Release of Biopolymers from Saccharomyces cerevisiae Biomass Through Thermal and Non-Thermal Technologies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Biomasses
2.2. Biomass Treatments
2.2.1. High-Pressure Homogenisation (HPH) Treatment
2.2.2. Pulsed Electric Field (PEF) Treatment
2.3. Microscopic Observation
2.4. Evaluation of the Material Composition After Different Treatments
2.4.1. Dry Matter
2.4.2. Carbohydrates (CH) Concentration
2.4.3. Proteins Concentration
2.4.4. β-(1,3)(1,6)-glucan Content
2.4.5. Mannoproteins Content
2.5. Determination of the Dispersible Index (DI%)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Microscopic Observations
3.2. Dry Matter Content of Baker’s and Brewer’s Spent Saccharomyces cerevisiae Yeast
3.3. Evaluation of the Composition of Intracellular Material After Different Treatments
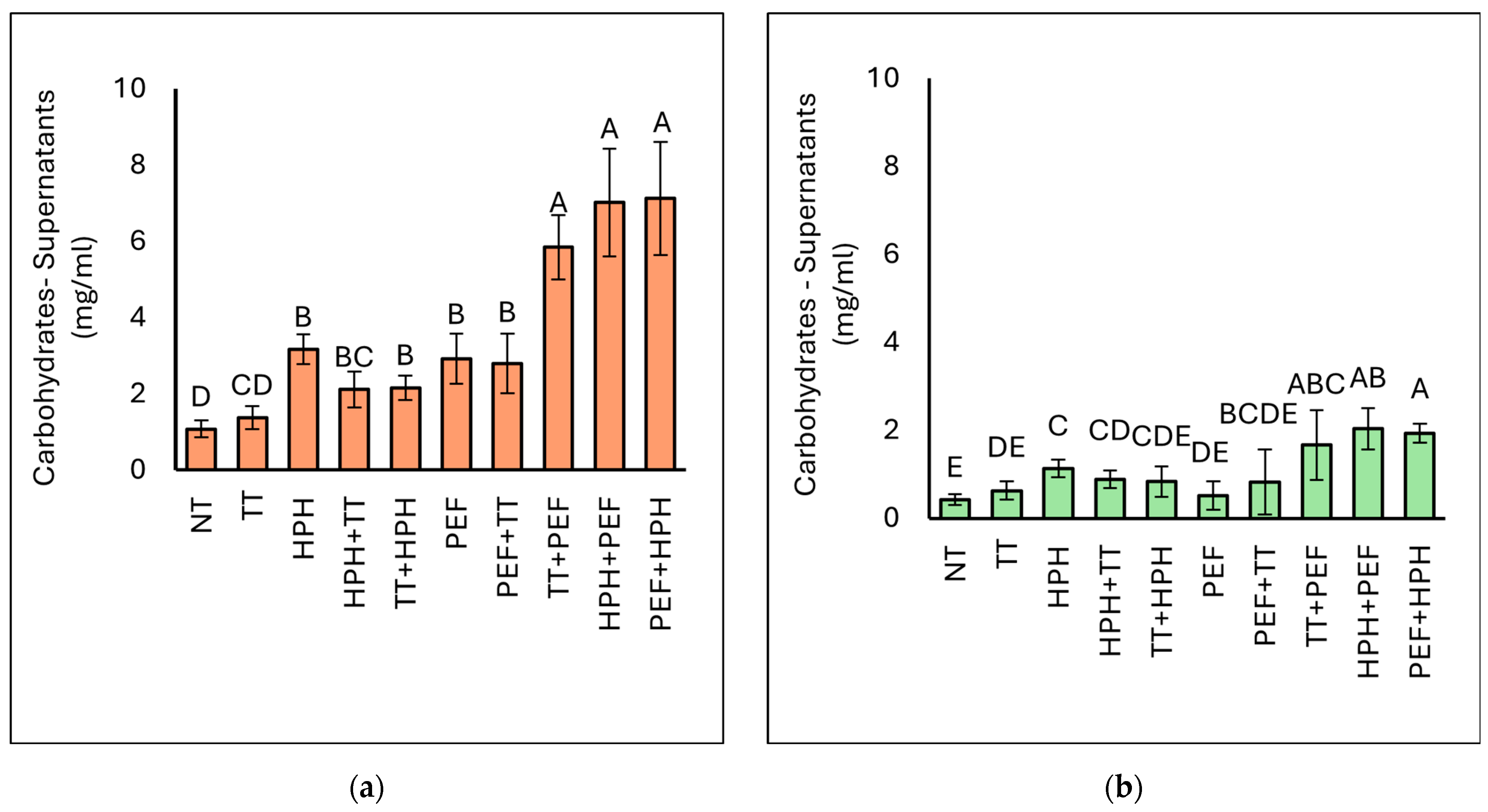
3.3.1. Carbohydrate (CH) Concentration
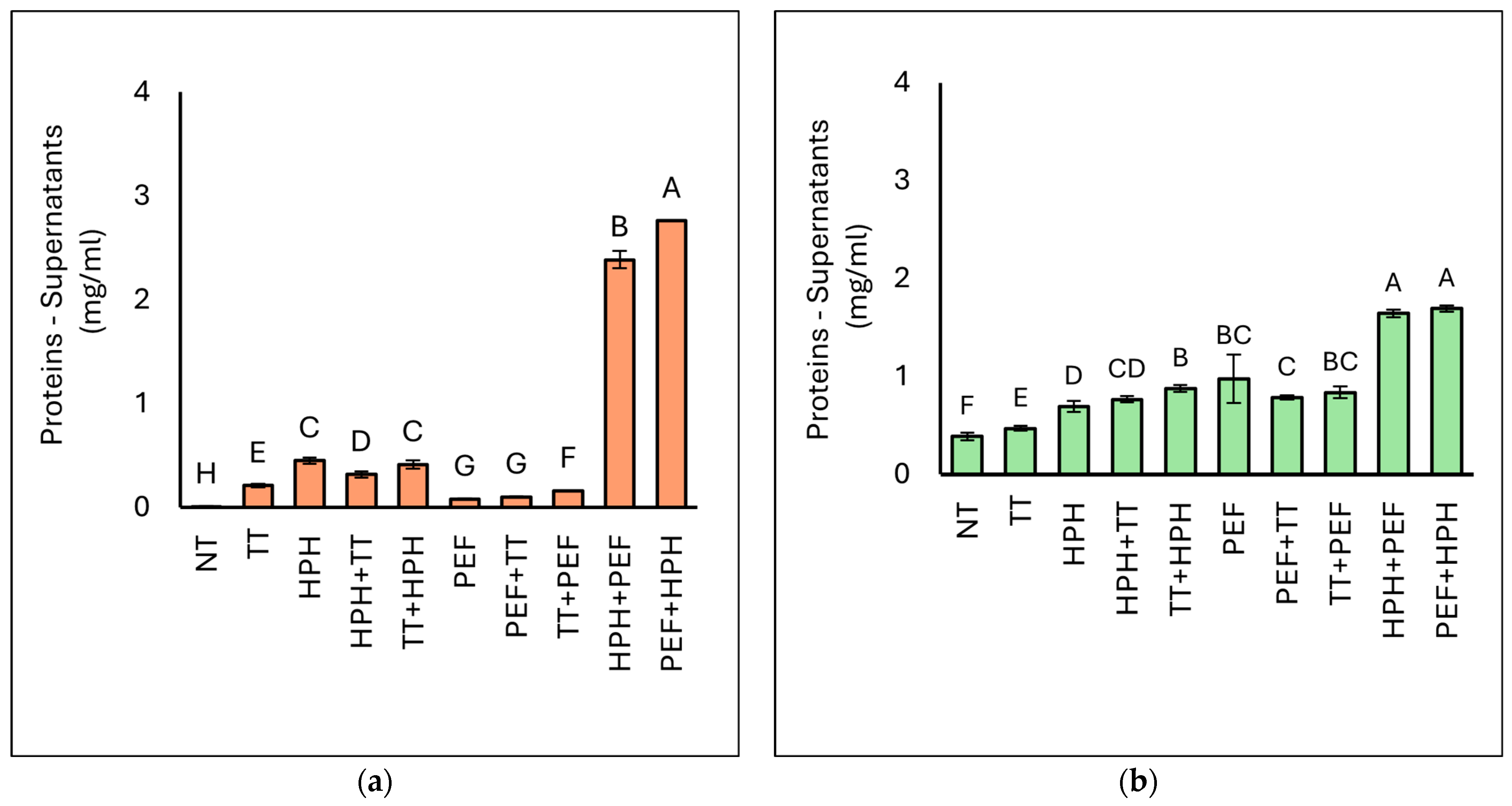
3.3.2. Protein Concentration
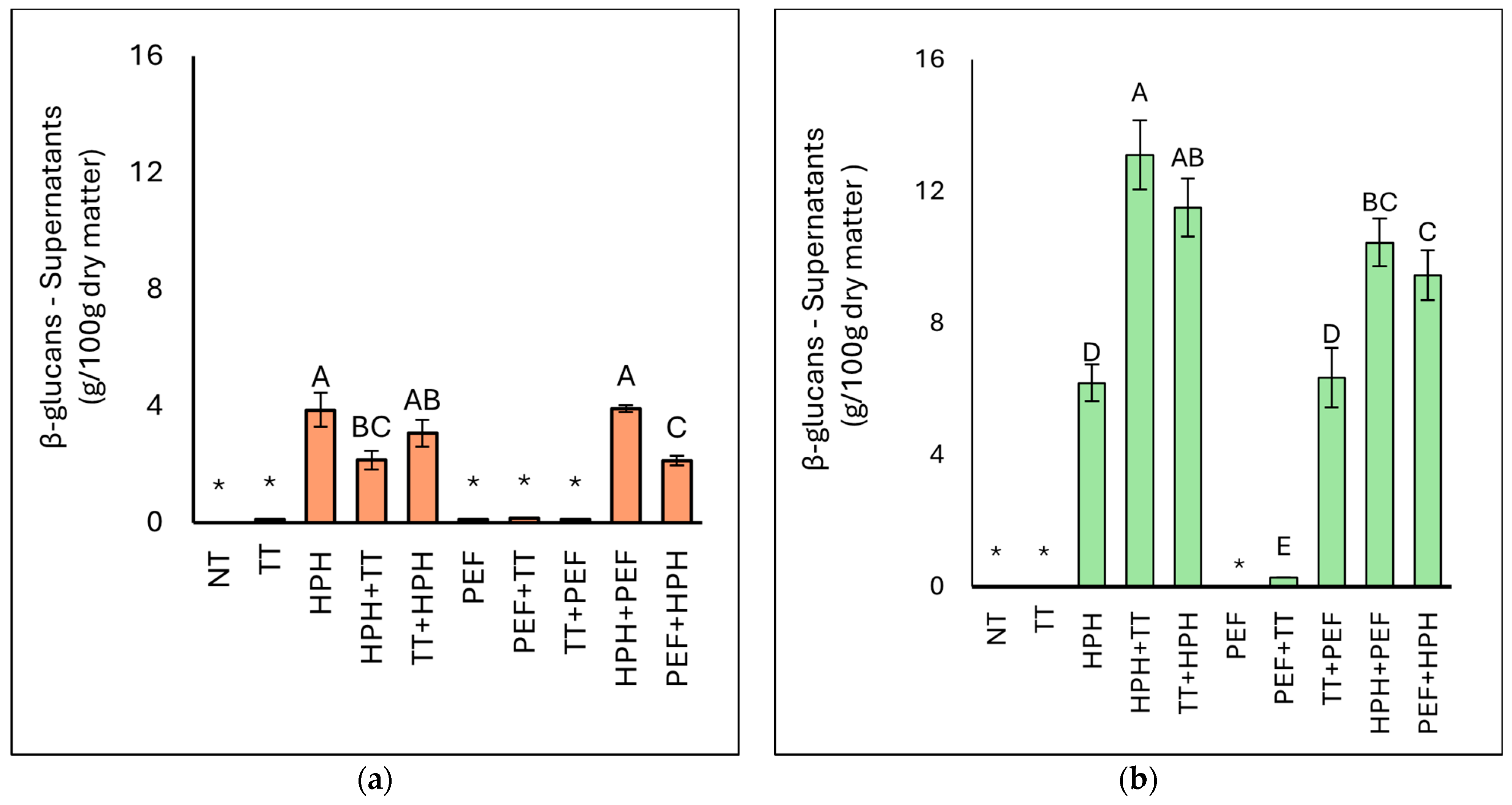
3.3.3. Determination of β-(1,3)(1,6)-Glucans Content
3.3.4. Determination of Mannoprotein/Mannose Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Crippa, M.; Solazzo, E.; Guizzardi, D.; Monforti-Ferrario, F.; Tubiello, F.N.; Leip, A. Food Systems Are Responsible for a Third of Global Anthropogenic GHG Emissions. Nat. Food 2021, 2, 198–209. [Google Scholar] [CrossRef]
- Jamwal, V.; Mittal, A.; Dhaundiyal, A. Valorization of Agro-Industrial Waste in Composite Films for Sustainable Packaging Applications. Mater. Today Proc. 2023, 113, 94–100. [Google Scholar] [CrossRef]
- Tardy, B.L.; Richardson, J.J.; Greca, L.G.; Guo, J.; Bras, J.; Rojas, O.J. Advancing Bio-Based Materials for Sustainable Solutions to Food Packaging. Nat. Sustain. 2023, 6, 360–367. [Google Scholar] [CrossRef]
- Peltzer, M.; Delgado, J.; Salvay, A.; Wagner, J. β-Glucan, a Promising Polysaccharide for Bio-Based Films Developments for Food Contact Materials and Medical Applications. Curr. Org. Chem. 2018, 22, 1249–1254. [Google Scholar] [CrossRef]
- Ferreira, I.M.P.L.V.O.; Pinho, O.; Vieira, E.; Tavarela, J.G. Brewer’s Saccharomyces Yeast Biomass: Characteristics and Potential Applications. Trends Food Sci. Technol. 2010, 21, 77–84. [Google Scholar] [CrossRef]
- França, R.A.D.; Rosa, A.C.F.D.S.; Braz, C.J.D.F.; Barbosa, R.; Alves, T.S. Development of Mulch Films from Biodegradable Polymer and Agro-Industrial Waste. Polímeros 2024, 34, e20230042. [Google Scholar] [CrossRef]
