Differences in the Microbial Composition and Function of the Arundo donax Rhizosphere Under Different Cultivation Conditions
Abstract
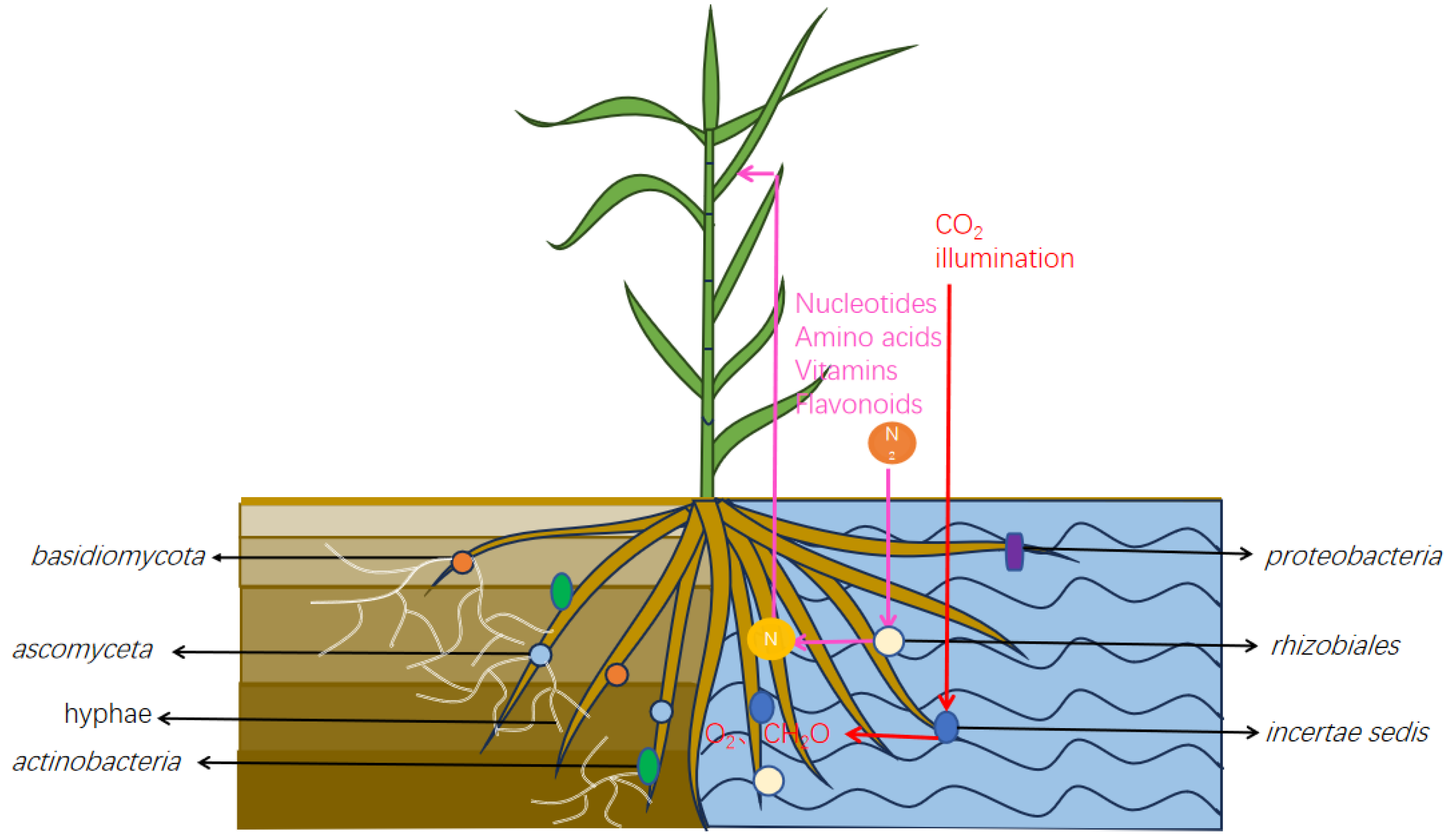
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. 16S-rRNA Gene and Ribosomal DNA ITS Region-End Amplification and Sequencing
2.3. Bioinformatic Analysis
3. Results
3.1. Effects of Different Cultivation Conditions on the Diversity and Community Composition of Rhizosphere Bacteria of Arundo donax
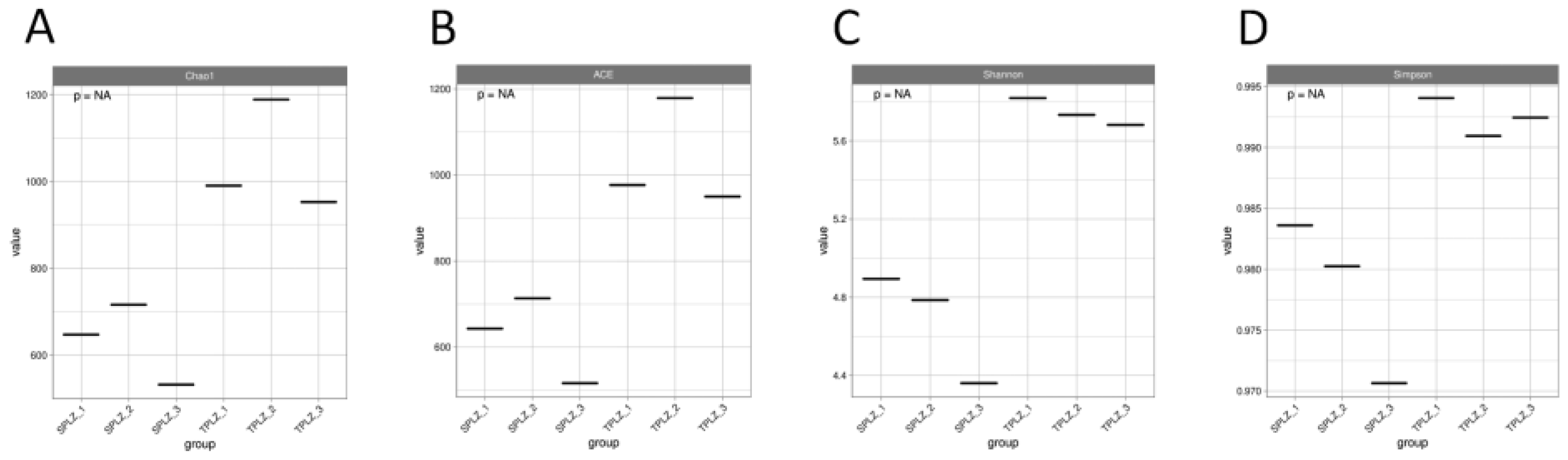
3.1.1. High-Throughput Sequencing Data and Diversity Analysis of Rhizosphere Bacteria
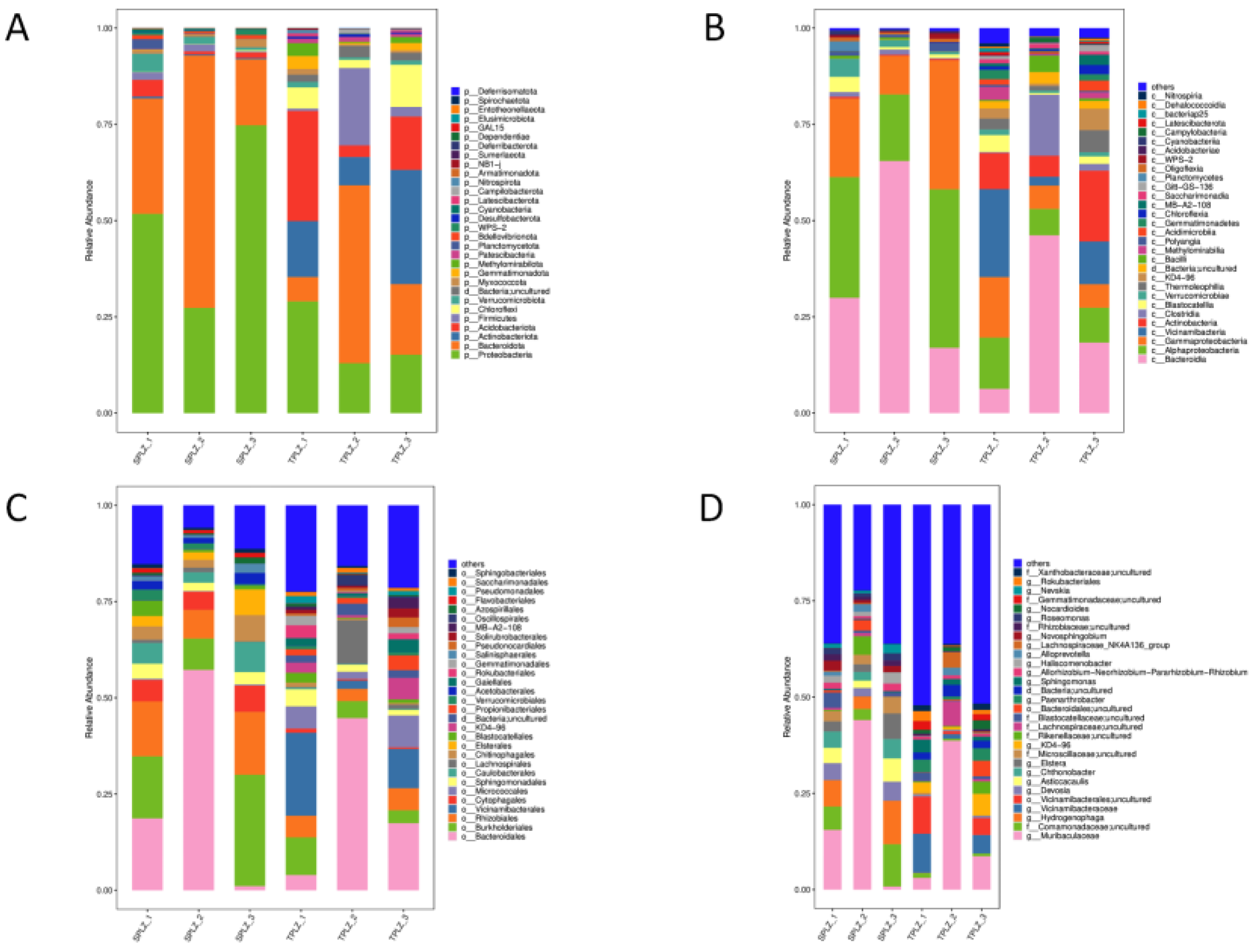
3.1.2. Community Composition and Dominant Species Abundance of Rhizosphere Bacteria
3.1.3. Clustering Analysis of Bacterial Community and Similarity and Difference in Its Structure
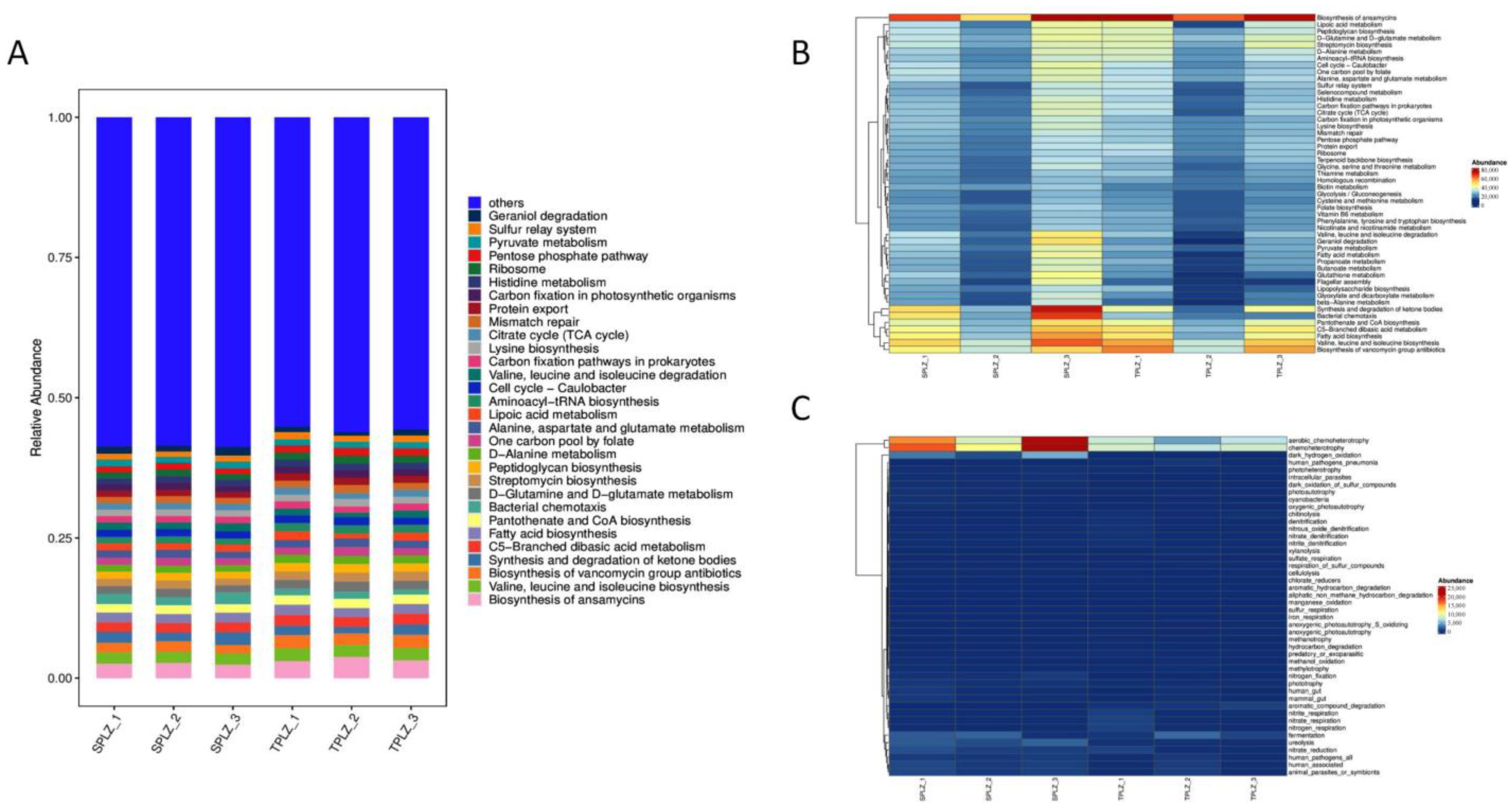
3.1.4. Prediction of Metabolic Functions of the Rhizosphere Bacteria
3.2. Effects of Different Cultivation Conditions on the Diversity and Community Composition of Rhizosphere Fungi of A. donax
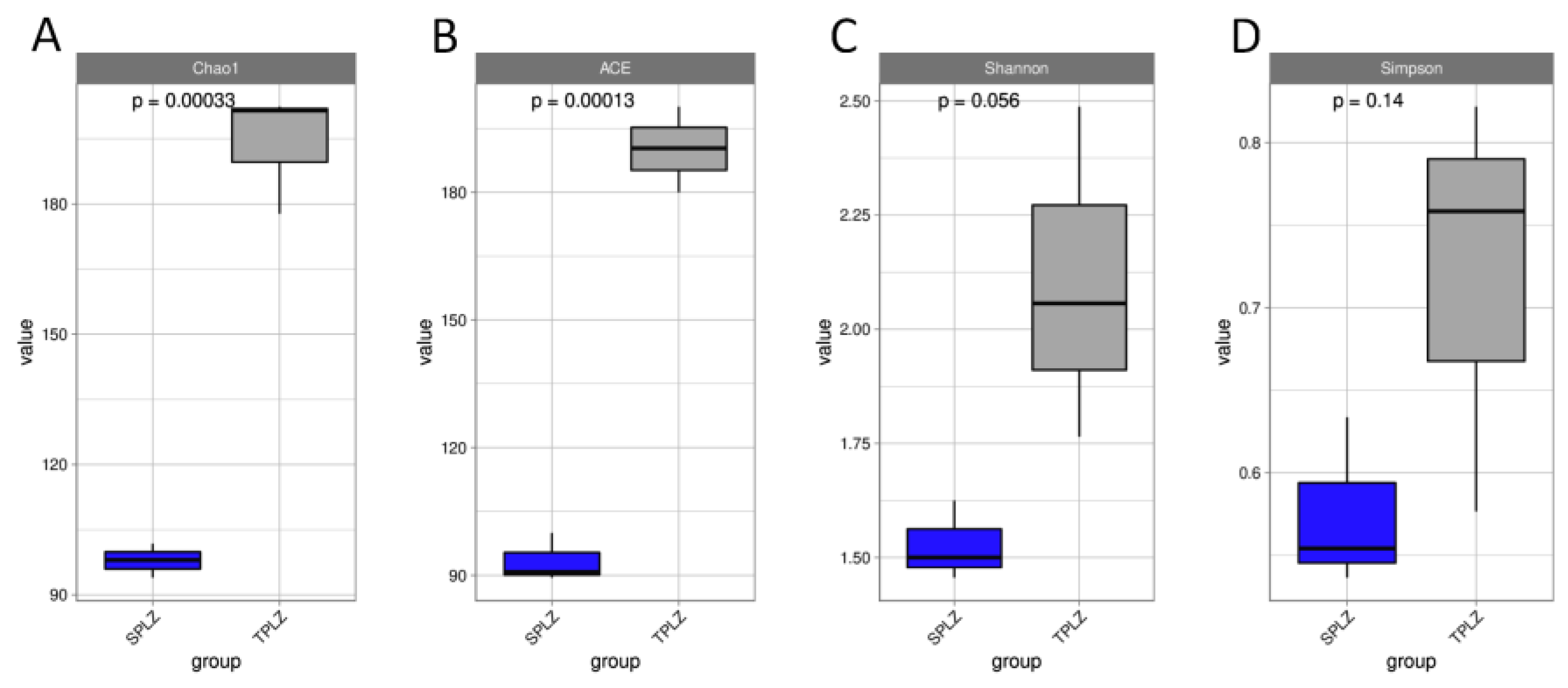
3.2.1. High-Throughput Sequencing Data and Diversity Analysis of Rhizosphere Fungi
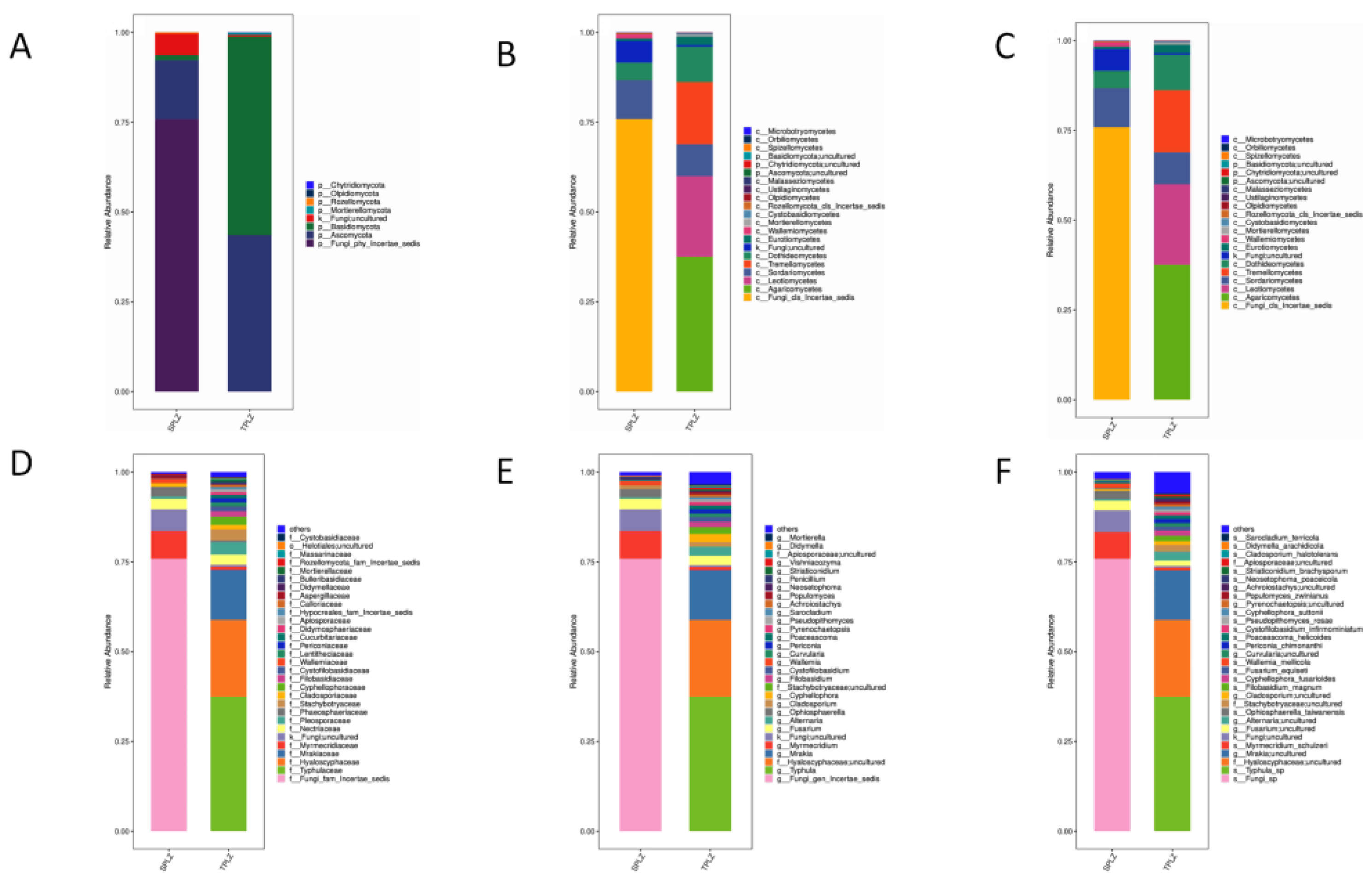
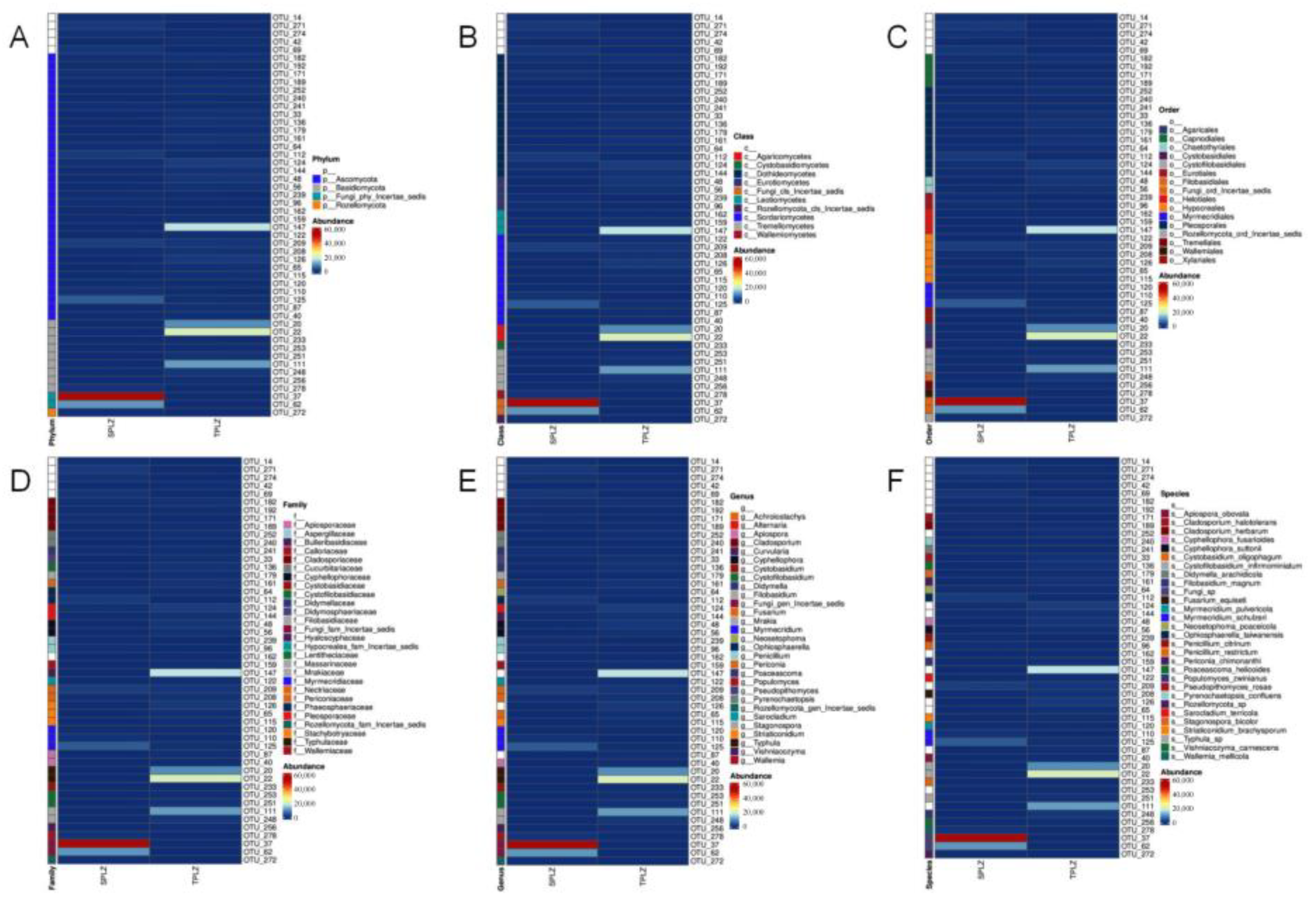
3.2.2. Community Composition and Dominant Species Abundance of Rhizosphere Fungi
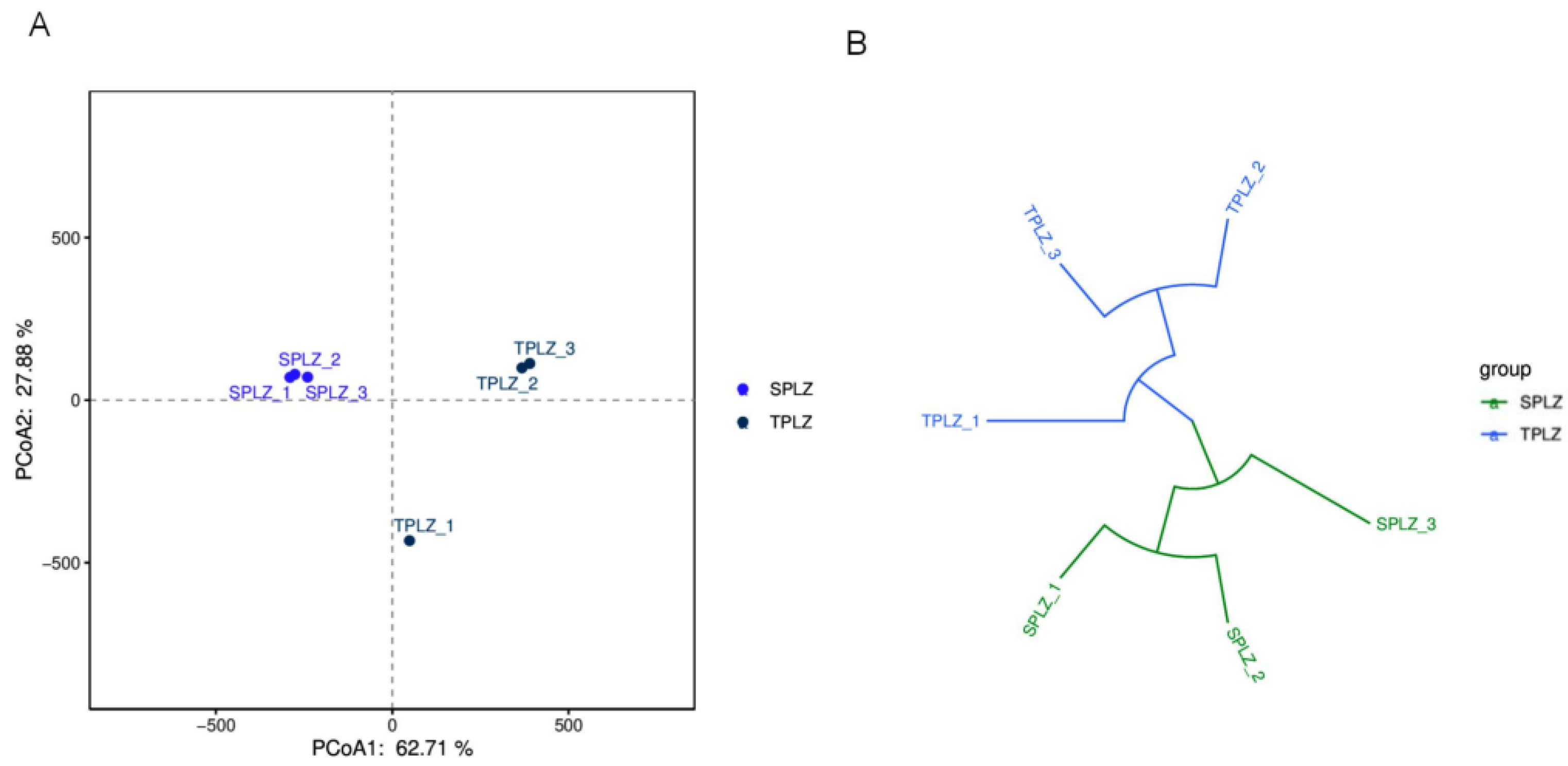
3.2.3. Clustering Analysis of the Fungal Communities and Their Structural Similarities and Differences
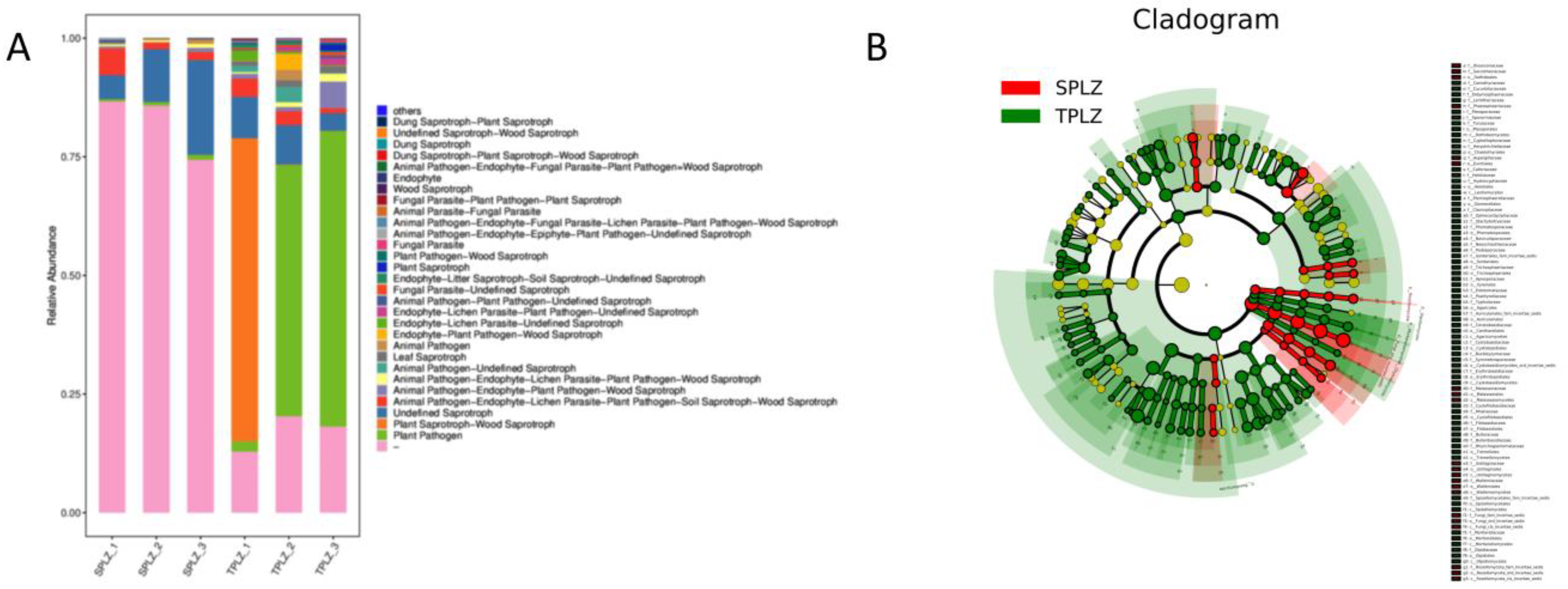
3.2.4. Prediction of the Metabolic Functions of Rhizosphere Fungal Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mochizuki, K.; Takikawa, H.; Mori, K. Synthesis of 2,2,4,4-tetramethyl-N,N’-bis(2,6-dimethylphenyl)cyclobutane-1,3-diimine, a unique compound from Arundo donax, and its analogues to test their antifeedant activity against the boll weevil, Anthonomus grandis. Biosci. Biotechnol. Biochem. 