Evaluation of the Immunity Responses in Mice to Recombinant Bacillus subtilis Displaying Newcastle Disease Virus HN Protein Truncations
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Vaccines, Cell, Plasmids, Primers Sequences, and Experimental Ethics
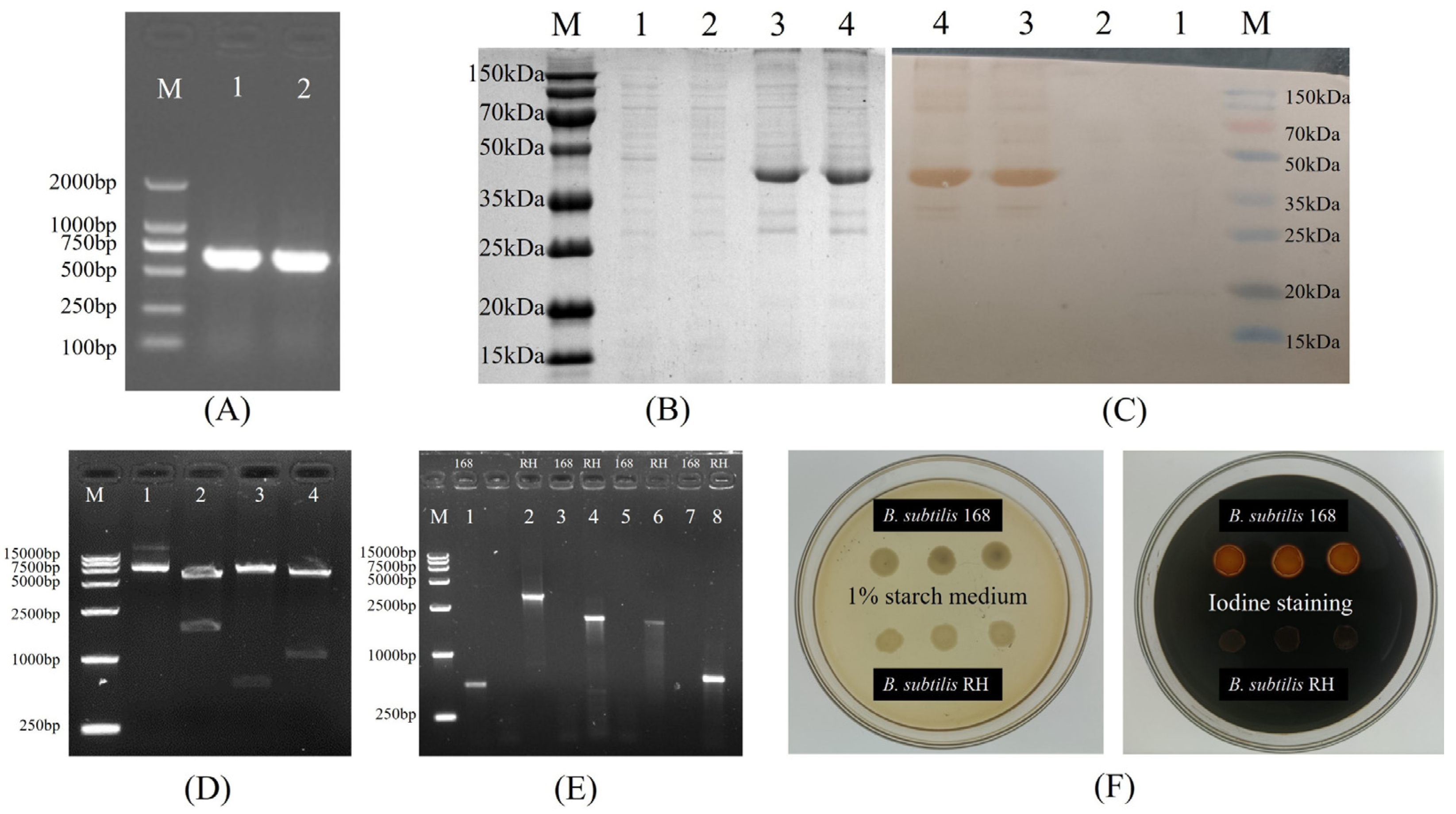
2.2. Cloning of Truncated HN Gene
2.3. Preparation of Hyperimmune Serum and Prokaryotic Expression of HNJD
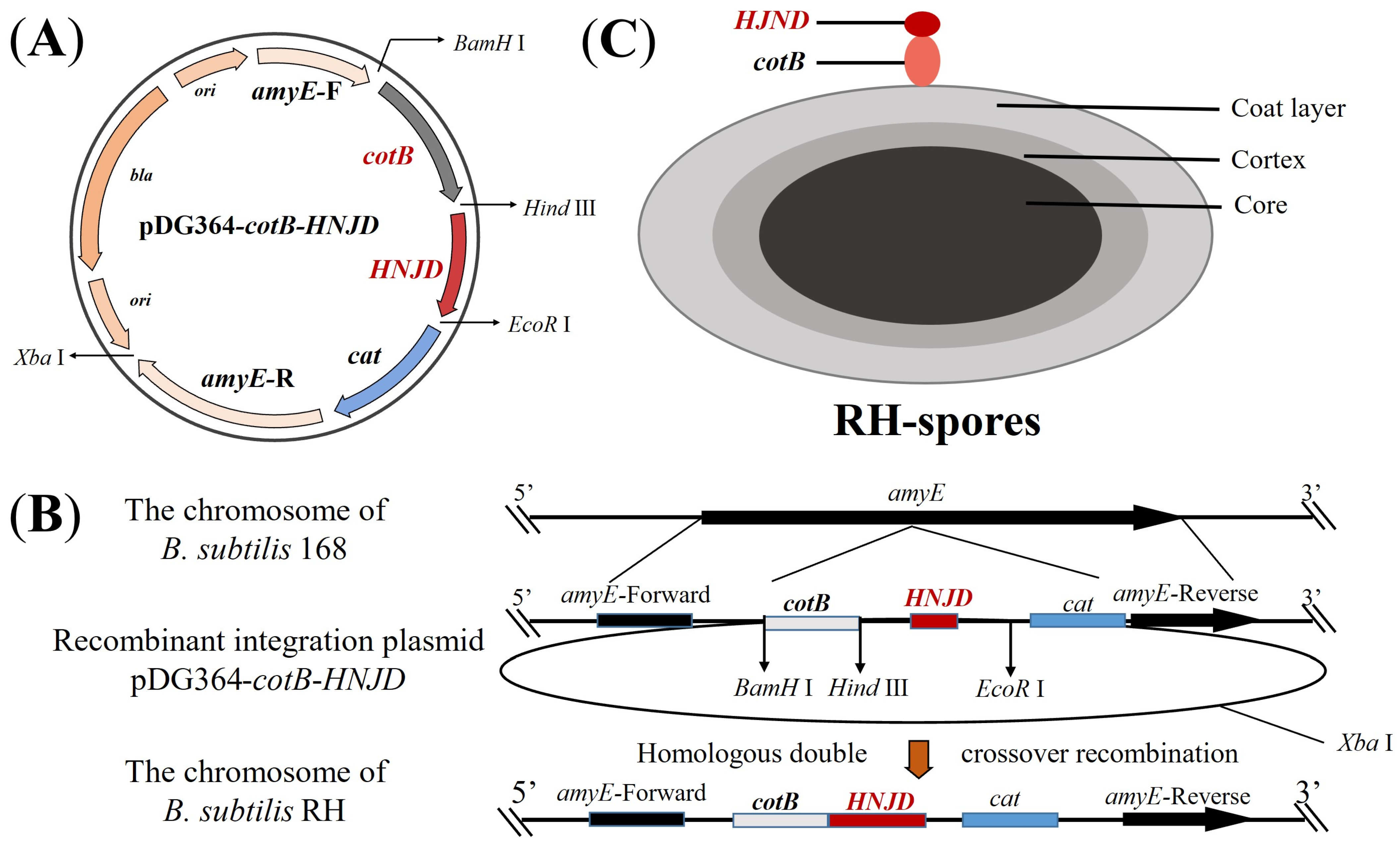
2.4. Construction and Transformation of Recombinant Integration Plasmid
2.5. Sporulation and Immunofluorescence Microscopy
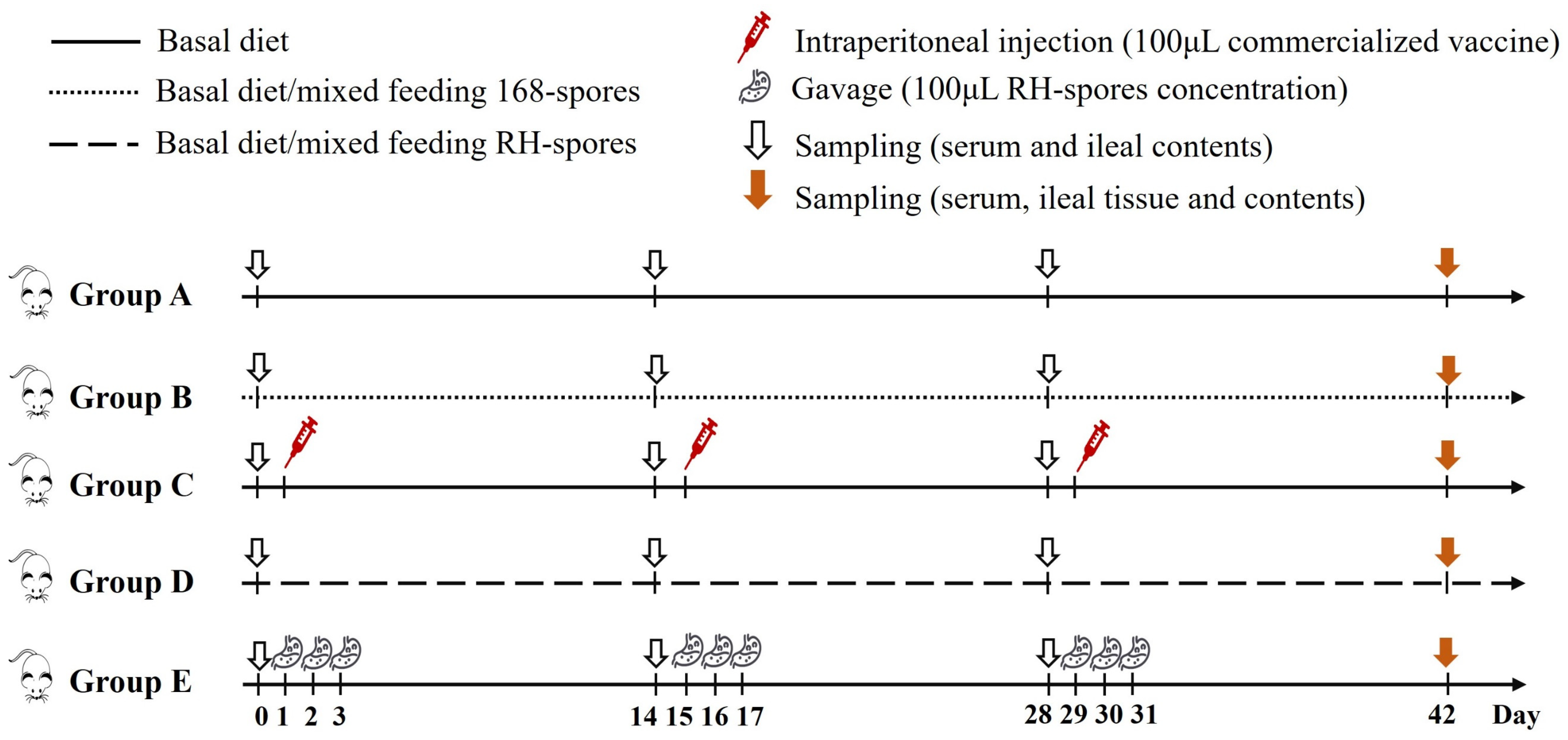
2.6. Immunization of Mice and Collection of Samples
2.7. Detection of NDV-Specific Serum and Mucosal Antibodies
2.8. Microneutralization Test
2.9. Hemagglutination Inhibition Test
2.10. Gene Expression of Cytokine in Ileum
2.11. The Morphology of Ileum
2.12. Statistical Analysis
3. Results
3.1. Construction and Transformation of Recombinant Plasmid
3.2. Sporulation and Immunofluorescence Microscopy
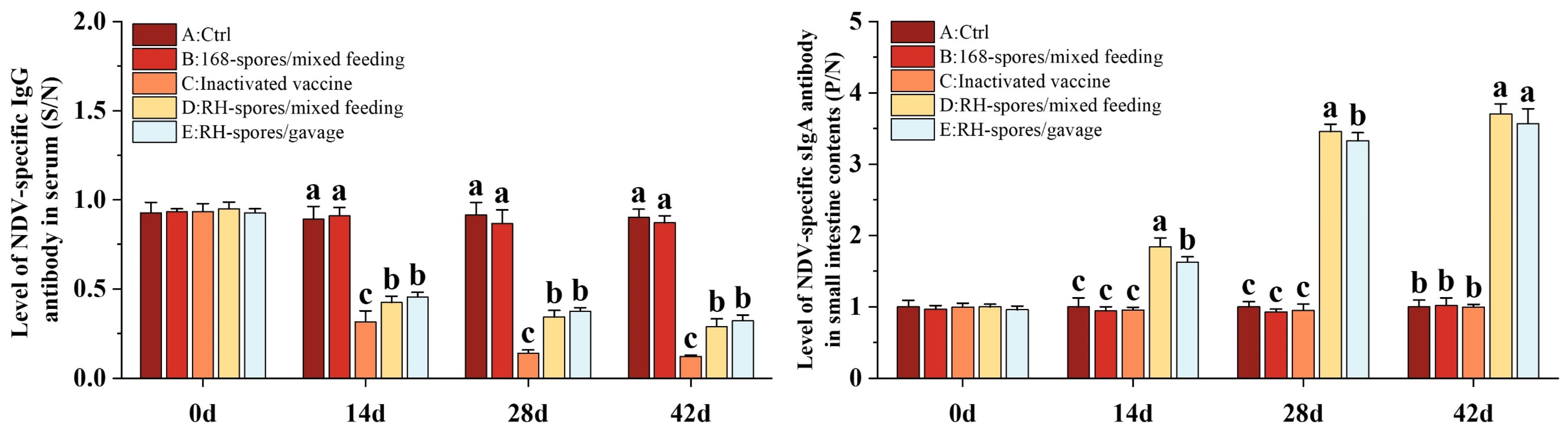
3.3. Detection of NDV-Specific Serum and Mucosal Antibodies
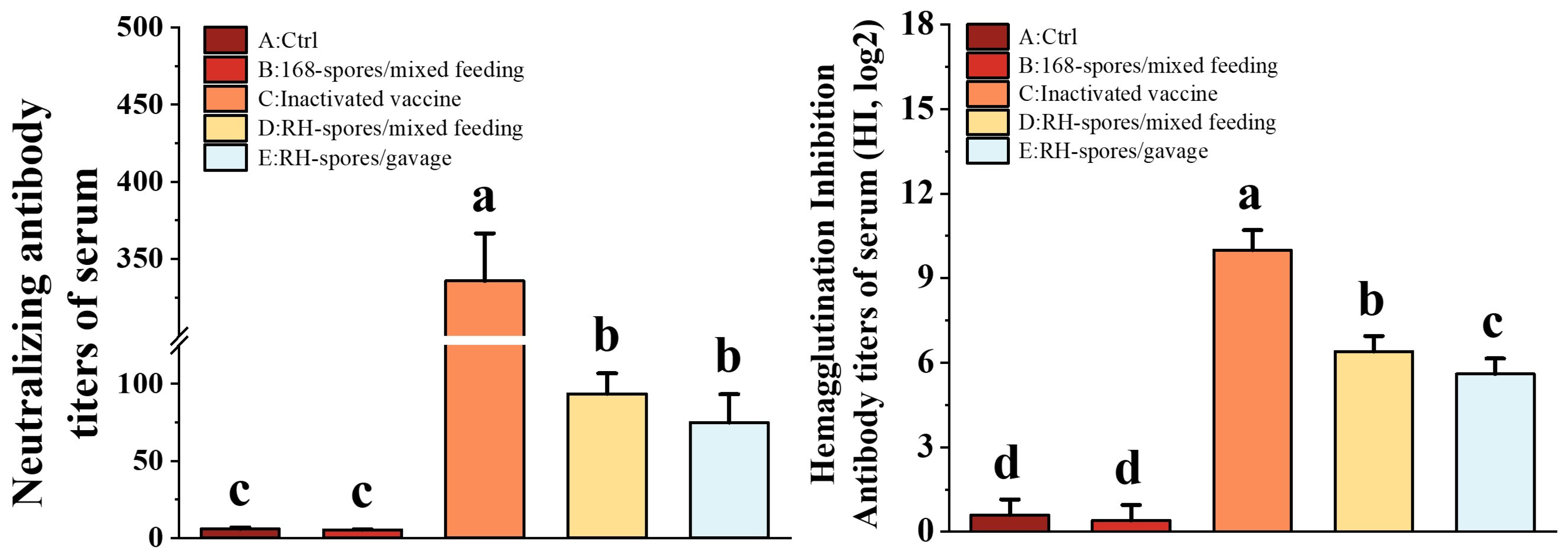
3.4. Detection of NA and HI Antibody Titers
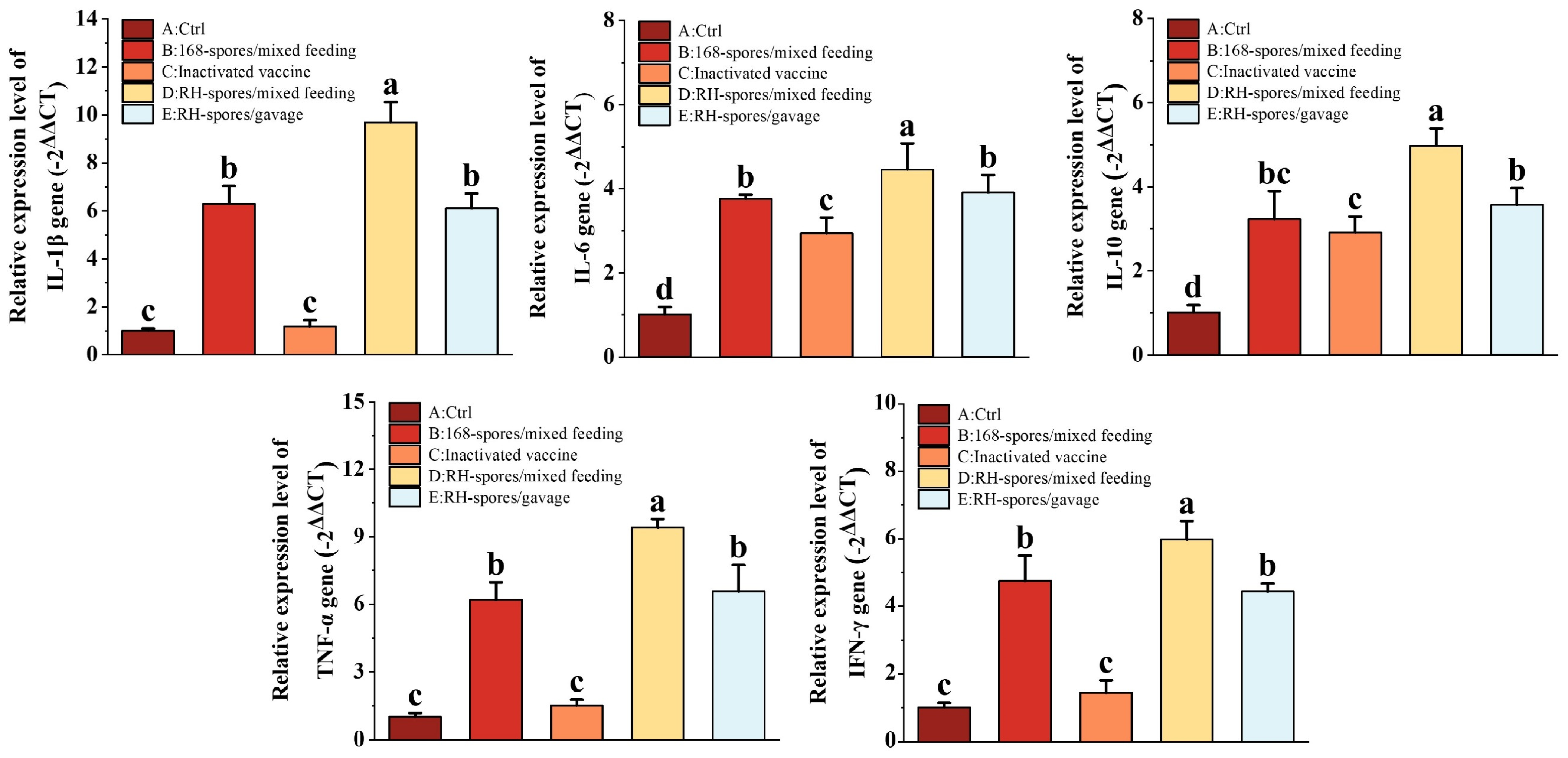
3.5. Gene Expression of Cytokine in the Ileum
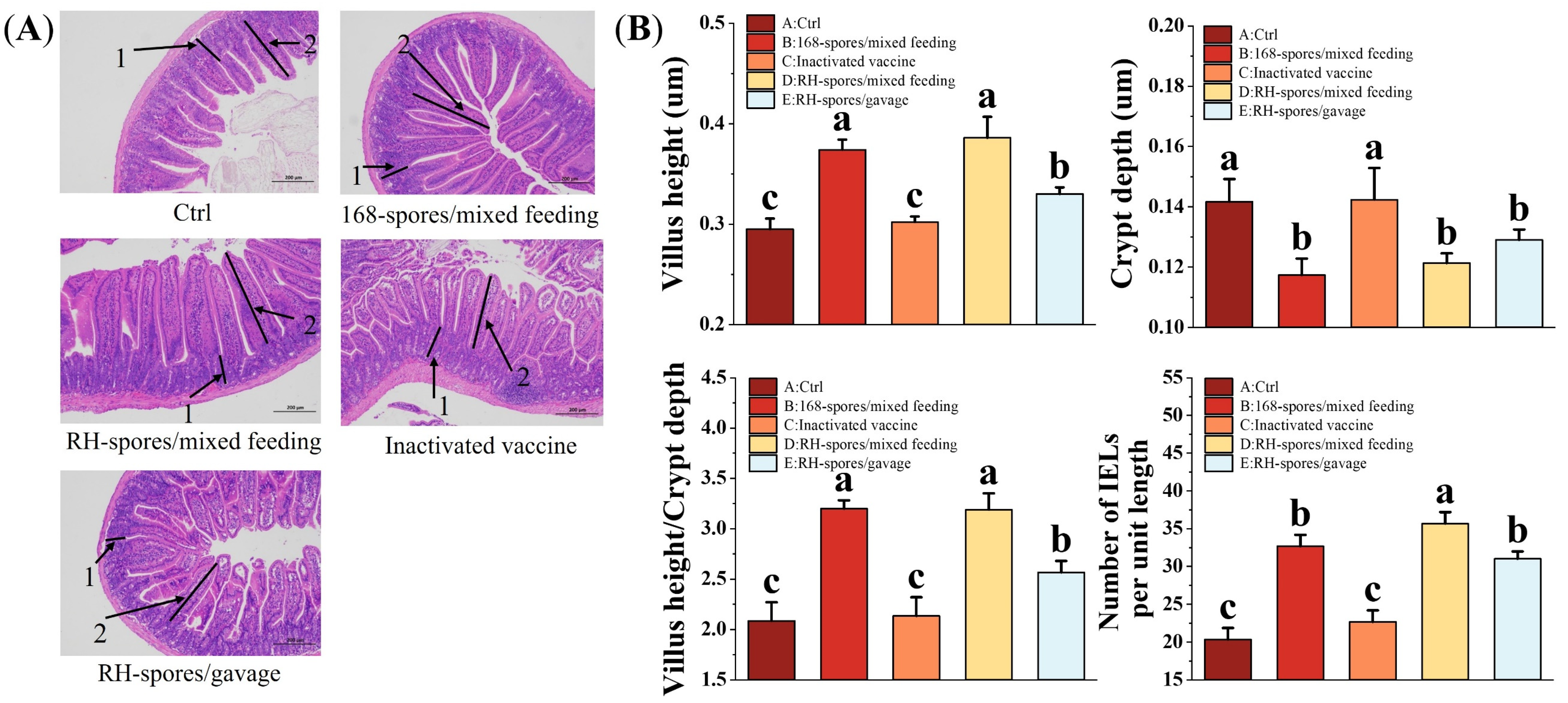
3.6. Histomorphology and Intraepithelial Lymphocytes of the Mouse Ileum
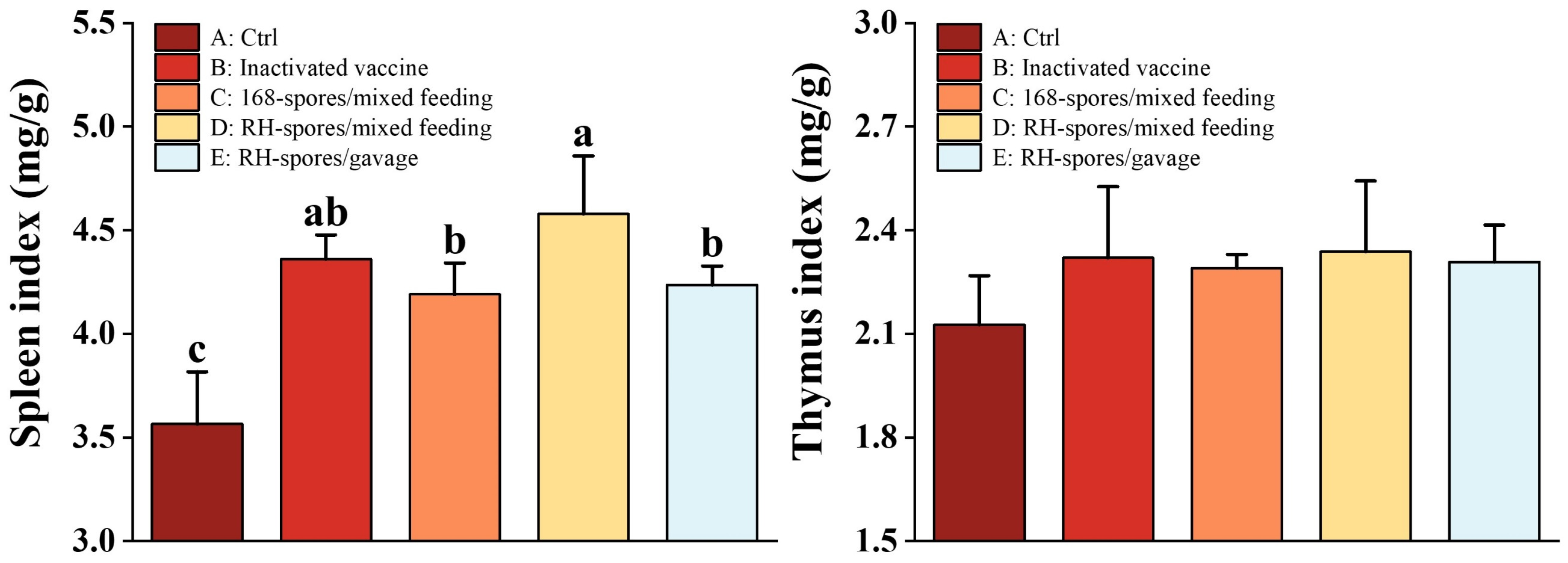
3.7. Mouse Immune Organ Index
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Piewngam, P.; Khongthong, S.; Roekngam, N.; Theapparat, Y.; Sunpaweravong, S.; Faroongsarng, D.; Otto, M. Probiotic for Pathogen-Specific Staphylococcus aureus Decolonisation in Thailand: A Phase 2, Double-Blind, Randomised, Placebo-Controlled Trial. Lancet Microbe 2023, 4, e75–e83. [Google Scholar] [CrossRef]
- Brophy, J.A.N.; Triassi, A.J.; Adams, B.L.; Renberg, R.L.; Stratis-Cullum, D.N.; Grossman, A.D.; Voigt, C.A. Engineered Integrative and Conjugative Elements for Efficient and Inducible DNA Transfer to Undomesticated Bacteria. Nat. Microbiol. 2018, 3, 1043–1053. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Yang, R. Recent Progress in Bacillus subtilis Spore-Surface Display: Concept, Progress, and Future. Appl. Microbiol. Biotechnol. 2017, 101, 933–949. [Google Scholar] [CrossRef]
- Sarao, L.K.; Arora, M. Probiotics, Prebiotics, and Microencapsulation: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 344–371. [Google Scholar] [CrossRef]
- Setlow, P. Spores of Bacillus subtilis: Their Resistance to and Killing by Radiation, Heat and Chemicals. J. Appl. Microbiol. 2006, 101, 514–525. [Google Scholar] [CrossRef]
- Saggese, A.; Baccigalupi, L.; Donadio, G.; Ricca, E.; Isticato, R. The Bacterial Spore as a Mucosal Vaccine Delivery System. Int. J. Mol. Sci. 2023, 24, 10880. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lin, Z.; Zhao, L.; Chen, T.; Shang, M.; Jiang, H.; Tang, Z.; Zhou, X.; Shi, M.; Zhou, L.; et al. Bacillus subtilis Spore with Surface Display of Paramyosin from Clonorchis sinensis Potentializes a Promising Oral Vaccine Candidate. Parasites Vectors 2018, 11, 156. [Google Scholar] [CrossRef]
