Molecular Testing of Zoonotic Bacteria in Cattle, Sheep, and Goat Abortion Cases in Botswana
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval and Participation Consent
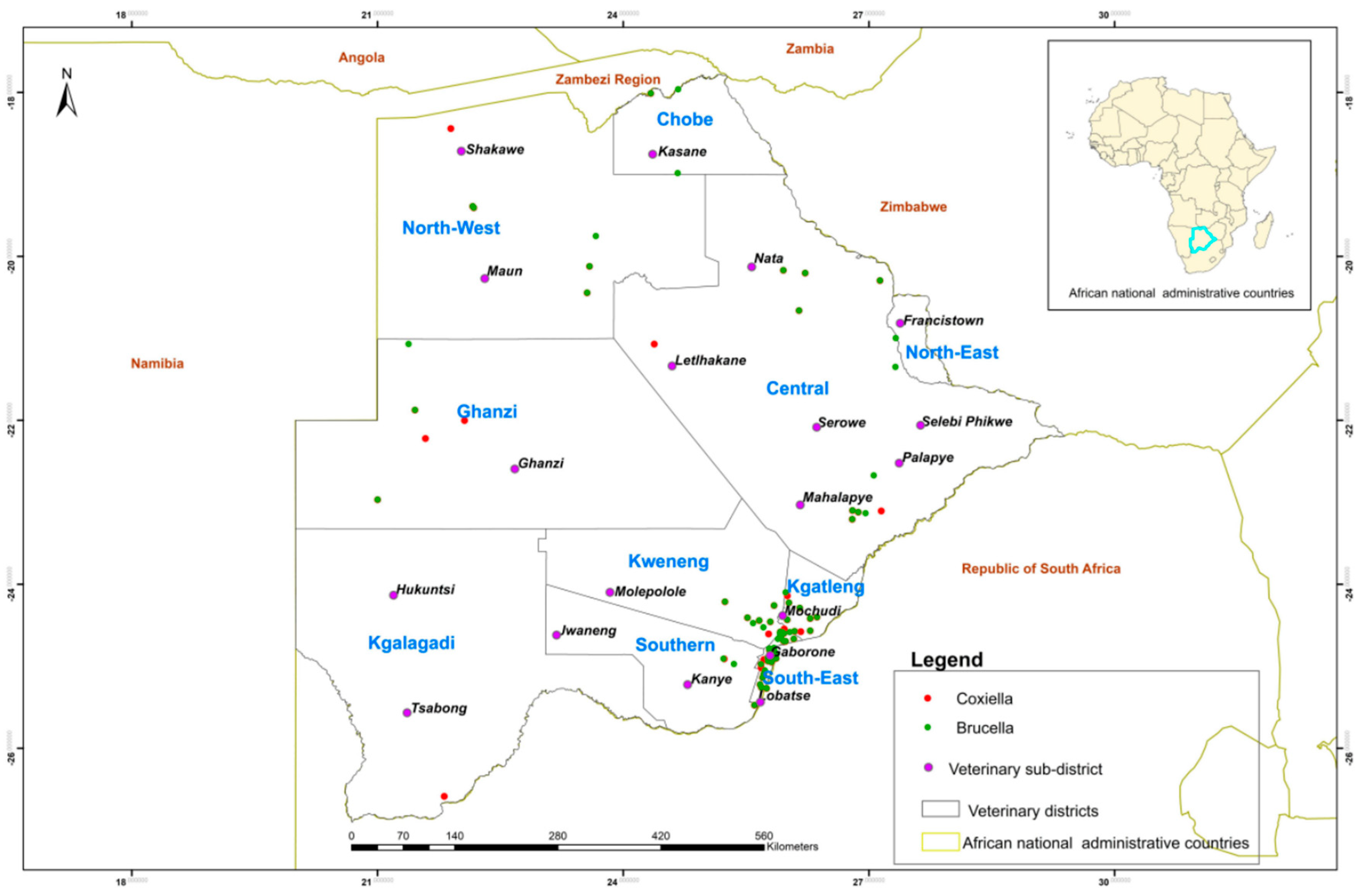
2.2. Study Areas and Sample Collection
2.3. Detection of Nucleic Acids
2.4. Data Analysis and Statistical Analysis
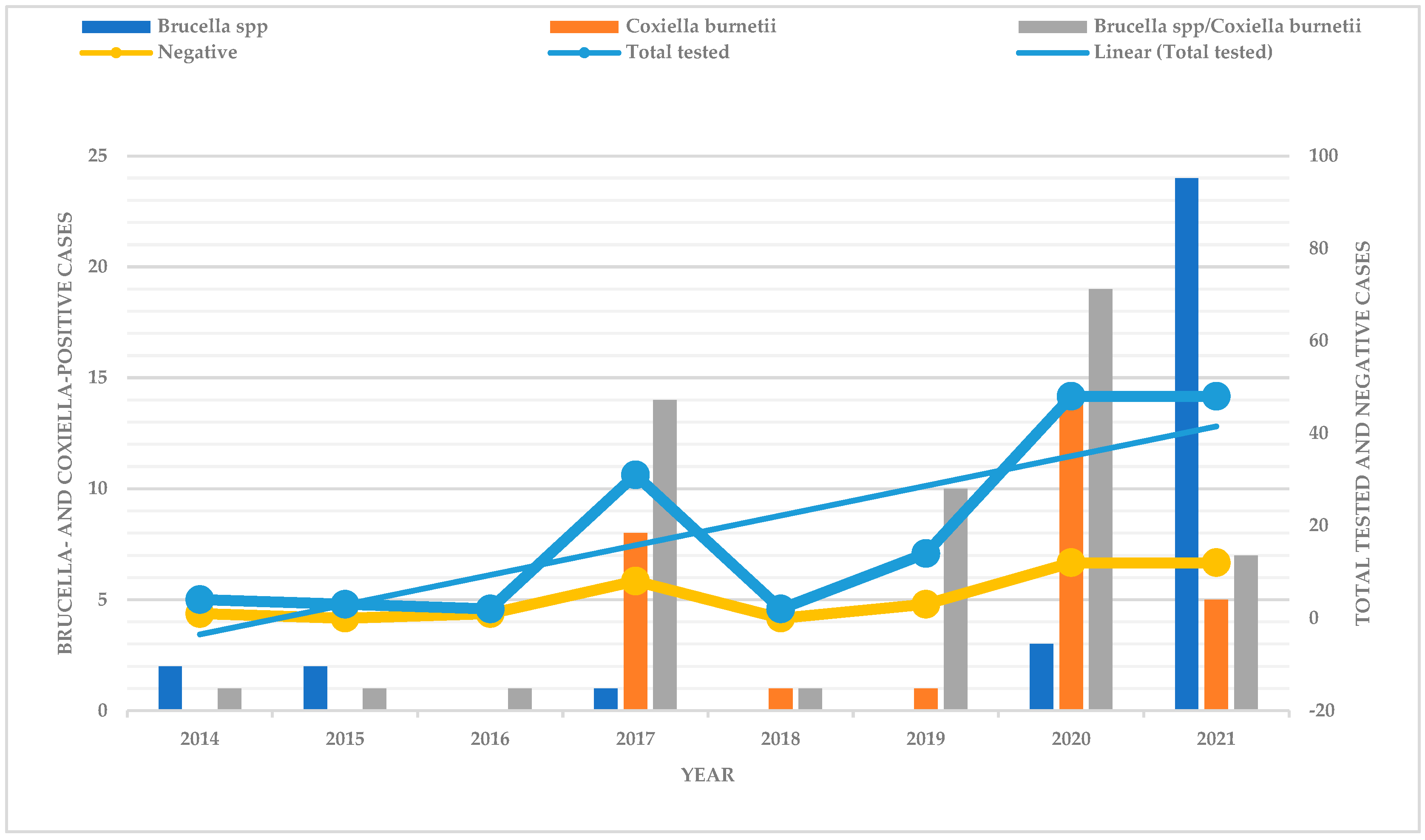
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahabile, M.; Lyne, M.; Panin, A. Factors Affecting the productivity of communal and private livestock farmers in Southern Botswana: A descriptive analysis of sample survey results. Agrekon 2002, 41, 326–338. [Google Scholar] [CrossRef]
- Mahabile, M.; Lyne, M.C.; Panin, A. Anempirical analysis of factors affecting the productivity of livestock in Southern Botswana. Agrekon 2005, 44, 99–117. [Google Scholar] [CrossRef]
- Mosalagae, D.; Mogotsi, K. Caught in a sandstorm: An assessment of pressures on communal pastoral livelihoods in the Kalahari Desert of Botswana. Pastor. Res. Policy Pract. 2013, 3, 18. [Google Scholar] [CrossRef]
- Statistics, Botswana. Annual Agricultural Survey Report 2019; Traditional Sector. Gaborone, Botswana. 2019. Available online: http://www.statsbots.org.bw/ (accessed on 25 October 2024).
