Association Between Diabetes Mellitus–Tuberculosis and the Generation of Drug Resistance
Abstract
1. Introduction
2. Epidemiology of the T2DM–TB Binomial
3. Peculiarities of M. tuberculosis
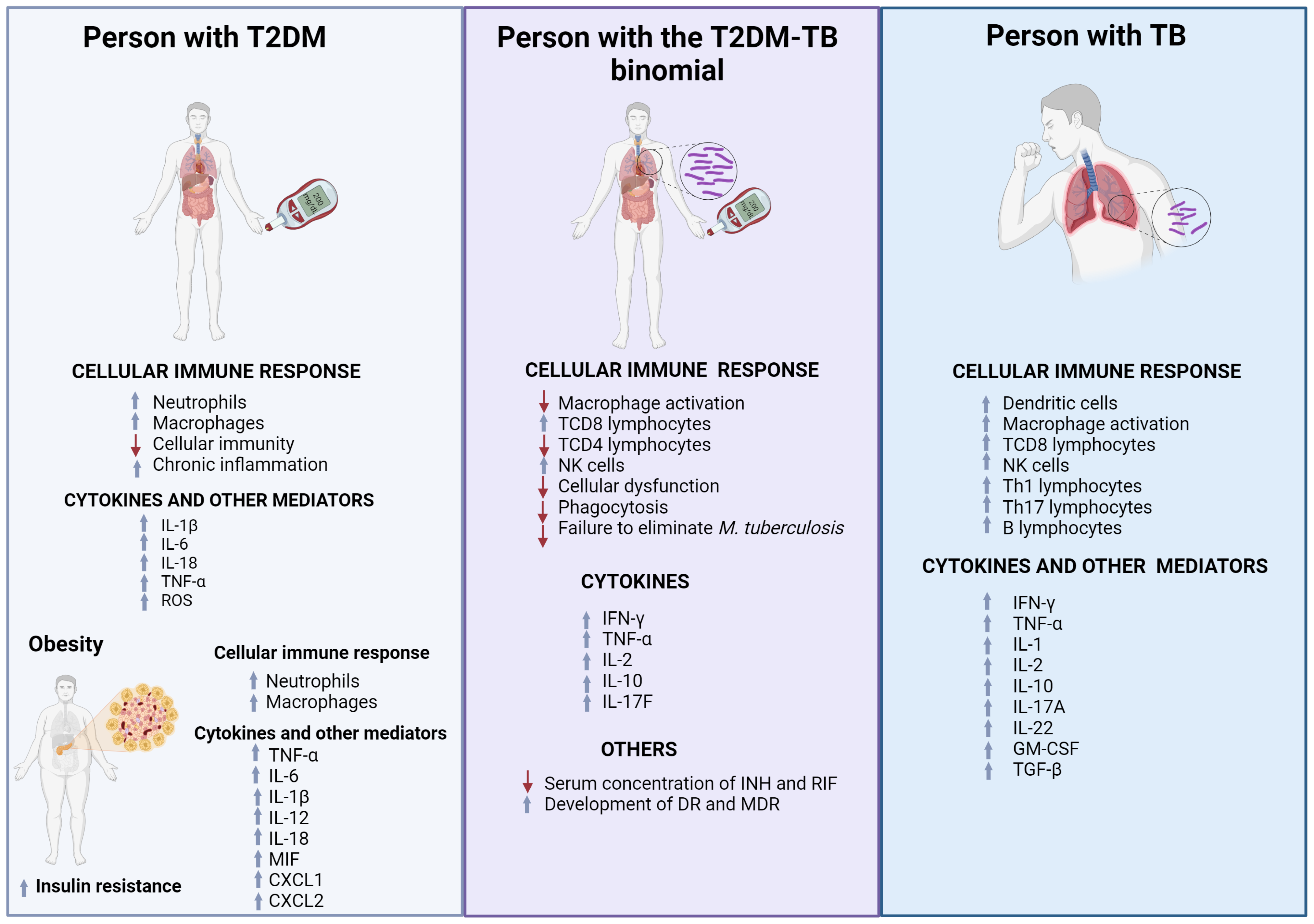
4. Physiology of the T2DM–TB Binomial
5. Innate Immune Response to M. tuberculosis
Phagosome Maturation
6. T2DM Promotes Chronic Inflammation
Alterations in Innate Immunity Induced by T2DM and Their Role in Susceptibility to M. tuberculosis
7. Influence of T2DM on the Modification of M. tuberculosis Genomes: Drug Resistance Generation
7.1. Role of Persistence, Tolerance, and Efflux Pumps Expression in the Development of Drug Resistance
7.2. Isoniazid Resistance (INH-R)
7.3. Rifampicin Resistance (RIF-R)
8. Potential Adjunctive Therapies for the Management of the T2DM–TB Comorbidity
8.1. Efflux Pumps Inhibitors
8.2. Metformin
9. Advances in Diagnostics and Immunization Strategies for Binomial T2DM–TB Control
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan American Health Organization. Panorama of Diabetes in the Americas. Pan American Health Organization. 2022. Available online: https://iris.paho.org/handle/10665.2/56643 (accessed on 9 January 2023).
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S15–S33. Available online: https://diabetesjournals.org/care/article/44/Supplement_1/S15/30859/2-Classification-and-Diagnosis-of-Diabetes (accessed on 31 January 2023). [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas. IDF Atlas 10th Edition. 2022. Available online: https://diabetesatlas.org/ (accessed on 9 January 2024).
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhao, Y.; Li, C.; Shao, R. The Expression of Programmed Death-1 on CD4+ and CD8+ T Lymphocytes in Patients with Type 2 Diabetes and Severe Sepsis. PLoS ONE 2016, 11, e0159383. [Google Scholar] [CrossRef] [PubMed]
- Kamei, J.; Yamamoto, S. Complicated urinary tract infections with diabetes mellitus. J. Infect. Chemother. 2021, 27, 1131–1136. [Google Scholar] [CrossRef]
- Shohaimi, S.; Salari, N.; Vaisi-Raygani, A.; Rasoulpoor, S.; Shabani, S.; Jalali, R.; Mohammadi, M. Candida albicans skin infection in patients with type 2 diabetes: A systematic review and meta-analysis. J. Diabetes Metab. Disord. 2021, 20, 665–672. [Google Scholar]
- Dryden, M.; Baguneid, M.; Eckmann, C.; Corman, S.; Stephens, J.; Solem, C.; Li, J.; Charbonneau, C.; Baillon-Plot, N.; Haider, S. Pathophysiology and burden of infection in patients with diabetes mellitus and peripheral vascular disease: Focus on skin and soft-tissue infections. Clin. Microbiol. Infect. 2015, 21, S27–S32. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.H.T.; Lareu, R.R.; Dix, B.R.; Hughes, J.D.; Parsons, R.W. A sequence symmetry analysis of the interrelationships between statins, diabetes and skin infections. Br. J. Clin. Pharmacol. 2019, 85, 2559–2567. [Google Scholar] [CrossRef]
- Falcone, M.; Meier, J.J.; Marini, M.G.; Caccialanza, R.; Aguado, J.M.; Del Prato, S.; Menichetti, F. Diabetes and acute bacterial skin and skin structure infections. Diabetes Res. Clin. Pr. 2021, 174, 108732. [Google Scholar] [CrossRef]
- Hulme, K.D.; Gallo, L.A.; Short, K.R. Influenza virus and glycemic variability in diabetes: A killer combination? Front Microbiol. 2017, 8, 861. [Google Scholar] [CrossRef]
- Gómez-Gómez, A.; Sánchez-Ramos, E.L.; Noyola, D.E. Diabetes is a major cause of influenza-associated mortality in Mexico. Rev. Epidemiol Sante Publique 2021, 69, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, J.E.; Bloxham, C.J.; Chiu, H.; Chew, K.Y.; Russell, J.; Yoshikawa, Y.; Bielefeldt-Ohmann, H.; Steele, L.E.; Hulme, K.D.; Verzele, N.A.; et al. Type I Diabetes Mellitus Increases the Cardiovascular Complications of Influenza Virus Infection. Front. Cell Infect. Microbiol. 2021, 11, 714440. [Google Scholar] [CrossRef] [PubMed]
- Gazzaz, Z.J. Diabetes and COVID-19. Open Life Sci. 2021, 16, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Abdi, A.; Jalilian, M.; Sarbarzeh, P.A.; Vlaisavljevic, Z. Diabetes and COVID-19: A systematic review on the current evidences. Diabetes Res. Clin. Pract. 2020, 166, 108347. [Google Scholar] [CrossRef] [PubMed]
- Hasan, T.H.; Shlash, S.A.; Jasim, S.A.; Hussein, E.F.; Alasedi, K.K.; Aljanaby, A.A. The epidemiology of Klebsiella pneumoniae: A review. Ann. Rom. Soc. Cell Biol. 2021, 25, 8848–8860. [Google Scholar]
- Huang, C.H.; Tsai, J.S.; Chen, I.W.; Hsu, B.R.S.; Huang, M.J.; Huang, Y.Y. Risk factors for in-hospital mortality in patients with type 2 diabetes complicated by community-acquired Klebsiella pneumoniae bacteremia. J. Formos. Med Assoc. 2015, 114, 916–922. [Google Scholar] [CrossRef]
- Langley, G.; Hao, Y.; Pondo, T.; Miller, L.; Petit, S.; Thomas, A.; Lindegren, M.L.; Farley, M.M.; Dumyati, G.; Como-Sabetti, K.; et al. The impact of obesity and diabetes on the risk of disease and death due to invasive group a streptococcus infections in adults. Clin. Infect. Dis. 2016, 62, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Jiang, J.Y.; Hsu, M.S.; Hsu, H.S.; Liao, C.H.; Hsueh, P.R. The prevalence of rectal carriage of Klebsiella pneumoniae amongst diabetic patients and their clinical relevance in Taiwan: A five-year prospective study. J. Microbiol. Immunol. Infect. 2018, 51, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Yen, F.S.; Wei, J.C.C.; Shih, Y.H.; Hsu, C.C.; Hwu, C.M. Metformin use and the risk of bacterial pneumonia in patients with type 2 diabetes. Sci. Rep. 2022, 12, 3270. [Google Scholar] [CrossRef] [PubMed]
