The Use of Chemical Flocculants and Chitosan as a Pre-Concentration Step in the Harvesting Process of Three Native Microalgae Species from the Canary Islands Cultivated Outdoors at the Pilot Scale
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgae Strains and Indoor Culture Conditions
2.2. Lab-Scale Flocculation
2.3. Pilot-Scale Flocculation
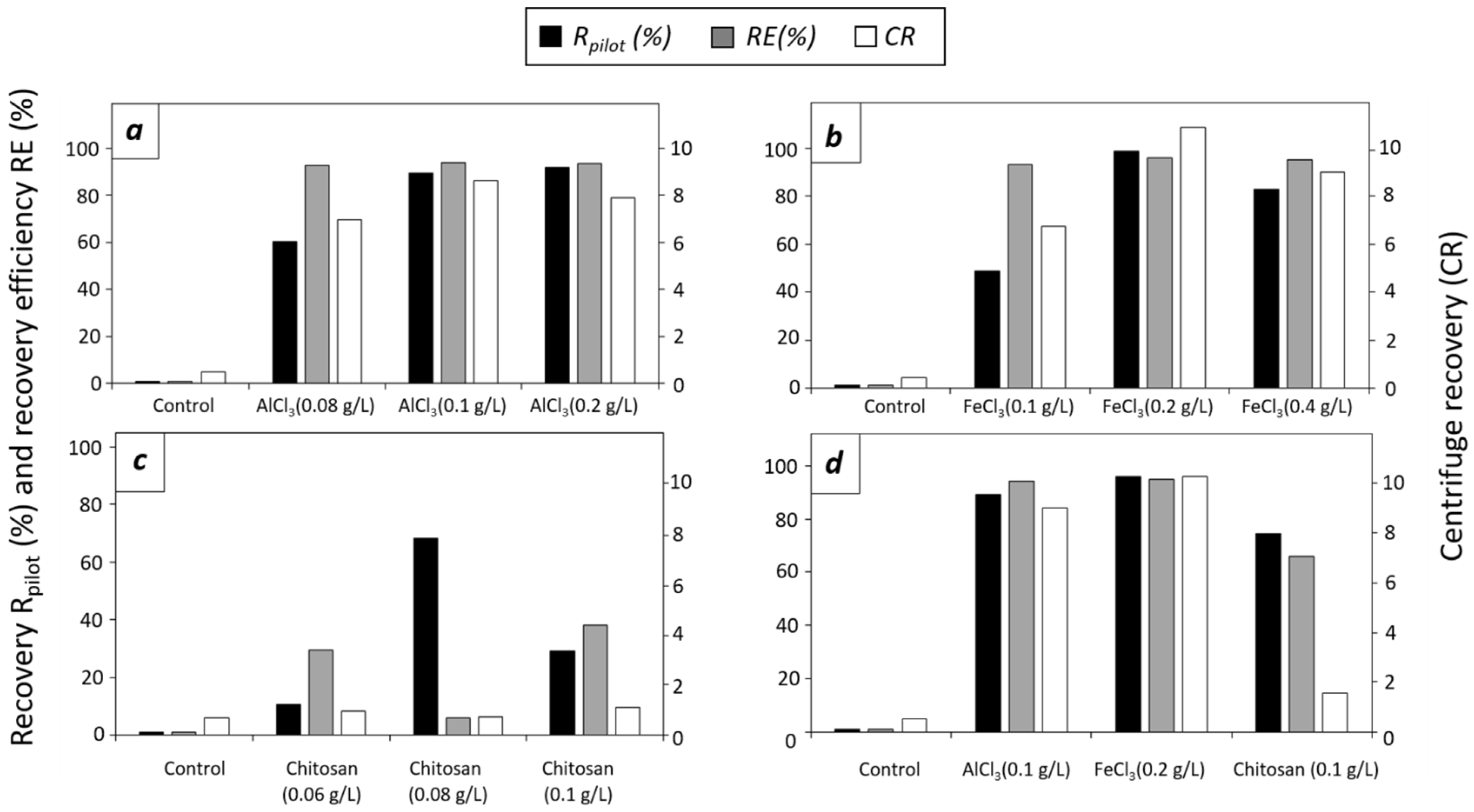
- (a)
- A single-step flocculation harvesting process was calculated as described above (Section 2.2), where the pilot-scale recovery equation iswhere OD750 (t0) is the optical density of the culture before the addition of the flocculant and OD750 (supernatant_at_t180) is the optical density of the supernatant at the end of the flocculation experiment (at t = 180 min).