- Zhang, H.; Deng, X.; Cui, B.; Shao, Z.; Zhao, X.; Yang, Q.; Song, S.; Wang, Z.; Wang, Y.; Wang, Y.; et al. Abortion and various associated risk factors in dairy cow andsheep in Ili, China. PLoS ONE 2020, 15, e0232568. [Google Scholar]
- Hajibemani, A.; Sheikhalislami, H. Zoonotic Pathogens Cause of Animal Abortion and Fetal Loss. J. Zoonotic Dis. 2020, 4, 1–19. [Google Scholar]
- da Silva, T.M.A.; Gonzaga De Oliveira, R.; Pinto Da Silva, J.; Ii, M.; Noyma, M.; Tatiane, X.I.; Da Paixão, A.; Cortez, A.; Marcos, I.; Heinemann, B.; et al. Etiologic Diagnosis of bovine infectious abortion by PCR Diagnóstico Etiológico de Aborto Infeccioso Bovino Por PCR. Cienc. Rural 2009, 39, 2563–2570. [Google Scholar] [CrossRef]
- Parthiban, S. Review on emerging and reemerging microbial causes in bovine abortion. Int. J. Food Sci. Nutr. 2015, 4, 1–6. [Google Scholar] [CrossRef]
- Barkallah, M.; Gharbi, Y.; Hassena, A.B.; Slima, A.B.; Mallek, Z.; Gautier, M.; Greub, G.; Gdoura, R.; Fendri, I. Survey of infectious etiologies of bovine abortion during mid- to late gestation in dairy herds. PLoS ONE. 2014, 9, e91549. [Google Scholar] [CrossRef] [PubMed]
- Njiro, S.M.; Kidanemariam, A.G.; Tsotetsi, A.M.; Katsande, C.; Mnisi, M.; Lubisi, A.; Potts, A.D.; Baloyi, F.; Moyo, G.; Mpofu, J.; et al. A study of some infectious causes of reproductive disorders in cattle owned by resource-poor farmers in Gauteng Province, South Africa. J. S. Afr. Vet. Assoc. 2011, 82, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Guesmi, K.; Kalthoum, S.; Mamlouk, A.; Baccar, M.N.; BelHajMohamed, B.; Hajlaoui, H.; Toumi, A.; Cherni, J.; Seghaier, C.; Messadi, L. Seroprevalence of zoonotic abortive diseases and their associated risk factors in Tunisian sheep. BMC Vet. Res. 2023, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Clothier, K.; Anderson, M. Evaluation of bovine abortion cses and tissue suitability for identification of infectious agents in California diagnostic laboratory cases from 2007 to 2012. Theriogenology 2016, 85, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Abdelhadi, F.Z.; Abdelhadi, S.A.; Niar, A.; Benallou, B.; Meliani, S.; Smail, N.L.; Mahmoud, D. Abortions in Cattle on the Level of Tiaret Area (Algeria). Glob. Vet. 2015, 14, 638–645. [Google Scholar]
- de Figueiredo, P.; Ficht, T.A.; Rice-Ficht, A.; Rossetti, C.A.; Adams, L.G. Pathogenesis and immunobiology of brucellosis: Review of Brucella-host interactions. Am. J. Clin. Pathol. 2015, 185, 1505–1517. [Google Scholar] [CrossRef]
- McDermott, J.; Grace, D.; Zinsstag, J. Economics of brucellosis impact and control in low-income countries. Rev. Sci. Tech. 2013, 32, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Ducrotoy, M.; Bertu, W.J.; Matope, G.; Cadmus, S.; Conde-Álvarez, R.; Gusi, A.M.; Welburn, S.; Ocholi, R.; Blasco, J.M.; Moriyón, I. Brucellosis in Sub-Saharan Africa: Current challenges for management, diagnosis and control. Acta Trop. 2017, 165, 179–193. [Google Scholar] [CrossRef]
- Poester, F.P.; Nielsen, K.; Samartino, L.E.; Yu, W.L. Diagnosis of brucellosis. Open Vet. Sci. J. 2010, 4, 46–60. [Google Scholar] [CrossRef]
- Troy, S.B.; Rickman, L.S.; Davis, C.E. Brucellosis in San Diego: Epidemiology and species-related differences in acute clinical presentations. Medicine 2005, 84, 174–187. [Google Scholar] [CrossRef]
- Godfroid, J.; Cloeckaert, A.; Liautard, J.P.; Kohler, S.; Fretin, D.; Walravens, K.; Garin-Bastuji, B.; Letesson, J.J. From the discovery of the Malta Fever’s agent to thediscovery of a marine mammalreservoir, brucellosis has continuously been a re-emerging zoonosis. Vet. Res. 2005, 36, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, M.C.; Hielpos, M.S.; Carvalho, N.B.; Barrionuevo, P.; Corsetti, P.P.; Giambartolomei, G.H.; Oliveira, S.C.; Baldi, P.C. Key role of toll-like receptor 2 in the inflammatory response and major histocompatibility complex class II downregulation in Brucella abortus-infected alveolar macrophages. Infect. Immun. 2014, 82, 626–639. [Google Scholar] [CrossRef]