- Riza, A.L.; Pearson, F.; Ugarte-Gil, C.; Alisjahbana, B.; van de Vijver, S.; Panduru, N.M.; Hill, P.C.; Ruslami, R.; Moore, D.; Aarnoutse, R.; et al. Clinical management of concurrent diabetes and tuberculosis and the implications for patient services. Lancet Diabetes Endocrinol. 2014, 2, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.M.; de Araújo, G.S.; Santos, C.A.d.S.T.; de Oliveira, M.G.; Barreto, M.L. Association between diabetes and tuberculosis: Case-control study. Rev. Saude Publica 2016, 50, 82. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, G.; Calderon, R.; Ugarte-Gil, C. Tuberculosis and comorbidities: Treatment challenges in patients with comorbid diabetes mellitus and depression. Ther. Adv. Infect. Dis. 2022, 9, 20499361221095831. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2023. 2023. Available online: https://iris.who.int/ (accessed on 3 March 2023).
- Lee, J.Y. Diagnosis and treatment of extrapulmonary tuberculosis. Tuberc. Respir. Dis. 2015, 78, 47–55. [Google Scholar] [CrossRef]
- Sousa, F.D.M.; Souza, I.P.; Amoras, E.D.S.G.; Lima, S.S.; Cayres-Vallinoto, I.M.V.; Ishak, R.; Vallinoto, A.C.R.; Queiroz, M.A.F. Low levels of TNFA gene expression seem to favor the development of pulmonary tuberculosis in a population from the Brazilian Amazon. Immunobiology 2023, 228, 152333. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2022. 2022. Available online: http://apps.who.int/bookorders (accessed on 8 March 2023).
- Cadena, J.; Rathinavelu, S.; Lopez-Alvarenga, J.C.; Restrepo, B.I. The re-emerging association between tuberculosis and diabetes: Lessons from past centuries. Tuberculosis 2019, 116, S89–S97. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. TB and Diabetes. 2024. Available online: https://www.who.int/publications/digital/global-tuberculosis-report-2021/featured-topics/tb-diabetes (accessed on 17 November 2024).
- Noubiap, J.J.; Nansseu, J.R.; Nyaga, U.F.; Nkeck, J.R.; Endomba, F.T.; Kaze, A.D.; Agbor, V.N.; Bigna, J.J. Global prevalence of diabetes in active tuberculosis: A systematic review and meta-analysis of data from 2·3 million patients with tuberculosis. Lancet Glob. Health 2019, 7, e448–e460. [Google Scholar] [CrossRef]
- Zenteno-Cuevas, R.; Cornejo-Báez, A.A.; Pérez-Martínez, D.E. Type 2 Diabetes Mellitus and Tuberculosis, the next syndemic. Microbes Infect. Chemother. 2023, 3, e1821. [Google Scholar] [CrossRef]
- Gautam, S.; Shrestha, N.; Mahato, S.; Nguyen, T.P.A.; Mishra, S.R.; Berg-Beckhoff, G. Diabetes among tuberculosis patients and its impact on tuberculosis treatment in South Asia: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 2113. [Google Scholar] [CrossRef] [PubMed]
- Tegegne, B.S.; Mengesha, M.M.; Teferra, A.A.; Awoke, M.A.; Habtewold, T.D. Association between diabetes mellitus and multi-drug-resistant tuberculosis: Evidence from a systematic review and meta-analysis. Syst. Rev. 2018, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, P.; Ugarte-Gil, C.; Golub, J.; Pearson, F.; Critchley, J. The effects of diabetes on tuberculosis treatment outcomes: An updated systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 2019, 23, 783–796. [Google Scholar] [CrossRef]
- Segura-Cerda, C.A.; López-Romero, W.; Flores-Valdez, M.A. Changes in Host Response to Mycobacterium tuberculosis Infection Associated With Type 2 Diabetes: Beyond Hyperglycemia. Front. Cell. Infect. Microbiol. 2019, 9, 342. [Google Scholar] [CrossRef]
- Gagneux, S. Ecology and evolution of Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2018, 16, 202–213. [Google Scholar] [CrossRef]
- Kanabalan, R.D.; Lee, L.J.; Lee, T.Y.; Chong, P.P.; Hassan, L.; Ismail, R.; Chin, V.K. Human tuberculosis and Mycobacterium tuberculosis complex: A review on genetic diversity, pathogenesis and omics approaches in host biomarkers discovery. Microbiol. Res. 2021, 246, 126674. [Google Scholar] [CrossRef]
- Camacho, L.R.; Constant, P.; Raynaud, C.; Lanéelle, M.-A.; Triccas, J.A.; Gicquel, B.; Daffe, M.; Guilhot, C. Analysis of the phthiocerol dimycocerosate locus of Mycobacterium tuberculosis. Evidence that this lipid is involved in the cell wall permeability barrier. J. Biol. Chem. 2001, 276, 19845–19854. [Google Scholar] [CrossRef]
- Chai, Q.; Zhang, Y.; Liu, C.H. Mycobacterium tuberculosis: An Adaptable Pathogen Associated With Multiple Human Diseases. Front. Cell. Infect. Microbiol. 2018, 8, 158. [Google Scholar] [CrossRef]
- Parbhoo, T.; Mouton, J.M.; Sampson, S.L. Phenotypic adaptation of Mycobacterium tuberculosis to host-associated stressors that induce persister formation. Front. Cell. Infect. Microbiol. 2022, 12, 956607. [Google Scholar] [CrossRef]
- Hoff, D.R.; Ryan, G.J.; Driver, E.R.; Ssemakulu, C.C.; De Groote, M.A.; Basaraba, R.J.; Lenaerts, A.J. Location of Intra- and Extracellular M. tuberculosis Populations in Lungs of Mice and Guinea Pigs during Disease Progression and after Drug Treatment. PLoS ONE 2011, 6, e17550. [Google Scholar] [CrossRef]
- Rohde, K.; Yates, R.M.; Purdy, G.E.; Russell, D.G. Mycobacterium tuberculosis and the environment within the phagosome. Immunol. Rev. 2007, 219, 37–54. [Google Scholar] [CrossRef]
- Magee, D.A.; Conlon, K.M.; Nalpas, N.C.; Browne, J.A.; Pirson, C.; Healy, C.; McLoughlin, K.E.; Chen, J.; Vordermeier, H.M.; Gormley, E.; et al. Innate cytokine profiling of bovine alveolar macrophages reveals commonalities and divergence in the response to Mycobacterium bovis and Mycobacterium tuberculosis infection. Tuberculosis 2014, 94, 441–450. [Google Scholar] [CrossRef]
