Elimination of Methicillin-Resistant Staphylococcus aureus from Mammary Glands of Dairy Cows by an Additional Antibiotic Treatment Prior to Dry Cow Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Dairy Herd and Dry-off Treatment
2.2. Sampling
2.3. Culture and Confirmation
2.4. WGS and Bioinformatics Analyses
2.5. Antimicrobial Susceptibility Testing
2.6. Statistical Analyses
3. Results
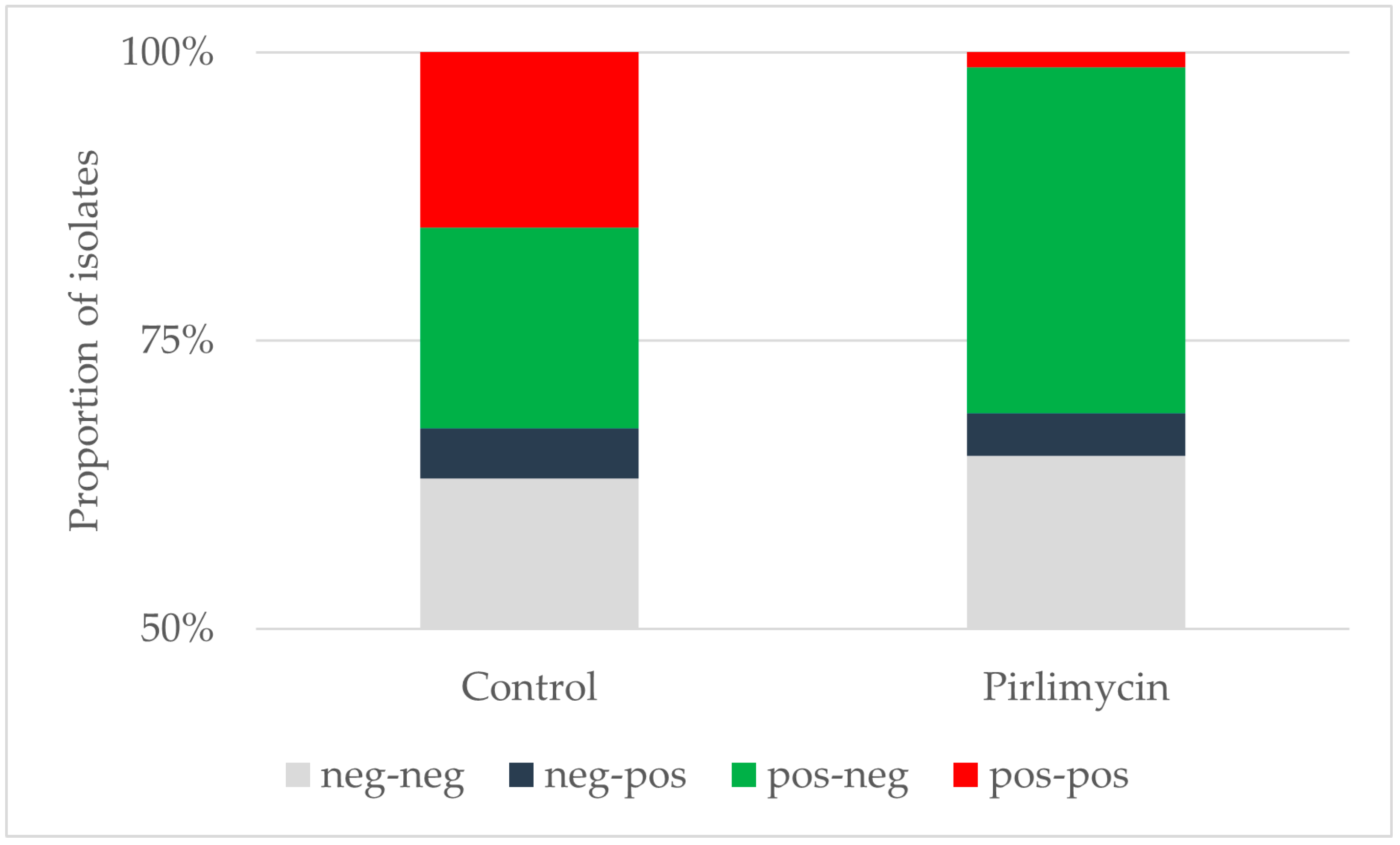
3.1. Efficiency of Pirlimycin Treatment
3.2. Typing Results
3.3. MRSA Colonization of Other Body Sites
3.4. Antimicrobial Susceptibility Testing and Genotypic Characterization
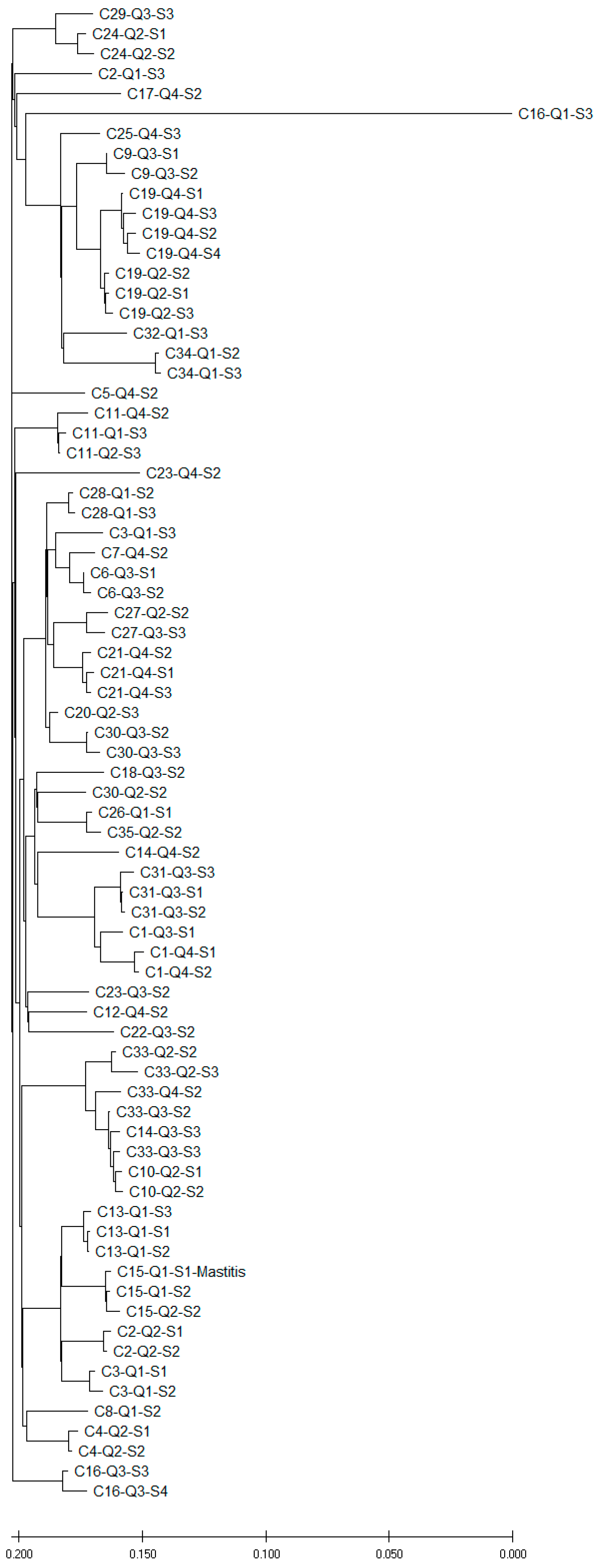
3.5. Phylogenetic Analysis of MRSA Isolated from Quarter Milk Samples Before and After Dry-off
3.6. Phylogenetic Comparison of MRSA from Nasal and Udder Cleft Swabs with MRSA from Quarter Milk Samples
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schnitt, A.; Tenhagen, B.A. Risk Factors for the Occurrence of Methicillin-Resistant Staphylococcus aureus in Dairy Herds: An Update. Foodborne Pathog. Dis. 2020, 17, 585–596. [Google Scholar] [CrossRef]
- Moawad, A.A.; El-Adawy, H.; Linde, J.; Jost, I.; Tanja, G.; Katja, H.; Karsten, D.; Neubauer, H.; Monecke, S.; Tomaso, H. Whole genome sequence-based analysis of Staphylococcus aureus isolated from bovine mastitis in Thuringia, Germany. Front. Microbiol. 2023, 14, 1216850. [Google Scholar] [CrossRef] [PubMed]
- Preziuso, S.; Attili, A.R.; Cuteri, V. Methicillin-resistant staphylococci in clinical bovine mastitis: Occurrence, molecular analysis, and biofilm production. Vet. Res. Commun. 2023, 48, 969–977. [Google Scholar] [CrossRef]
- Alves, J.S.; de Moura Souza, R.; Lima Moreira, J.P.; Gonzalez, A.G.M. Antimicrobial resistance of Enterobacteriaceae and Staphylococcus spp. isolated from raw cow’s milk from healthy, clinical and subclinical mastitis udders. Prev. Vet. Med. 2024, 227, 106205. [Google Scholar] [CrossRef] [PubMed]