- Negri, A.; Potocki, W.; Iwanicki, A.; Obuchowski, M.; Hinc, K. Expression and Display of Clostridium difficile Protein FliD on the Surface of Bacillus subtilis Spores. J. Med. Microbiol. 2013, 62, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, Y.L.; Lv, F.L.; Xu, L.D.; Huang, Y.W. Surface Display of Peptides Corresponding to the Heptad Repeat 2 Domain of the Feline Enteric Coronavirus Spike Protein on Bacillus subtilis Spores Elicits Protective Immune Responses against Homologous Infection in a Feline Aminopeptidase-N-Transduced Mouse Model. Front. Immunol. 2022, 13, 925922. [Google Scholar]
- Barnes, A.G.C.; Cerovic, V.; Hobson, P.S.; Klavinskis, L.S. Bacillus subtilis Spores: A Novel Microparticle Adjuvant Which Can Instruct a Balanced Th1 and Th2 Immune Response to Specific Antigen. Eur. J. Immunol. 2007, 37, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Amuguni, H.; Lee, S.; Kerstein, K.; Brown, D.; Belitsky, B.; Herrmann, J.; Keusch, G.; Sonenshein, A.; Tzipori, S. Sublingual Immunization with an Engineered Bacillus subtilis Strain Expressing Tetanus Toxin Fragment C Induces Systemic and Mucosal Immune Responses in Piglets. Microbes Infect. 2012, 14, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Cuburu, N.; Kweon, M.N.; Song, J.H.; Hervouet, C.; Luci, C.; Sun, J.B.; Hofman, P.; Holmgren, J.; Anjuère, F.; Czerkinsky, C. Sublingual Immunization Induces Broad-Based Systemic and Mucosal Immune Responses in Mice. Vaccine 2007, 25, 8598–8610. [Google Scholar] [CrossRef] [PubMed]
- Mou, C.; Zhu, L.; Xing, X.; Lin, J.; Yang, Q. Immune Responses Induced by Recombinant Bacillus subtilis Expressing the Spike Protein of Transmissible Gastroenteritis Virus in Pigs. Antivir. Res. 2016, 131, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Mou, C.; Zhu, L.; Yang, J.; Xu, W.; Cheng, X.; Yang, Q. Immune Responses Induced by Recombinant Bacillus subtilis Expressing the Hemagglutinin Protein of H5N1 in Chickens. Sci. Rep. 2016, 6, 38403. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.; Santos, R.A.; Coutinho, F.; Pedrosa, N.; Curado, M.; Machado, M.; Costas, B.; Bonneville, L.; Serrano, M.; Carvalho, A.P.; et al. Oral Vaccination of Fish against Vibriosis Using Spore-Display Technology. Front. Immunol. 2022, 13, 1012301. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortázar Schmidt, C.; Herskin, M.; et al. Assessment of the Control Measures of the Category a Diseases of Animal Health Law: Newcastle Disease. EFSA J. 2021, 19, e06946. [Google Scholar]
- Absalón, A.E.; Cortés-Espinosa, D.V.; Lucio, E.; Miller, P.J.; Afonso, C.L. Epidemiology, Control, and Prevention of Newcastle Disease in Endemic Regions: Latin America. Trop. Anim. Health Prod. 2019, 51, 1033–1048. [Google Scholar] [CrossRef]
- Dimitrov, K.M.; Afonso, C.L.; Yu, Q.; Miller, P.J. Newcastle Disease Vaccines-A Solved Problem or a Continuous Challenge? Vet. Microbiol. 2017, 206, 126–136. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, J.; Xu, H.; Li, J.; Hu, Z.; Hu, S.; And, X.W.; Liu, X. Effects of the HN Antigenic Difference between the Vaccine Strain and the Challenge Strain of Newcastle Disease Virus on Virus Shedding and Transmission. Viruses 2017, 9, 225. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Kwon, H.J.; Kim, T.E.; Kim, J.H.; Yoo, H.S.; Kim, S.J. Variation of a Newcastle Disease Virus Hemagglutinin-Neuraminidase Linear Epitope. J. Clin. Microbiol. 2008, 46, 1541–1544. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iorio, R.M.; Syddall, R.J.; Sheehan, J.P.; Bratt, M.A.; Glickman, R.L.; Riel, A.M. Neutralization Map of the Hemagglutinin-Neuraminidase Glycoprotein of Newcastle Disease Virus: Domains Recognized by Monoclonal Antibodies That Prevent Receptor Recognition. J. Virol. 1991, 65, 4999–5006. [Google Scholar] [CrossRef] [PubMed]
