Exploring Metal Ions as Potential Antimicrobial Agents to Combat Future Drug Resistance in Mycoplasma bovis
Abstract
1. Introduction
2. Materials and Methods
2.1. Mycoplasma Isolates (Identification and Culture)
2.2. Antimicrobial Susceptibility Testing
Interpretation of the MIC Testing
2.3. Metal Solvent Toxicity Testing
2.4. Time–Kill Kinetics Assay of Metal Ions
2.5. Statistical Analysis
3. Results and Discussion
3.1. Mycoplasma Isolates Selection
| Tested Agents | Quality Control Strain M. bovis (PG45) | Acceptable Range of MICs Tested by Others * |
|---|---|---|
| Phenicol (Florphenicol) | 2.00 | 1.00–32.00 |
| Tetracyclines (chlor- and oxy-tetracycline) | 0.25 | ≤0.12–32.00 |
| Macrolide–tylosin Macrolide–tulathromycin | 1.00 0.50 | 0.06–128.00 ND |
| Cobalt | 3.12 | 0.78–12.50 |
| Copper | 1.56 | 0.19–12.50 |
| Silver | 6.25 | 0.19–12.50 |
| Zinc | 1.56 | 1.56–12.50 |
| Colloidal silver * | 1.56 | 0.78–1.56 |
| Tested Antimicrobials | Percentage of Mycoplasma bovis Isolates Showing MIC Values at Tested Antimicrobial Concentrations (mg/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | |
| Florphenicol | 0 | 6.25 | 6.25 | 18.75 | 25 | 43.75 | 0 | 0 | 0 | 0 |
| Tetracyclines | 6.25 | 6.25 | 6.25 | 18.75 | 0 | 31.25 | 25 | 6.25 | 0 | 0 |
| Tulathromycin | 0 | 6.25 | 12.5 | 25 | 25 | 31.25 | 0 | 0 | 0 | 0 |
| Tylosin | 0 | 0 | 6.25 | 0 | 37.5 | 31.25 | 25 | 0 | 0 | 0 |
3.2. In Vitro Susceptibility Testing
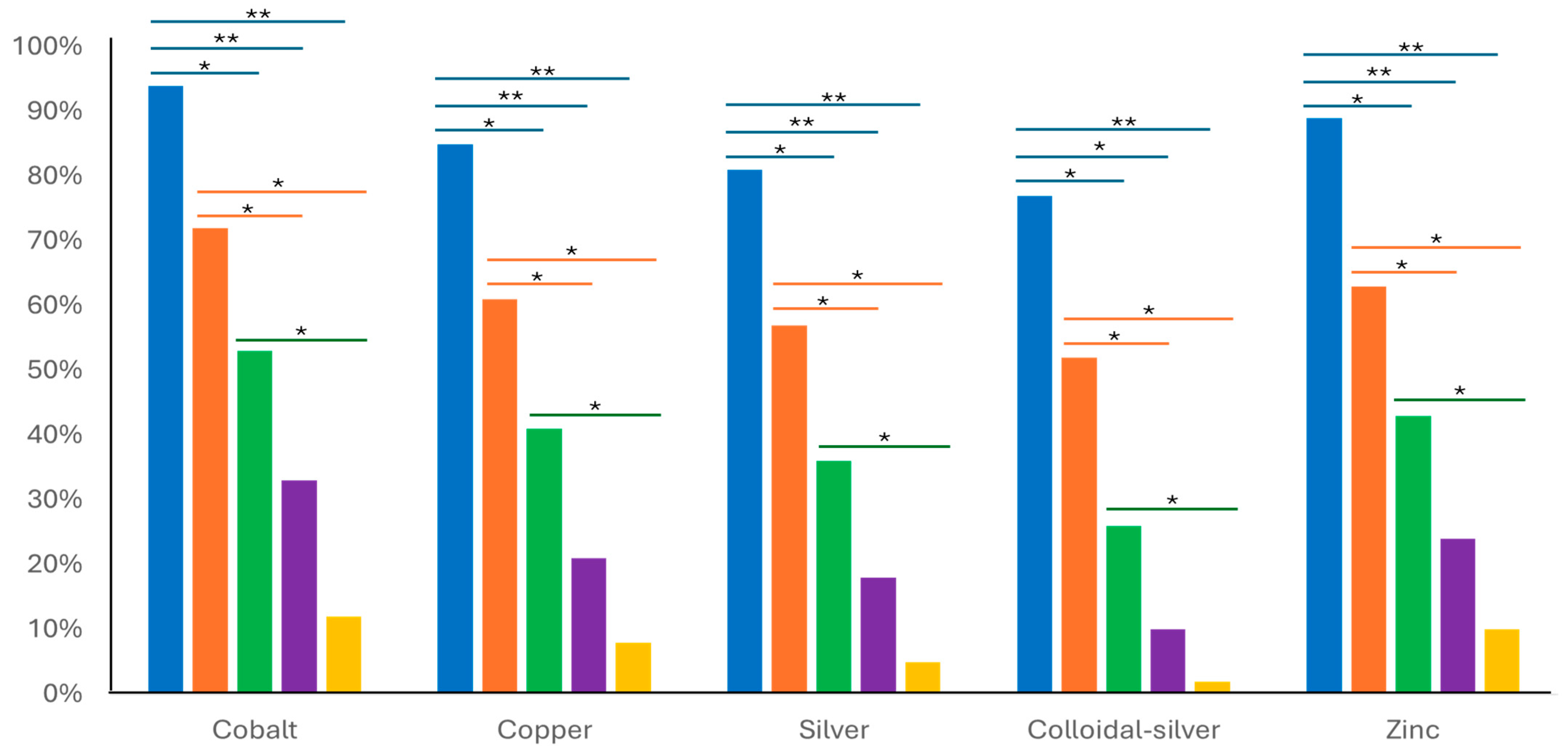
| Tested Metal Agent | Percentage of Mycoplasma bovis Isolates Showing MIC Values at the Tested Metal Ion Concentrations (mg/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.3 | 0.7 | 1.5 | 3.1 | 6.2 | 12.5 | 25 | 50 | 100 | |
| Cobalt | 0 | 0 | 12.5 | 0 | 6.25 | 56.25 | 25 | 0 | 0 | 0 |
| Copper | 6.25 | 0 | 0 | 37.5 | 18.75 | 31.25 | 6.25 | 0 | 0 | 0 |
| Silver | 6.25 | 0 | 0 | 0 | 6.25 | 43.75 | 43.75 | 0 | 0 | 0 |
| Colloidal silver | 0 | 0 | 18.75 | 81.25 | 0 | 0 | 0 | 0 | 0 | 0 |
| Zinc | 0 | 0 | 0 | 12.5 | 25 | 31.25 | 31.25 | 0 | 0 | 0 |
3.3. Time–Kill Kinetic Assay of Metal Ions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lysnyansky, I.; Ayling, R.D. Mycoplasma bovis: Mechanisms of Resistance and Trends in Antimicrobial Susceptibility. Front. Microbiol. 2016, 7, 595. [Google Scholar] [CrossRef] [PubMed]
- Rottem, S. Interaction of mycoplasmas with host cells. Physiol. Rev. 2003, 83, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Badger, S.M.; Sullivan, K.F.; Jordan, D.; Caraguel, C.G.B.; Page, S.W.; Cusack, P.M.V.; Frith, D.; Trott, D.J. Antimicrobial use and stewardship practices on Australian beef feedlots. Aust. Vet. J. 2020, 98, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Sulyok, K.M.; Kreizinger, Z.; Fekete, L.; Hrivnák, V.; Magyar, T.; Jánosi, S.; Schweitzer, N.; Turcsányi, I.; Makrai, L.; Erdélyi, K.; et al. Antibiotic susceptibility profiles of Mycoplasma bovis strains isolated from cattle in Hungary, Central Europe. BMC Vet. Res. 2014, 10, 256. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Hasoon, M.F.; Jarocki, V.M.; Mohammed, M.H.; Djordjevic, S.P.; Yip, H.Y.E.; Carr, M.; Khabiri, A.; Azari, A.A.; Amanollahi, R.; Jozani, R.J.; et al. Antimicrobial Susceptibility and Molecular Characteristics of Mycoplasma bovis Isolated from Cases of Bovine Respiratory Disease in Australian Feedlot Cattle. Vet. Microbiol. 2023, 283, 109779. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Taha-Abdelaziz, K.; Hawwas, H.A.E.-H.; Ghosh, S.; AlKafaas, S.S.; Moawad, M.M.M.; Saied, E.M.; Kassem, I.I.; Mawad, A.M.M. Antimicrobial Resistance and Recent Alternatives to Antibiotics for the Control of Bacterial Pathogens with an Emphasis on Foodborne Pathogens. Antibiotics 2023, 12, 274. [Google Scholar] [CrossRef]
- Frei, A.; Verderosa, A.D.; Elliott, A.G.; Zuegg, J.; Blaskovich, M.A.T. Metals to Combat Antimicrobial Resistance. Nat. Rev. Chem. 2023, 7, 202–224. [Google Scholar] [CrossRef]
- Raja, F.N.S.; Worthington, T.; Martin, R.A. The Antimicrobial Efficacy of Copper, Cobalt, Zinc, and Silver Nanoparticles: Alone and in Combination. Biomed. Mater. 2023, 18, 045003. [Google Scholar] [CrossRef]
- Cervantes-Cervantes, M.P.; Calderón-Salinas, J.V.; Albores, A.; Muñoz-Sánchez, J.L. Copper Increases the Damage to DNA and Proteins Caused by Reactive Oxygen Species. Biol. Trace Elem. Res. 2005, 103, 229–248. [Google Scholar] [CrossRef]
- Hood, M.I.; Skaar, E.P. Nutritional Immunity: Transition Metals at the Pathogen–Host Interface. Nat. Rev. Microbiol. 2012, 10, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.; Rensing, C.; Solioz, M. Metallic Copper as an Antimicrobial Surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhao, J.; Zeng, Z.; Wan, D.; Yu, P.; Cheng, D.; Gong, D.; Deng, S. Antibacterial Activity and Membrane-Disrupting Mechanism of Monocaprin against Escherichia coli and Its Application in Apple and Carrot Juices. LWT 2020, 131, 109794. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, A. Copper-instigated modulatory cell mortality mechanisms and progress in oncological treatment investigations. Front. Immunol. 2023, 14, 1236063. [Google Scholar] [CrossRef]
- Eckhardt, S.; Brunetto, P.S.; Gagnon, J.; Priebe, M.; Giese, B.; Fromm, K.M. Nanobio Silver: Its Interactions with Peptides and Bacteria, and Its Uses in Medicine. Chem. Rev. 2013, 113, 4708–4754. [Google Scholar] [CrossRef]
- Sim, W.; Barnard, R.T.; Blaskovich, M.A.T.; Ziora, Z.M. Antimicrobial Silver in Medicinal and Consumer Applications: A Patent Review of the Past Decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef]