- Schnitt, A.; Lienen, T.; Wichmann-Schauer, H.; Cuny, C.; Tenhagen, B.A. The occurrence and distribution of livestock-associated methicillin-resistant Staphylococcus aureus ST398 on German dairy farms. J. Dairy Sci. 2020, 103, 11806–11819. [Google Scholar] [CrossRef]
- Tomao, P.; Pirolo, M.; Agnoletti, F.; Pantosti, A.; Battisti, A.; Di Martino, G.; Visaggio, D.; Monaco, M.; Franco, A.; Pimentel de Araujo, F.; et al. Molecular epidemiology of methicillin-resistant Staphylococcus aureus from dairy farms in North-eastern Italy. Int. J. Food Microbiol. 2020, 332, 108817. [Google Scholar] [CrossRef] [PubMed]
- Nobrega, D.B.; French, J.E.; Kelton, D.F. A scoping review of the testing of bulk tank milk to detect nonbacterial pathogens or herd exposure to nonbacterial pathogens in dairy cattle. J. Dairy Sci. 2023, 106, 5636–5658. [Google Scholar] [CrossRef]
- Spohr, M.; Rau, J.; Friedrich, A.; Klittich, G.; Fetsch, A.; Guerra, B.; Hammerl, J.A.; Tenhagen, B.A. Methicillin-resistant Staphylococcus aureus (MRSA) in three dairy herds in southwest Germany. Zoonoses Public. Health 2011, 58, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Lienen, T.; Schnitt, A.; Cuny, C.; Maurischat, S.; Tenhagen, B.A. Phylogenetic Tracking of LA-MRSA ST398 Intra-Farm Transmission among Animals, Humans and the Environment on German Dairy Farms. Microorganisms 2021, 9, 1119. [Google Scholar] [CrossRef]
- Neave, F.K.; Dodd, F.H.; Kingwill, R.G.; Westgarth, D.R. Control of Mastitis in the Dairy Herd by Hygiene and Management. J. Dairy Sci. 1969, 52, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Ruegg, P.L. A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef] [PubMed]