- Boursnell, M.E.; Green, P.F.; Samson, A.C.; Campbell, J.I.; Deuter, A.; Peters, R.W.; Millar, N.S.; Emmerson, P.T.; Binns, M.M. A Recombinant Fowlpox Virus Expressing the Hemagglutinin-Neuraminidase Gene of Newcastle Disease Virus (NDV) Protects Chickens against Challenge by NDV. Virology 1990, 178, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Mayahi, V.; Esmaelizad, M.; Harzandi, N. Designing a Novel Recombinant HN Protein with Multi Neutralizing Antigenic Sites and Auto Tag Removal Ability Based on NDV-VIIj for Diagnosis and Vaccination Application. Indian J. Microbiol. 2018, 58, 326–331. [Google Scholar] [CrossRef]
- Hu, S.; Wang, T.; Liu, Y.; Meng, C.; Wang, X.; Wu, Y.; Liu, X. Identification of a Variable Epitope on the Newcastle Disease Virus Hemagglutinin-Neuraminidase Protein. Vet. Microbiol. 2010, 140, 92–97. [Google Scholar] [CrossRef]
- Pedersen, K.; Marks, D.R.; Arsnoe, D.M.; Afonso, C.L.; Bevins, S.N.; Miller, P.J.; Randall, A.R.; DeLiberto, T.J. Avian Paramyxovirus Serotype 1 (Newcastle Disease Virus), Avian Influenza Virus, and Salmonella spp. In Mute Swans (Cygnus olor) in the Great Lakes Region and Atlantic Coast of the United States. Avian Dis. 2014, 58, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Young, J.K.; Li, D.; Abramowitz, M.C.; Morrison, T.G. Interaction of Peptides with Sequences from the Newcastle Disease Virus Fusion Protein Heptad Repeat Regions. J. Virol. 1999, 73, 5945–5956. [Google Scholar] [CrossRef]
- Ju, A.; Duan, A.; Zhang, Y.; Qin, Y.; Xue, L.; Ma, X.; Luan, W.; Yang, S. The Construction of Recombinant Lactobacillus casei Expressing Hemagglutinin-Neuraminidase Protein and Its Immune Response in Chickens. Microb. Pathog. 2021, 158, 105091. [Google Scholar] [CrossRef]
- Kaiser, A.; Willer, T.; Sid, H.; Petersen, H.; Baumgärtner, W.; Steinberg, P.; Rautenschlein, S. Susceptibility of Primary Chicken Intestinal Epithelial Cells for Low Pathogenic Avian Influenza Virus and Velogenic Viscerotropic Newcastle Disease Virus. Virus Res. 2016, 225, 50–63. [Google Scholar] [CrossRef]
- Dong, B.; Tang, N.; Guan, Y.; Qu, G.; Miao, L.; Han, W.; Shen, Z. Type and Abundance of Sialic Acid Receptors on Host Cell Membrane Affect Infectivity and Viral Titer of Different Strains of Newcastle Disease Virus. J. Virol. Methods 2022, 302, 114488. [Google Scholar] [CrossRef]
- Zhang, D.; Ding, Z.; Xu, X. Pathologic Mechanisms of the Newcastle Disease Virus. Viruses 2023, 15, 864. [Google Scholar] [CrossRef]
- Mao, Q.; Ma, S.; Schrickel, P.L.; Zhao, P.; Wang, J.; Zhang, Y.; Li, S.; Wang, C. Review Detection of Newcastle Disease Virus. Front. Vet. Sci. 2022, 9, 936251. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, C.; Zhang, O. Prokaryotic Expression of Structural Domains of the Newcastle Disease Virus Hemagglutinin-Neuraminidase. Chin. J. Prev. Vet. Med. 2007, 29, 32–35. [Google Scholar]