- Augenstreich, J.; Briken, V. Host Cell Targets of Released Lipid and Secreted Protein Effectors of Mycobacterium tuberculosis. Front. Cell. Infect. Microbiol. 2020, 10, 595029. [Google Scholar] [CrossRef]
- Nisa, A.; Kipper, F.C.; Panigrahy, D.; Tiwari, S.; Kupz, A.; Subbian, S. Different modalities of host cell death and their impact on Mycobacterium tuberculosis infection. Am. J. Physiol. Cell Physiol. 2022, 323, C1444–C1474. [Google Scholar] [CrossRef]
- Chandra, P.; Grigsby, S.J.; Philips, J.A. Immune evasion and provocation by Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2022, 20, 750–766. [Google Scholar] [CrossRef]
- Lachmandas, E.; Vrieling, F.; Wilson, L.G.; Joosten, S.A.; Netea, M.G.; Ottenhoff, T.H.; van Crevel, R. The effect of hyperglycaemia on in vitro cytokine production and macrophage infection with Mycobacterium tuberculosis. PLoS ONE 2015, 10, e0117941. [Google Scholar] [CrossRef]
- Ayala, T.S.; Tessaro, F.H.G.; Jannuzzi, G.P.; Bella, L.M.; Ferreira, K.S.; Martins, J.O. High Glucose Environments Interfere with Bone Marrow-Derived Macrophage Inflammatory Mediator Release, the TLR4 Pathway and Glucose Metabolism. Sci. Rep. 2019, 9, 11447. [Google Scholar] [CrossRef]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J. Diabetes Res. 2020, 2020, 7489795. [Google Scholar] [CrossRef]
- Podell, B.K.; Ackart, D.F.; Obregon-Henao, A.; Eck, S.P.; Henao-Tamayo, M.; Richardson, M.; Orme, I.M.; Ordway, D.J.; Basaraba, R.J. Increased Severity of Tuberculosis in Guinea Pigs with Type 2 Diabetes. Am. J. Pathol. 2014, 184, 1104–1118. [Google Scholar] [CrossRef]
- Lin, J.N.; Lai, C.H.; Chen, Y.H.; Lee SS, J.; Tsai, S.S.; Huang, C.K.; Chung, H.C.; Liang, S.H.; Lin, H.H. Risk factors for extra-pulmonary tuberculosis compared to pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 2009, 13, 620–625. [Google Scholar]
- Baker, M.A.; Harries, A.D.; Jeon, C.Y.; Hart, J.E.; Kapur, A.; Lönnroth, K.; Ottmani, S.E.; Goonesekera, S.D.; Murray, M.B. The impact of diabetes on tuberculosis treatment outcomes: A systematic review. BMC Med. 2011, 9, 81. [Google Scholar] [CrossRef]
- Novita, B.D. Metformin: A review of its potential as enhancer for anti tuberculosis efficacy in diabetes mellitus-tuberculosis coinfection patients. Indian J. Tuberc. 2019, 66, 294–298. [Google Scholar] [CrossRef]
- Perez-Navarro, L.M.; Restrepo, B.I.; Fuentes-Dominguez, F.J.; Duggirala, R.; Morales-Romero, J.; López-Alvarenga, J.C.; Comas, I.; Zenteno-Cuevas, R. The effect size of type 2 diabetes mellitus on tuberculosis drug resistance and adverse treatment outcomes. Tuberculosis 2017, 103, 83–91. [Google Scholar] [CrossRef]
- Song, C.; Xie, W.; Gong, L.; Ren, M.; Pan, P.; Luo, B. The relationship between HbA1c control levels and antituberculosis treatment effects: A meta-analysis. J. Chin. Med. Assoc. 2019, 82, 915–921. [Google Scholar] [CrossRef]
- Moreno-Martínez, A.; Casals, M.; Orcau Gorrindo, P.; Masdeu, E.; Caylà, J.A. Factors associated with diabetes mellitus among adults with tuberculosis in a large European city, 2000–2013. Int. J. Tuberc. Lung Dis. 2015, 19, 1507–1512. [Google Scholar] [CrossRef]
- Lyu, M.; Wang, D.; Zhao, J.; Yang, Z.; Chong, W.; Zhao, Z.; Ming, L.; Ying, B. A novel risk factor for predicting anti-tuberculosis drug resistance in patients with tuberculosis complicated with type 2 diabetes mellitus. Int. J. Infect. Dis. 2020, 97, 69–77. [Google Scholar] [CrossRef]
- Tireh, H.; Heidarian Miri, H.; Khajavian, N.; Samiei, A.; Afzalaghaee, M. Prevalence of Diabetes Mellitus and its Related Factors in Patients with Tuberculosis. Arch. Iran. Med. 2022, 25, 835–840. [Google Scholar] [CrossRef]
- Smallwood, H.S.; Shi, L.; Squier, T.C. Increases in calmodulin abundance and stabilization of activated inducible nitric oxide synthase mediate bacterial killing in RAW 264.7 macrophages. Biochemistry 2006, 45, 9717–9726. [Google Scholar] [CrossRef] [PubMed]
- de Martino, M.; Lodi, L.; Galli, L.; Chiappini, E. Immune Response to Mycobacterium tuberculosis: A Narrative Review. Front. Pediatr. 2019, 7, 350. [Google Scholar] [CrossRef]
- Natarajan, K.; Kundu, M.; Sharma, P.; Basu, J. Innate immune responses to M. tuberculosis infection. Tuberculosis 2011, 91, 427–431. [Google Scholar] [CrossRef]
- Firoz, A.; Malik, A.; Ali, H.M.; Akhter, Y.; Manavalan, B.; Kim, C.B. PRR-HyPred: A two-layer hybrid framework to predict pattern recognition receptors and their families by employing sequence encoded optimal features. Int. J. Biol. Macromol. 2023, 234, 123622. [Google Scholar] [CrossRef]
- Juárez Carvajal, E.; López González, J.S.; Torres Rojas, M.; Sada Diaz, E. Receptores de la inmunidad innata en procesos infecciosos pulmonares. Rev. Inst. Nac. Enfermedades Respir. 2009, 22, 366–378. [Google Scholar]
- Sahoo, B.R. Structure of fish Toll-like receptors (TLR) and NOD-like receptors (NLR). Int. J. Biol. Macromol. 2020, 161, 1602–1617. [Google Scholar] [CrossRef]
- Thada, S.; Horvath, G.L.; Müller, M.M.; Dittrich, N.; Conrad, M.L.; Sur, S.; Hussain, A.; Pelka, K.; Gaddam, S.L.; Latz, E.; et al. Interaction of TLR4 and TLR8 in the innate immune response against Mycobacterium tuberculosis. Int. J. Mol. Sci. 2021, 22, 1560. [Google Scholar] [CrossRef]
- Lerner, T.R.; Borel, S.; Gutierrez, M.G. The innate immune response in human tuberculosis. Cell. Microbiol. 2015, 17, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell. 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Elinav, E.; Strowig, T.; Henao-Mejia, J.; Flavell, R.A. Regulation of the Antimicrobial Response by NLR Proteins. Immunity 2011, 34, 665–679. [Google Scholar] [CrossRef]
