pietzka, A.; Ruppitsch, W.; Schmitz-Esser, S.; Wagner, M.; Rychli, K. Virulence characterization and comparative genomics of Listeria monocytogenes sequence type 155 strains. BMC Genom. 2020, 21, 847. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.; Rengarajan, M.; Chavez, N.; Radhakrishnan, P.; Gloerich, M.; Bianchini, J.; Siemers, K.; Luckett, W.; Lauer, P.; Nelson, W.; et al. Listeria monocytogenes Adhesion to the host cell surface is sufficient to mediate entry into epithelial cells. Mol. Biol. Cell 2017, 28, 2945–2957. [Google Scholar] [CrossRef] [PubMed]
- Lyautey, E.; Hartmann, A.; Pagotto, F.; Tyler, K.; Lapen, D.R.; Wilkes, G.; Piveteau, P.; Rieu, A.; Robertson, W.J.; Medeiros, D.T. Characteristics and frequency of detection of fecal Listeria monocytogenes shed by livestock, wildlife, and humans. Can. J. Microbiol. 2007, 53, 1158–1167. [Google Scholar] [CrossRef]
- Chen, Y.; Ross, W.H.; Whiting, R.C.; Stelten, A.V.; Nightingale, K.K.; Wiedmann, M.; Scott, V.N. Variation in Listeria monocytogenes dose responses in relation to subtypes encoding a full-length or truncated internalin A. Appl. Environ. Microbiol. 2011, 77, 1171. [Google Scholar] [CrossRef] [PubMed]
- Van Stelten, A.; Nightingale, K.K. Development and Implementation of a Multiplex Single-Nucleotide Polymorphism Genotyping Assay for Detection of Virulence-Attenuating Mutations in the Listeria monocytogenes Virulence-Associated Gene inlA. Appl. Environ. Microbiol. 2008, 74, 7365–7375. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Molina, A.; Cobo-Díaz, J.F.; López, M.; Prieto, M.; de Toro, M.; Alvarez-Ordóez, A. Unraveling the emergence and population diversity of Listeria monocytogenes in a newly built meat facility through whole genome sequencing. Int. J. Food Microbiol. 2021, 340, 109043. [Google Scholar] [CrossRef] [PubMed]
- Orsi, R.H.; Bakker, H.C.D.; Wiedmann, M. Listeria monocytogenes lineages: Genomics, evolution, ecology, and phenotypic characteristics. Int. J. Med. Microbiol. IJMM 2011, 301, 79–96. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.F.; Ferreira, V.; Magalhaes, R.; Almeida, G.; Alves, A.; Teixeira, P. Detection of premature stop codons leading to truncated internalin A among food and clinical strains of Listeria monocytogenes. Food Microbiol. 2017, 63, 6–11. [Google Scholar] [CrossRef]
- Chen, M.; Cheng, J.; Zhang, J.; Chen, Y.; Wu, Q. Isolation, Potential Virulence, and Population Diversity of Listeria monocytogenes From Meat and Meat Products in China. Front. Microbiol. 2019, 10, 946. [Google Scholar] [CrossRef]
- Song, Z.; Ji, S.; Wang, Y.; Luo, L.; Wang, Y.; Mao, P.; Li, L.; Jiang, H.; Ye, C. The population structure and genetic diversity of Listeria monocytogenes ST9 strains based on genomic analysis. Front. Microbiol. 2022, 13, 982220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.; Li, Z.; Xu, C.; Wang, H.; Yang, R.; Liu, L. Clinical and Genomic Characteristics of a Clinical Listeria monocytogenes ST120 Isolate Recovered from a Pregnant Woman. Infect. Drug Resist. 2024, 17, 229–237. [Google Scholar] [CrossRef]
- Muchaamba, F.; Eshwar, A.; Stevens, M.; Stephan, R.; Tasara, T. Different Shades of Listeria monocytogenes: Strain, Serotype, and Lineage-Based Variability in Virulence and Stress Tolerance Profiles. Front. Microbiol. 2021, 12, 792162. [Google Scholar] [CrossRef]
- Gyanwali, G.C.; Herath, T.U.B.; Gianfelice, A.; Ireton, K. Listeria monocytogenes Co-Opts the Host Exocyst Complex to Promote Internalin A-Mediated Entry. Infect. Immun. 2022, 90, e0032622. [Google Scholar] [CrossRef]