- Li, W.; Feng, J.; Li, J.; Li, J.; Wang, Z.; Khalique, A.; Yang, M.; Ni, X.; Zeng, D.; Zhang, D.; et al. Surface Display of Antigen Protein VP8* of Porcine Rotavirus on Bacillus subtilis Spores Using CotB as a Fusion Partner. Molecules 2019, 24, 3793. [Google Scholar] [CrossRef]
- Julkowska, D.; Obuchowski, M.; Holland, I.B.; Séror, S.J. Comparative Analysis of the Development of Swarming Communities of Bacillus subtilis 168 and a Natural Wild Type: Critical Effects of Surfactin and the Composition of the Medium. J. Bacteriol. 2005, 187, 65–76. [Google Scholar] [CrossRef]
- Stasiłojć, M.; Hinc, K.; Peszyńska-Sularz, G.; Obuchowski, M.; Iwanicki, A. Recombinant Bacillus subtilis Spores Elicit Th1/Th17-Polarized Immune Response in a Murine Model of Helicobacter pylori Vaccination. Mol. Biotechnol. 2015, 57, 685–691. [Google Scholar] [CrossRef]
- Wang, H.; Yang, R.; Hua, X.; Zhao, W.; Zhang, W. Functional Display of Active β-Galactosidase on Bacillus subtilis Spores Using Crust Proteins as Carriers. Food Sci. Biotechnol. 2015, 24, 1755–1759. [Google Scholar] [CrossRef]
- Hinc, K.; Isticato, R.; Dembek, M.; Karczewska, J.; Iwanicki, A.; Peszyńska-Sularz, G.; De Felice, M.; Obuchowski, M.; Ricca, E. Expression and Display of UreA of Helicobacter acinonychis on the Surface of Bacillus subtilis Spores. Microb. Cell Fact. 2010, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Duc, L.H.; Hong, H.A.; Fairweather, N.; Ricca, E.; Cutting, S.M. Bacterial Spores as Vaccine Vehicles. Infect. Immun. 2003, 71, 2810–2818. [Google Scholar] [CrossRef] [PubMed]
- Chumbe, A.; Izquierdo-Lara, R.; Calderón, K.; Fernández-Díaz, M.; Vakharia, V.N. Development of a Novel Newcastle Disease Virus (NDV) Neutralization Test Based on Recombinant NDV Expressing Enhanced Green Fluorescent Protein. Virol. J. 2017, 14, 232. [Google Scholar] [CrossRef] [PubMed]
- GB/T 16550-2020: Diagnostic Techniques for Newcastle Disease. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=DAA58A6FCC696F91339EED5BF8460CAB (accessed on 14 December 2020).
- Xin, J.; Wang, H.; Sun, N.; Bughio, S.; Zeng, D.; Li, L.; Wang, Y.; Khalique, A.; Zeng, Y.; Pan, K.; et al. Probiotic Alleviate Fluoride-Induced Memory Impairment by Reconstructing Gut Microbiota in Mice. Ecotox. Environ. Safe. 2021, 215, 112108. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Dai, X.; Wang, Z.; Ni, X.; Zeng, D.; Zeng, Y.; Zhang, D.; Pan, K. Effects of Antimicrobial Peptides Gal-13 on the Growth Performance, Intestinal Microbiota, Digestive Enzyme Activities, Intestinal Morphology, Antioxidative Activities, and Immunity of Broilers. Probiotics Antimicrob. Proteins 2023, 15, 694–705. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; de Oliveira Coelho, B.; Magalhães Júnior, A.I.; Thomaz-Soccol, V.; Soccol, C.R. How to Select a Probiotic? A Review and Update of Methods and Criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.A.; Huang, J.M.; Khaneja, R.; Hiep, L.V.; Urdaci, M.C.; Cutting, S.M. The Safety of Bacillus subtilis and Bacillus indicus as Food Probiotics. J. Appl. Microbiol. 2008, 105, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Á.T. Bacillus subtilis . Trends Microbiol. 2019, 27, 724–725. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Xu, D.; Xie, D.; Wang, M.; Li, Z.; Guo, X. Effects of Antibacterial Peptide-Producing Bacillus subtilis and Lactobacillus buchneri on Fermentation, Aerobic Stability, and Microbial Community of Alfalfa Silage. Bioresour. Technol. 2020, 315, 123881. [Google Scholar] [CrossRef] [PubMed]