- Khurana, S.K.; Sehrawat, A.; Tiwari, R.; Prasad, M.; Gulati, B.; Shabbir, M.Z.; Chhabra, R.; Karthik, K.; Patel, S.K.; Pathak, M.; et al. Bovine brucellosis–A comprehensive review. Vet. Q. 2021, 41, 61–88. [Google Scholar] [CrossRef] [PubMed]
- Ducrotoy, M.J.; Bertu, W.J.; Ocholi, R.A.; Gusi, A.M.; Bryssinckx, W.; Welburn, S.; Moriyón, I. Brucellosis as an emerging threat in developing economies: Lessons from Nigeria. PLoS Negl. Trop. Dis. 2014, 8, e3008. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.P.; Patil, S.S.; Nayak, A.; Dhanze, H.; Rajamani, S.; Shivamallu, C.; Cull, C.A.; Amachawadi, R.G. Prevalence of brucellosis in livestock of African and Asian continents: A systematic review and meta-analysis. Front. Vet. Sci. 2022, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Glazunova, O.; Roux, V.; Freylikman, O.; Sekeyova, Z.; Fournous, G.; Tyczka, J.; Tokarevich, N.; Kovacova, E.; Marrie, T.J.; Raoult, D. Coxiella burnetii genotyping. Emerg. Infect. Dis. 2005, 11, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Nokhodian, Z.; Feizi, A.; Ataei, B.; Hoseini, S.G.; Mostafavi, E. Epidemiology of Q Fever in Iran: A systematic review and metaanalysis for estimating serological and molecular prevalence. J. Res. Med. Sci. 2017, 22, 121. [Google Scholar] [PubMed]
- Muema, J.; Nyamai, M.; Wheelhouse, N.; Njuguna, J.; Jost, C.; Oyugi, J.; Bukania, Z.; Oboge, H.; Ogoti, B.; Makori, A.; et al. Endemicity of Coxiella burnetii infection among people and their livestock in pastoral communities in Northern Kenya. Heliyon 2022, 8, e11133. [Google Scholar] [CrossRef] [PubMed]
- Bwatota, S.F.; Cook, E.A.J.; de Clare Bronsvoort, B.M.; Wheelhouse, N.; Hernandez-Castor, L.E.; Shirima, G.M. Epidemiology of Q-Fever in domestic ruminants and humans in Africa. A systematic review. CABI One Health 2022, 1–17. [Google Scholar] [CrossRef]
- Maurin, M.; Raoult, D. Q Fever. Clin. Microbiol. Rev. 1999, 12, 518–553. [Google Scholar] [CrossRef]
- Ortega-Mora, L.M. IS Q Fever a significant cause of reproductive failure in cattle? Vet. Rec. 2012, 170, 260. [Google Scholar] [CrossRef]
- Freick, M.; Enbergs, H.; Walraph, J.; Diller, R.; Weber, J.; Konrath, A. Coxiella burnetii: Serological reactions and bacterial shedding in primiparous dairy cows in an endemically infected herd—Impact on milk yield and fertility. Reprod. Domest. Anim. 2017, 52, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Agerholm, J.S. Coxiella burnetii Associated reproductive disorders in domestic animals-A critical review. Acta. Vet. Scand. 2013, 55, 13. [Google Scholar] [CrossRef] [PubMed]
- Kazar, J. Coxiella burnetii Infection. Ann. N. Y. Acad. Sci. 2005, 1063, 105–114. [Google Scholar] [CrossRef] [PubMed]
- García-Seco, T.; Pérez-Sancho, M.; Martínez-Nevado, E.; Álvarez, J.; Santiago-Moreno, J.; Goyache, J.; Domínguez, L.; García, N. Detection of Coxiella burnetii infection in a Saharawi Dorcas Gazelle (Gazella Dorcas Neglecta). J. Zoo Wildl. Med. 2016, 47, 939–941. [Google Scholar] [CrossRef]
- Robi, D.T.; Demissie, W.; Temteme, S. Coxiellosis in livestock: Epidemiology, public health significance, andprevalence of Coxiella burnetii infection in Ethiopia. Vet. Med. Res. Rep. 2023, 14, 145–158. [Google Scholar] [CrossRef] [PubMed]
- de Vries, S.G.; Visser, B.J.; Nagel, I.M.; Goris, M.G.A.; Hartskeerl, R.A.; Grobusch, M.P. Leptospirosis in Sub-Saharan Africa: A systematic review. Int. J. Infect. Dis. 2014, 28, e47–e64. [Google Scholar] [CrossRef] [PubMed]