- (b)
- Two-step flocculation harvesting is flocculation followed by a centrifugation process based on the calculation of the concentration factor (CF), centrifuge recovery (CR), and recovery efficiency (RE), as follows:where V0 is the initial culture volume of the PBRs (100 L) and Vf is the volume of the flocs after the 180 min flocculation period.where Charvest (g) is the weight of the paste harvested by the centrifuge, Cflocculants (g) is the weight of the flocculants added, Vharvest is the total harvested volume, and Cx0 is the biomass concentration of the control culture without the flocculants.where CR (f) is the centrifuge recovery of the concentrated phase (flocs) and CR (0) is the centrifuge recovery of the untreated culture (control) at the end of the flocculation experiments.
2.4. Biomass Analysis
2.5. Economic Viability Assessment
2.6. Statistical Analysis
3. Results
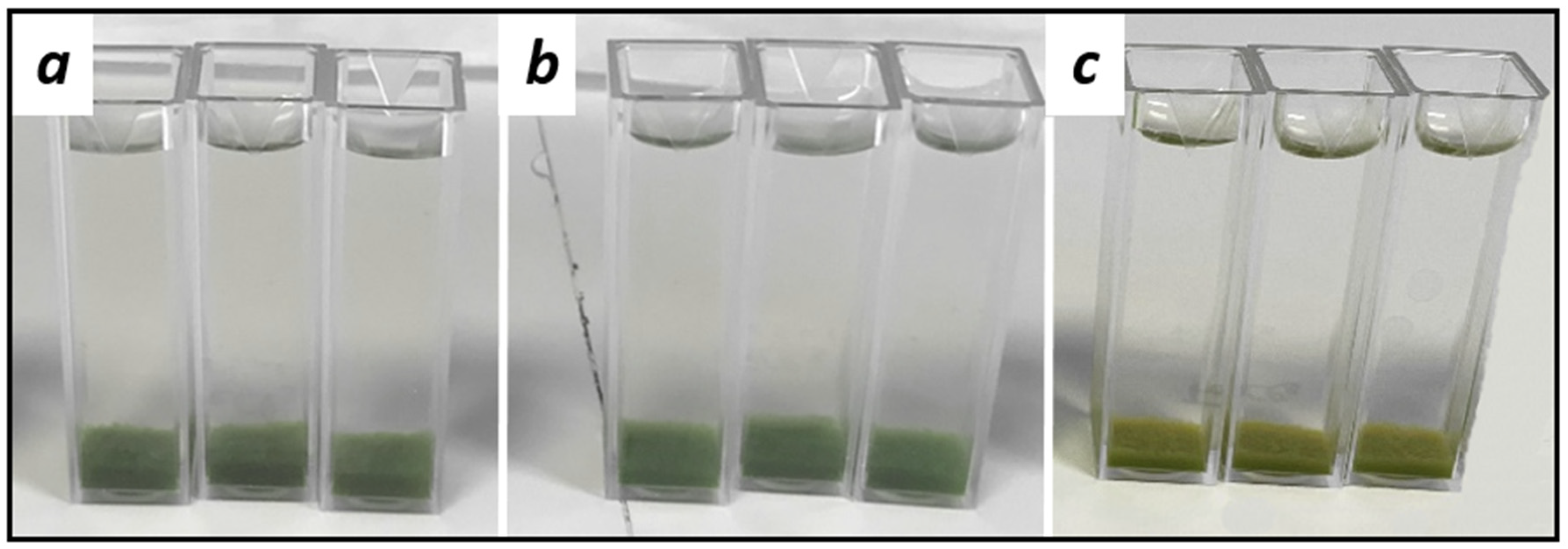