- Ramlucken, U.; Ramchuran, S.O.; Moonsamy, G.; Lalloo, R.; Thantsha, M.S.; Jansen van Rensburg, C. A Novel Bacillus Based Multi-Strain Probiotic Improves Growth Performance and Intestinal Properties of Clostridium perfringens Challenged Broilers. Poult. Sci. 2020, 99, 331–341. [Google Scholar] [CrossRef]
- Azimirad, M.; Alebouyeh, M.; Naji, T. Inhibition of Lipopolysaccharide-Induced Interleukin 8 in Human Adenocarcinoma Cell Line HT-29 by Spore Probiotics: B. coagulans and B. subtilis (natto). Probiotics Antimicrob. Proteins 2017, 9, 56–63. [Google Scholar] [CrossRef]
- Pi, X.; Teng, W.; Fei, D.; Zhao, G.; Liu, W. Effects of Live Combined Bacillus subtilis and Enterococcus faecium on Gut Microbiota Composition in C57BL/6 Mice and in Humans. Front. Cell. Infect. Microbiol. 2022, 12, 821662. [Google Scholar] [CrossRef]
- Clemente, J.C.; Manasson, J.; Scher, J.U. The Role of the Gut Microbiome in Systemic Inflammatory Disease. BMJ 2018, 360, j5145. [Google Scholar] [CrossRef]
- Isticato, R.; Cangiano, G.; Tran, H.T.; Ciabattini, A.; Medaglini, D.; Oggioni, M.R.; De Felice, M.; Pozzi, G.; Ricca, E. Surface Display of Recombinant Proteins on Bacillus subtilis Spores. J. Bacteriol. 2001, 183, 6294–6301. [Google Scholar] [CrossRef]
- Dai, X.; Liu, M.; Pan, K.; Yang, J. Surface Display of OmpC of Salmonella Serovar Pullorum on Bacillus subtilis Spores. PLoS ONE 2018, 13, e0191627. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Dai, X.; Liu, M.; Khalique, A.; Wang, Z.; Zeng, Y.; Zhang, D.; Ni, X.; Zeng, D.; et al. Surface Display of Porcine Circovirus Type 2 Antigen Protein Cap on the Spores of Bacillus subtilis 168: An Effective Mucosal Vaccine Candidate. Front. Immunol. 2022, 13, 1007202. [Google Scholar] [CrossRef]
- Hoa, T.T.; Duc, L.H.; Isticato, R.; Baccigalupi, L.; Ricca, E.; Van, P.H.; Cutting, S.M. Fate and Dissemination of Bacillus subtilis Spores in a Murine Model. Appl. Environ. Microbiol. 2001, 67, 3819–3823. [Google Scholar] [CrossRef]
- Hoa, N.T.; Baccigalupi, L.; Huxham, A.; Smertenko, A.; Van, P.H.; Ammendola, S.; Ricca, E.; Cutting, A.S. Characterization of Bacillus Species Used for Oral Bacteriotherapy and Bacterioprophylaxis of Gastrointestinal Disorders. Appl. Environ. Microbiol. 2000, 66, 5241–5247. [Google Scholar] [CrossRef]
- Olivares-Villagómez, D.; Van Kaer, L. Intestinal Intraepithelial Lymphocytes: Sentinels of the Mucosal Barrier. Trends Immunol. 2018, 39, 264–275. [Google Scholar] [CrossRef]
- Shan, C.; Sun, B.; Dalloul, R.A.; Zhai, Z.; Sun, P.; Li, M.; Yang, S.; Luan, W. Effect of the Oral Administration of Astragalus Polysaccharides on Jejunum Mucosal Immunity in Chickens Vaccinated against Newcastle Disease. Microb. Pathog. 2019, 135, 103621. [Google Scholar] [CrossRef]
- Cerutti, A.; Chen, K.; Chorny, A. Immunoglobulin Responses at the Mucosal Interface. Annu. Rev. Immunol. 2011, 29, 273–293. [Google Scholar] [CrossRef]
