Subclinical Mastitis in Small-Holder Dairy Herds of Gansu Province, Northwest China: Prevalence, Bacterial Pathogens, Antimicrobial Susceptibility, and Risk Factor Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Data Collection, and Milk Sampling
2.1.1. Study Design
2.1.2. Data Collection
2.1.3. Milk Sampling
2.2. Isolation and Identification of Bacterial Species
2.3. Antimicrobial Susceptibility Testing
2.4. Statistical Analysis
3. Results
3.1. Prevalence of SCM and Isolated Bacterial Species
3.2. Antimicrobial Susceptibility of Isolated Bacterial Species
3.3. Risk Factor Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emon, A.A.; Hossain, H.; Chowdhury, M.S.R.; Rahman, M.A.; Tanni, F.Y.; Asha, M.N.; Akter, H.; Hossain, M.M.; Islam, M.R.; Rahman, M.M. Prevalence, antimicrobial susceptibility profiles and resistant gene identification of bovine subclinical mastitis pathogens in Bangladesh. Heliyon 2024, 10, e34567. [Google Scholar] [CrossRef]
- Silva, A.C.; Laven, R.; Benites, N.R. Risk Factors Associated with Mastitis in Smallholder Dairy Farms in Southeast Brazil. Animals 2021, 11, 2089. [Google Scholar] [CrossRef] [PubMed]
- Haxhiaj, K.; Wishart, D.S.; Ametaj, B.N. Mastitis: What It Is, Current Diagnostics, and the Potential of Metabolomics to Identify New Predictive Biomarkers. Dairy 2022, 3, 722–746. [Google Scholar] [CrossRef]
- Puerto, M.A.; Shepley, E.; Cue, R.I.; Warner, D.; Dubuc, J.; Vasseur, E. The hidden cost of disease: I. Impact of the first incidence of mastitis on production and economic indicators of primiparous dairy cows. J. Dairy Sci. 2021, 104, 7932–7943. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Xu, J.; Meng, X.; Wu, Z.; Hou, X.; He, Z.; Shang, R.; Zhang, H.; Pu, W. Molecular epidemiology and characterization of antimicrobial-resistant Staphylococcus haemolyticus strains isolated from dairy cattle milk in Northwest, China. Front. Cell Infect. Microbiol. 2023, 13, 1183390. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Aqib, A.I.; Muzammil, I.; Majeed, N.; Bhutta, Z.A.; Kulyar, M.F.; Fatima, M.; Zaheer, C.F.; Muneer, A.; Murtaza, M.; et al. MRSA compendium of epidemiology, transmission, pathophysiology, treatment, and prevention within one health framework. Front. Microbiol. 2022, 13, 1067284. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.A.; Aziz, S.; Ashfaq, K.; Aqib, A.I.; Shoaib, M.; Naseer, M.A.; Alvi, M.A.; Muzammil, I.; Bhutta, Z.A.; Sattar, H. Trends in frequency, potential risks and antibiogram of E. coli isolated from semi-intensive dairy systems. Pak. Vet. J. 2022, 42, 162–172. [Google Scholar]
- Ijaz, M.; Javed, M.U.; Ahmed, A.; Rasheed, H.; Shah, S.F.A.; Ali, M. Evidence-based identification and characterization of methicillin-resistant Staphylococcus aureus isolated from subclinical mastitis in dairy buffaloes of Pakistan. Iran. J. Vet. Res. 2023, 24, 215–226. [Google Scholar] [CrossRef]