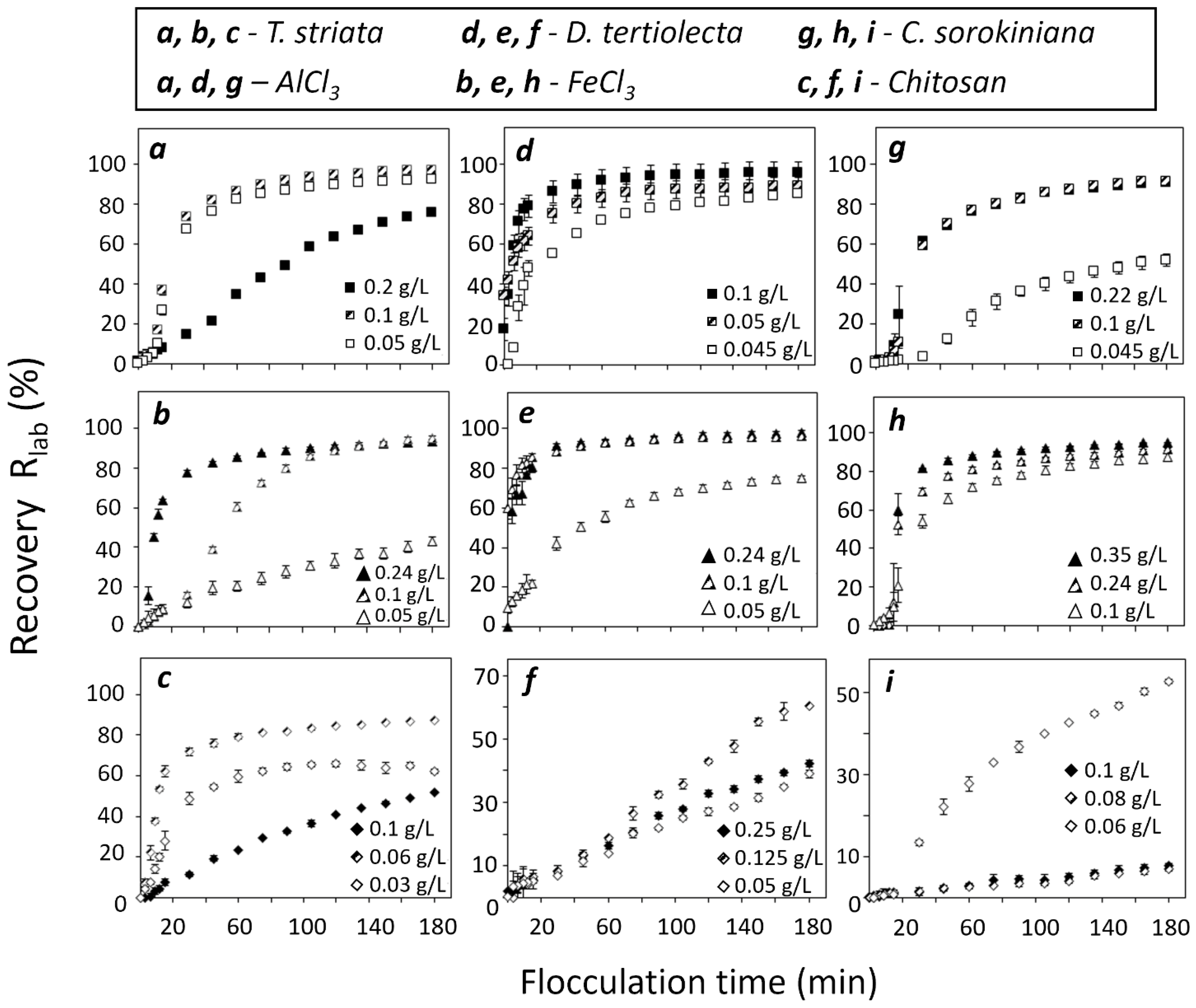
3.1. Evaluation of Flocculants in Laboratory Scale
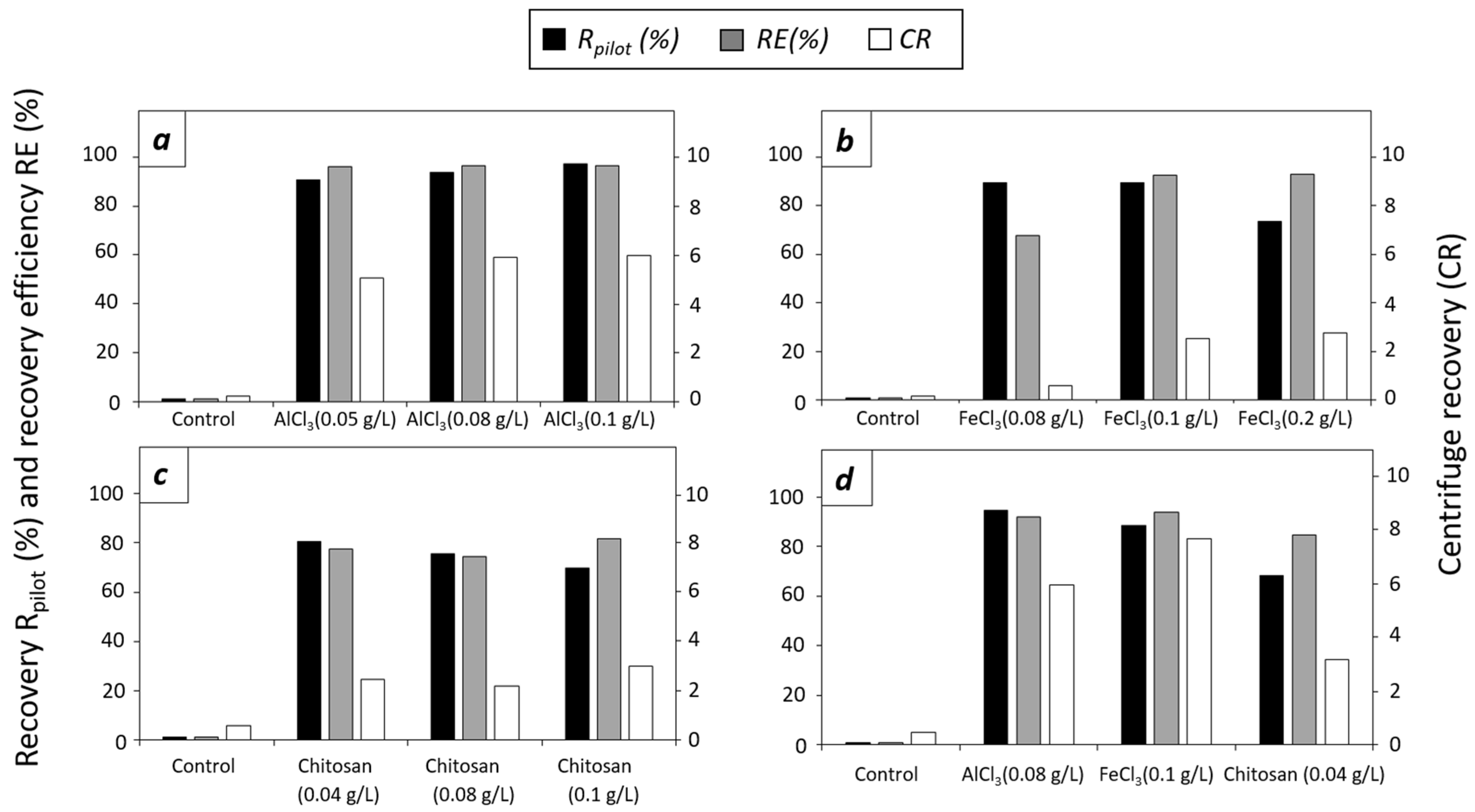
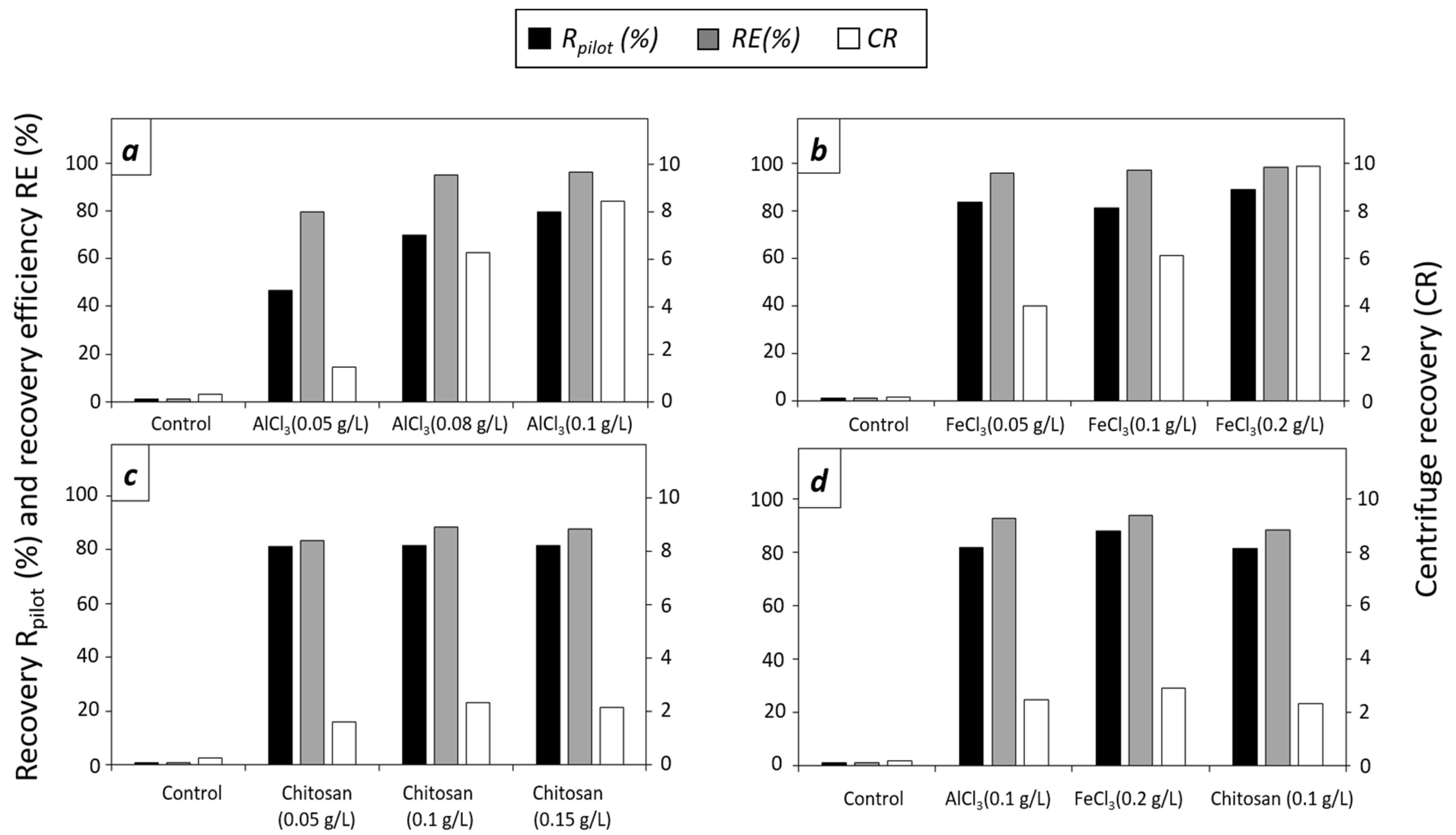
3.2. Evaluation of Flocculants in Pilot Scale
3.2.1. Effect of Flocculants in T. striata at Pilot Scale
3.2.2. Effect of Flocculants in D. tertiolecta at Pilot Scale
3.2.3. Effect of Flocculants in C. sorokiniana at Pilot Scale
3.3. Quality of the Flocculated Biomass
3.4. Economic Viability Assessment of Down-Streaming in One-Step and Two-Step Process
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Elements | Untreated | AlCl3 Treated | FeCl3 Treated | Chitosan Treated | |
|---|---|---|---|---|---|
| Mineral (ppm) | K | 11,173.0 ± 175.7 | 9442.3 ± 144.8 | 10,512.7 ± 471.2 | 9675.7 ± 158.5 |
| Na | 17,274.0 ± 673.7 | 32,002.0 ± 482.9 | 39,074.7 ± 495.9 | 22,005.7 ± 498.5 | |
| P | 3933.3 ± 152.8 | 3366.7 ± 152.8 | 3166.7 ± 251.7 | 4233.3 ± 513.2 | |