- Allan, K.J.; Halliday, J.E.B.; Moseley, M.; Carter, R.W.; Ahmed, A.; Goris, M.G.A.; Hartskeerl, R.A.; Keyyu, J.; Kibona, T.; Maro, V.P.; et al. Assessment of animal hosts of pathogenic Leptospira in Northern Tanzania. PLoS Negl. Trop. Dis. 2018, 12, e0006444. [Google Scholar] [CrossRef] [PubMed]
- Underwood, W.J.; Blauwiekel, R.; Delano, M.L.; Gillesby, R.; Mischler, S.A.; Schoell, A. Biology and diseases of ruminants (Sheep, Goats, and Cattle). In Laboratory Animal Medicine; Academic Press: Cambridge, MA, USA, 2015; pp. 623–694. ISBN 9780124095274. [Google Scholar]
- Hartskeerl, R.A.; Collares-Pereira, M.; Ellis, W.A. Emergence, control and re-Emerging Leptospirosis: Dynamics of infection in the changing world. Clin. Microbiol. Infect. 2011, 17, 494–501. [Google Scholar] [CrossRef]
- Minter, A.; Diggle, P.J.; Costa, F.; Childs, J.; Ko, A.I.; Begon, M. Evidence of multiple intraspecific transmission routes for Leptospira acquisition in Norway rats (Rattus norvegicus). Epidemiol. Infect. 2017, 145, 3438–3448. [Google Scholar] [CrossRef] [PubMed]
- Adler, B.; de la Peña Moctezuma, A. Leptospira and Leptospirosis. Vet. Microbiol. 2010, 140, 287–296. [Google Scholar] [CrossRef]
- Hage, E.; Mpamugo, O.; Ohai, C.; Sapkota, S.; Swift, C.; Wooldridge, D.; Amar, C.F.L. Identification of six Listeria species by real-time PCR assay. Lett Appl. Microbiol. 2014, 58, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Weller, D.; Andrus, A.; Wiedmann, M.; den Bakker, H.C. Listeria booriae sp. Nov. and Listeria newyorkensis sp. nov., from food processing environments in the USA. Int. J. Syst. Evol. Microbiol. 2015, 65, 286–292. [Google Scholar] [CrossRef]
- Hagos, H.A. Review of animal listeriosis, diagnosis, etiology and its public health. World J. Adv. Healthc. Res. 2017, 1, 9–24. [Google Scholar]
- Dhama, K.; Karthik, K.; Tiwari, R.; Shabbir, M.Z.; Barbuddhe, S.; Malik, S.V.S.; Singh, R.K. Listeriosis in animals, Its public health significance (Food-Borne Zoonosis) and advances in diagnosis and control: A comprehensive review. Vet. Q. 2015, 35, 211–235. [Google Scholar] [CrossRef]
- Hunt, K.; Drummond, N.; Murphy, M.; Butler, F.; Buckley, J.; Jordan, K. A case of bovineraw milkcontamination with Listeria monocytogenes. Ir. Vet. J. 2012, 65, 13. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.V.S.; Barbuddhe, S.B.; Chaudhari, S.P. Listeric infections in humans and animals in the Indian subcontinent: A review. Trop. Anim. Health Prod. 2002, 34, 359–381. [Google Scholar] [CrossRef] [PubMed]
- Zakir, S.; Abdo, S.; Bushra, M.M.B.; Hussein, J.A. Current epidemiologic status and public health importance of Listeriosis: A Review. J. Vet. Physiol. Pathol. 2022, 1, 61–68. [Google Scholar] [CrossRef]
- Fieseler, L.; Doyscher, D.; Loessner, M.J.; Schuppler, M. ETH Library Acanthamoeba release compounds which promote growth of Listeria monocytogenes and other bacteria. Appl. Microbiol. Biotechnol. 2014, 98, 3091–3097. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.J.; Freitag, N.E.; Boor, K.J. How the bacterial pathogen Listeria monocytogenes mediates the switch from environmental Dr. Jekyll topathogenic Mr. Hyde. Infect. Immun. 2006, 74, 2506–2512. [Google Scholar] [CrossRef] [PubMed]
- Letchumanan, V.; Wong, P.-C.; Goh, B.-H.; Chiau Ming, L.; Pus-parajah, P.; Hei Wong, S.; Ab Mutalib, N.-S.; Lee, L.-H.; Ka Shing, L. Progress in microbes and molecular biology A Review on the characteristics, taxanomy and prevalence of Listeria monocytogenes. Prog. Microbes Mol. Biol. 2018, 1, a0000007. [Google Scholar] [CrossRef]
- Tchatchouang, C.D.K.; Fri, J.; De Santi, M.; Brandi, G.; Schiavano, G.F.; Amagliani, G.; Ateba, C.N. Listeriosis Outbreak in South Africa: A comparative analysis with previously reported cases worldwide. Microorganisms 2020, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- The ARRIVE guidelines 2.0. Available online: https://arriveguidelines.org (accessed on 16 December 2020).