- Stanford, W.; Rappole, B.W.; Fox, C.L., Jr. Clinical Experience with Silver Sulfadiazine, a New Topical Agent for Control of Pseudomonas Infections in Burns. J. Trauma 1969, 9, 377–388. [Google Scholar] [CrossRef]
- Xiu, Z.M.; Zhang, Q.B.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J. Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef]
- Chen, M.; Yang, Z.; Wu, H.; Pan, X.; Xie, X.; Wu, C. Antimicrobial Activity and the Mechanism of Silver Nanoparticle Thermosensitive Gel. Int. J. Nanomed. 2011, 6, 2873–2877. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial Activity and Mechanism of Action of the Silver Ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef]
- More, P.R.; Pandit, S.; De Filippis, A.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach Against Drug-Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Cuajungco, M.P.; Ramirez, M.S.; Tolmasky, M.E. Zinc: Multidimensional effects on living organisms. Biomedicines 2021, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- González-Montaña, J.R.; Escalera-Valente, F.; Alonso, A.J.; Lomillos, J.M.; Robles, R.; Alonso, M.E. Relationship between vitamin B12 and cobalt metabolism in domestic ruminants: An update. Animals 2020, 10, 1855. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.; Kavanagh, K.A. Evaluation of metal-based antimicrobial compounds for the treatment of bacterial pathogens. J. Med. Microbiol. 2021, 70, 001363. [Google Scholar] [CrossRef]
- Lansdown, A.B. Silver in health care: Antimicrobial effects and safety in use. Curr. Probl. Dermatol. 2006, 33, 17–34. [Google Scholar] [CrossRef]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of bacterial efflux pumps in antibiotic resistance, virulence, and strategies to discover novel efflux pump inhibitors. Microbiology 2023, 169, 001333. [Google Scholar] [CrossRef]
- Huang, L.; Wu, C.; Gao, H.; Xu, C.; Dai, M.; Huang, L.; Hao, H.; Wang, X.; Cheng, G. Bacterial Multidrug Efflux Pumps at the Frontline of Antimicrobial Resistance: An Overview. Antibiotics 2022, 11, 520. [Google Scholar] [CrossRef]
- Chetri, S. The culmination of multidrug-resistant efflux pumps vs. meager antibiotic arsenal era: Urgent need for an improved new generation of EPIs. Front. Microbiol. 2023, 14, 1149418. [Google Scholar] [CrossRef]
- Subramaniam, S.; Bergonier, D.; Poumarat, F.; Capaul, Y.; Schlatter, J.; Nicolet, J.; Frey, J. Species identification of Mycoplasma bovis and Mycoplasma agalactiae based on the uvrC genes by PCR. Mol. Cell. Probes 1998, 12, 161–169. [Google Scholar] [CrossRef]
- Hannan, P.C. Guidelines and recommendations for antimicrobial minimum inhibitory concentration (MIC) testing against veterinary Mycoplasma species. Int. Res. Programme Comp. Mycoplasmol. Vet. Res. 2000, 31, 373–395. [Google Scholar] [CrossRef] [PubMed]
- Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacterial Isolated from Animals; The Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- Eleftheriadou, I.; Giannousi, K.; Protonotariou, E.; Skoura, L.; Arsenakis, M.; Dendrinou-Samara, C.; Sivropoulou, A. Cocktail of CuO, ZnO, or CuZn nanoparticles and antibiotics for combating multidrug-resistant Pseudomonas aeruginosa via efflux pump inhibition. ACS Appl. Nano Mater. 2021, 4, 9799–9810. [Google Scholar] [CrossRef]
- Hata, E.; Harada, T.; Itoh, M. Relationship between antimicrobial susceptibility and multilocus sequence type of Mycoplasma bovis isolates and development of a method for rapid detection of point mutations involved in decreased susceptibility to macrolides, lincosamides, tetracyclines, and spectinomycin. Appl. Environ. Microbiol. 2019, 85, 13. [Google Scholar] [CrossRef]
- Kong, L.-C.; Gao, D.; Jia, B.-Y.; Wang, Z.; Gao, Y.-H.; Pei, Z.-H.; Liu, S.-M.; Xin, J.-Q. Antimicrobial susceptibility and molecular characterization of macrolide resistance of Mycoplasma bovis isolates from multiple provinces in China. J. Vet. Med. Sci. 2016, 78, 293–296. [Google Scholar] [CrossRef]
- Totten, A.H.; Crawford, C.L.; Dalecki, A.G.; Xiao, L.; Wolschendorf, F.; Atkinson, T.P. Differential susceptibility of Mycoplasma and Ureaplasma species to compound-enhanced copper toxicity. Front. Microbiol. 2019, 10, 1720. [Google Scholar] [CrossRef]
- Ross, J.I.; Eady, E.A.; Cove, J.H.; Cunliffe, W.J. 16S rRNA mutation associated with tetracycline resistance in a gram-positive bacterium. Antimicrob. Agents Chemother. 1998, 42, 1702–1705. [Google Scholar] [CrossRef]
- Serwecińska, L. Antimicrobials and antibiotic-resistant bacteria: A risk to the environment and public health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- van Duijkeren, E.; Schink, A.K.; Roberts, M.C.; Wang, Y.; Schwarz, S. Mechanisms of bacterial resistance to antimicrobial agents. Microbiol. Spectr. 2017, 6, ARBA-0019-2017. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance. Microbiol. Spectr. 2016, 4, VMBF-0016-2015. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente YJ, D.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef]
- Perálvarez-Marín, A.; Baranowski, E.; Bierge, P.; Pich, O.Q.; Lebrette, H. Metal utilization in genome-reduced bacteria: Do human mycoplasmas rely on iron? Comput. Struct. Biotechnol. J. 2021, 19, 5752–5761. [Google Scholar] [CrossRef] [PubMed]
- Margaryan, A.; Panosyan, H.; Birkeland, N.K. Heavy metal resistance in prokaryotes: Mechanism and application. In Microbial Communities and Their Interactions in the Extreme Environment; Springer: Singapore, 2021; pp. 273–313. [Google Scholar]