| Mg | 6752.7 ± 57.8 | 7676.0 ± 101.2 | 9548.3 ± 287.8 | 6456.0 ± 265.9 | |
| Ca | 23,605.7 ± 1042.2 | 8725.7 ± 853.5 | 9637.7 ± 211.6 | 13,003.3 ± 1812.4 | |
| Trace element (ppm) | Fe | 1219.7 ± 33.3 | 1282.0 ± 162.8 | 40,838.3 ± 2280.9 | 1953.0 ± 68.8 |
| Al | 524.7 ± 17.8 | 14,442.0 ± 2870.9 | 562.7 ± 63.8 | 673.0 ± 53.1 | |
| Mn | 64.3 ± 4.7 | 49.3 ± 7.6 | 46.3 ± 8.4 | 62.0 ± 6.6 | |
| Cu | 195.3 ± 6.0 | 183.0 ± 7.2 | 205.3 ± 14.0 | 230.3 ± 15.6 | |
| Zn | 184.3 ± 5.7 | 130.7 ± 2.5 | 120.7 ± 2.1 | 168.7 ± 3.2 | |
| Heavy metals (ppb) | Cr | 29.3 ± 3.5 | 16.7 ± 1.5 | 113.0 ± 7.0 | 42.7 ± 5.0 |
| Pb | 4.7 ± 0.6 | 10.0 ± 1.0 | 21.3 ± 2.1 | 18.7 ± 2.1 | |
| Cd | 4.0 ± 0.5 | 3.3 ± 0.2 | 0.0 ± 0.0 | 2.8 ± 0.2 | |
| Co, Hg, As, Se | n.d. * | n.d. | n.d. | n.d. | |
| Elements | Control | AlCl3 | FeCl3 | Chitosan | |
|---|---|---|---|---|---|
| Minerals (ppm) | K | 9748.0 ± 112.2 | 5825.0 ± 301.7 | 4442.0 ± 199.3 | 8087.0 ± 454.6 |