- Settypalli, T.B.K.; Lamien, C.E.; Spergser, J.; Lelenta, M.; Wade, A.; Gelaye, E.; Loitsch, A.; Minoungou, G.; Thiaucourt, F.; Diallo, A. One-Step multiplex RT-qPCR assay for the detection of Peste des petits ruminants virus, Capripoxvirus, Pasteurella multocida and Mycoplasma capricolum subspecies (ssp.) capripneumoniae. PLoS ONE 2016, 11, e0153688. [Google Scholar] [CrossRef]
- Modise, B.M.; Mpoloka, S.W.; Settypalli, T.B.K.; Hyera, J.; Natale, A.; Ceglie, L.; Gcebe, N.; Marobela-Raborokgwe, C.; Viljoen, G.J.; Cattoli, G.; et al. A Novel Multiplex qPCR-HRM assay for the simultaneous detection of four abortive zoonotic agents in cattle, sheep, and goats. Sci. Rep. 2023, 13, 12282. [Google Scholar] [CrossRef] [PubMed]
- Loftis, A.D.; Reeves, W.K.; Szumlas, D.E.; Abbassy, M.M.; Helmy, I.M.; Moriarity, J.R.; Dasch, G.A. Surveillance of Egyptian fleas for agents of public health significance: Anaplasma, Bartonella, Coxiella, Ehrlichia, Rickettsia, and Yersinia pestis. Am. J. Trop. Med. Hyg. 2006, 75, 41–48. [Google Scholar] [CrossRef]
- Liu, J.; Ochieng, C.; Wiersma, S.; Ströher, U.; Towner, J.S.; Whitmer, S.; Nichol, S.T.; Moore, C.C.; Kersh, G.J.; Kato, C.; et al. Development of a TaqMan Array Card for acute-febrile-illness outbreak investigation and surveillance of emerging pathogens, including Ebola virus. J. Clin. Microbiol. 2016, 54, 49–58. [Google Scholar] [CrossRef]
- Jin, D.; Luo, Y.; Zhang, Z.; Fang, W.; Ye, J.; Wu, F.; Ding, G. Rapid moleculariIdentification of Listeria species by use of real-time PCR and high-resolution melting analysis. FEMS Microbiol. Lett. 2012, 330, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Giavarina, D. Understanding Bland Altman Analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Jodełko, A.; Szymańska-Czerwińska, M.; Rola, J.G.; Niemczuk, K. Molecular detection of Coxiella burnetii in small ruminants and genotyping of specimens collected from goats in Poland. BMC Vet. Res. 2021, 17, 341. [Google Scholar] [CrossRef]
- Cardinale, E.; Esnault, O.; Beral, M.; Naze, F.; Michault, A. Emergence of Coxiella burnetii in ruminants on Reunion Island? Prevalence and risk factors. PLoS Negl. Trop. Dis. 2014, 8, e3055. [Google Scholar] [CrossRef]
- Clemente, L.; Barahona, M.J.; Andrade, M.F.; Botelho, A. Diagnosis by PCR of Coxiella burnetii in aborted fetuses of domestic ruminants in Portugal. Vet. Rec. 2009, 164, 373–374. [Google Scholar] [CrossRef] [PubMed]
- Hardi, F.; Sherzad Rauf, H.; Latif Mahmood, S.; Bahir Ahmad, R.; Abdulwahid Ali, B.; Omar Baba Sheikh, M. Molecular detection and identification of Coxiella Burnetii in aborted sheep and goats in Sulaimani Province, Kurdistan-Iraq. Assiut Vet. Med. J. 2020, 66, 133–139. [Google Scholar] [CrossRef]
- Eibach, R.; Bothe, F.; Runge, M.; Fischer, S.F.; Philipp, W.; Ganter, M. Q Fever: Baseline monitoring of a sheep and a goat flock associated with human infections. Epidemiol. Infect. 2012, 140, 1939–1949. [Google Scholar] [CrossRef]
- de los Angeles Ramo, M.; Benito, A.A.; Quílez, J.; Monteagudo, L.V.; Baselga, C.; Tejedor, M.T. Coxiella burnetii and co-infections with other major pathogens causing abortion in small ruminant flocks in the Iberian Peninsula. Animals 2022, 12, 3454. [Google Scholar] [CrossRef]
- Adamu, S.G.; Kabir, J.; Umoh, J.U.; Raji, M.A. Seroprevalence of brucellosis and q Fever (Coxiellosis) in cattle herds in Maigana and Birnin Gwari Agro-Ecological zone of Kaduna State, Nigeria. Trop. Anim. Health Prod. 2018, 50, 1583–1589. [Google Scholar] [CrossRef]