2000, 64, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, G. Arundo donax as a potential biomonitor of trace element contamination in water and sediment. Ecotoxicol. Environ. Saf. 2012, 80, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Pilon, G.; Lavoie, J.M. Pyrolysis of switchgrass (Panicum virgatum L.) at low temperatures in N2 and CO2 environments; a study on chemical composition of chars extracts and biooils. J. Anal. Appl. Pyrolysis 2013, 101, 122–131. [Google Scholar] [CrossRef]
- Corno, L.; Pilu, R.; Adani, F. Arundo donax L: A non-food crop for bioenergy and biocompound production. Biotechnol. Adv. 2014, 32, 1535–1549. [Google Scholar] [CrossRef] [PubMed]
- Verónica, C.; Alejandra, M.; Estela, S. Thermal Behaviour and Emission Characteristics of Arundo donax L. as Potential Biofuel. BioEnergy Res. 2022, 16, 1618–1628. [Google Scholar] [CrossRef]
- Lin, L. Evaluation of Nutritional Value of Reeds and Its Application in Mutton Sheep Production; Chinese Academy of Agricultural: Guangzhou, China, 2020. [Google Scholar]
- Maduro Dias, C.S.; Nunes, H.; Vouzela, C.; Madruga, J.; Borba, A. In Vitro Rumen Fermentation Kinetics Determination and Nutritional Evaluation of Several Non-Conventional Plants with Potential for Ruminant Feeding. Fermentation 2023, 9, 416. [Google Scholar] [CrossRef]
- Kaur, A.; Singh, J.; Kamboj, S.S.; Sexana, A.K.; Pandita, R.M.; Shamnugavel, M. Isolation of an N-acetyl-D-glucosamine specific lectin from the rhizomes of Arundo donax with antiproliferative activity. Phytochemistry 2005, 66, 1933–1940. [Google Scholar] [CrossRef]
- Liu, Q.; Peng, W.; Xie, B.; Li, S. Advances in studies on chemical constituents and biological activities of Arundo donax. Chin. Herb. Med. 2014, 37, 1892–1895. [Google Scholar]
- Long, X.; Deguan, W. Study on nitrogen removal performance of horizontal subsurface flow constructed wetland of Arundinaria fargesii. Environ. Eng. 2009, 3, 1759–1762. [Google Scholar]