- Malik, G.; Zhou, Y. Innate immune sensing of influenza a virus. Viruses 2020, 12, 755. [Google Scholar] [CrossRef]
- Li, K.; Underhill, D.M. C-Type Lectin Receptors in Phagocytosis. Curr. Top. Microbiol. Immunol. 2020, 429, 1–18. [Google Scholar]
- Drouin, M.; Saenz, J.; Chiffoleau, E. C-Type Lectin-Like Receptors: Head or Tail in Cell Death Immunity. Front. Immunol. 2020, 11, 251. [Google Scholar] [CrossRef]
- Mucke, L.; Oldstone, M.B.A. The expression of major histocompatibility complex (MHC) class I antigens in the brain differs markedly in acute and persistent infections with lymphocytic choriomeningitis virus (LCMV). J. Neuroimmunol. 1992, 36, 193–198. [Google Scholar] [CrossRef]
- Hargrave, K.E.; MacLeod, M.K.L.; Worrell, J.C. Antigen presenting cells: Professionals, amateurs, and spectators in the “long game” of lung immunity. Int. J. Biochem. Cell Biol. 2022, 153, 106331. [Google Scholar] [CrossRef]
- Herrera, M.T.; Guzmán-Beltrán, S.; Bobadilla, K.; Santos-Mendoza, T.; Flores-Valdez, M.A.; Gutiérrez-González, L.H.; González, Y. Human Pulmonary Tuberculosis: Understanding the Immune Response in the Bronchoalveolar System. Biomolecules 2022, 12, 1148. [Google Scholar] [CrossRef]
- Ravesloot-Chávez, M.M.; Dis EVan Stanley, S.A. The Innate Immune Response to Mycobacterium tuberculosis Infection. Annu. Rev. Immunol. 2021, 39, 611–637. [Google Scholar] [CrossRef] [PubMed]
- Flesch, I.E.A.; Kaufmann, S.H.E. Role of Cytokines in Tuberculosis. Immunobiology 1993, 189, 316–339. [Google Scholar] [CrossRef]
- Tan, S.; Sukumar, N.; Abramovitch, R.B.; Parish, T.; Russell, D.G. Mycobacterium tuberculosis Responds to Chloride and pH as Synergistic Cues to the Immune Status of its Host Cell. PLoS Pathog. 2013, 9, e1003282. [Google Scholar] [CrossRef]
- Combaluzier, B.; Pieters, J. Chemotaxis and Phagocytosis in Neutrophils Is Independent of Coronin 1. J. Immunol. 2009, 182, 2745–2752. [Google Scholar] [CrossRef] [PubMed]
- Schuller, S.; Neefjes, J.; Ottenhoff, T.; Thole, J.; Young, D. Coronin is involved in uptake of Mycobacterium bovis BCG in human macrophages but not in phagosome maintenance. Cell Microbiol. 2001, 3, 785–793. [Google Scholar] [CrossRef]
- Sinha, S.; Gupta, G.; Biswas, S.; Gupta, K.; Singh, P.; Jain, R.; Sharma, S.K.; Das, B.K. Coronin-1 levels in patients with tuberculosis. Indian J. Med. Res. 2021, 154, 866. [Google Scholar] [CrossRef] [PubMed]
- Ferluga, J.; Yasmin, H.; Al-Ahdal, M.N.; Bhakta, S.; Kishore, U. Natural and trained innate immunity against Mycobacterium tuberculosis. Immunobiology 2020, 225, 151951. [Google Scholar] [CrossRef] [PubMed]
- Roca, F.J.; Whitworth, L.J.; Prag, H.A.; Murphy, M.P.; Ramakrishnan, L. Tumor necrosis factor induces pathogenic mitochondrial ROS in tuberculosis through reverse electron transport. Science 2022, 376, eabh2841. [Google Scholar] [CrossRef]
- Dabla, A.; Liang, Y.C.; Rajabalee, N.; Irwin, C.; Moonen, C.G.J.; Willis, J.V.; Berton, S.; Sun, J. TREM2 Promotes Immune Evasion by Mycobacterium tuberculosis in Human Macrophages. mBio 2022, 13, e0145622. [Google Scholar] [CrossRef]
- Krakauer, T. Inflammasomes, Autophagy, and Cell Death: The Trinity of Innate Host Defense against Intracellular Bacteria. Mediat. Inflamm. 2019, 2019, 2471215. [Google Scholar]
- Rendra, E.; Riabov, V.; Mossel, D.M.; Sevastyanova, T.; Harmsen, M.C.; Kzhyshkowska, J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology 2019, 224, 242–253. [Google Scholar] [CrossRef]
- Huang, L.; Nazarova, E.V.; Russell, D.G. Mycobacterium tuberculosis: Bacterial Fitness within the Host Macrophage. Microbiol. Spectr. 2019, 7, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.S.; Calder, B.; Gonnelli, G.; Degroeve, S.; Rajaonarifara, E.; Mulder, N.; Soares, N.C.; Martens, L.; Blackburn, J.M. Identification of Quantitative Proteomic Differences between Mycobacterium tuberculosis Lineages with Altered Virulence. Front Microbiol. 2016, 7, 813. [Google Scholar] [CrossRef]
- He, C.; Cheng, X.; Kaisaier, A.; Wan, J.; Luo, S.; Ren, J.; Sha, Y.; Peng, H.; Zhen, Y.; Liu, W.; et al. Effects of Mycobacterium tuberculosis lineages and regions of difference (RD) virulence gene variation on tuberculosis recurrence. Ann. Transl. Med. 2022, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.; Gaffney, K.J.; Rodgers, K.E. Improving the Innate Immune Response in Diabetes by Modifying the Renin Angiotensin System. Front. Immunol. 2019, 10, 2885. [Google Scholar] [CrossRef] [PubMed]
- Park, M.D.; Silvin, A.; Ginhoux, F.; Merad, M. Macrophages in health and disease. Cell 2022, 185, 4259–4279. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Dinarello, C.A.; Mandrup-Poulsen, T. Targeting innate immune mediators in type 1 and type 2 diabetes. Nat. Rev. Immunol. 2019, 19, 734–746. [Google Scholar] [CrossRef]
- Chen, G.Y.; Nuñez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef]
- Nwadiugwu, M.C. Inflammatory Activities in Type 2 Diabetes Patients With Co-morbid Angiopathies and Exploring Beneficial Interventions: A Systematic Review. Front. Public Health 2021, 8, 600427. [Google Scholar] [CrossRef] [PubMed]
- Sholeye, A.R.; Williams, A.A.; Loots, D.T.; Tutu van Furth, A.M.; van der Kuip, M.; Mason, S. Tuberculous Granuloma: Emerging Insights From Proteomics and Metabolomics. Front. Neurol. 2022, 13, 804838. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 3 March 2024).