- Hejna, A. More than Just a Beer—The Potential Applications of by-Products from Beer Manufacturing in Polymer Technology. Emergent. Mater. 2022, 5, 765–783. [Google Scholar] [CrossRef]
- Rachwał, K.; Waśko, A.; Gustaw, K.; Polak-Berecka, M. Utilization of Brewery Wastes in Food Industry. PeerJ 2020, 8, e9427. [Google Scholar] [CrossRef] [PubMed]
- Cottet, C.; Ramirez-Tapias, Y.A.; Delgado, J.F.; de la Osa, O.; Salvay, A.G.; Peltzer, M.A. Biobased Materials from Microbial Biomass and Its Derivatives. Materials 2020, 13, 1263. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.F.; Salvay, A.G.; de la Osa, O.; Wagner, J.R.; Peltzer, M.A. Impact of the Film-Forming Dispersion PH on the Properties of Yeast Biomass Films. J. Sci. Food Agric. 2021, 101, 5636–5644. [Google Scholar] [CrossRef]
- Faustino, M.; Durão, J.; Pereira, C.F.; Oliveira, A.S.; Pereira, J.O.; Pereira, A.M.; Ferreira, C.; Pintado, M.E.; Carvalho, A.P. Comparative Analysis of Mannans Extraction Processes from Spent Yeast Saccharomyces cerevisiae. Foods 2022, 11, 3753. [Google Scholar] [CrossRef] [PubMed]
- Avramia, I.; Amariei, S. Spent Brewer’s Yeast as a Source of Insoluble β-Glucans. Int. J. Mol. Sci. 2021, 22, 825. [Google Scholar] [CrossRef] [PubMed]
- Tam, T.M.; Duy, N.Q.; Minh, N.P.; Dao, D.T.A. Optimization of Βeta-Glucan Extraction from Waste Brewer’s Yeast Saccharomyces cerevisiae Using Autolysis, Enzyme, Ultrasonic and Combined Enzyme—Ultrasonic Treatment. Am. J. Res. Commun. 2013, 1, 149–158. [Google Scholar]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A Concise Review on the Molecular Structure and Function Relationship of β-Glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef] [PubMed]
- Novák, M.; Synytsya, A.; Gedeon, O.; Slepička, P.; Procházka, V.; Synytsya, A.; Blahovec, J.; Hejlová, A.; Čopíková, J. Yeast β(1-3),(1-6)-d-Glucan Films: Preparation and Characterization of Some Structural and Physical Properties. Carbohydr. Polym. 2012, 87, 2496–2504. [Google Scholar] [CrossRef]
- Razzaq, H.A.A.; Pezzuto, M.; Santagata, G.; Silvestre, C.; Cimmino, S.; Larsen, N.; Duraccio, D. Barley β-Glucan-Protein Based Bioplastic Film with Enhanced Physicochemical Properties for Packaging. Food Hydrocoll. 2016, 58, 276–283. [Google Scholar] [CrossRef]
- Sherafatkhah Azari, S.; Alizadeh, A.; Roufegarinejad, L.; Asefi, N.; Hamishehkar, H. Preparation and Characterization of Gelatin/β-Glucan Nanocomposite Film Incorporated with ZnO Nanoparticles as an Active Food Packaging System. J. Polym. Environ. 2021, 29, 1143–1152. [Google Scholar] [CrossRef]