- Danelli, T.; Sepulcri, A.; Masetti, G.; Colombo, F.; Sangiorgio, S.; Cassani, E.; Anelli, S.; Adani, F.; Pilu, R. Arundo donax L. Biomass Production in a Polluted Area: Effects of Two Harvest Timings on Heavy Metals Uptake. Appl. Sci. 2021, 11, 1147. [Google Scholar] [CrossRef]
- Cano-Ruiz, J.; Galea, M.R.; Amorós, M.C.; Alonso, J.; Mauri, P.V.; Lobo, M.C. Assessing Arundo donax L. in vitrotolerance for phytoremediation purposes. Chemosphere 2020, 252, 126576. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, L.; Teng, Y.; Luo, Y.; Zhao, Q. Effects of cadmium on mercury accumulation and transformation by Arundo donax L. Environ. Sci. Pollut. Res. Int. 2023, 30, 62461–62469. [Google Scholar] [CrossRef] [PubMed]
- Cristaldi, A.; Conti, G.O.; Cosentino, S.L.; Mauromicale, G.; Copat, C.; Grasso, A.; Zuccarello, P.; Fiore, M.; Restuccia, C.; Ferrante, M. Phytoremediation potential of Arundo donax (Giant Reed) in contaminated soil by heavy metals. Environ. Res. 2020, 185, 109427. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.; Wen, S.; Xie, Y. Study on rapid propagation of Arundo donax. Study Econ. For. 1998, 16, 13–15. [Google Scholar]
- Ran, L.; Lin, X.; Wen, S.; Xie, J. Study on Arundo donax’s tissue culture technique. J. Cent. South Univ. For. Technol. 1998, 18, 49–52. [Google Scholar]
- Martin, B.D.; Schwab, E. Current Usage of Symbiosis and Associated Terminology. Int. J. Biol. 2013, 5, 32. [Google Scholar] [CrossRef]
- Zolla, G.; Badri, D.V.; Bakker, M.; Manter, D.K.; Vivanco, J.M. Soil microbiomes vary in their ability to confer drought tolerance to Arabidopsis. Appl. Soil. Ecol. 2013, 68, 1–9. [Google Scholar] [CrossRef]
- Selvakumar, G.; Panneerselvam, P.; Ganeshamurthy, A.N. Bacterial Mediated Alleviation of Abiotic Stress in Crops. In Bacteria in Agrobiology: Stress Management; Springer: Berlin/Heidelberg, Germany, 2012; pp. 205–224. [Google Scholar]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; Van Der Voort, M.; Schneider, J.H.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.; et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Lu, X.; Bai, Y.; Wang, G. Two diversities meet in the rhizosphere: Root specialized metabolites and microbiome. J. Genet. Genom. 2023, 51, 467–478. [Google Scholar] [CrossRef]