- Jeong, J.W.; Lee, B.; Kim, D.H.; Jeong, H.O.; Moon, K.M.; Kim, M.J.; Yokozawa, T.; Chung, H.Y. Mechanism of Action of Magnesium Lithospermate B against Aging and Obesity-Induced ER Stress, Insulin Resistance, and Inflammsome Formation in the Liver. Molecules 2018, 23, 2098. [Google Scholar] [CrossRef]
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and type 2 diabetes mellitus: Connections in epidemiology, pathogenesis, and treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, Z.; Gomez-Salazar, M.; Alexaki, V.I. Innate Immune Cells in the Adipose Tissue in Health and Metabolic Disease. J. Innate Immun. 2022, 14, 4–30. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, M.; Jia, S. Macrophage: Key player in the pathogenesis of autoimmune diseases. Front. Immunol. 2023, 14, 1080310. [Google Scholar] [CrossRef]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2009, 72, 219–246. [Google Scholar] [CrossRef] [PubMed]
- Sabio, G.; Das, M.; Mora, A.; Zhang, Z.; Jun, J.Y.; Ko, H.J.; Barrett, T.; Kim, J.K.; Davis, R.J. A Stress Signaling Pathway in Adipose Tissue Regulates Hepatic Insulin Resistance. Science 2008, 322, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Flores-Aldana, M.; Peralta-Zaragosa, O.; Barquera-Cervera, S. El paradigma inmune Th1-Th2: Un vínculo entre obesidad, aterosclerosis y diabetes mellitus. Clin. Investig. Arterioscl. 2005, 17, 232–248. [Google Scholar] [CrossRef]
- Lu, S.; Li, Y.; Qian, Z.; Zhao, T.; Feng, Z.; Weng, X.; Yu, L. Role of the inflammasome in insulin resistance and type 2 diabetes mellitus. Front. Immunol. 2023, 14, 1052756. [Google Scholar]
- Petrilli, V.; Stephanie, P.; Jürg, T. The inflammasome. Curr. Biol. 2005, 15, R581. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.P.; Leal, V.N.C.; Souza de Lima, D.; Reis, E.C.; Pontillo, A. Inflammasome genetics and complex diseases: A comprehensive review. Eur. J. Hum. Genet. 2020, 28, 1307–1321. [Google Scholar] [CrossRef]
- von Moltke, J.; Ayres, J.S.; Kofoed, E.M.; Chavarría-Smith, J.; Vance, R.E. Recognition of Bacteria by Inflammasomes. Annu. Rev. Immunol. 2013, 31, 73–106. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.R.; Kanneganti, T.D. NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 2021, 22, 550–559. [Google Scholar] [CrossRef]
- Rathinam, V.A.K.; Vanaja, S.K.; Fitzgerald, K.A. Regulation of inflammasome signaling. Nat. Immunol. 2012, 13, 333–342. [Google Scholar] [CrossRef]
- Gong, Z.; Zhao, S.; Zhou, J.; Yan, J.; Wang, L.; Du, X.; Li, H.; Chen, Y.; Cai, W.; Wu, J. Curcumin alleviates DSS-induced colitis via inhibiting NLRP3 inflammsome activation and IL-1β production. Mol. Immunol. 2018, 104, 11–19. [Google Scholar] [CrossRef]
- Guarda, G.; Zenger, M.; Yazdi, A.S.; Schroder, K.; Ferrero, I.; Menu, P.; Tardivel, A.; Mattmann, C.; Tschopp, J. Differential Expression of NLRP3 among Hematopoietic Cells. J. Immunol. 2011, 186, 2529–2534. [Google Scholar] [CrossRef] [PubMed]
- Olona, A.; Leishman, S.; Anand, P.K. The NLRP3 inflammasome: Regulation by metabolic signals. Trends Immunol. 2022, 43, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Sutterwala, F.S.; Haasken, S.; Cassel, S.L. Mechanism of NLRP3 inflammasome activation. Ann. N. Y. Acad. Sci. 2014, 1319, 82–95. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Lee, S.G.; Kim, S.H.; Kim, J.H.; Choi, E.; Cho, W.; Rim, J.H.; Hwang, I.; Lee, C.J.; Lee, M.; et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat. Commun. 2020, 11, 2127. [Google Scholar] [CrossRef]
- Hardaway, A.L.; Podgorski, I. Il-1β, Rage and Fabp4: Targeting the Dynamic Trio in Metabolic Inflammation and Related Pathologies. Future Med. Chem. 2013, 5, 1089–1108. [Google Scholar] [CrossRef] [PubMed]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 Diabetes and its Impact on the Immune System. Curr. Diabetes Rev. 2019, 16, 442–449. [Google Scholar]
- Al-Attiyah, R.J.; Mustafa, A.S. Mycobacterial antigen-induced T helper type 1 (Th1) and Th2 reactivity of peripheral blood mononuclear cells from diabetic and non-diabetic tuberculosis patients and Mycobacterium bovis bacilli Calmette-Guérin (BCG)-vaccinated healthy subjects. Clin. Exp. Immunol. 2009, 158, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.P.; Sridhar, R.; Nair, D.; Banurekha, V.V.; Nutman, T.B.; Babu, S. Type 2 diabetes mellitus is associated with altered CD8+ T and natural killer cell function in pulmonary tuberculosis. Immunology 2015, 144, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Nandy, D.; Janardhanan, R.; Mukhopadhyay, D.; Basu, A. Effect of Hyperglycemia on Human Monocyte Activation. J. Investig. Med. 2011, 59, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, J.; Wei, X.; Zhang, X.; You, L.; Liu, Y.; Chen, L.; Liu, C.; Sun, C.; Tian, X.; et al. C-type lectin (CTL) and sialic acid-binding lectin (SABL) from Venerupis philippinarum: Function on PAMP binding and opsonic activities in immune responses. Fish Shellfish. Immunol. 2023, 133, 108554. [Google Scholar] [CrossRef]
- Ebbecke, T.; Diersing, C.; Lindenwald, D.L.; Stegmann, F.; Lepenies, B. C-Type Lectins and Their Roles in Disease and Immune Homeostasis. In Comprehensive Glycoscience: Second Edition; Elsevier: Amsterdam, The Netherlands, 2021; Volume 5, pp. 185–214. [Google Scholar]
- Brown, G.D.; Willment, J.A.; Whitehead, L. C-type lectins in immunity and homeostasis. Nat. Rev. Immunol. 2018, 18, 374–389. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.; Wallis, R.; Soilleux, E.J.; Townsend, P.; Zehnder, D.; Tan, B.K.; Sim, R.B.; Lehnert, H.; Randeva, H.S.; Mitchell, D.A. High glucose disrupts oligosaccharide recognition function via competitive inhibition: A potential mechanism for immune dysregulation in diabetes mellitus. Immunobiology 2011, 216, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.Q.; Rentfro, A.R.; Lu, Y.; Nair, S.; Hanis, C.L.; McCormick, J.B.; Fisher-Hoch, S.P. Host susceptibility to tuberculosis: Insights from a longitudinal study of gene expression in diabetes. Int. J. Tuberc. Lung Dis. 2012, 16, 370–372. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wong, K.; Jacobs, W.R., Jr. Critical role for NLRP3 in necrotic death triggered by Mycobacterium tuberculosis. Cell. Microbiol. 2011, 13, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.B.; Moura-Alves, P.; Sonawane, A.; Hacohen, N.; Griffiths, G.; Moita, L.F.; Anes, E. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell. Microbiol. 2010, 12, 1046–1063. [Google Scholar] [CrossRef] [PubMed]
- Simeone, R.; Bobard, A.; Lippmann, J.; Bitter, W.; Majlessi, L.; Brosch, R.; Enninga, J. Phagosomal Rupture by Mycobacterium tuberculosis Results in Toxicity and Host Cell Death. PLoS Pathog. 2012, 8, e1002507. [Google Scholar] [CrossRef]
- Jager, J.; Grémeaux, T.; Cormont, M.; Le Marchand-Brustel, Y.; Tanti, J.F. Interleukin-1β-Induced Insulin Resistance in Adipocytes through Down-Regulation of Insulin Receptor Substrate-1 Expression. Endocrinology 2007, 148, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, R.; Wang, H.; Liang, F. Mechanisms Linking Inflammation to Insulin Resistance. Int. J. Endocrinol. 2015, 2015, 508409. [Google Scholar] [CrossRef]
- Lara-Gómez, R.E.; Moreno-Cortes, M.L.; Muñiz-Salazar, R.; Zenteno-Cuevas, R. Association of polymorphisms at −174 in IL-6, and −308 and −238 in TNF-α in the development of tuberculosis and type 2 diabetes mellitus in the Mexican population. Gene 2019, 702, 1–7. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Consolidated Guidelines on Tuberculosis Module 4: Treatment Drug-Susceptible Tuberculosis Treatment. 2022. Available online: https://www.who.int/publications/i/item/9789240048126 (accessed on 24 July 2023).