- Podpora, B.; Świderski, F.; Sadowska, A.; Piotrowska, A.; Rakowska, R. Spent Brewer’s Yeast Autolysates as a New and Valuable Component of Functional Food and Dietary Supplements. J. Food Process. Technol. 2015, 6, 12. [Google Scholar] [CrossRef]
- Chakka, A.K.; Babu, A.S. Bioactive Compounds of Winery By-Products: Extraction Techniques and Their Potential Health Benefits. Appl. Food Res. 2022, 2, 100058. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Cui, S.; Liu, H. A New Isolation Method of β-d-Glucans from Spent Yeast Saccharomyces cerevisiae. Food Hydrocoll. 2008, 22, 239–247. [Google Scholar] [CrossRef]
- Utama, G.L.; Oktaviani, L.; Balia, R.L.; Rialita, T. Potential Application of Yeast Cell Wall Biopolymers as Probiotic Encapsulants. Polymers 2023, 15, 3481. [Google Scholar] [CrossRef] [PubMed]
- Patrignani, F.; Lanciotti, R. Applications of High and Ultra High Pressure Homogenization for Food Safety. Front. Microbiol. 2016, 7, 1132. [Google Scholar] [CrossRef]
- Liu, D.; Ding, L.; Sun, J.; Boussetta, N.; Vorobiev, E. Yeast Cell Disruption Strategies for Recovery of Intracellular Bio-Active Compounds—A Review. Innov. Food Sci. Emerg. Technol. 2016, 36, 181–192. [Google Scholar] [CrossRef]
- Gottardi, D.; Siroli, L.; Braschi, G.; Rossi, S.; Ferioli, F.; Vannini, L.; Patrignani, F.; Lanciotti, R. High-Pressure Homogenization and Biocontrol Agent as Innovative Approaches Increase Shelf Life and Functionality of Carrot Juice. Foods 2021, 10, 2998. [Google Scholar] [CrossRef]
- Dimopoulos, G.; Tsantes, M.; Taoukis, P. Effect of High Pressure Homogenization on the Production of Yeast Extract via Autolysis and Beta-Glucan Recovery. Innov. Food Sci. Emerg. Technol. 2020, 62, 102340. [Google Scholar] [CrossRef]
- Spiden, E.M.; Scales, P.J.; Kentish, S.E.; Martin, G.J.O. Critical Analysis of Quantitative Indicators of Cell Disruption Applied to Saccharomyces cerevisiae Processed with an Industrial High Pressure Homogenizer. Biochem. Eng. J. 2013, 70, 120–126. [Google Scholar] [CrossRef]
- Dimopoulos, G.; Stefanou, N.; Andreou, V.; Taoukis, P. Effect of Pulsed Electric Fields on the Production of Yeast Extract by Autolysis. Innov. Food Sci. Emerg. Technol. 2018, 48, 287–295. [Google Scholar] [CrossRef]
- Liu, D.; Lebovka, N.I.; Vorobiev, E. Impact of Electric Pulse Treatment on Selective Extraction of Intracellular Compounds from Saccharomyces cerevisiae Yeasts. Food Bioproc. Tech. 2013, 6, 576–584. [Google Scholar] [CrossRef]