- Peric, L.; Sabadi, D.; Rubil, I.; Bogdan, M.; Guzvinec, M.; Rode, O.D.; Kaic, B.; Tabain, I.; Vilibic-Cavlek, T. Imported brucellosis and q-Fever co-infection in Croatia: A Case Report. J. Infect. Dev. Ctries. 2018, 12, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Hu, X.; Li, X.; Chen, Y.; Yan, X.; Zhu, W.; Ding, Y.; Zhou, J. Rickettsia burneti and Brucella melitensis co-Infection: A case report and literature review. BMC Microbiol. 2021, 21, 270. [Google Scholar] [CrossRef]
- Middlebrook, E.A.; Romero, A.T.; Bett, B.; Nthiwa, D.; Oyola, S.O.; Fair, J.M.; Bartlow, A.W. Identification and distribution of pathogens coinfecting with Brucella spp., Coxiella burnetii and Rift Valley fever virus in humans, livestock and wildlife. Zoonoses Public Health 2022, 69, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Picardeau, M. Diagnosis and Epidemiology of Leptospirosis. Med. Mal. Infect. 2013, 43, 1–9. [Google Scholar] [CrossRef]
- Di Azevedo, M.I.N.; Lilenbaum, W. An Overview on the molecular diagnosis of animal leptospirosis. Lett Appl. Microbiol. 2021, 72, 496–508. [Google Scholar] [CrossRef]
- Noomy, B.S.; Anwar, S.A.; Salih, S.M. Detection of Listeria monocytogenes in raw milk and aborted cow cases at Salahudeen Province. Iraqi J. Agric. Sci. 2021, 52, 315–321. [Google Scholar] [CrossRef]
- Whitman, K.J.; Bono, J.L.; Clawson, M.L.; Loy, J.D.; Bosilevac, J.M.; Arthur, T.M.; Ondrak, J.D. Genomic-based identification of environmental and clinical Listeria monocytogenes strains associated with an abortion outbreak in beef heifers. BMC Vet. Res. 2020, 16, 70. [Google Scholar] [CrossRef]
- Farag, H.; Abdellah, M.; Nossair, M. Prevalence of listeriosis in some farm animals. Damanhour J. Vet. Sci. 2019, 2, 17–20. [Google Scholar] [CrossRef]
- McGahey, D.J. Livestock Mobility and Animal Health Policy in Southern Africa: The Impact of Veterinary Cordon Fences on Pastoralists. Pastoralism 2011, 1, 14. [Google Scholar] [CrossRef]
- Gupta, C. A Genealogy of Conservation in Botswana. Pula Botsw. J. Afr. Stud. 2013, 27, 45–67. [Google Scholar]
- Mokopasetso, M.; Derah, N. Recent Outbreaks of Foot and Mouth Disease in Botswana and Zimbabwe. Tropicultura 2005, 23, 8–12. [Google Scholar]
- Marobela-Raborokgwe, C. Contagious bovine pleuropneumonia in Botswana: Experience with control, eradication, prevention and surveillance. Vet. Ital. 2011, 47, 397–405. [Google Scholar]
- Botswana Daily News. 2016. Available online: https://allafrica.com/stories/201603090213.html (accessed on 10 March 2023).
- Minas, A.; Minas, M.; Stournara, A.; Tselepidis, S. The “Effects” of Rev-1 vaccination of sheep and goats on human brucellosis in Greece. Prev. Vet. Med. 2004, 64, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Grantiņa-Ieviņa, L.; Šteingolde, Ž.; Boikmanis, G.; Laizāne, L.; Ringa-Ošleja, G.; Bubula, I.; Sergejeva, M.; Mališevs, A.; Ķibilds, J.; Cvetkova, S.; et al. Shedding of Coxiella burnetii in milk ofdairy cattle and evidence of q Fever in domestic ruminants with emphasis on abortion cases in Latvia. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2022, 76, 295–306. [Google Scholar]
- Hamdy, M.E.R.; Amin, A.S. Detection of Brucella species in the milk of infected cattle, sheep, goats and camels by PCR. Vet. J. 2002, 163, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Islam, M.A.; Rahman, M.M.; Islam, K.; Islam, M.M.; Kamal, M.M.; Islam, M.N. Presence of Brucella spp. in milk and dairy products: A comprehensive review anditsperspectives. J. Food Qual. 2023, 2023, 1–19. [Google Scholar] [CrossRef]
- Gwida, M.; El-Ashker, M.; Melzer, F.; El-Diasty, M.; El-Beskawy, M.; Neubauer, H. Use of Serology and real time PCR to control an outbreak of bovine brucellosis at a Dairy Cattle farm in the Nile Delta Region, Egypt. Ir. Vet. J. 2016, 69, 3–10. [Google Scholar] [CrossRef]