- Grace, A.G.; Mittal, A.; Jain, S.; Tripathy, J.P.; Satyanarayana, S.; Tharyan, P.; Kirubakaran, R. Shortened treatment regimens versus the standard regimen for drug-sensitive pulmonary tuberculosis. Cochrane Database Syst. Rev. 2019, 12, CD012918. [Google Scholar] [CrossRef]
- Rumende, C.M. Risk Factors for Multidrug-resistant Tuberculosis. Acta Med. Indones-Indones J. Intern. Med. 2018, 50, 1–2. [Google Scholar]
- Martínez-Aguilar, G.; Serrano, C.J.; Castañeda-Delgado, J.E.; Macías-Segura, N.; Hernández-Delgadillo, N.; Enciso-Moreno, L.; de Lira, Y.G.; Valenzuela-Méndez, E.; Gándara-Jasso, B.; Correa-Chacón, J.; et al. Associated Risk Factors for Latent Tuberculosis Infection in Subjects with Diabetes. Arch. Med. Res. 2015, 46, 221–227. [Google Scholar] [CrossRef]
- Espinosa-Pereiro, J.; Sánchez-Montalvá, A.; Aznar, M.L.; Espiau, M. MDR Tuberculosis Treatment. Medicina 2022, 58, 188. [Google Scholar] [CrossRef] [PubMed]
- Zenteno-Cuevas, R.; Xochihua-Gonzalez, O.; Cuevas-Córdoba, B.; Victoria-Cota, N.L.; Muñiz-Salazar, R.; Montero, H.; Hamsho-Díaz, P.; Lauzardo, M. Mutations conferring resistance to first- and second-line drugs in multidrug-resistant Mycobacterium tuberculosis clinical isolates in southeast Mexico. Int. J. Antimicrob. Agents. 2015, 45, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, J.; Boeree, M.J.; Cambau, E.; Chesov, D.; Conradie, F.; Cox, V.; Dheda, K.; Dudnyk, A.; Farhat, M.R.; Gagneux, S.; et al. Clinical implications of molecular drug resistance testing for Mycobacterium tuberculosis: A 2023 TBnet/RESIST-TB consensus statement. Lancet Infect. Dis. 2023, 23, e122–e137. [Google Scholar] [CrossRef] [PubMed]
- Zenteno-Cuevas, R. New molecular mechanisms related to drug resistance in tuberculosis. Microbes Infect. Chemother. 2022, 2, e1318. [Google Scholar] [CrossRef]
- Medellín-Garibay, S.E.; Cortez-Espinosa, N.; Milán-Segovia, R.C.; Magaña-Aquino, M.; Vargas-Morales, J.M.; González-Amaro, R.; Portales-Pérez, D.P.; Romano-Moreno, S. Clinical Pharmacokinetics of Rifampin in Patients with Tuberculosis and Type 2 Diabetes Mellitus: Association with Biochemical and Immunological Parameters. Antimicrob. Agents Chemother. 2015, 59, 7707–7714. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, D.; Wang, Y.; Wang, Y.; Liang, Z. The impact of diabetes mellitus on drug resistance in patients with newly diagnosed tuberculosis: A systematic review and meta-analysis. Ann. Palliat. Med. 2020, 9, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Alame Emane, A.K.; Guo, X.; Takiff, H.E.; Liu, S. Drug resistance, fitness and compensatory mutations in Mycobacterium tuberculosis. Tuberculosis 2021, 129, 102091. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Hernández, G.A.; Pérez-Martínez, D.E.; Madrazo-Moya, C.F.; Cancino-Muñoz, I.; Comas, I.; Zenteno-Cuevas, R. Whole genome sequencing analysis to evaluate the influence of T2DM on polymorphisms associated with drug resistance in M. tuberculosis. BMC Genom. 2022, 23, 465. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Martínez, D.E.; Bermúdez-Hernández, G.A.; Madrazo-Moya, C.F.; Cancino-Muñoz, I.; Montero, H.; Licona-Cassani, C.; Muñiz-Salazar, R.; Comas, I.; Zenteno-Cuevas, R. SNPs in Genes Related to DNA Damage Repair in Mycobacterium Tuberculosis: Their Association with Type 2 Diabetes Mellitus and Drug Resistance. Genes 2022, 13, 609. [Google Scholar] [CrossRef]
- Flynn, J.A.L.; Chan, J. Immune cell interactions in tuberculosis. Cell 2022, 185, 4682–4702. [Google Scholar] [CrossRef] [PubMed]
- Goossens, S.N.; Sampson, S.L.; Van Rie, A. Mechanisms of Drug-Induced Tolerance in Mycobacterium tuberculosis. Clin. Microbiol. Rev. 2020, 34, e00141–20. [Google Scholar] [CrossRef]
- Boldrin, F.; Provvedi, R.; Cioetto Mazzabò, L.; Segafreddo, G.; Manganelli, R. Tolerance and Persistence to Drugs: A Main Challenge in the Fight Against Mycobacterium tuberculosis. Front. Microbiol. 2020, 11, 1924. [Google Scholar] [CrossRef] [PubMed]
- Shultis, M.W.; Mulholland, C.V.; Berney, M. Are all antibiotic persisters created equal? Front. Cell. Infect. Microbiol. 2022, 12, 933458. [Google Scholar] [CrossRef] [PubMed]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Van Bambeke, F.; Glupczynski, Y.; Plésiat, P.; Pechère, J.C.; Tulkens, P.M. Antibiotic efflux pumps in prokaryotic cells: Occurence, impact on resistance and strategies for the future of antimicrobial therapy. J. Antimicrob. Chemother. 2003, 51, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Kabra, R.; Chauhan, N.; Kumar, A.; Ingale, P.; Singh, S. Efflux pumps and antimicrobial resistance: Paradoxical components in systems genomics. Prog. Biophys. Mol. Biol. 2019, 141, 15–24. [Google Scholar] [CrossRef]
- Silva, P.E.; Bigi, F.; de la Paz Santangelo, M.; Romano, M.I.; Martín, C.; Cataldi, A.; Aínsa, J.A. Characterization of P55, a multidrug efflux pump in Mycobacterium bovis and Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2001, 45, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Schmalstieg, A.M.; Srivastava, S.; Belkaya, S.; Deshpande, D.; Meek, C.; Leff, R.; van Oers, N.S.; Gumbo, T. The antibiotic resistance arrow of time: Efflux pump induction is a general first step in the evolution of mycobacterial drug resistance. Antimicrob. Agents Chemother. 2012, 56, 4806–4815. [Google Scholar] [CrossRef] [PubMed]
- AlMatar, M.; Var, I.; Kayar, B.; Köksal, F. Differential Expression of Resistant and Efflux Pump Genes in MDR-TB Isolates. Endocr. Metab. Immune Disord. Drug Targets. 2019, 20, 271–287. [Google Scholar] [CrossRef]
- Li, H.; Guo, H.; Chen, T.; Li, Y.; Chen, Y.; Zhao, J.; Yan, H.; Chen, M.; Sun, Q.; Zhang, C.; et al. Genome-wide SNP and InDel mutations in Mycobacterium tuberculosis associated with rifampicin and isoniazid resistance. Int. J. Clin. Exp. Pathol. 2018, 11, 3903–3914. [Google Scholar]