- Parra-Flores, J.; Holý, O.; Bustamante, F.; Lepuschitz, S.; Pietzka, A.; Contreras-Fernández, A.; Castillo, C.; Ovalle, C.; Alarcón-Lavín, M.; Cruz-Córdova, A.; et al. Virulence and Antibiotic Resistance Genes in Listeria monocytogenes Strains Isolated from Ready-to-Eat Foods in Chile. Front. Microbiol. 2021, 12, 796040. [Google Scholar] [CrossRef]
- Agostinho Davanzo, E.; Dos Santos, R.; Castro, V.; Palma, J.; Pribul, B.; Dallago, B.; Fuga, B.; Medeiros, M.; Titze de Almeida, S.; da Costa, H.; et al. Molecular characterization of Salmonella spp. and Listeria monocytogenes strains from biofilms in cattle and poultry slaughterhouses located in the federal District and State of Goiás, Brazil. PLoS ONE 2021, 16, e0259687. [Google Scholar] [CrossRef]
- Cruz, C.; Pitman, A.; Harrow, S.; Fletcher, G. Listeria monocytogenes associated with New Zealand seafood production and clinical cases: Unique sequence types, truncated InlA, and attenuated invasiveness. Appl. Environ. Microbiol. 2014, 80, 1489–1497. [Google Scholar] [CrossRef]
- Li, L.L.; Ji, S.S.; Wang, Y.; Mao, P.; Chen, J.N.; Liu, L.Y.; Ye, C.Y. Genetic polymorphism of inlA in Listeria monocytogenes. Chin. J. Zoonoses 2023, 39, 240–248. (In Chinese) [Google Scholar] [CrossRef]
- Magagna, G.; Gori, M.; Russini, V.; De Angelis, V.; Spinelli, E.; Filipello, V.; Tranquillo, V.; De Marchis, M.; Bossù, T.; Fappani, C.; et al. Evaluation of the Virulence Potential of Listeria monocytogenes through the Characterization of the Truncated Forms of Internalin A. Int. J. Mol. Sci. 2023, 24, 10141. [Google Scholar] [CrossRef]
- Ji, S.; Song, Z.; Luo, L.; Wang, Y.; Li, L.; Mao, P.; Ye, C.; Wang, Y. Whole-genome sequencing reveals genomic characterization of Listeria monocytogenes from food in China. Front. Microbiol. 2022, 13, 1049843. [Google Scholar] [CrossRef]
- Medeiros, M.; Castro, V.; Mota, A.; Pereira, M.G.; Santana, A.P. Assessment of Internalin A Gene Sequences and Cell Adhesion and Invasion Capacity of Listeria monocytogenes Strains Isolated from Foods of Animal and Related Origins. Foodborne Pathog. Dis. 2020, 18. [Google Scholar] [CrossRef]
- Dellafiora, L.; Filipello, V.; Dall’Asta, C.; Finazzi, G.; Galaverna, G.; Losio, M.N. A Structural Study on the Listeria monocytogenes Internalin A—Human E-cadherin Interaction: A Molecular Tool to Investigate the Effects of Missense Mutations. Toxins 2020, 12, 60. [Google Scholar] [CrossRef]
- Schmitz-Esser, S.; Anast, J.M.; Cortes, B.W. A Large-Scale Sequencing-Based Survey of Plasmids in Listeria monocytogenes Reveals Global Dissemination of Plasmids. Front. Microbiol. 2021, 12, 653155. [Google Scholar] [CrossRef]
- Gelbíčová, T.; Koláčková, I.; Pantůček, R.; Karpíšková, R. A novel mutation leading to a premature stop codon in inlA of Listeria monocytogenes isolated from neonatal listeriosis. New Microbiol. 2015, 38, 293–296. [Google Scholar]
- Kanki, M.; Naruse, H.; Taguchi, M.; Kumeda, Y. Characterization of specific alleles in InlA and PrfA of Listeria monocytogenes isolated from foods in Osaka, Japan and their ability to invade Caco-2 cells. Int. J. Food Microbiol. 2015, 211, 18–22. [Google Scholar] [CrossRef]
- Mao, P.; Wang, Y.; Gan, L.; Liu, L.; Chen, J.; Li, L.; Sun, H.; Luo, X.; Ye, C. Large-scale genetic analysis and biological traits of two SigB factors in Listeria monocytogenes: Lineage correlations and differential functions. Front. Microbiol. 2023, 14, 1268709. [Google Scholar] [CrossRef]
- Schubert, W.D.; Urbanke, C.; Ziehm, T.; Beier, V.; Heinz, D.W. Structure of internalin, a major invasion protein of Listeria monocytogenes, in complex with its human receptor E-cadherin. Cell 2002, 111, 825–836. [Google Scholar] [CrossRef]