- Rahman, M.M.; Uyangaa, E.; Han, Y.W.; Hur, J.; Park, S.Y.; Lee, J.H.; Kim, K.; Eo, S.K. Modulation of Systemic and Mucosal Immunity against an Inactivated Vaccine of Newcastle Disease Virus by Oral Co-Administration of Live Attenuated Salmonella enterica Serovar Typhimurium Expressing Chicken Interleukin-18 and Interferon-α. J. Vet. Med. Sci. 2015, 77, 395–403. [Google Scholar] [CrossRef]
- Xu, X.; Qian, J.; Qin, L.; Li, J.; Xue, C.; Ding, J.; Wang, W.; Ding, W.; Yin, R.; Jin, N.; et al. Chimeric Newcastle Disease Virus-Like Particles Containing DC-Binding Peptide-Fused Haemagglutinin Protect Chickens from Virulent Newcastle Disease Virus and H9N2 Avian Influenza Virus Challenge. Virol. Sin. 2020, 35, 455–467. [Google Scholar] [CrossRef]
- Marasini, N.; Skwarczynski, M.; Toth, I. Oral Delivery of Nanoparticle-Based Vaccines. Expert Rev. Vaccines 2014, 13, 1361–1376. [Google Scholar] [CrossRef]
- Zhao, K.; Xie, Y.; Lin, X.; Xu, W. The Mucoadhesive Nanoparticle-Based Delivery System in the Development of Mucosal Vaccines. Int. J. Nanomed. 2022, 17, 4579–4598. [Google Scholar] [CrossRef]
- Oh, Y.; Kim, J.A.; Kim, C.H.; Choi, S.K.; Pan, J.G. Bacillus subtilis Spore Vaccines Displaying Protective Antigen Induce Functional Antibodies and Protective Potency. BMC Vet. Res. 2020, 16, 259. [Google Scholar] [CrossRef]
- Latorre, J.D.; Hernandez-Velasco, X.; Kallapura, G.; Menconi, A.; Pumford, N.R.; Morgan, M.J.; Layton, S.L.; Bielke, L.R.; Hargis, B.M.; Téllez, G. Evaluation of Germination, Distribution, and Persistence of Bacillus subtilis Spores through the Gastrointestinal Tract of Chickens. Poult. Sci. 2014, 93, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chen, T.; Sun, H.; Tang, Z.; Yu, J.; Lin, Z.; Ren, P.; Zhou, X.; Huang, Y.; Li, X.; et al. Immune Response Induced by Oral Delivery of Bacillus subtilis Spores Expressing Enolase of Clonorchis sinensis in Grass Carps (Ctenopharyngodon idellus). Fish Shellfish Immunol. 2017, 60, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Mendoza, S.; Angulo, C. Bacillus subtilis Comes of Age as a Vaccine Production Host and Delivery Vehicle. Expert Rev. Vaccines 2015, 14, 1135–1148. [Google Scholar] [PubMed]
- Lee, J.E.; Kye, Y.C.; Park, S.M.; Shim, B.S.; Yoo, S.; Hwang, E.; Kim, H.; Kim, S.J.; Han, S.H.; Park, T.S.; et al. Bacillus subtilis Spores as Adjuvants against Avian Influenza H9N2 Induce Antigen-Specific Antibody and T Cell Responses in White Leghorn Chickens. Vet. Res. 2020, 51, 68. [Google Scholar] [CrossRef] [PubMed]
- Xun, W.; Shi, L.; Zhou, H.; Hou, G.; Cao, T.; Zhao, C. Effects of Curcumin on Growth Performance, Jejunal Mucosal Membrane Integrity, Morphology and Immune Status in Weaned Piglets Challenged with Enterotoxigenic Escherichia coli. Int. Immunopharmacol. 2015, 27, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Ghareeb, K.; Abdel-Raheem, S.; Böhm, J. Effects of Dietary Inclusion of Probiotic and Synbiotic on Growth Performance, Organ Weights, and Intestinal Histomorphology of Broiler Chickens. Poult. Sci. 2009, 88, 49–56. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.P.; Martino, H.S.D.; Tako, E. Plant Origin Prebiotics Affect Duodenal Brush Border Membrane Functionality and Morphology, in Vivo (Gallus Gallus). Food Funct. 2021, 12, 6157–6166. [Google Scholar] [CrossRef] [PubMed]