| Na | 22,823.7 ± 302.9 | 55,522.7 ± 684.2 | 35,855.7 ± 636.0 | 30,604.3 ± 529.3 | |
| P | 2800.0 ± 100.0 | 2633.3 ± 57.7 | 2500.0 ± 100.0 | 2400.0 ± 100.0 | |
| Mg | 3866.7 ± 81.8 | 10,903.3 ± 334.5 | 7746.0 ± 189.0 | 5612.7 ± 84.8 | |
| Ca | 866.0 ± 67.5 | 3198.0 ± 316.2 | 2670.0 ± 111.2 | 1145.3 ± 114.5 | |
| Trace elements (ppm) | Fe | 456.0 ± 47.1 | 605.3 ± 41.0 | 53,237.0 ± 4847.4 | 766.3 ± 100.3 |
| Al | 182.3 ± 21.7 | 34,335.7 ± 5041.1 | 528.0 ± 43.3 | 96.3 ± 6.7 | |
| Mn | 13.7 ± 1.5 | 15.0 ± 1.0 | 12.7 ± 2.1 | 30.3 ± 1.5 | |
| Cu | 202.0 ± 3.6 | 341.0 ± 32.2 | 330.3 ± 18.8 | 49.0 ± 2.6 | |
| Zn | 202.3 ± 8.1 | 213.3 ± 9.0 | 180.3 ± 6.0 | 62.7 ± 6.8 | |
| Heavy metals (ppb) | Cr | 15.7 ± 0.6 | 21.3 ± 2.1 | 197.3 ± 11.0 | 55.7 ± 0.6 |
| Pb | 5.2 ± 1.1 | 3.7 ± 0.5 | 20.0 ± 5.6 | 3.5 ± 1.0 | |
| Cd | 3.7 ± 0.5 | 1.0 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| Co, Hg, As, Se | n.d. * | n.d. | n.d. | n.d. | |