- Shynkaryk, M.V.; Lebovka, N.I.; Lanoisellé, J.-L.; Nonus, M.; Bedel-Clotour, C.; Vorobiev, E. Electrically-Assisted Extraction of Bio-Products Using High Pressure Disruption of Yeast Cells (Saccharomyces cerevisiae). J. Food Eng. 2009, 92, 189–195. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed Electric Field-Assisted Extraction of Valuable Compounds from Microorganisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 530–552. [Google Scholar] [CrossRef] [PubMed]
- Ganeva, V.; Galutzov, B.; Teissie, J. Evidence That Pulsed Electric Field Treatment Enhances the Cell Wall Porosity of Yeast Cells. Appl. Biochem. Biotechnol. 2014, 172, 1540–1552. [Google Scholar] [CrossRef] [PubMed]
- Bzducha-Wróbel, A.; Błażejak, S.; Kawarska, A.; Stasiak-Różańska, L.; Gientka, I.; Majewska, E. Evaluation of the Efficiency of Different Disruption Methods on Yeast Cell Wall Preparation for β-Glucan Isolation. Molecules 2014, 19, 20941–20961. [Google Scholar] [CrossRef] [PubMed]
- Paniagua-Martínez, I.; Ramírez-Martínez, A.; Serment-Moreno, V.; Rodrigues, S.; Ozuna, C. Non-Thermal Technologies as Alternative Methods for Saccharomyces cerevisiae Inactivation in Liquid Media: A Review. Food Bioproc. Tech. 2018, 11, 487–510. [Google Scholar] [CrossRef]
- Marín-Sánchez, J.; Berzosa, A.; Álvarez, I.; Sánchez-Gimeno, C.; Raso, J. Selective Extraction of Biomolecules from Saccharomyces cerevisiae Assisted by High-Pressure Homogenization, Pulsed Electric Fields, and Heat Treatment: Exploring the Effect of Endogenous Enzymes. LWT 2024, 207, 116614. [Google Scholar] [CrossRef]
- Berzosa, A.; Delso, C.; Sanz, J.; Sánchez-Gimeno, C.; Raso, J. Sequential Extraction of Compounds of Interest from Yeast Biomass Assisted by Pulsed Electric Fields. Front. Bioeng. Biotechnol. 2023, 11, 1197710. [Google Scholar] [CrossRef] [PubMed]
- Carullo, D.; Abera, B.D.; Scognamiglio, M.; Donsì, F.; Ferrari, G.; Pataro, G. Application of Pulsed Electric Fields and High-Pressure Homogenization in Biorefinery Cascade of C. vulgaris Microalgae. Foods 2022, 11, 471. [Google Scholar] [CrossRef]
- Carullo, D.; Abera, B.D.; Casazza, A.A.; Donsì, F.; Perego, P.; Ferrari, G.; Pataro, G. Effect of Pulsed Electric Fields and High Pressure Homogenization on the Aqueous Extraction of Intracellular Compounds from the Microalgae Chlorella vulgaris. Algal Res. 2018, 31, 60–69. [Google Scholar] [CrossRef]
- Delgado, J.F.; Sceni, P.; Peltzer, M.A.; Salvay, A.G.; de la Osa, O.; Wagner, J.R. Development of Innovative Biodegradable Films Based on Biomass of Saccharomyces cerevisiae. Innov. Food Sci. Emerg. Technol. 2016, 36, 83–91. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Ganeva, V.; Angelova, B.; Galutzov, B.; Goltsev, V.; Zhiponova, M. Extraction of Proteins and Other Intracellular Bioactive Compounds From Baker’s Yeasts by Pulsed Electric Field Treatment. Front. Bioeng. Biotechnol. 2020, 8, 552335. [Google Scholar] [CrossRef]