- Wang, Z.; Solanki, M.K.; Kumar, A.; Solanki, A.C.; Pang, F.; Ba, Z.-X.; Niu, J.-Q.; Ren, Z.-X. Promoting plant resilience against stress by engineering root microenvironment with Streptomyces inoculants. Microbiol. Res. 2023, 277, 127509. [Google Scholar] [CrossRef]
- Jamil, F.; Mukhtar, H.; Fouillaud, M.; Dufossé, L. Rhizosphere signaling: Insights into plant-rhizomicrobiome interactions for sustainable agronomy. Microorganisms 2022, 10, 899. [Google Scholar] [CrossRef] [PubMed]
- Chamkhi, I.; El Omari, N.; Balahbib, A.; El Menyiy, N.; Benali, T.; Ghoulam, C. Is—The rhizosphere a source of applicable multi-beneficial microorganisms for plant enhancement? Saudi J. Biol. Sci. 2022, 29, 1246–1259. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xiong, W.; Dini-Andreote, F.; Wang, B.; Tao, C.; Ruan, Y.; Shen, Z.; Li, R.; Shen, Q. Changes in bulk soil affect the disease-suppressive rhizosphere microbiome against Fusarium wilt disease. Front. Agric. Sci. Eng. 2020, 7, 307–316. [Google Scholar] [CrossRef]
- Birt, H.W.G.; Tharp, C.L.; Custer, G.F.; Dini-Andreote, F. Root phenotypes as modulators of microbial microhabitats. Front. Plant Sci. 2022, 13, 1003868. [Google Scholar] [CrossRef]
- Gruet, C.; Oudot, A.; Abrouk, D.; Moënne-Loccoz, Y.; Muller, D. Rhizophere analysis of auxin producers harboring the phenylpyruvate decarboxylase pathway. Appl. Soil Ecol. 2022, 173, 104363. [Google Scholar] [CrossRef]
- Farag, M.A.; Song, G.C.; Park, Y.-S.; Audrain, B.; Lee, S.; Ghigo, J.-M.; Kloepper, J.W.; Ryu, C.-M. Biological and chemical strategies for exploring inter- and intra-kingdom communication mediated via bacterial volatile signals. Nat. Protoc. 2017, 12, 1359–1377. [Google Scholar] [CrossRef]