- Leal-klevezas, D.S.; Martínez-Vázquez, I.O.; Lopez-Merino, A.; Martínez-Soriano, J.P. Single-Step PCR for detection of Brucella spp. from blood and milk of infected animals. J. Clin. Microbiol. 1995, 33, 3087–3090. [Google Scholar] [CrossRef]
- Khamesipour, F.; Nejat Dehkordi, S.; Taktaz Hafshejani, T.; Tajbakhsh, E.; Azizi, S. Determine the prevalence of Brucella spp. and Leptospira spp. in blood samples by multiplex polymerase chainreaction collected from cattle, sheep and goats in herds located in Provinces of Iran. Vet. Sci. Dev. 2014, 4, 5351. [Google Scholar] [CrossRef]
- Wareth, G.; Melzer, F.; Tomaso, H.; Roesler, U.; Neubauer, H. Detection of Brucella abortus DNA in aborted goats and sheep in Egypt by Real-Time PCR. BMC Res. Notes 2015, 8, 212. [Google Scholar] [CrossRef] [PubMed]
| Specimen | Brucella spp./ C. burnetii | Brucella spp. | C. burnetii | Negative | Total |
|---|---|---|---|---|---|
| Stomach contents | 20 | 14 | 5 | 3 | 42 |
| Liver/spleen | 12 | 2 | 12 | 17 | 43 |
| Pooled foetal tissues | 4 | 8 | 5 | 9 | 26 |
| Serum | 2 | 2 | 0 | 4 | 8 |
| Whole blood | 3 | 1 | 1 | 1 | 6 |
| Placenta | 4 | 0 | 1 | 1 | 6 |
| Brain | 1 | 0 | 0 | 0 | 1 |
| Foetal brain | 0 | 2 | 0 | 1 | 3 |
| Uterus | 1 | 1 | 0 | 0 | 2 |
| Lymph node | 2 | 0 | 1 | 0 | 3 |
| Abdominal fluid | 0 | 0 | 1 | 0 | 1 |
| Abomasum | 0 | 0 | 1 | 0 | 1 |
| liver | 0 | 0 | 1 | 1 | 2 |
| Liver/kidney | 1 | 0 | 0 | 0 | 1 |
| Lung/liver/kidney | 1 | 0 | 0 | 0 | 1 |
| Lung/liver/spleen | 0 | 1 | 0 | 0 | 1 |
| Liver/spleen/kidney | 0 | 1 | 0 | 0 | 1 |
| Vaginal swab | 2 | 0 | 1 | 0 | 3 |
| Ovary tubes | 1 | 0 | 0 | 0 | 1 |
| Total | 54 | 32 | 29 | 37 | 152 |
| Pathogen(s) Detected | Cattle (%) | Sheep (%) | Goats (%) | Total (%) |
|---|---|---|---|---|
| Brucella spp. | 9 (34.6) | 3 (21.4) | 20 (17.9) | 32 (21.1) |
| C. burnetii | 2 (7.7) | 3 (21.4) | 24 (21.4) | 29 (19.1) |
| Brucella spp./C. burnetii | 11 (42.3) | 6 (42.8) | 37 (33.0) | 54 (35.5) |
| Negative | 4 (15.4) | 2 (14.3) | 31 (27.7) | 37 (24.3) |
| Total | 26 | 14 | 112 | 152 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modise-Tlotleng, B.M.; Mpoloka, S.W.; Settypalli, T.B.K.; Hyera, J.; Kgotlele, T.; Kumile, K.; Sechele, M.E.; Raboloko, O.O.; Marobela-Raborokgwe, C.; Viljoen, G.J.; et al. Molecular Testing of Zoonotic Bacteria in Cattle, Sheep, and Goat Abortion Cases in Botswana. Microorganisms 2024, 12, 2644. https://doi.org/10.3390/microorganisms12122644
Modise-Tlotleng BM, Mpoloka SW, Settypalli TBK, Hyera J, Kgotlele T, Kumile K, Sechele ME, Raboloko OO, Marobela-Raborokgwe C, Viljoen GJ, et al. Molecular Testing of Zoonotic Bacteria in Cattle, Sheep, and Goat Abortion Cases in Botswana. Microorganisms. 2024; 12(12):2644. https://doi.org/10.3390/microorganisms12122644
Chicago/Turabian StyleModise-Tlotleng, Boitumelo M., Sununguko W. Mpoloka, Tirumala B. K. Settypalli, Joseph Hyera, Tebogo Kgotlele, Kago Kumile, Mosarwa E. Sechele, Obuile O. Raboloko, Chandapiwa Marobela-Raborokgwe, Gerrit J. Viljoen, and et al. 2024. "Molecular Testing of Zoonotic Bacteria in Cattle, Sheep, and Goat Abortion Cases in Botswana" Microorganisms 12, no. 12: 2644. https://doi.org/10.3390/microorganisms12122644
APA StyleModise-Tlotleng, B. M., Mpoloka, S. W., Settypalli, T. B. K., Hyera, J., Kgotlele, T., Kumile, K., Sechele, M. E., Raboloko, O. O., Marobela-Raborokgwe, C., Viljoen, G. J., Cattoli, G., & Lamien, C. E. (2024). Molecular Testing of Zoonotic Bacteria in Cattle, Sheep, and Goat Abortion Cases in Botswana. Microorganisms, 12(12), 2644. https://doi.org/10.3390/microorganisms12122644