- Aliakbarpour, H.R.; Chamani, M.; Rahimi, G.; Sadeghi, A.A.; Qujeq, D. The Bacillus subtilis and Lactic Acid Bacteria Probiotics Influences Intestinal Mucin Gene Expression, Histomorphology and Growth Performance in Broilers. Asian Australas. J. Anim. Sci. 2012, 25, 1285–1293. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, L.; Liu, H.; Shen, J.; Zhang, Y.; Lu, L.; Zhang, X.; Ma, X. Bacillus subtilis M6 Improves Intestinal Barrier, Antioxidant Capacity and Gut Microbial Composition in AA Broiler. Front. Nutr. 2022, 9, 965310. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; Li, C.L.; Wang, J.; Qi, G.H.; Gao, J.; Zhang, H.J.; Wu, S.G. Effects of Dietary Supplementation with Bacillus subtilis, as an Alternative to Antibiotics, on Growth Performance, Serum Immunity, and Intestinal Health in Broiler Chickens. Front. Nutr. 2021, 8, 786878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, Z.; Gu, X.; Zhao, J.; Guo, T.; Kong, J. Probiotic Bacillus subtilis LF11 Protects Intestinal Epithelium against Salmonella Infection. Front. Cell. Infect. Microbiol. 2022, 12, 837886. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, R.; Liu, Y.; Ma, L.; Zha, J.; Qiao, X.; Chai, T.; Wu, B. Benefit of Dietary Supplementation with Bacillus subtilis BYS2 on Growth Performance, Immune Response, and Disease Resistance of Broilers. Probiotics Antimicrob. Proteins 2020, 12, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, S.; Das, P.P.; Saini, P.C.; Roy, B.; Chatterjee, P.N. Use of Bacillus subtilis PB6 as a Potential Antibiotic Growth Promoter Replacement in Improving Performance of Broiler Birds. Poult. Sci. 2017, 96, 2614–2622. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Peng, V.; Sudan, R.; Ulezko Antonova, A.; Di Luccia, B.; Ohara, T.E.; Fachi, J.L.; Grajales-Reyes, G.E.; Jaeger, N.; Trsan, T.; et al. Repression of the Aryl-Hydrocarbon Receptor Prevents Oxidative Stress and Ferroptosis of Intestinal Intraepithelial Lymphocytes. Immunity 2023, 56, 797–812. [Google Scholar] [CrossRef]
- Villagrán-de la Mora, Z.; Vázquez-Paulino, O.; Avalos, H.; Ascencio, F.; Nuño, K.; Villarruel-López, A. Effect of a Synbiotic Mix on Lymphoid Organs of Broilers Infected with Salmonella typhimurium and Clostridium perfringens. Animals 2020, 10, 886. [Google Scholar] [CrossRef]
- Sun, S.; Li, B.; Wu, M.; Deng, Y.; Li, J.; Xiong, Y.; He, S. Effect of Dietary Supplemental Vitamin C and Betaine on the Growth Performance, Humoral Immunity, Immune Organ Index, and Antioxidant Status of Broilers under Heat Stress. Trop. Anim. Health Prod. 2023, 55, 96. [Google Scholar] [CrossRef]
- Gui, J.; Mustachio, L.M.; Su, D.M.; Craig, R.W. Thymus Size and Age-Related Thymic Involution: Early Programming, Sexual Dimorphism, Progenitors and Stroma. Aging Dis. 2012, 3, 280–290. [Google Scholar]
- Xu, Y.; Yu, Y.; Shen, Y.; Li, Q.; Lan, J.; Wu, Y.; Zhang, R.; Cao, G.; Yang, C. Effects of Bacillus subtilis and Bacillus licheniformis on Growth Performance, Immunity, Short Chain Fatty Acid Production, Antioxidant Capacity, and Cecal Microflora in Broilers. Poult. Sci. 2021, 100, 101358. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, H.; Yu, Y.; Zhang, R.; Wu, Y.; Yue, M.; Yang, C. Effects of Bacillus coagulans on Growth Performance, Antioxidant Capacity, Immunity Function, and Gut Health in Broilers. Poult. Sci. 2021, 100, 101168. [Google Scholar] [CrossRef]