- Philpot, W.N. Control of mastitis by hygiene and therapy. J. Dairy Sci. 1979, 62, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Barkema, H.W.; Schukken, Y.H.; Zadoks, R.N. Invited Review: The role of cow, pathogen, and treatment regimen in the therapeutic success of bovine Staphylococcus aureus mastitis. J. Dairy Sci. 2006, 89, 1877–1895. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. European Public MRL Assessment Report (EPMAR): Neomycin (Including Framycetin); EMEA/CVMP/417488/2006; European Medicines Agency: London, UK, 2013; p. 14. [Google Scholar]
- European Medicines Agency. EPAR Summary for the Public: Pirsue, Pirlimycin Hydrochloride; EMEA/CVMP/417488/2006; European Medicines Agency: London, UK, 2013; p. 3. [Google Scholar]
- BVL. Berichte zur Resistenzmonitoringstudie 2021—Resistenzsituation Bei Klinisch Wichtigen Tierpathogenen Bakterien; Bundesamt für Verbraucherschutz und Lebensmittelsicherheit: Berlin, Germany, 2023; Volume 17, p. 134. [Google Scholar]
- Karell, J.; Petzl, W.; Gangl, A.; Huber-Schlenstedt, R.; Sorge, U.S. Changes in antimicrobial resistance of Staphylococcus aureus in bovine quarter milk samples from Southern Germany between 2012 and 2022. J. Dairy Sci. 2023, 107, 3802–3812. [Google Scholar] [CrossRef]
- Pillar, C.M.; Stoneburner, A.; Shinabarger, D.L.; Abbeloos, E.; Goby, L.; Bradley, A.J. In vitro susceptibility of bovine mastitis pathogens to a combination of penicillin and framycetin: Development of interpretive criteria for testing by broth microdilution and disk diffusion. J. Dairy Sci. 2014, 97, 6594–6607. [Google Scholar] [CrossRef] [PubMed]
- Deneke, C.; Brendebach, H.; Uelze, L.; Borowiak, M.; Malorny, B.; Tausch, S.H. Species-Specific Quality Control, Assembly and Contamination Detection in Microbial Isolate Sequences with AQUAMIS. Genes 2021, 12, 644. [Google Scholar] [CrossRef]