- Akoijam, N.; Joshi, S.R. Bioprospecting acid- and arsenic-tolerant plant growth-promoting rhizobacteria for mitigation of arsenic toxicity in acidic agricultural soils. Arch. Microbiol. 2023, 205, 229. [Google Scholar] [CrossRef]
- Silva, E.R.; Zoz, J.; Oliveira, C.E.S.; Zuffo, A.M.; Steiner, F.; Zoz, T.; Vendruscolo, E.P. Can co-inoculation of Bradyrhizobium and Azospirillum alleviate adverse effects of drought stress on soybean (Glycine max L. Merrill.)? Arch. Microbiol. 2019, 201, 325–335. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Pinto, A.P.; Teixeira, D.M.; Barrulas, P.; Brito, I.; Carvalho, M. Subcellular element distribution in shoots of wheat grown in an acidic soil with native AMF extraradical mycelium. Agronomy 2022, 12, 2173. [Google Scholar] [CrossRef]
- Palmieri, D.; Vitale, S.; Lima, G.; Di Pietro, A.; Turrà, D. A bacterial endophyte exploits chemotropism of a fungal pathogen for plant colonization. Nat. Commun. 2020, 11, 5264. [Google Scholar] [CrossRef]
- Ge, A.H.; Liang, Z.H.; Xiao, J.L.; Zhang, Y.; Zeng, Q.; Xiong, C.; Han, L.L.; Wang, J.T.; Zhang, L.M. Microbial assembly and association network in watermelon rhizosphere after soil fumigation for Fusarium wilt control. Agric. Ecosyst. Environ. 2021, 312, 107336. [Google Scholar] [CrossRef]
- Stringlis, I.A.; Yu, K.E.; Feussner, K.; de Jonge, R.; Van Bentum, S.; Van Verk, M.C.; Berendsen, R.L.; Bakker, P.A.; Feussner, I.; Pieterse, C.M. MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc. Natl. Acad. Sci. USA 2018, 115, E5213–E5222. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G.; Shen, R.F.; He, J.Z.; Wang, Y.F.; Han, X.G.; Jia, Z.J. China soil microbiome initiative: Progress and perspective. Bull. Chin. Acad. Sci. 2017, 32, 554–565. [Google Scholar]
- Sarathambal, C.; Khankhane, P.J.; Gharde, Y.; Kumar, B.; Varun, M.; Arun, S. The effect of plant growth-promoting rhizobacteria on the growth, physiology, and Cd uptake of Arundo donax L. Int. J. Phytoremediation 2017, 19, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Youfa, L. Effects of Phytoremediation on Heavy Metal Biogeochemistry in Zinc Smelting Waste Residue; Guizhou University: Guiyang, China, 2018. [Google Scholar]