- Shoaib, M.; Tang, M.; Aqib, A.I.; Zhang, X.; Wu, Z.; Wen, Y.; Hou, X.; Xu, J.; Hao, R.; Wang, S.; et al. Dairy farm waste: A potential reservoir of diverse antibiotic resistance and virulence genes in aminoglycoside- and beta-lactam-resistant Escherichia coli in Gansu Province, China. Environ. Res. 2024, 263, 120190. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; He, Z.; Geng, X.; Tang, M.; Hao, R.; Wang, S.; Shang, R.; Wang, X.; Zhang, H.; Pu, W. The emergence of multi-drug resistant and virulence gene carrying Escherichia coli strains in the dairy environment: A rising threat to the environment, animal, and public health. Front. Microbiol. 2023, 14, 1197579. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Zhang, W.; Chen, S.; Wen, X.; Ran, X.; Wang, H.; Zhao, J.; Qi, Y.; Xue, N. Prevalence of subclinical mastitis among dairy cattle and associated risks factors in China during 2012–2021: A systematic review and meta-analysis. Res. Vet. Sci. 2022, 148, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wang, Y.; Yuan, Y.; Xie, Y. A systematic review on antibiotics misuse in livestock and aquaculture and regulation implications in China. Sci. Total Environ. 2021, 798, 149205. [Google Scholar] [CrossRef]
- Roberts, J. CPD article: The California mastitis test: What is the value? Livestock 2024, 29, 184–193. [Google Scholar] [CrossRef]
- Huang, C.-H.; Kusaba, N. Association between differential somatic cell count and California Mastitis Test results in Holstein cattle. JDS Commun. 2022, 3, 441–445. [Google Scholar] [CrossRef]
- Abed, A.H.; Menshawy, A.M.S.; Zeinhom, M.M.A.; Hossain, D.; Khalifa, E.; Wareth, G.; Awad, M.F. Subclinical Mastitis in Selected Bovine Dairy Herds in North Upper Egypt: Assessment of Prevalence, Causative Bacterial Pathogens, Antimicrobial Resistance and Virulence-Associated Genes. Microorganisms 2021, 9, 1175. [Google Scholar] [CrossRef] [PubMed]
- Abdulkhader, A.; Meganath, K.; Vignesh, S.; Rojan, P.; Muhammed, E.; Rathish, R.; Asaf, V.M.; Dinesh, C. Comparison of California mastitis test and somatic cell counts for detection of subclinical mastitis in crossbred cattle. J. Vet.-Anim. Sci. 2022, 53, 725–730. [Google Scholar] [CrossRef]
- Zhang, M.L.; Shen, Q.Y. Review and prospect of Gansu dairy industry in 2015. Chin. Cattle Sci. 2016, 42, 43–47. [Google Scholar] [CrossRef]
- Kumar, R.; Thakur, A.; Sharma, A. Comparative prevalence assessment of subclinical mastitis in two crossbred dairy cow herds using the California mastitis test. J. Dairy Vet. Anim. Res. 2023, 12, 98–102. [Google Scholar] [CrossRef]
- Adkins, P.; Fox, L.; Godden, S.; Jayarao, B.M.; Keefe, G.; Kelton, D.; Lago, A.; Middleton, J.; Owens, W.; Wolfe, C.P.; et al. Laboratory Handbook of Bovine Mastitis, 3rd ed.; National Mastitis Council: Madison, WI, USA, 2017. [Google Scholar]
- Verbeke, J.; Piepers, S.; Supré, K.; De Vliegher, S. Pathogen-specific incidence rate of clinical mastitis in Flemish dairy herds, severity, and association with herd hygiene. J. Dairy Sci. 2014, 97, 6926–6934. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, M100, 31st ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- EUCAST. The European Committee on Antimicrobial Susceptibility Testing; EUCAST: Copenhagen, Denmark, 2018. [Google Scholar]