- Feldgarden, M.; Brover, V.; Haft, D.H.; Prasad, A.B.; Slotta, D.J.; Tolstoy, I.; Tyson, G.H.; Zhao, S.; Hsu, C.H.; McDermott, P.F.; et al. Validating the AMRFinder Tool and Resistance Gene Database by Using Antimicrobial Resistance Genotype-Phenotype Correlations in a Collection of Isolates. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Ito, T.; Ma, X.X.; Watanabe, S.; Kreiswirth, B.N.; Etienne, J.; Hiramatsu, K. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: Rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob. Agents Chemother. 2007, 51, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- IWG-SCC. Classification of staphylococcal cassette chromosome mec (SCCmec): Guidelines for reporting novel SCCmec elements. Antimicrob. Agents Chemother. 2009, 53, 4961–4967. [Google Scholar] [CrossRef] [PubMed]
- Bartels, M.D.; Petersen, A.; Worning, P.; Nielsen, J.B.; Larner-Svensson, H.; Johansen, H.K.; Andersen, L.P.; Jarløv, J.O.; Boye, K.; Larsen, A.R.; et al. Comparing whole-genome sequencing with Sanger sequencing for spa typing of methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 2014, 52, 4305–4308. [Google Scholar] [CrossRef] [PubMed]
- Kaas, R.S.; Leekitcharoenphon, P.; Aarestrup, F.M.; Lund, O. Solving the problem of comparing whole bacterial genomes across different sequencing platforms. PLoS ONE 2014, 9, e104984. [Google Scholar] [CrossRef]
- ISO 20776-1:2006; Clinical Laboratory Testing and In Vitro Diagnostic Test Systems—Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices—Part 1: Reference Method for Testing the In Vitro Activity of Antimicrobial Agents Against Rapidly Growing Aerobic Bacteria Involved in Infectious Diseases. International Organization for Standardization: Geneva, Switzerland, 2006.