- Gottardi, D.; Ciccone, M.; Siroli, L.; Lanciotti, R.; Patrignani, F. Use of Yarrowia lipolytica to obtain fish waste functional hydrolysates rich in flavoring compounds. Fermentation 2022, 8, 708. [Google Scholar] [CrossRef]
- Vaithanomsat, P.; Boonlum, N.; Trakunjae, C.; Apiwatanapiwat, W.; Janchai, P.; Boondaeng, A.; Phalinphattharakit, K.; Nimitkeatkai, H.; Jarerat, A. Functionality of Yeast β-Glucan Recovered from Kluyveromyces marxianus by Alkaline and Enzymatic Processes. Polymers 2022, 14, 1582. [Google Scholar] [CrossRef] [PubMed]
- Varelas, V.; Liouni, M.; Calokerinos, A.C.; Nerantzis, E.T. An Evaluation Study of Different Methods for the Production of β-D-Glucan from Yeast Biomass. Drug Test Anal. 2016, 8, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Quirós, M.; Gonzalez, R.; Morales, P. A Simple Method for Total Quantification of Mannoprotein Content in Real Wine Samples. Food. Chem. 2012, 134, 1205–1210. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Maza, M.A.; Álvarez, I.; Raso, J. Pulsed Electric Fields Accelerate Release of Mannoproteins from Saccharomyces cerevisiae during Aging on the Lees of Chardonnay Wine. Food Res. Int. 2019, 116, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.A.; Wagner, J.R.; Vasallo, M.C.; García, L.; Añón, M.C. Thermal Behavior and Hydration Properties of Yeast Proteins from Saccharomyces cerevisiae and Kluyveromyces fragilis. Food Chem. 2000, 69, 161–165. [Google Scholar] [CrossRef]
- Ekpeni, L.E.N. Investigation and Disruption of Baker’s Yeast/Chlorella vulgaris in High-Pressure Homogenizer (HPH) to Improve Cost-Effective Protein Yield. Ph.D. Thesis, Dublin City University, Dublin, Ireland, 2015. [Google Scholar]
- Huang, Z.; Zhang, J.; Zhang, G.; Gao, F.; Bi, C. The Impact of High-Pressure Homogenization and Thermal Processing on the Functional Properties of De-Fatted Chickpea Flour Dispersion. Foods 2023, 12, 1513. [Google Scholar] [CrossRef] [PubMed]
- Middelberg, A.P.J. Process-Scale Disruption of Microorganisms. Biotechnol. Adv. 1995, 13, 491–551. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.M.; Cebrián, G.; Álvarez, I.; Raso, J. Release of Mannoproteins during Saccharomyces cerevisiae Autolysis Induced by Pulsed Electric Field. Front. Microbiol. 2016, 7, 1435. [Google Scholar] [CrossRef]