- Ren, Q.; Liao, G.; Wu, Z.; Lv, J.; Chen, W. Prevalence and characterization of Staphylococcus aureus isolates from subclinical bovine mastitis in southern Xinjiang, China. J. Dairy Sci. 2020, 103, 3368–3380. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, Y.; Bai, R.; Pei, X.; Xu, H.; Zhu, K.; Wu, C. Antimicrobial resistance profiles of common mastitis pathogens on large Chinese dairy farms. JDS Commun. 2024, 5, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, P.; Goudar, A.L.; Suresh, K.P.; Roy, P. Global and countrywide prevalence of subclinical and clinical mastitis in dairy cattle and buffaloes by systematic review and meta-analysis. Res. Vet. Sci. 2021, 136, 561–586. [Google Scholar] [CrossRef] [PubMed]
- Bari, M.S.; Rahman, M.M.; Persson, Y.; Derks, M.; Sayeed, M.A.; Hossain, D.; Singha, S.; Hoque, M.A.; Sivaraman, S.; Fernando, P.; et al. Subclinical mastitis in dairy cows in south-Asian countries: A review of risk factors and etiology to prioritize control measures. Vet. Res. Commun. 2022, 46, 621–640. [Google Scholar] [CrossRef]
- Song, X.; Huang, X.; Xu, H.; Zhang, C.; Chen, S.; Liu, F.; Guan, S.; Zhang, S.; Zhu, K.; Wu, C. The prevalence of pathogens causing bovine mastitis and their associated risk factors in 15 large dairy farms in China: An observational study. Vet. Microbiol. 2020, 247, 108757. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Huang, X.; Yang, H.; He, Y.; He, X.; Huang, J.; Li, S.; Wang, X.; Tang, S.; Liu, G.; et al. Molecular epidemiology, antimicrobial activity, and virulence gene clustering of Streptococcus agalactiae isolated from dairy cattle with mastitis in China. J. Dairy Sci. 2021, 104, 4893–4903. [Google Scholar] [CrossRef]
- Demil, E.; Teshome, L.; Kerie, Y.; Habtamu, A.; Kumilachew, W.; Andualem, T.; Mekonnen, S.A. Prevalence of subclinical mastitis, associated risk factors and antimicrobial susceptibility of the pathogens isolated from milk samples of dairy cows in Northwest Ethiopia. Prev. Vet. Med. 2022, 205, 105680. [Google Scholar] [CrossRef]
- Jensen, V.F.; Damborg, P.; Norström, M.; Nonnemann, B.; Slettemeås, J.S.; Smistad, M.; Sølverød, L.; Turnidge, J.; Urdahl, A.M.; Veldman, K.; et al. Estimation of epidemiological cut-off values for eight antibiotics used for treatment of bovine mastitis caused by Streptococcus uberis and Streptococcus dysgalactiae subsp. dysgalactiae. Vet. Microbiol. 2024, 290, 109994. [Google Scholar] [CrossRef]
- Aarestrup, F.M.; Jensen, N.E. Genotypic and phenotypic diversity of Streptococcus dysgalactiae strains isolated from clinical and subclinical cases of bovine mastitis. Vet. Microbiol. 1996, 53, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Das Mitra, S.; Bandopadhyay, S.; Jadhao, S.; Shome, R.; Shome, B.R. Genetic characterization and comparative genomics of a multi drug resistant (MDR) Escherichia coli SCM-21 isolated from a subclinical case of bovine mastitis. Comp. Immunol. Microbiol. Infect. Dis. 2022, 85, 101799. [Google Scholar] [CrossRef]