- Purkan Ihsanawati Natalia, D.; Syah, Y.M.; Retnoningrum, D.S.; Kusuma, H.S. Mutation of katG in a clinical isolate of Mycobacterium tuberculosis: Effects on catalase-peroxidase for isoniazid activation. Ukr. Biochem. J. 2016, 88, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Kreutzfeldt, K.M.; Jansen, R.S.; Hartman, T.E.; Gouzy, A.; Wang, R.; Krieger, I.V.; Zimmerman, M.D.; Gengenbacher, M.; Sarathy, J.P.; Xie, M.; et al. CinA mediates multidrug tolerance in Mycobacterium tuberculosis. Nat. Commun. 2022, 13, 2203. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, M.J.; Haeili, M.; Ghazi, M.; Goudarzi, H.; Pormohammad, A.; Imani Fooladi, A.A.; Feizabadi, M.M. New Insights in to the Intrinsic and Acquired Drug Resistance Mechanisms in Mycobacteria. Front. Microbiol. 2017, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Lavender, C.; Globan, M.; Sievers, A.; Billman-Jacobe, H.; Fyfe, J. Molecular Characterization of Isoniazid-Resistant Mycobacterium tuberculosis Isolates Collected in Australia. Antimicrob. Agents Chemother. 2005, 49, 4068–4074. [Google Scholar] [CrossRef] [PubMed]
- Purkan, P.; Ihsanawati, I.; Natalia, D.; Syah, Y.; Retnoningrum, D.; Siswanto, I. Molecular Analysis of katG Encoding Catalase-Peroxidase from Clinical Isolate of Isoniazid-Resistant Mycobacterium tuberculosis. J. Med. Life 2018, 11, 160–167. [Google Scholar]
- De Maio, F.; Cingolani, A.; Bianco, D.M.; Salustri, A.; Palucci, I.; Sanguinetti, M.; Delogu, G.; Sali, M. First description of the katG gene deletion in a Mycobacterium tuberculosis clinical isolate and its impact on the mycobacterial fitness. Int. J. Med. Microbiol. 2021, 311, 151506. [Google Scholar] [CrossRef]
- Hsu, L.Y.; Lai, L.Y.; Hsieh, P.F.; Lin, T.L.; Lin, W.H.; Tasi, H.Y.; Lee, W.-T.; Jou, R.; Wang, J.-T. Two Novel katG Mutations Conferring Isoniazid Resistance in Mycobacterium tuberculosis. Front. Microbiol. 2020, 11, 1644. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Liao, J.; Gong, Y.; Han, X.; Chen, Y.; Tang, Z.; Ma, Q. Diabetes and multidrug-resistance gene mutation: Tuberculosis in Zunyi, Southwest China. Ann. Palliat. Med. 2020, 9, 3152–3161. [Google Scholar] [CrossRef] [PubMed]
- Sahiratmadja, E.; Rini, I.A.; Penggoam, S.; Charles, A.; Maskoen, A.M.; Parwati, I. Acetylator Status Among Newly Diagnosed and Recurrent Tuberculosis Patients from Kupang, Eastern Part of Indonesia. Pharmgenomics Pers. Med. 2021, 14, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Pasipanodya, J.G.; Srivastava, S.; Gumbo, T. Meta-Analysis of Clinical Studies Supports the Pharmacokinetic Variability Hypothesis for Acquired Drug Resistance and Failure of Antituberculosis Therapy. Clin. Infect. Dis. 2012, 55, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Gafar, F.; Wasmann, R.E.; McIlleron, H.M.; Aarnoutse, R.E.; Schaaf, H.S.; Marais, B.J.; Agarwal, D.; Antwi, S.; Bang, N.D.; Bekker, A.; et al. Global estimates and determinants of antituberculosis drug pharmacokinetics in children and adolescents: A systematic review and individual patient data meta-analysis. Eur. Respir. J. 2023, 61, 2201596. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Aglitti, A.; Bucci, T.; Caruso, R.; Salvatore, T.; Sasso, F.C.; Tripodi, M.F.; Persico, M. Liver biopsy in type 2 diabetes mellitus: Steatohepatitis represents the sole feature of liver damage. PLoS ONE 2017, 12, e0178473. [Google Scholar] [CrossRef]
- Perwitasari, D.A.; Irham, L.M.; Darmawan, E.; Mulyani, U.A.; Atthobari, J. CYP2E1 polymorphism, acetylator profiles and drug-induced liver injury incidence of Indonesian tuberculosis patients. Indian J. Tuberc. 2016, 63, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Wattanapokayakit, S.; Mushiroda, T.; Yanai, H.; Wichukchinda, N.; Chuchottawon, C.; Nedsuwan, S.; Rojanawiwat, A.; Denjanta, S.; Kantima, T.; Wongyai, J.; et al. NAT2 slow acetylator associated with anti-tuberculosis drug-induced liver injury in Thai patients. Int. J. Tuberc. Lung Dis. 2016, 20, 1364–1369. [Google Scholar] [CrossRef]
- Zaw, M.T.; Emran, N.A.; Lin, Z. Mutations inside rifampicin-resistance determining region of rpoB gene associated with rifampicin-resistance in Mycobacterium tuberculosis. J. Infect. Public Health 2018, 11, 605–610. [Google Scholar] [CrossRef]
- da Silva, P.E.A.; Palomino, J.C. Molecular basis and mechanisms of drug resistance in Mycobacterium tuberculosis: Classical and new drugs. J. Antimicrob. Chemother. 2011, 66, 1417–1430. [Google Scholar] [CrossRef] [PubMed]
- Singpanomchai, N.; Akeda, Y.; Tomono, K.; Tamaru, A.; Santanirand, P.; Ratthawongjirakul, P. Rapid detection of multidrug-resistant tuberculosis based on allele-specific recombinase polymerase amplification and colorimetric detection. PLoS ONE 2021, 16, e0253235. [Google Scholar] [CrossRef]
- Majumdar, T.; Banik, A.; Allada, V.; Das, B. Molecular analysis of rpoB gene mutation in MTB detected isolates in a tertiary care centre (AGMC) of North-East, India. Indian J. Med. Microbiol. 2023, 45, 100399. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Navarro, L.M.; Fuentes-Domínguez, F.J.; Zenteno-Cuevas, R. Type 2 diabetes mellitus and its influence in the development of multidrug resistance tuberculosis in patients from southeastern Mexico. J. Diabetes Complicat. 2015, 29, 77–82. [Google Scholar] [CrossRef]
- Metwally, A.S.; El-Sheikh, S.M.A.; Galal, A.A.A. The impact of diabetes mellitus on the pharmacokinetics of rifampicin among tuberculosis patients: A systematic review and meta-analysis study. Diabetes Metab. Syndr. Clin. Res. Rev. 2022, 16, 102410. [Google Scholar] [CrossRef]
- Mehta, S.; Yu, E.A.; Ahamed, S.F.; Bonam, W.; Kenneth, J. Rifampin resistance and diabetes mellitus in a cross-sectional study of adult patients in rural South India. BMC Infect. Dis. 2015, 15, 451. [Google Scholar] [CrossRef]
- Perea-Jacobo, R.; Muniz-Salazar, R.; Laniado-Laborín, R.; Cabello-Pasini, A.; Zenteno-Cuevas, R.; Ochoa-Terán, A. Rifampin pharmacokinetics in tuberculosis-diabetes mellitus patients: A pilot study from Baja California, Mexico. Int. J. Tuberc. Lung Dis. 2019, 23, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Hernández, G.A.; Pérez-Martínez, D.; Cristina Ortiz-León, M.; Muñiz-Salazar, R.; Licona-Cassani, C.; Zenteno-Cuevas, R. Mutational Dynamics Related to Antibiotic Resistance in M. tuberculosis Isolates from Serial Samples of Patients with Tuberculosis and Type 2 Diabetes Mellitus. Microorganisms 2024, 12, 324. [Google Scholar] [CrossRef] [PubMed]