- CLSI M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard-Ninth Edition. CLSI document M07 Ed11. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- EFSA. Technical specifications on the harmonised monitoring and reporting of antimicrobial resistance in methicillin-resistant Staphylococcus aureus in food-producing animals and food. EFSA J. 2012, 2012, 2897. [Google Scholar] [CrossRef]
- WHO. WHO List of Medically Important Antimicrobials; World Health Organisation: Geneva, Switzerland, 2024; p. 41. [Google Scholar]
- EMA. Categorisation of antibiotics in the >European Union. In Answer to the Request from the European Commission for Updating the Scientific Advice on the Impact on Public Health and Animal Health of the Use of Antibiotics in Animals; European Medicines Agency: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Roy, J.P.; Keefe, G. Systematic review: What is the best antibiotic treatment for Staphylococcus aureus intramammary infection of lactating cows in North America? Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 39–50. [Google Scholar] [CrossRef]
- Gillespie, B.E.; Moorehead, H.; Lunn, P.; Dowlen, H.H.; Johnson, D.L.; Lamar, K.C.; Lewis, M.J.; Ivey, S.J.; Hallberg, J.W.; Chester, S.T.; et al. Efficacy of extended pirlimycin hydrochloride therapy for treatment of environmental Streptococcus spp and Staphylococcus aureus intramammary infections in lactating dairy cows. Vet. Ther. 2002, 3, 373–380. [Google Scholar] [PubMed]
- Skoulikas, S.; Dufour, S.; Haine, D.; Perreault, J.Y.; Roy, J.P. Early-lactation extended pirlimycin therapy against naturally acquired Staphylococcus aureus intramammary infections in heifers: A randomized controlled trial. J. Dairy Sci. 2018, 101, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Donahue, M.; Godden, S.M.; Bey, R.; Wells, S.; Oakes, J.M.; Sreevatsan, S.; Stabel, J.; Fetrow, J. Heat treatment of colostrum on commercial dairy farms decreases colostrum microbial counts while maintaining colostrum immunoglobulin G concentrations. J. Dairy Sci. 2012, 95, 2697–2702. [Google Scholar] [CrossRef] [PubMed]
- Wörmann, M.E.; Bhatte, A.; Wichmann-Schauer, H.; Tenhagen, B.A.; Lienen, T. Heat Inactivation of Methicillin-Resistant Staphylococcus aureus Strains from German Dairy farms in Colostrum and Raw Milk. Animals 2023, 13, 3549. [Google Scholar] [CrossRef]
- Keefe, G. Update on control of Staphylococcus aureus and Streptococcus agalactiae for management of mastitis. Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Feßler, A.; Kadlec, K.; Wang, Y.; Zhang, W.J.; Wu, C.; Shen, J.; Schwarz, S. Small Antimicrobial Resistance Plasmids in Livestock-Associated Methicillin-Resistant Staphylococcus aureus CC398. Front. Microbiol. 2018, 9, 2063. [Google Scholar] [CrossRef]
| Target | Primer/Probe | Nucleotide Sequence |
|---|---|---|
| tuf | Tuf-P1 | AAACAACTGTTACTGGTGTAGAAATG |
| Tuf-P2 | AGTACGGAAATAGAATTGTG | |
| Tuf-Probe | TCCGTAAATTATTAGACTACGCTGAAGC | |
| nuc | Nuc-P1 | GTTGCTTAGTGTTAACTTTAGTTGTA |