- Mohammed, A.N.; Radi, A.M.; Khaled, R.; Abo El-Ela, F.I.; Kotp, A.A. Exploitation of new approach to control of environmental pathogenic bacteria causing bovine clinical mastitis using novel anti-biofilm nanocomposite. Environ. Sci. Pollut. Res. 2020, 27, 42791–42805. [Google Scholar] [CrossRef]
- Paramasivam, R.; Gopal, D.R.; Dhandapani, R.; Subbarayalu, R.; Elangovan, M.P.; Prabhu, B.; Veerappan, V.; Nandheeswaran, A.; Paramasivam, S.; Muthupandian, S. Is AMR in Dairy Products a Threat to Human Health? An Updated Review on the Origin, Prevention, Treatment, and Economic Impacts of Subclinical Mastitis. Infect. Drug Resist. 2023, 16, 155–178. [Google Scholar] [CrossRef]
- Sweeney, M.T.; Gunnett, L.; Kumar, D.M.; Lunt, B.L.; Moulin, V.; Barrett, M.; Gurjar, A.; Doré, E.; Pedraza, J.R.; Bade, D.; et al. Antimicrobial susceptibility of mastitis pathogens isolated from North American dairy cattle, 2011–2022. Vet. Microbiol. 2024, 291, 110015. [Google Scholar] [CrossRef] [PubMed]
- Bag, M.A.S.; Khan, M.S.R.; Sami, M.D.H.; Begum, F.; Islam, M.S.; Rahman, M.M.; Rahman, M.T.; Hassan, J. Virulence determinants and antimicrobial resistance of E. coli isolated from bovine clinical mastitis in some selected dairy farms of Bangladesh. Saudi J. Biol. Sci. 2021, 28, 6317–6323. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.C.; Ribeiro-Almeida, M.; Ribeiro, J.; Silveira, L.; Prata, J.C.; Pista, A.; Martins da Costa, P. Occurrence of multidrug-resistant bacteria resulting from the selective pressure of antibiotics: A comprehensive analysis of ESBL K. pneumoniae and MRSP isolated in a dog with rhinorrhea. Vet. Sci. 2023, 10, 326. [Google Scholar] [CrossRef]
- Shoaib, M.; Tang, M.; Awan, F.; Aqib, A.I.; Hao, R.; Ahmad, S.; Wang, S.; Shang, R.; Pu, W. Genomic Characterization of Extended-Spectrum β-Lactamase (ESBL) Producing E. coli Harboring blaOXA−1-catB3-arr-3 Genes Isolated from Dairy Farm Environment in China. Transbound. Emerg. Dis. 2024, 2024, 3526395. [Google Scholar] [CrossRef]
- Shoaib, M.; Aqib, A.I.; Ali, M.M.; Ijaz, M.; Sattar, H.; Ghaffar, A.; Sajid Hasni, M.; Bhutta, Z.A.; Ashfaq, K.; Kulyar, M.F.-e.-A. Tracking infection and genetic divergence of methicillin-resistant Staphylococcus aureus at pets, pet owners, and environment interface. Front. Vet. Sci. 2022, 9, 900480. [Google Scholar] [CrossRef]
- Ruegg, P.L. What is success? A narrative review of research evaluating outcomes of antibiotics used for treatment of clinical mastitis. Front. Vet. Sci. 2021, 8, 639641. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.F.; Stephen, J.; Lekshmi, M.; Ojha, M.; Wenzel, N.; Sanford, L.M.; Hernandez, A.J.; Parvathi, A.; Kumar, S.H. Bacterial resistance to antimicrobial agents. Antibiotics 2021, 10, 593. [Google Scholar] [CrossRef]