- Pham, K.C.; Tran, H.T.T.; Van Doan, C.; Le, P.H.; Van Nguyen, A.T.; Nguyen, H.A.; Hong, H.A.; Cutting, S.M.; Phan, T.N. Protection of Penaeus monodon against White Spot Syndrome by Continuous Oral Administration of a Low Concentration of Bacillus subtilis Spores Expressing the VP28 Antigen. Lett. Appl. Microbiol. 2017, 64, 184–191. [Google Scholar] [CrossRef]
- Chen, H.; Wu, B.; Zhang, T.; Jia, J.; Lu, J.; Chen, Z.; Ni, Z.; Tan, T. Effect of Linker Length and Flexibility on the Clostridium thermocellum Esterase Displayed on Bacillus subtilis Spores. Appl. Biochem. Biotechnol. 2017, 182, 168–180. [Google Scholar] [CrossRef]
- Hinc, K.; Iwanicki, A.; Obuchowski, M. New Stable Anchor Protein and Peptide Linker Suitable for Successful Spore Surface Display in B. subtilis. Microb. Cell Fact. 2013, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Iwanicki, A.; Piątek, I.; Stasiłojć, M.; Grela, A.; Lęga, T.; Obuchowski, M.; Hinc, K. A System of Vectors for Bacillus subtilis Spore Surface Display. Microb. Cell Fact. 2014, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Ullah, J.; Chen, H.; Vastermark, A.; Jia, J.; Wu, B.; Ni, Z.; Le, Y.; Wang, H. Impact of Orientation and Flexibility of Peptide Linkers on T. maritima Lipase Tm1350 Displayed on Bacillus subtilis Spores Surface Using CotB as Fusion Partner. World J. Microbiol. Biotechnol. 2017, 33, 166. [Google Scholar] [CrossRef] [PubMed]
- Potocki, W.; Negri, A.; Peszyńska-Sularz, G.; Hinc, K.; Obuchowski, M.; Iwanicki, A. The Combination of Recombinant and Non-Recombinant Bacillus subtilis Spore Display Technology for Presentation of Antigen and Adjuvant on Single Spore. Microb. Cell Fact. 2017, 16, 151. [Google Scholar] [CrossRef] [PubMed]
- Ghaedmohammadi, S.; Rigi, G.; Zadmard, R.; Ricca, E.; Ahmadian, G. Immobilization of Bioactive Protein a from Staphylococcus aureus (SpA) on the Surface of Bacillus subtilis Spores. Mol. Biotechnol. 2015, 57, 756–766. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Yang, M.; Chen, B.; Wang, Z.; Cao, Y.; Yang, Y.; Zhang, M.; Zhang, D.; Ni, X.; Zeng, Y.; et al. Evaluation of the Immunity Responses in Mice to Recombinant Bacillus subtilis Displaying Newcastle Disease Virus HN Protein Truncations. Microorganisms 2024, 12, 439. https://doi.org/10.3390/microorganisms12030439
Li J, Yang M, Chen B, Wang Z, Cao Y, Yang Y, Zhang M, Zhang D, Ni X, Zeng Y, et al. Evaluation of the Immunity Responses in Mice to Recombinant Bacillus subtilis Displaying Newcastle Disease Virus HN Protein Truncations. Microorganisms. 2024; 12(3):439. https://doi.org/10.3390/microorganisms12030439
Chicago/Turabian StyleLi, Jianzhen, Miao Yang, Bin Chen, Zhenhua Wang, Yuheng Cao, Yang Yang, Mengwei Zhang, Dongmei Zhang, Xueqin Ni, Yan Zeng, and et al. 2024. "Evaluation of the Immunity Responses in Mice to Recombinant Bacillus subtilis Displaying Newcastle Disease Virus HN Protein Truncations" Microorganisms 12, no. 3: 439. https://doi.org/10.3390/microorganisms12030439
APA StyleLi, J., Yang, M., Chen, B., Wang, Z., Cao, Y., Yang, Y., Zhang, M., Zhang, D., Ni, X., Zeng, Y., & Pan, K. (2024). Evaluation of the Immunity Responses in Mice to Recombinant Bacillus subtilis Displaying Newcastle Disease Virus HN Protein Truncations. Microorganisms, 12(3), 439. https://doi.org/10.3390/microorganisms12030439