- Lecuit, M.; Ohayon, H.; Braun, L.; Mengaud, J.; Cossart, P. Internalin of Listeria monocytogenes with an intact leucine-rich repeat region is sufficient to promote internalization. Infect. Immun. 1997, 65, 5309–5319. [Google Scholar] [CrossRef]
- Osek, J.; Lachtara, B.; Wieczorek, K. How This Pathogen Survives in Food-Production Environments? Front. Microbiol. 2022, 13, 866462. [Google Scholar] [CrossRef]
- Liu, X.; Chen, W.; Fang, Z.; Yu, Y.; Bi, J.; Wang, J.; Dong, Q.; Zhang, H. Persistence of Listeria monocytogenes ST5 in Ready-to-Eat Food Processing Environment. Foods 2022, 11, 2561. [Google Scholar] [CrossRef]
- Frédérique, P.; Federica, P.; Laurent, G.; Alex, L.; Alessandra, D.C.; Gerardo, M. Listeria monocytogenes sequence Types 121 and 14 Repeatedly Isolated Within One Year of Sampling in a Rabbit Meat Processing Plant: Persistence and Ecophysiology. Front. Microbiol. 2018, 9, 596. [Google Scholar]
- Koutsoumanis, K.; Allende, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Herman, L.; Hilbert, F.; Lindqvist, R.; Nauta, M.; et al. Persistence of microbiological hazards in food and feed production and processing environments. EFSA J. Eur. Food Saf. Auth. 2024, 22, e8521. [Google Scholar] [CrossRef]
- Mao, P.; Wang, Y.; Li, L.; Ji, S.; Li, P.; Liu, L.; Chen, J.; Sun, H.; Luo, X.; Ye, C. ListeriaThe Isolation, Genetic Analysis and Biofilm Characteristics of spp. from the Marine Environment in China. Microorganisms 2023, 11, 2166. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, L.; Ji, S.; Li, Q.; Wang, H.; Zhang, Z.; Mao, P.; Sun, H.; Li, L.; Wang, Y.; et al. Dissecting Listeria monocytogenes Persistent Contamination in a Retail Market Using Whole-Genome Sequencing. Microbiol. Spectr. 2022, 10, e0018522. [Google Scholar] [CrossRef]
- Lisa, G.; Parker, C.T.; Liang, A.S.; Samarpita, W.; Romanolo, K.F.; Raymond, S. The Majority of Genotypes of the Virulence Gene inlA Are Intact among Natural Watershed Isolates of Listeria monocytogenes from the Central California Coast. PLoS ONE 2016, 11, e0167566. [Google Scholar]
- Ireton, K.; Mortuza, R.; Gyanwali, G.; Gianfelice, A.; Hussain, M. Role of internalin proteins in the pathogenesis of Listeria monocytogenes. Mol. Microbiol. 2021, 116, 1407–1419. [Google Scholar] [CrossRef]
- Gómez Borrego, J.; Torrent Burgas, M. Analysis of Host-Bacteria Protein Interactions Reveals Conserved Domains and Motifs That Mediate Fundamental Infection Pathways. Int. J. Mol. Sci. 2022, 23, 11489. [Google Scholar] [CrossRef]
- Jackson, B.; Salter, M.; Tarr, C.; Conrad, A.; Harvey, E.; Steinbock, L.; Saupe, A.; Sorenson, A.; Katz, L.; Stroika, S.; et al. Notes from the field: Listeriosis associated with stone fruit—United States, 2014. MMWR. Morb. Mortal. Wkly. Rep. 2015, 64, 282–283. [Google Scholar]
- Maurella, C.; Gallina, S.; Ru, G.; Adriano, D.; Decastelli, L. Outbreak of febrile gastroenteritis caused by Listeria monocytogenes 1/2a in sliced cold beef ham, Italy, May 2016. Euro. Surveill. 2018, 23, 17-00155. [Google Scholar] [CrossRef] [PubMed]
- Acciari, V.; Iannetti, L.; Gattuso, A.; Sonnessa, M.; Scavia, G.; Montagna, C.; Addante, N.; Torresi, M.; Zocchi, L.; Scattolini, S.; et al. Tracing sources of Listeria contamination in traditional Italian cheese associated with a US outbreak: Investigations in Italy. Epidemiol. Infect. 2016, 144, 2719–2727. [Google Scholar] [CrossRef]
- Thomas, J.; Govender, N.; Mccarthy, K.M.; Erasmus, L.K.; Blumberg, L.H. Outbreak of Listeriosis in South Africa Associated With Processed Meat. New Engl. J. Med. 2020, 382, 632–643. [Google Scholar] [CrossRef]
- Chen, Y.; Strain, E.A.; Allard, M.; Brown, E.W. Genome sequences of Listeria monocytogenes strains J1816 and J1-220, associated with human outbreaks. J. Bacteriol. 2011, 193, 3424–3425. [Google Scholar] [CrossRef]