- Kamel, M.S.; Bakry, N.M. Clinical and subclinical mastitis. In The Microbiology, Pathogenesis and Zoonosis of Milk Borne Diseases; Elsevier: Amsterdam, The Netherlands, 2024; pp. 153–190. [Google Scholar]
- Kovačević, Z.; Samardžija, M.; Horvat, O.; Tomanić, D.; Radinović, M.; Bijelić, K.; Vukomanović, A.G.; Kladar, N. Is there a relationship between antimicrobial use and antibiotic resistance of the most common mastitis pathogens in dairy cows? Antibiotics 2022, 12, 3. [Google Scholar] [CrossRef]
- Schunig, R.; Busanello, M.; Nogara, K.F.; Zopollatto, M. Cow-level risk factors associated with the increase in somatic cell count and the occurrence of subclinical mastitis in Brazilian Holstein and Jersey dairy cows. Prev. Vet. Med. 2024, 227, 106208. [Google Scholar] [CrossRef] [PubMed]
- Hiitiö, H.; Vakkamäki, J.; Simojoki, H.; Autio, T.; Junnila, J.; Pelkonen, S.; Pyörälä, S. Prevalence of subclinical mastitis in Finnish dairy cows: Changes during recent decades and impact of cow and herd factors. Acta Vet. Scand. 2017, 59, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fesseha, H.; Mathewos, M.; Aliye, S.; Wolde, A. Study on prevalence of bovine mastitis and associated risk factors in dairy farms of Modjo town and suburbs, central Oromia, Ethiopia. Vet. Med. Res. Rep. 2021, 12, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, H.; Zhai, J.; Wang, H.; Chen, X.; Qi, Y. Prevalence of clinical mastitis and its associated risk factors among dairy cattle in mainland China during 1982–2022: A systematic review and meta-analysis. Front. Vet. Sci. 2023, 10, 1185995. [Google Scholar] [CrossRef] [PubMed]
- Singha, S.; Koop, G.; Persson, Y.; Hossain, D.; Scanlon, L.; Derks, M.; Hoque, M.A.; Rahman, M.M. Incidence, Etiology, and Risk Factors of Clinical Mastitis in Dairy Cows under Semi-Tropical Circumstances in Chattogram, Bangladesh. Animals 2021, 11, 2255. [Google Scholar] [CrossRef] [PubMed]
- Kok, A.; Tsousis, G.; Niozas, G.; Kemp, B.; Kaske, M.; van Knegsel, A.T.M. Short communication: Variance and autocorrelation of deviations in daily milk yield are related with clinical mastitis in dairy cows. Animal 2021, 15, 100363. [Google Scholar] [CrossRef] [PubMed]
- Palii, A.; Kovalchuk, Y.; Boyko, Y.; Bondaruk, Y.; Diachuk, P.; Duka, T.; Pidlypniak, I.; Kalabska, V.; Kovalenko, A.; Tarasuk, L. Impact of various milking equipment on incidence of mastitis in dairy herd. Ukr. J. Ecol. 2020, 10, 160–165. [Google Scholar] [CrossRef] [PubMed]
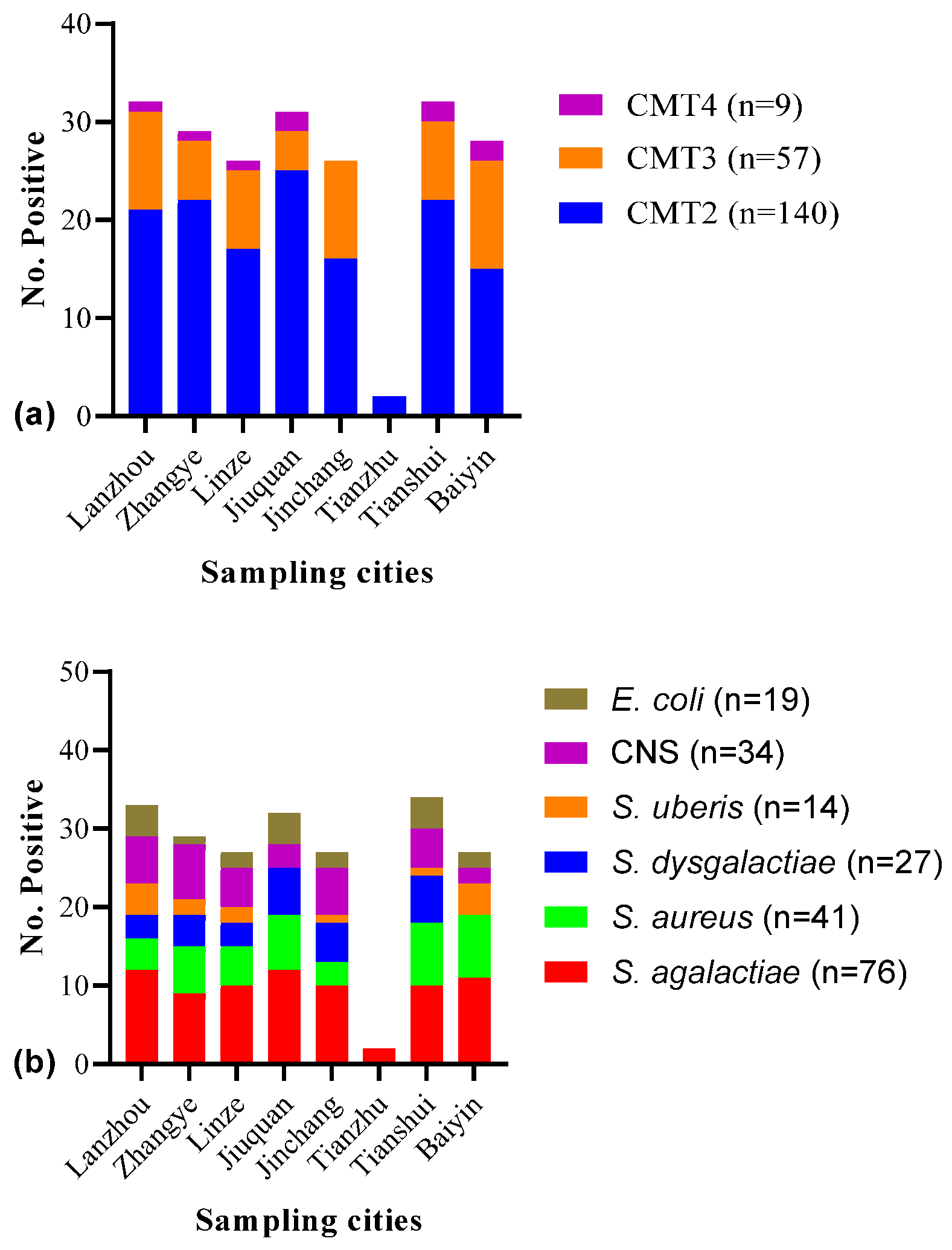

| Sampling Areas | No. of Samples | No. of SCM-Positive Samples | % Age of SCM-Positive Samples | C.I. (95%) | p-Value |
|---|---|---|---|---|---|
| Lanzhou | 80 | 32 | 40.0 a | 29.39–51.58 | 0.936 |
| Zhangye | 83 | 29 | 34.94 a | 25.02–46.27 | |
| Linze | 68 | 26 | 38.24 a | 26.96–50.86 | |
| Jiuquan | 80 | 31 | 38.75 a | 28.26–50.33 | |
| Jinchang | 71 | 26 | 36.62 a | 25.75–48.95 | |
| Tianzhu | 08 | 02 | 25.0 a | 4.450–64.42 | |
| Tianshui | 76 | 32 | 42.11 a | 31.05–53.97 | |