| Nuc-P2 | AATGTCGCAGGTTCTTTATGTAATTT | |
| Nuc-Probe | AAGTCTAAGTAGCTCAGCAAATGCA | |
| mecA | MecA-P1 | AAATATTATTAGCTGATTCAGGTTAC |
| MecA-P2 | CGTTAATATTGCCATTATTTCTAAT | |
| MecA-Probe | CAAGGTGAAATACTGATTAACCCAGTA |
| Control | Pirlimycin | Total | ||||
|---|---|---|---|---|---|---|
| spa Type | Prior | Post | Prior | Post | Prior | Post |
| t011 | 4 | 1 | 0 | 1 | 4 | 2 |
| t034 | 21 | 14 | 22 | 3 | 43 | 17 |
| t571 | 2 | 1 | 0 | 0 | 2 | 1 |
| t588 | 2 | 2 | 0 | 0 | 2 | 2 |
| t19084 | 1 | 0 | 0 | 0 | 1 | 0 |
| Not available | 0 | 0 | 1 | 0 | 1 | 0 |
| Total | 30 | 18 | 23 | 4 | 53 | 22 |
| MIC (mg/L) | 0.016 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | %res |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial | |||||||||||||||
| Clindamycin | 15 | 54 | 3 | 1 | 2 | 8 | |||||||||
| Pirlimycin | 63 | 10 | 2 | 2.7 | |||||||||||
| Ciprofloxacin | 48 | 26 | 1 | 0 | |||||||||||
| Erythromycin | 52 | 21 | 1 | 1 | 1.3 | ||||||||||
| Cefoxitin | 5 | 48 | 22 | 100 | |||||||||||
| Fusidic Acid | 75 | 0 | |||||||||||||
| Gentamicin | 75 | 0 | |||||||||||||
| Chloramphenicol | 69 | 5 | 1 | 1.3 | |||||||||||
| Kanamycin | 74 | 1 | 1.3 | ||||||||||||
| Linezolid | 72 | 3 | 0 | ||||||||||||
| Mupirocin | 75 | 0 | |||||||||||||
| Penicillin | 1 | 74 | 100 | ||||||||||||
| Rifampicin | 75 | 0 | |||||||||||||
| Sulfamethoxazol | 75 | 0 | |||||||||||||
| Streptomycin | 58 | 17 | 0 | ||||||||||||
| Quinupristin/ Dalfopristin | 28 | 45 | 2 | 2.7 | |||||||||||
| Tetracycline | 75 | 100 | |||||||||||||
| Tiamulin | 5 | 70 | 100 | ||||||||||||
| Trimethoprim | 1 | 74 | 98.7 | ||||||||||||
| Vancomycin | 74 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenhagen, B.-A.; Wörmann, M.E.; Gretzschel, A.; Grobbel, M.; Maurischat, S.; Lienen, T. Elimination of Methicillin-Resistant Staphylococcus aureus from Mammary Glands of Dairy Cows by an Additional Antibiotic Treatment Prior to Dry Cow Treatment. Microorganisms 2024, 12, 2651. https://doi.org/10.3390/microorganisms12122651
Tenhagen B-A, Wörmann ME, Gretzschel A, Grobbel M, Maurischat S, Lienen T. Elimination of Methicillin-Resistant Staphylococcus aureus from Mammary Glands of Dairy Cows by an Additional Antibiotic Treatment Prior to Dry Cow Treatment. Microorganisms. 2024; 12(12):2651. https://doi.org/10.3390/microorganisms12122651
Chicago/Turabian StyleTenhagen, Bernd-Alois, Mirka Elisabeth Wörmann, Anja Gretzschel, Mirjam Grobbel, Sven Maurischat, and Tobias Lienen. 2024. "Elimination of Methicillin-Resistant Staphylococcus aureus from Mammary Glands of Dairy Cows by an Additional Antibiotic Treatment Prior to Dry Cow Treatment" Microorganisms 12, no. 12: 2651. https://doi.org/10.3390/microorganisms12122651
APA StyleTenhagen, B.-A., Wörmann, M. E., Gretzschel, A., Grobbel, M., Maurischat, S., & Lienen, T. (2024). Elimination of Methicillin-Resistant Staphylococcus aureus from Mammary Glands of Dairy Cows by an Additional Antibiotic Treatment Prior to Dry Cow Treatment. Microorganisms, 12(12), 2651. https://doi.org/10.3390/microorganisms12122651