- Ganeva, V.; Galutzov, B.; Teissié, J. High Yield Electroextraction of Proteins from Yeast by a Flow Process. Anal Biochem. 2003, 315, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R. Impact of Emerging Technologies on the Cell Disruption and Fractionation of Microalgal Biomass. Ph.D. Thesis, Université de Technologie de Compiègne, Compiègne, France, 2020. [Google Scholar]
- Balasundaram, B.; Harrison, S.T.L. Influence of the Extent of Disruption of Bakers’ Yeast on Protein Adsorption in Expanded Beds. J. Biotechnol. 2008, 133, 360–369. [Google Scholar] [CrossRef]
- Gentès, M.-C.; Caron, A.; Champagne, C.P. Potential Applications of Pulsed Electric Field in Cheesemaking. Int J. Dairy Technol. 2022, 75, 270–288. [Google Scholar] [CrossRef]
- Suphantharika, M.; Khunrae, P.; Thanardkit, P.; Verduyn, C. Preparation of Spent Brewer’s Yeast B-Glucans with a Potential Application as an Immunostimulant for Black Tiger Shrimp, Penaeus Monodon. Bioresour. Technol. 2003, 88, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Dallies, N.; François, J.; Paquet, V. A New Method for Quantitative Determination of Polysaccharides in the Yeast Cell Wall. Application to the Cell Wall Defective Mutants of Saccharomyces cerevisiae. Yeast 1998, 14, 1297–1306. [Google Scholar] [CrossRef]
- Marinescu, G.; Stoicescu, A. Researches concerning the preparation of spent brewer’s yeast β-glucans. J. Agroaliment. Process. Technol. 2009, 15, 547–553. [Google Scholar]
- Giancone, T.; Torrieri, E.; Masi, P.; Michon, C. Protein–Polysaccharide Interactions: Phase Behaviour of Pectin–Soy Flour Mixture. Food Hydrocoll. 2009, 23, 1263–1269. [Google Scholar] [CrossRef]
- Hunter Jr, K.W.; Gault, R.A.; Berner, M.D. Preparation of Microparticulate Β-glucan from Saccharomyces cerevisiae for Use in Immune Potentiation. Lett. Appl. Microbiol. 2002, 35, 267–271. [Google Scholar] [CrossRef]
- Zechner-Krpan, V.; Petravić-Tominac, V.; Galović, P.; Galović, V.; Filipović-Grčić, J.; Srečec, S. Application of Different Drying Methods on β-Glucan Isolated from Spent Brewer’s Yeast Using Alkaline Procedure. Agric. Conspec. Sci. 2010, 75, 45–50. [Google Scholar]
- Ganeva, V.; Kranz, A. Selective Extraction of Recombinant Membrane Proteins from Hansenula Polymorpha by Pulsed Electric Field and Lytic Enzyme Pretreatment. Microb. Cell. Fact. 2023, 22, 251. [Google Scholar] [CrossRef] [PubMed]
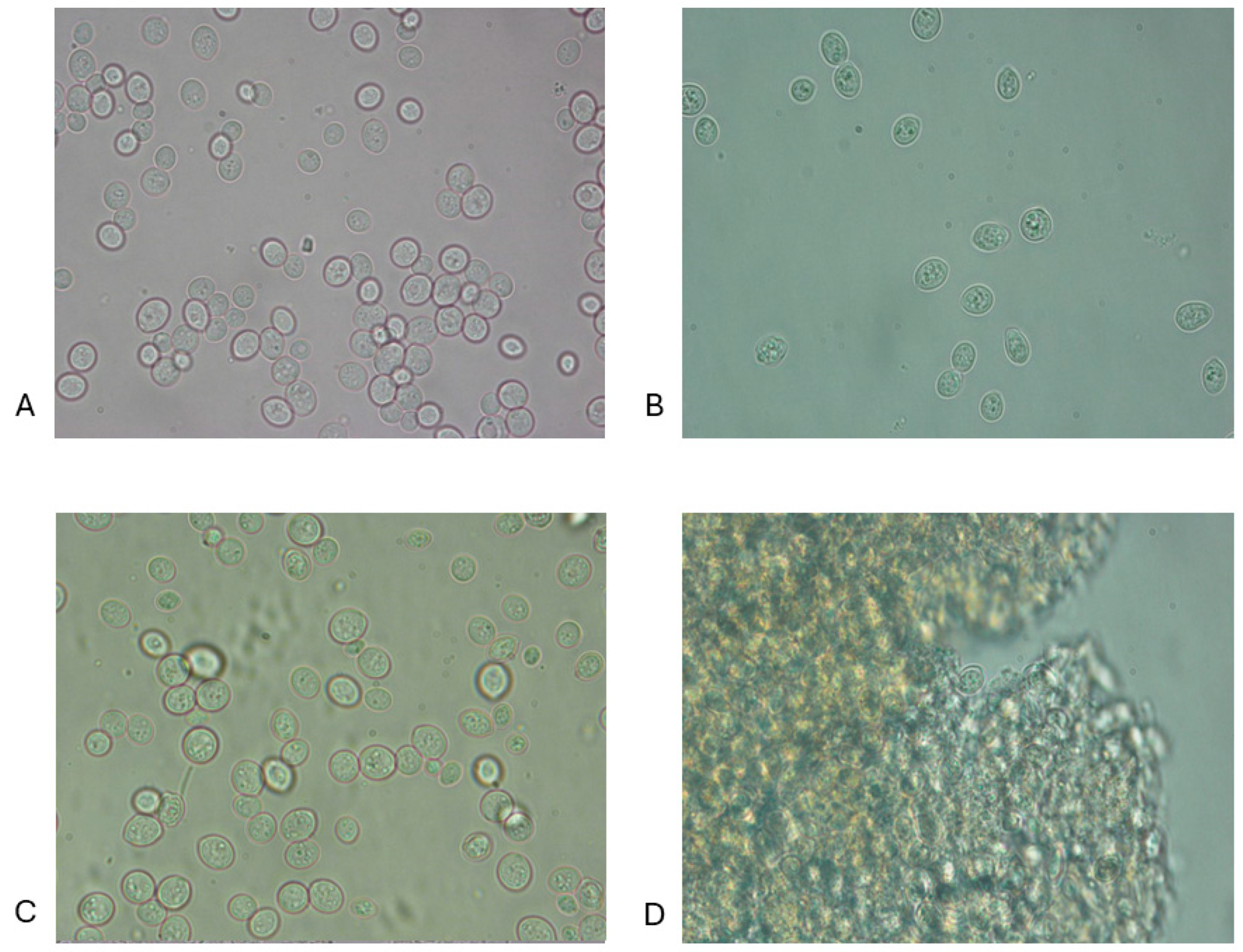


| Treatments Description | Performed Treatments |
|---|---|
| NT | Not treated |
| TT | Heat treatment (TT: 90 °C for 20 min) |
| HPH | 3 cycles of HPH at a pressure of 125 MPa |
| HPH+TT | 3 cycles of HPH at a pressure of 125 MPa followed by heat treatment (TT: 90 °C for 20 min) |