| Baiyin | 64 | 28 | 43.75 a | 31.58–56.67 | |
| Total | 530 | 206 | 38.87 a | 34.72–43.18 |
| Antimicrobial Agent/Disk Content (µg or U) | Bacterial Species | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. agalactiae (n = 76) | S. aureus (n = 41) | S. dysgalactiae (n = 27) | S. uberis (n = 14) | CNS (n = 34) | E. coli (n = 19) | |||||||||||||
| R, No. (%) | I, No. (%) | S, No. (%) | R, No. (%) | I, No. (%) | S, No. (%) | R, No. (%) | I, No. (%) | S, No. (%) | R, No. (%) | I, No. (%) | S, No. (%) | R, No. (%) | I, No. (%) | S, No. (%) | R, No. (%) | I, No. (%) | S, No. (%) | |
| Penicillin (10 U) | 76 (100.00) | 0 (0.0) | 0 (0.0) | 36 (87.80) | 0 (0.0) | 5 (12.20) | 27 (100.00) | 0 (0.0) | 0 (0.0) | 14 (100.00) | 0 (0.0) | 0 (0.0) | 32 (94.12) | 2 (5.88) | 0 (0.0) | 19 (100.00) | 0 (0.0) | 0 (0.0) |
| Ampicillin (10 µg) | 0 (0.0) | 0 (0.0) | 76 (100.00) | 2 (4.88) | 9 (21.95) | 30 (73.17) | 4 (14.81) | 0 (0.0) | 23 (85.19) | 0 (0.0) | 0 (0.0) | 14 (100.00) | 0 (0.0) | 4 (11.76) | 30 (88.24) | 0 (0.0) | 4 (21.05) | 15 (78.95) |
| Amoxicillin– Sulbactam (10 µg) | 0 (0.0) | 8 (10.53) | 68 (89.47) | 0 (0.0) | 5 (12.2) | 36 (87.80) | 0 (0.0) | 0 (0.0) | 27 (100.00) | 0 (0.0) | 0 (0.0) | 14 (100.00) | 0 (0.0) | 0 (0.0) | 34 (100.00) | 0 (0.0) | 5 (26.32) | 14 (73.68) |
| Ceftazidime (30 µg) | 0 (0.0) | 0 (0.0) | 76 (100.00) | 0 (0.0) | 4 (9.76) | 37 (90.24) | 0 (0.0) | 3 (11.11) | 24 (88.89) | 0 (0.0) | 2 (14.29) | 12 (85.71) | 0 (0.0) | 0 (0.0) | 34 (100.00) | 0 (0.0) | 1 (5.26) | 18 (94.74) |
| Streptomycin (10 µg) | 76 (100.00) | 0 (0.0) | 0 (0.0) | 41 (100.00) | 0 (0.0) | 0 (0.0) | 27 (100.00) | 0 (0.0) | 0 (0.0) | 14 (100.00) | 0 (0.0) | 0 (0.0) | 34 (100.00) | 0 (0.0) | 0 (0.0) | 17 (89.47) | 0 (0.0) | 2 (10.53) |
| Neomycin (30 µg) | 0 (0.0) | 0 (0.0) | 76 (100.00) | 0 (0.0) | 2 (4.88) | 39 (95.12) | 0 (0.0) | 0 (0.0) | 27 (100.00) | 0 (0.0) | 0 (0.0) | 14 (100.00) | 0 (0.0) | 3 (8.82) | 31 (91.18) | 0 (0.0) | 2 (10.53) | 17 (89.47) |
| Kanamycin (30 µg) | 0 (0.0) | 0 (0.0) | 76 (100.00) | 3 (7.32) | 5 (12.2) | 33 (80.49) | 0 (0.0) | 0 (0.0) | 27 (100.00) | 0 (0.0) | 0 (0.0) | 14 (100.00) | 0 (0.0) | 4 (11.76) | 30 (88.24) | 4 (21.05) | 2 (10.53) | 13 (68.42) |
| Gentamicin (10 µg) | 70 (92.11) | 6 (7.89) | 0 (0.0) | 7 (17.07) | 0 (0.0) | 34 (82.93) | 21 (77.78) | 3 (11.11) | 3 (11.11) | 12 (85.71) | 0 (0.0) | 2 (14.29) | 0 (0.0) | 0 (0.0) | 34 (100.00) | 11 (57.89) | 0 (0.0) | 8 (42.11) |
| Spectinomycin (100 µg) | 0 (0.0) | 0 (0.0) | 76 (100.00) | 0 (0.0) | 3 (7.32) | 38 (92.68) | 0 (0.0) | 0 (0.0) | 27 (100.00) | 0 (0.0) | 0 (0.0) | 14 (100.00) | 0 (0.0) | 0 (0.0) | 34 (100.00) | 0 (0.0) | 2 (10.53) | 17 (89.47) |
| SXT (25 µg) | 76 (100.00) | 0 (0.0) | 0 (0.0) | 41 (100.00) | 0 (0.0) | 0 (0.0) | 27 (100.00) | 0 (0.0) | 0 (0.0) | 14 (100.00) | 0 (0.0) | 0 (0.0) | 30 (88.24) | 0 (0.0) | 4 (11.76) | 19 (100.00) | 0 (0.0) | 0 (0.0) |