- Guarino, F.; Miranda, A.; Castiglione, S.; Cicatelli, A. Arsenic phytovolatilization and epigenetic modifications in Arundo donax L. assisted by a PGPR consortium. Chemosphere 2020, 251, 126310. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, X.; Mo, L.; Zeng, J.; Pu, G. Effect of microbial inoculants on remediation of PB contaminated soil by Arundo donax. J. Plant Resour. Environ. 2023, 32, 43–49. [Google Scholar]
- Ondreičková, K.; Gubišová, M.; Gubiš, J.; Klčová, L.; Horník, M. Rhizosphere Bacterial Communities of Arundo Donax Grown in Soil Fertilised with Sewage Sludge and Agricultural by-Products. Agriculture 2019, 65, 37–41. [Google Scholar] [CrossRef]
- Liang, M.; Li, Y.; Xie, S.; Guo, F.; Zou, J.; Li, Z. AFLP and ISSR analysis of genetic diversity of Arundo donax provenances. Mol. Plant Breed. 2014, 1–9. [Google Scholar]
- Xian, K.; Su, J.; Fu, C.; Huang, N.; Gong, Q.; He, J. Studies on tissue culture and rapid propagation of Arundo donax. Plants Guangxi 2018, 38, 128–134. [Google Scholar]
- Ercoli, L.; Rossetto, R.; Di Giorgi, S.; Raffaelli, A.; Nuti, M.; Pellegrino, E. Effective bioremediation of clarithromycin and diclofenac in wastewater by microbes and Arundo donax L. Environ. Sci. Pollut. Res. Int. 2023, 30, 77193–77209. [Google Scholar] [CrossRef] [PubMed]
- Maria Sabeen, M.S.; Qaisar Mahmood, Q.M.; Muhammad Irshad, M.I.; Iftikhar Fareed, I.F.; Afsar Khan, A.K.; Farid Ullah, F.U.; Jamshaid Hussain, J.H.; Yousaf Hayat, Y.H.; Sobia Tabassum, S.T. Cadmium phytoremediation by Arundo donax L. from contaminated soil and water. BioMed Res. Int. 2013, 2013, 324830. [Google Scholar]
- Jianhong, C. Environmental Engineering Microbiology; The Wuhan University of Technology Press: Wuhan, China, 2003; pp. 21–23. [Google Scholar]
- Yang, Y.; Li, H.; Ma, K.; Wang, Y.; Niu, S. Advances in actinomycetes and their metabolites based on CiteSpace visualization analysis. J. Microbiol. 2022, 62, 3825–3843. [Google Scholar] [CrossRef]