| TT+HPH | Heat treatment (TT: 90 °C for 20 min) followed by 3 cycles of HPH at a pressure of 125 MPa |
| PEF | Pulsed electric field treatment (1.5 kV/cm—45 pulses) |
| PEF+TT | Pulsed electric field treatment (1.5 kV/cm—45 pulses) followed by heat treatment (TT: 90 °C for 20 min) |
| TT+PEF | Heat treatment (TT: 90 °C for 20 min) followed by a pulsed electric field treatment (1.5 kV/cm—45 pulses) |
| HPH+PEF | 3 cycles of HPH at a pressure of 125 MPa followed by a pulsed electric field treatment (1.5 kV/cm—45 pulses) |
| PEF+HPH | 3 cycles of HPH at a pressure of 125 MPa followed by a pulsed electric field treatment (1.5 kV/cm—45 pulses) |
| Yeast Biomass | Treatment | Dispersions ± DS (%) | Supernatants ± DS (%) |
|---|---|---|---|
| BY | NT | 2.82 ± 0.25 ab | 0.04 ± 0.03 c |
| TT | 2.70 ± 0.49 ab | 0.30 ± 0.12 ab | |
| HPH | 2.68 ± 0.21 ab | 0.95 ± 0.02 a | |
| HPH+TT | 3.31 ± 0.71 a | 0.94 ± 0.02 a | |
| TT+HPH | 2.81 ± 0.63 ab | 0.95 ± 0.02 a | |
| PEF | 2.05 ± 0.56 bc | 0.11 ± 0.01 c | |
| PEF+TT | 2.59 ± 0.01 b | 0.52 ± 0.09 ab | |
| TT+PEF | 2.59 ± 0.01 b | 0.54 ± 0.04 ab | |
| HPH+PEF | 2.12 ± 0.08 bc | 0.70 ± 0.23 ab | |
| PEF+HPH | 2.24 ± 0.01 bc | 0.78 ± 0.20 ab | |
| BYS | NT | 3.08 ± 0.11 d | 0.01 ± 0.00 g |
| TT | 4.38 ± 0.83 abc | 0.05 ± 0.01 f | |
| HPH | 4.09 ± 1.26 abcd | 0.37 ± 0.07 bc | |
| HPH+TT | 2.73 ± 0.28 d | 0.36 ± 0.03 b | |
| TT+HPH | 3.31 ± 0.36 cd | 0.30 ± 0.02 c | |
| PEF | 3.12 ± 0.07 d | 0.24 ± 0.03 d | |
| PEF+TT | 3.43 ± 0.50 cd | 0.23 ± 0.01 d | |
| TT+PEF | 5.50 ± 0.39 a | 0.13 ± 0.01 e | |
| HPH+PEF | 3.96 ± 0.78 bcd | 0.89 ± 0.00 a | |
| PEF+HPH | 5.68 ± 0.57 a | 0.39 ± 0.10 bc |
| Samples | Dispersible Index of BY (%) | Dispersible Index of BSY (%) |
|---|---|---|
| NT | 6.67 ± 1.91 e | 14.76 ± 5.99 d |
| TT | 8.54 ± 2.61 de | 21.82 ± 10.32 cd |
| HPH | 19.59 ± 3.38 c | 39.25 ± 9.78 bc |
| HPH+TT | 13.09 ± 4.08 cd | 30.75 ± 9.74 cd |
| TT+HPH | 13.39 ± 2.80 cd | 28.88 ± 16.95 bcd |
| PEF | 18.02 ± 5.75 cd | 17.93 ± 15.79 cd |
| PEF+TT | 17.35 ± 6.83 cd | 28.44 ± 26.41 bcd |
| TT+PEF | 36.16 ± 7.34 a | 57.55 ± 34.23 abc |
| HPH+PEF | 43.40 ± 12.40 a | 70.37 ± 22.71 ab |
| PEF+HPH | 44.07 ± 12.98 a | 66.75 ± 10.37 a |
| Samples | Dispersible Index of BY (%) | Dispersible Index of BSY (%) |
|---|---|---|
| NT | 0.31 ± 0.17 h | 7.30 ± 1.06 d |
| TT | 7.00 ± 0.68 e | 8.81 ± 0.66 d |
| HPH | 14.94 ± 1.40 c | 13.05 ± 1.49 c |
| HPH+TT | 10.55 ± 1.38 d | 14.37 ± 0.82 bc |
| TT+HPH | 13.70 ± 1.77 cd | 16.49 ± 0.93 b |
| PEF | 2.73 ± 0.11 g | 18.37 ± 6.54 bc |