| Norfloxacin (10 µg) | 0 (0.0) | 7 (9.22) | 69 (90.78) | 0 (0.0) | 3 (7.32) | 38 (92.68) | 0 (0.0) | 3 (11.11) | 24 (88.89) | 0 (0.0) | 3 (21.43) | 11 (78.57) | 0 (0.0) | 0 (0.0) | 34 (100.00) | 0 (0.0) | 0 (0.0) | 19 (100.00) |
| Ciprofloxacin (5 µg) | 0 (0.0) | 0 (0.0) | 76 (100.00) | 0 (0.0) | 2 (4.88) | 39 (95.12) | 0 (0.0) | 0 (0.0) | 27 (100.00) | 0 (0.0) | 0 (0.0) | 14 (100.00) | 0 (0.0) | 0 (0.0) | 34 (100.00) | 0 (0.0) | 0 (0.0) | 19 (100.00) |
| Vancomycin (30 µg) | 71 (93.42) | 5 (6.58) | 0 (0.0) | 35 (85.37) | 0 (0.0) | 6 (14.63) | 27 (100.00) | 0 (0.0) | 0 (0.0) | 13 (92.86) | 0 (0.0) | 1 (7.14) | 31 (91.18) | 3 (8.82) | 0 (0.0) | 19 (100.00) | 0 (0.0) | 0 (0.0) |
| Tetracycline (30 µg) | 76 (100.00) | 0 (0.0) | 0 (0.0) | 2 (4.88) | 6 (19.52) | 33 (80.48) | 27 (100.00) | 0 (0.0) | 0 (0.0) | 14 (100.00) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 34 (100.00) | 0 (0.0) | 3 (15.79) | 16 (84.21) |
| Doxycycline (30 µg) | 0 (0.0) | 8 (10.53) | 68 (89.47) | 0 (0.0) | 4 (9.76) | 37 (90.24) | 0 (0.0) | 0 (0.0) | 27 (100.00) | 0 (0.0) | 0 (0.0) | 14 (100.00) | 0 (0.0) | 0 (0.0) | 34 (100.00) | 0 (0.0) | 1 (5.26) | 18 (94.74) |
| Erythromycin (15 µg) | 76 (100.00) | 0 (0.0) | 0 (0.0) | 35 (85.37) | 2 (4.88) | 4 (9.76) | 27 (100.00) | 0 (0.0) | 0 (0.0) | 14 (100.00) | 0 (0.0) | 0 (0.0) | 29 (85.29) | 0 (0.0) | 5 (14.71) | 17 (89.47) | 0 (0.0) | 2 (10.53) |
| Variables | Total | CMT | Binary Logistic Regression Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive (n = 206), No. (%) | Negative (n = 324) No. (%) | B | S.E. | Wald | df | p-Value | O.R. | 95% C.I. | ||
| Age (years) | ||||||||||
| 2–5 | 146 | 32 (15.5) | 114 (35.2) | 1 | ||||||
| 6–8 | 340 | 148 (71.9) | 192 (59.3) | 0.628 | 0.326 | 3.721 | 1 | 0.054 | 1.871 | 0.990–3.547 |
| ≥9 | 44 | 26 (12.6) | 18(5.5) | 1.638 | 0.366 | 20.02 | 1 | 0.000 | 5.146 | 2.511–10.546 |
| Parity | ||||||||||
| 1–2 | 97 | 18 (8.7) | 79 (24.4) | 1 | ||||||
| 3–4 | 177 | 67 (32.5) | 110 (34.0) | 0.644 | 0.424 | 2.306 | 1 | 0.129 | 1.905 | 0.829–4.375 |
| 5–6 | 230 | 105 (51.0) | 125 (38.6) | 0.966 | 0.432 | 5.001 | 1 | 0.025 | 2.627 | 1.127–6.124 |
| ≥7 | 26 | 16 (7.8) | 10 (3.0) | 1.949 | 0.480 | 16.46 | 1 | 0.000 | 7.022 | 2.739–18.002 |
| Lactation months | ||||||||||
| 1–3 | 168 | 55 (26.7) | 113 (34.9) | 1 | ||||||
| 4–6 | 217 | 83 (40.3) | 134 (41.3) | 0.355 | 0.217 | 2.665 | 1 | 0.103 | 1.426 | 0.931–2.183 |
| 7–9 | 145 | 68 (33.0) | 77 (23.8) | 0.596 | 0.234 | 6.486 | 1 | 0.011 | 1.814 | 1.147–2.870 |
| Teat lesion | ||||||||||
| Absent | 482 | 175 (85.0) | 307 (94.8) | 1 | ||||||
| Present | 48 | 31 (15.0) | 17 (5.2) | 1.163 | 0.316 | 13.51 | 1 | 0.000 | 3.199 | 1.721–5.946 |
| CM history | ||||||||||
| No | 497 | 183 (88.8) | 314 (96.9) | 1 | ||||||
| Yes | 33 | 23 (11.2) | 10 (3.1) | 1.373 | 0.390 | 12.38 | 1 | 0.000 | 3.946 | 1.837–8.476 |
| Milk yield (kg/d) | ||||||||||
| <25.0 | 229 | 105 (51.0) | 124 (38.3) | 1 | ||||||
| ≥25.0 | 301 | 101 (49.0) | 200 (61.7) | 0.517 | 0.180 | 8.223 | 1 | 0.004 | 1.677 | 1.178–2.387 |
| Milking training | ||||||||||
| Yes | 436 | 177 (85.9) | 259 (79.9) | 1 | ||||||