- Christena, L.R.; Mangalagowri, V.; Pradheeba, P.; Ahmed, K.B.A.; Shalini, B.I.S.; Vidyalakshmi, M.; Anbazhagan, V. Copper nanoparticles as an efflux pump inhibitor to tackle drug resistant bacteria. RSC Adv. 2015, 5, 12899–12909. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Amin, B.H.; Hagras, F.A.; Ramadan, A.A.; Kamel, M.R.; Ahmed, M.A.; Atia, K.H.; Salem, S.S. Potential antimicrobial and antibiofilm properties of copper oxide nanoparticles: Time-kill kinetic essay and ultrastructure of pathogenic bacterial cells. Appl. Biochem. Biotechnol. 2023, 195, 467–485. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lin, W.; Ruan, Y.; Lu, H.; Fan, S.; Chen, D.; Huang, Y.; Zhang, T.; Pi, J.; Xu, J.-F. Advances of Cobalt Nanomaterials as Anti-Infection Agents, Drug Carriers, and Immunomodulators for Potential Infectious Disease Treatment. Pharmaceutics 2022, 14, 2351. [Google Scholar] [CrossRef]
- Roberts, M.C. Tetracyclines: Mode of action and their bacterial mechanisms of resistance. In Bacterial Resistance to Antibiotics—From Molecules to Man; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 101–124. [Google Scholar]
- Gautier-Bouchardon, A.V.; Ferre, S.; Le Grand, D.; Paoli, A.; Gay, E.; Poumarat, F. Overall decrease in the susceptibility of Mycoplasma bovis to antimicrobials over the past 30 years in France. PLoS ONE 2014, 9, e87672. [Google Scholar] [CrossRef]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential metals in health and disease. Chem.-Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; Conti, R.D.; Alves, O.L.; Costa, F.; Brocchi, M. Potential Use of Silver Nanoparticles on Pathogenic Bacteria, Their Toxicity and Possible Mechanisms of Action. J. Braz. Chem. Soc. 2010, 21, 949–959. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhallawi, M.F.H.; Mohammed, M.H.; Hemmatzadeh, F.; Petrovski, K. Exploring Metal Ions as Potential Antimicrobial Agents to Combat Future Drug Resistance in Mycoplasma bovis. Microorganisms 2025, 13, 169. https://doi.org/10.3390/microorganisms13010169
Alkhallawi MFH, Mohammed MH, Hemmatzadeh F, Petrovski K. Exploring Metal Ions as Potential Antimicrobial Agents to Combat Future Drug Resistance in Mycoplasma bovis. Microorganisms. 2025; 13(1):169. https://doi.org/10.3390/microorganisms13010169
Chicago/Turabian StyleAlkhallawi, Mauida F. Hasoon, Majed H. Mohammed, Farhid Hemmatzadeh, and Kiro Petrovski. 2025. "Exploring Metal Ions as Potential Antimicrobial Agents to Combat Future Drug Resistance in Mycoplasma bovis" Microorganisms 13, no. 1: 169. https://doi.org/10.3390/microorganisms13010169
APA StyleAlkhallawi, M. F. H., Mohammed, M. H., Hemmatzadeh, F., & Petrovski, K. (2025). Exploring Metal Ions as Potential Antimicrobial Agents to Combat Future Drug Resistance in Mycoplasma bovis. Microorganisms, 13(1), 169. https://doi.org/10.3390/microorganisms13010169