- Pule, C.M.; Sampson, S.L.; Warren, R.M.; Black, P.A.; van Helden, P.D.; Victor, T.C.; Louw, G.E. Efflux pump inhibitors: Targeting mycobacterial efflux systems to enhance TB therapy. J. Antimicrob. Chemother. 2016, 71, 17–26. [Google Scholar] [CrossRef]
- Gupta, S.; Tyagi, S.; Almeida, D.V.; Maiga, M.C.; Ammerman, N.C.; Bishai, W.R. Acceleration of Tuberculosis Treatment by Adjunctive Therapy with Verapamil as an Efflux Inhibitor. Am. J. Respir. Crit. Care Med. 2013, 188, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Niazi, A.K.; Kalra, S. Diabetes and tuberculosis: A review of the role of optimal glycemic control. J. Diabetes Metab. Disord. 2012, 11, 28. [Google Scholar] [CrossRef]
- Rodrigues, L.; Cravo, P.; Viveiros, M. Efflux pump inhibitors as a promising adjunct therapy against drug resistant tuberculosis: A new strategy to revisit mycobacterial targets and repurpose old drugs. Expert Rev. Anti-infective Ther. 2020, 18, 741–757. [Google Scholar] [CrossRef]
- Viveiros, M.; Martins, M.; Rodrigues, L.; Machado, D.; Couto, I.; Ainsa, J.; Amaral, L. Inhibitors of mycobacterial efflux pumps as potential boosters for anti-tubercular drugs. Expert Rev. Anti-infective Ther. 2012, 10, 983–998. [Google Scholar] [CrossRef]
- Boadu, A.A.; Yeboah-Manu, M.; Osei-Wusu, S.; Yeboah-Manu, D. Tuberculosis and diabetes mellitus: The complexity of the comorbid interactions. Int. J. Infect. Dis. 2024, 146, 107140. [Google Scholar] [CrossRef]
- Gupta, S.; Tyagi, S.; Bishaia, W.R. Verapamil increases the bactericidal activity of bedaquiline against Mycobacterium tuberculosis in a mouse model. Antimicrob. Agents Chemother. 2015, 59, 673–676. [Google Scholar] [CrossRef]
- Sutter, A.; Landis, D.; Nugent, K. The potential role for metformin in the prevention and treatment of tuberculosis. J. Thorac. Dis. 2022, 14, 1758–1765. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, B.I. Metformin: Candidate host-directed therapy for tuberculosis in diabetes and non-diabetes patients. Tuberculosis 2016, 101, S69–S72. [Google Scholar] [CrossRef] [PubMed]
- Naseri, A.; Sanaie, S.; Hamzehzadeh, S.; Seyedi-Sahebari, S.; Hosseini, M.-S.; Gholipour-Khalili, E.; Majidazar, R.; Seraji, P.; Daneshvar, S.; Rezazadeh-Gavgani, E. Metformin: New applications for an old drug. J. Basic Clin. Physiol. Pharmacol. 2023, 34, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Oglesby, W.; Kara, A.M.; Granados, H.; Cervantes, J.L. Metformin in tuberculosis: Beyond control of hyperglycemia. Infection 2019, 47, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Novita, B.D.; Pranoto, A.; Wuryani Soediono, E.I.; Mertaniasih, N.M. A case risk study of lactic acidosis risk by metformin use in type 2 diabetes mellitus tuberculosis coinfection patients. Indian J. Tuberc. 2018, 65, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Han, S.K.; Park, J.H.; Lee, J.K.; Kim, D.K.; Chung, H.S.; Heo, E.Y. The effect of metformin on culture conversion in tuberculosis patients with diabetes mellitus. Korean J. Intern. Med. 2018, 33, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Lee, C.H.; Lee, M.R.; Wang, J.Y.; Chen, S.M. Impact of metformin use among tuberculosis close contacts with diabetes mellitus in a nationwide cohort study. BMC Infect. Dis. 2019, 19, 936. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.K.H.; Kadam, A.; Karunaianantham, R.; Tamizhselvan, M.; Padmapriyadarsini, C.; Mohan, A.; Jeyadeepa, B.; Radhakrishnan, A.; Singh, U.B.; Bapat, S.; et al. Effect of Metformin on Plasma Exposure of Rifampicin, Isoniazid, and Pyrazinamide in Patients on Treatment for Pulmonary Tuberculosis. Ther. Drug Monit. 2023, 46, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-J.; Kim, T.Y.; Kim, G.; Shim, H.J.; Kang, O.K.; Kim, S.; Kim, C.-K.; Jhun, B.W.; Lee, N.Y.; Huh, H.J.; et al. Performance Evaluation of the BACTEC MGIT 960 System for Rifampin Drug-Susceptibility Testing of Mycobacterium tuberculosis Using the Current WHO Critical Concentration. J. Clin. Microbiol. 2023, 61, e0108622. [Google Scholar] [CrossRef]
- Achan, B.; Asiimwe, B.B.; Joloba, M.L.; Gumusboga, M.; Ssengooba, W.; Bwanga, F. The simple direct slide method is comparable to indirect Lowenstein Jensen proportion culture for detecting rifampicin resistant tuberculosis. J. Med Microbiol. 2021, 70, 001331. [Google Scholar] [CrossRef]
- Flandrois, J.P.; Lina, G.; Dumitrescu, O. MUBII-TB-DB: A database of mutations associated with antibiotic resistance in Mycobacterium tuberculosis. BMC Bioinform. 2014, 15, 107. [Google Scholar] [CrossRef]
- Radhakrishnan, R.K.; Thandi, R.S.; Tripathi, D.; Paidipally, P.; McAllister, M.K.; Mulik, S.; Samten, B.; Vankayalapati, R. BCG vaccination reduces the mortality of Mycobacterium tuberculosis-infected type 2 diabetes mellitus mice. JCI Insight 2020, 5, e133788. [Google Scholar] [CrossRef] [PubMed]
- Bouzeyen, R.; Javid, B. Therapeutic Vaccines for Tuberculosis: An Overview. Front. Immunol. 2022, 13, 878471. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornejo-Báez, A.A.; Zenteno-Cuevas, R.; Luna-Herrera, J. Association Between Diabetes Mellitus–Tuberculosis and the Generation of Drug Resistance. Microorganisms 2024, 12, 2649. https://doi.org/10.3390/microorganisms12122649
Cornejo-Báez AA, Zenteno-Cuevas R, Luna-Herrera J. Association Between Diabetes Mellitus–Tuberculosis and the Generation of Drug Resistance. Microorganisms. 2024; 12(12):2649. https://doi.org/10.3390/microorganisms12122649
Chicago/Turabian StyleCornejo-Báez, Axhell Aleid, Roberto Zenteno-Cuevas, and Julieta Luna-Herrera. 2024. "Association Between Diabetes Mellitus–Tuberculosis and the Generation of Drug Resistance" Microorganisms 12, no. 12: 2649. https://doi.org/10.3390/microorganisms12122649
APA StyleCornejo-Báez, A. A., Zenteno-Cuevas, R., & Luna-Herrera, J. (2024). Association Between Diabetes Mellitus–Tuberculosis and the Generation of Drug Resistance. Microorganisms, 12(12), 2649. https://doi.org/10.3390/microorganisms12122649