| No | 94 | 29 (14.1) | 65 (20.1) | 0.426 | 0.244 | 3.062 | 1 | 0.080 | 1.532 | 0.950–2.469 |
| Udder cleanliness | ||||||||||
| Yes | 395 | 144 (69.9) | 251 (77.5) | 1 | ||||||
| No | 115 | 62 (30.1) | 73 (22.5) | 0.392 | 0.202 | 3.776 | 1 | 0.052 | 1.480 | 0.997–2.199 |
| Risk Factors | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| B | S.E. | Wald | df | p-Value | O.R. | 95% C.I. | |
| Age (years) | |||||||
| 2–5 | 1 | ||||||
| 6–8 | 1.010 | 0.228 | 19.629 | 1 | 0.000 | 2.746 | 1.756–4.293 |
| ≥9 | 1.638 | 0.366 | 20.021 | 1 | 0.000 | 5.146 | 2.511–10.546 |
| Parity | |||||||
| 1–2 | 1 | ||||||
| 3–4 | 0.983 | 0.304 | 10.483 | 1 | 0.001 | 2.673 | 1.474–4.848 |
| 5–6 | 1.305 | 0.293 | 19.855 | 1 | 0.000 | 3.687 | 2.077–6.544 |
| ≥7 | 1.949 | 0.480 | 16.466 | 1 | 0.000 | 7.022 | 2.739–18.002 |
| Lactation months | |||||||
| 1–3 | 1 | ||||||
| 4–6 | 0.241 | 0.216 | 1.248 | 1 | 0.264 | 1.273 | 0.834–1.942 |
| 7–9 | 0.596 | 0.234 | 6.486 | 1 | 0.011 | 1.814 | 1.147–2.870 |
| Teat lesion | |||||||
| Absent | 1 | ||||||
| Present | 1.163 | 0.316 | 13.515 | 1 | 0.000 | 3.199 | 1.721–5.946 |
| CM history | |||||||
| No | 1 | ||||||
| Yes | 1.373 | 0.390 | 12.388 | 1 | 0.000 | 3.946 | 1.837–8.476 |
| Milk yield (kg/d) | |||||||
| <25.0 | Reference | ||||||
| ≥25.0 | −0.517 | 0.180 | 8.223 | 1 | 0.004 | 0.596 | 0.419–0.849 |
| Milking training | |||||||
| Yes | 1 | ||||||
| No | −0.426 | 0.244 | 3.062 | 1 | 0.000 | 0.653 | 0.405–1.053 |
| Udder cleanliness | |||||||
| Yes | 1 | ||||||
| No | 0.392 | 0.202 | 3.776 | 1 | 0.052 | 1.480 | 0.997–2.199 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Haq, S.U.; Shoaib, M.; He, J.; Guo, W.; Wei, X.; Zheng, X. Subclinical Mastitis in Small-Holder Dairy Herds of Gansu Province, Northwest China: Prevalence, Bacterial Pathogens, Antimicrobial Susceptibility, and Risk Factor Analysis. Microorganisms 2024, 12, 2643. https://doi.org/10.3390/microorganisms12122643
Wang L, Haq SU, Shoaib M, He J, Guo W, Wei X, Zheng X. Subclinical Mastitis in Small-Holder Dairy Herds of Gansu Province, Northwest China: Prevalence, Bacterial Pathogens, Antimicrobial Susceptibility, and Risk Factor Analysis. Microorganisms. 2024; 12(12):2643. https://doi.org/10.3390/microorganisms12122643
Chicago/Turabian StyleWang, Ling, Shahbaz Ul Haq, Muhammad Shoaib, Jiongjie He, Wenzhu Guo, Xiaojuan Wei, and Xiaohong Zheng. 2024. "Subclinical Mastitis in Small-Holder Dairy Herds of Gansu Province, Northwest China: Prevalence, Bacterial Pathogens, Antimicrobial Susceptibility, and Risk Factor Analysis" Microorganisms 12, no. 12: 2643. https://doi.org/10.3390/microorganisms12122643
APA StyleWang, L., Haq, S. U., Shoaib, M., He, J., Guo, W., Wei, X., & Zheng, X. (2024). Subclinical Mastitis in Small-Holder Dairy Herds of Gansu Province, Northwest China: Prevalence, Bacterial Pathogens, Antimicrobial Susceptibility, and Risk Factor Analysis. Microorganisms, 12(12), 2643. https://doi.org/10.3390/microorganisms12122643