| PEF+TT | 3.42 ± 0.09 g | 14.75 ± 0.62 bc |
| TT+PEF | 5.30 ± 0.09 f | 15.70 ± 1.58 bc |
| HPH+PEF | 78.85 ± 3.99 b | 30.87 ± 1.03 a |
| PEF+HPH | 91.17 ± 0.14 a | 31.78 ± 0.82 a |
| Samples | Dispersible Index of BY (%) | Dispersible Index of BSY (%) |
|---|---|---|
| NT | 0.00 | 0.00 |
| TT | 0.26 ± 0.01 f | 0.00 |
| HPH | 14.92 ± 3.17 a | 5.66 ± 0.72 d |
| HPH+TT | 8.26 ± 1.75 cd | 11.70 ± 1.33 b |
| TT+HPH | 11.88 ± 2.52 abc | 8.54 ± 0.93 c |
| PEF | 0.05 ± 0.01 g | 0.00 |
| PEF+TT | 0.32 ± 0.01 e | 0.16 ± 0.01 f |
| TT+PEF | 0.22 ± 0.01 f | 2.06 ± 0.41 e |
| HPH+PEF | 11.19 ± 0.46 b | 23.19 ± 2.29 a |
| PEF+HPH | 6.74 ± 0.79 d | 9.23 ± 1.04 c |
| Samples | Dispersible Index of BY (%) | Dispersible Index of BSY (%) |
|---|---|---|
| NT | 0.13 ± 0.01 i | 0.32 ± 0.01 h |
| TT | 30.58 ± 3.99 d | 1.05 ± 0.10 g |
| HPH | 58.02 ± 1.24 a | 8.87 ± 0.34 c |
| HPH+TT | 14.07 ± 0.28 g | 7.06 ± 2.07 cd |
| TT+HPH | 44.46 ± 1.53 c | 5.58 ± 0.48 d |
| PEF | 6.56 ± 0.31 h | 8.29 ± 1.75 c |
| PEF+TT | 21.14 ± 0.23 f | 4.09 ± 0.48 e |
| TT+PEF | 23.07 ± 0.94 e | 2.83 ± 0.10 f |
| HPH+PEF | 45.75 ± 0.62 c | 38.23 ± 1.00 a |
| PEF+HPH | 48.03 ± 0.11 b | 27.52 ± 1.17 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciccone, M.; Khan, M.R.; Hernandez, J.B.M.; Njieukam, J.A.; Siroli, L.; Gottardi, D.; Lanciotti, R.; Rocculi, P.; Patrignani, F. Release of Biopolymers from Saccharomyces cerevisiae Biomass Through Thermal and Non-Thermal Technologies. Microorganisms 2024, 12, 2596. https://doi.org/10.3390/microorganisms12122596
Ciccone M, Khan MR, Hernandez JBM, Njieukam JA, Siroli L, Gottardi D, Lanciotti R, Rocculi P, Patrignani F. Release of Biopolymers from Saccharomyces cerevisiae Biomass Through Thermal and Non-Thermal Technologies. Microorganisms. 2024; 12(12):2596. https://doi.org/10.3390/microorganisms12122596
Chicago/Turabian StyleCiccone, Marianna, Muhammad Rehan Khan, Junior Bernardo Molina Hernandez, Joel Armando Njieukam, Lorenzo Siroli, Davide Gottardi, Rosalba Lanciotti, Pietro Rocculi, and Francesca Patrignani. 2024. "Release of Biopolymers from Saccharomyces cerevisiae Biomass Through Thermal and Non-Thermal Technologies" Microorganisms 12, no. 12: 2596. https://doi.org/10.3390/microorganisms12122596
APA StyleCiccone, M., Khan, M. R., Hernandez, J. B. M., Njieukam, J. A., Siroli, L., Gottardi, D., Lanciotti, R., Rocculi, P., & Patrignani, F. (2024). Release of Biopolymers from Saccharomyces cerevisiae Biomass Through Thermal and Non-Thermal Technologies. Microorganisms, 12(12), 2596. https://doi.org/10.3390/microorganisms12122596