- Cui, X.; Lin, X.; Li, J.; Zhang, H.; Han, Y. Diversity and functional properties of stress-resistant actinomycetes and their application in environmental remediation. J. Microbiol. 2023, 63, 1930–1943. [Google Scholar] [CrossRef]
- Xiuqin, Y. “The 13th Five-Year Plan”, Microbiology and Immunology, 2nd ed.; Jiangsu Fenghuang Science and Technology Press: Wuhan, China, 2018; pp. 135–137. [Google Scholar]
- Xu, R.; Sun, X.; Häggblom, M.M.; Dong, Y.; Zhang, M. Metabolic potentials of members of the class Acidobacteriia in metal-contaminated soils revealed by metagenomic analysis. Environ. Microbiol. 2022, 24, 803–818. [Google Scholar] [CrossRef]
- Gqozo, M.P.; Bill, M.; Siyoum, N.; Labuschagne, N.; Korsten, L. Fungal diversity and community composition of wheat rhizosphere and non-rhizosphere soils from three different agricultural production regions of South Africa. Appl. Soil Ecol. 2020, 151, 103543. [Google Scholar] [CrossRef]
- Flombaum, P.; Gallegos, J.L.; Gordillo, R.A.; Rincón, J.; Zabala, L.L.; Jiao, N.; Karl, D.M.; Li, W.K.; Lomas, M.W.; Veneziano, D.; et al. Present and Future Global Distributions of the Marine Cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl. Acad. Sci. USA 2013, 110, 9824–9829. [Google Scholar] [CrossRef]
- Haolin, L.; Bing, Y.; Jinghua, F. Effects of cyanobacteria colonization on microplastics on marine ecosystems. Environ. Ecol. 2024, 6, 41–49. [Google Scholar]
- Renuka, N.; Sood, A.; Ratha, S.K.; Prasanna, R.; Ahluwalia, A.S. Evaluation of Microalgal Consortia for Treatment of Primary Treated Sewage Effluent and Biomass Production. J. Appl. Phycol. 2013, 25, 1529–1537. [Google Scholar] [CrossRef]
- Xue, Z.; Tina, C.; Yanhui, N. Illumina high-throughput sequencing revealed the composition and diversity of soil fungal communities in the rhizosphere of six halophytes in Aibi Lake Wetland. J. Microbiol. 2021, 61, 3965–3976. [Google Scholar]