- Kovacevic, J.; Arguedas-Villa, C.; Wozniak, A.; Tasara, T.; Allen, K. Examination of food chain-derived Listeria monocytogenes strains of different serotypes reveals considerable diversity in inlA genotypes, mutability, and adaptation to cold temperatures. Appl. Environ. Microbiol. 2013, 79, 1915–1922. [Google Scholar] [CrossRef]
- Pirone-Davies, C.; Chen, Y.; Pightling, A.; Ryan, G.; Wang, Y.; Yao, K.; Hoffmann, M.; Allard, M. Genes significantly associated with lineage II food isolates of Listeria monocytogenes. BMC Genom. 2018, 19, 708. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Pouillot, R.; Dennis, S.; Brown, E.W. Genetic diversity and profiles of genes associated with virulence and stress resistance among isolates from the 2010–2013 interagency Listeria monocytogenes market basket survey. PLoS ONE 2020, 15, e0231393. [Google Scholar] [CrossRef]
- Magagna, G.; Finazzi, G.; Filipello, V. inlANewly Designed Primers for the Sequencing of the Gene of Lineage I and II Isolates. Int. J. Mol. Sci. 2022, 23, 14106. [Google Scholar] [CrossRef]
- Holch, A.; Ingmer, H.; Licht, T.R.; Gram, L. Listeria monocytogenes strains encoding premature stop codons in inlA invade mice and guinea pig fetuses in orally dosed dams. J. Med. Microbiol. 2013, 62, 1799–1806. [Google Scholar] [CrossRef]
- Sévellec, Y.; Torresi, M.; Félix, B.; Palma, F.; Roussel, S. First Report on the Finding of Listeria monocytogenes ST121 Strain in a Dolphin Brain. Pathogens 2020, 9, 802. [Google Scholar] [CrossRef]
- Thalacker-Mercer, A.R.; Riddle, E.; Barre, L. Protein and amino acids for skeletal muscle health in aging. Adv. Food Nutr. Res. 2020, 91, 29–64. [Google Scholar]
- Sechrest, E.; Ma, X.; Cahill, M.; Barbera, R.; Wang, Y.; Deng, W. Structural and functional rescue of cones carrying the most common cone opsin C203R missense mutation. JCI Insight 2023, 23, e172834. [Google Scholar] [CrossRef]

| Site of Infection | B-1 | B-3 | B-4 | B-5 | Strains | PMSC Type | Lineage | Dominant ST | Dominant CC |
|---|---|---|---|---|---|---|---|---|---|
| SS | LRR | IR | B-Repeat | ||||||
| Breast milk | 36 | 36 | 6 | II | 121 | CC121 | |||
| Blood | 3 | 4 | 3 | 8 | 18 | 1, 4, 6, 12, 19, 23, 26 | II (16) I (2) | 9 | CC9 |
| Human | 2 | 1 | 3 | 6 | 1, 5, 6, 12, 19 | II (5) I (1) | 9 | CC9 | |
| Cerebrospinal fluid | 2 | 2 | 26, 19 | II | 325 | CC31 | |||
| Vaginal swab | 2 | 2 | 6 | II | 121 | CC121 | |||
| Throat swab | 2 | 2 | 11, 12 | II | 9 | CC9 | |||
| Feces of a pregnant | 1 | 1 | 12 | II | 9 | CC9 | |||
| Total | 3 | 8 | 42 | 14 | 67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Wang, Y.; Pu, J.; Chen, J.; Liu, L.; Mao, P.; Sun, H.; Luo, X.; Ye, C. Unveiling the Mutations and Conservation of InlA in Listeria monocytogenes. Microorganisms 2024, 12, 485. https://doi.org/10.3390/microorganisms12030485
Li L, Wang Y, Pu J, Chen J, Liu L, Mao P, Sun H, Luo X, Ye C. Unveiling the Mutations and Conservation of InlA in Listeria monocytogenes. Microorganisms. 2024; 12(3):485. https://doi.org/10.3390/microorganisms12030485
Chicago/Turabian StyleLi, Lingling, Yan Wang, Ji Pu, Jinni Chen, Lingyun Liu, Pan Mao, Hui Sun, Xia Luo, and Changyun Ye. 2024. "Unveiling the Mutations and Conservation of InlA in Listeria monocytogenes" Microorganisms 12, no. 3: 485. https://doi.org/10.3390/microorganisms12030485
APA StyleLi, L., Wang, Y., Pu, J., Chen, J., Liu, L., Mao, P., Sun, H., Luo, X., & Ye, C. (2024). Unveiling the Mutations and Conservation of InlA in Listeria monocytogenes. Microorganisms, 12(3), 485. https://doi.org/10.3390/microorganisms12030485