- Xu, X.N.; Quan, F.; Wei, Y.Q.; Yang, C.; Zhang, H.; Lan, G.Y. Diversity of rhizosphere fungal community of Hevea brasiliensis in rubber planting areas of Hainan and Xishuangbanna. J. South. Agric. 2022, 53, 2392–2402. [Google Scholar]
- Wu, Y.T.; Wubet, T.; Trogisch, S.; Both, S.; Scholten, T.; Bruelheide, H.; Buscot, F. Forest age and plant species composition determine the soil fungal community composition in a Chinese subtropical forest. PLoS ONE 2013, 8, e66829. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rosas-Medina, M.; Maciá-Vicente, J.G.; Piepenbring, M. Diversity of fungi in soils with different degrees of degradation in Germany and Panama. Mycobiology 2019, 48, 20–28. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Liu, M.; Wang, X.; Hong, Y.; Yao, Q.; Chang, X.; Shi, G.; Chen, W.; Tian, B.; Hegazy, A. Differences in the Microbial Composition and Function of the Arundo donax Rhizosphere Under Different Cultivation Conditions. Microorganisms 2024, 12, 2642. https://doi.org/10.3390/microorganisms12122642
Yang F, Liu M, Wang X, Hong Y, Yao Q, Chang X, Shi G, Chen W, Tian B, Hegazy A. Differences in the Microbial Composition and Function of the Arundo donax Rhizosphere Under Different Cultivation Conditions. Microorganisms. 2024; 12(12):2642. https://doi.org/10.3390/microorganisms12122642
Chicago/Turabian StyleYang, Fan, Miaomiao Liu, Xin Wang, Yuting Hong, Qiuju Yao, Xiaoke Chang, Gongyao Shi, Weiwei Chen, Baoming Tian, and Abeer Hegazy. 2024. "Differences in the Microbial Composition and Function of the Arundo donax Rhizosphere Under Different Cultivation Conditions" Microorganisms 12, no. 12: 2642. https://doi.org/10.3390/microorganisms12122642
APA StyleYang, F., Liu, M., Wang, X., Hong, Y., Yao, Q., Chang, X., Shi, G., Chen, W., Tian, B., & Hegazy, A. (2024). Differences in the Microbial Composition and Function of the Arundo donax Rhizosphere Under Different Cultivation Conditions. Microorganisms, 12(12), 2642. https://doi.org/10.3390/microorganisms12122642