| Elements | Control | AlCl3 | FeCl3 | Chitosan | |
|---|---|---|---|---|---|
| Minerals (ppm) | K | 10,241.0 ± 1182.6 | 8775.0 ± 109.1 | 7658.0 ± 199.0 | 7732.3 ± 203.3 |
| Na | 3914.0 ± 61.5 | 8541.3 ± 111.0 | 8757.0 ± 88.1 | 7767.3 ± 83.9 | |
| P | 9966.7 ± 208.2 | 19,633.3 ± 1021.4 | 18,300.0 ± 500.0 | 8400.0 ± 100.0 | |
| Mg | 3278.3 ± 83.1 | 3317.7 ± 36.6 | 3199.7 ± 93.4 | 3641.0 ± 54.7 | |
| Ca | 1417.7 ± 50.1 | 2851.0 ± 115.9 | 2210.0 ± 62.6 | 2076.0 ± 66.0 | |
| Trace elements (ppm) | Fe | 1250.3 ± 75.1 | 1368.3 ± 119.8 | 59,462.0 ± 2339.3 | 1515.3 ± 67.0 |
| Al | 164.7 ± 21.4 | 40,429.3 ± 585.7 | 202.7 ± 11.8 | 256.0 ± 10.1 | |
| Mn | 121.7 ± 3.1 | 114.3 ± 4.0 | 94.3 ± 5.0 | 118.0 ± 6.6 | |
| Cu | 20.0 ± 1.0 | 21.7 ± 2.5 | 29.7 ± 1.5 | 17.7 ± 1.5 | |
| Zn | 112.7 ± 12.0 | 110.7 ± 4.0 | 89.3 ± 3.1 | 120.0 ± 3.0 | |
| Heavy metals (ppb) | Cr | 10.0 ± 1.0 | 7.7 ± 0.6 | 131.0 ± 17.1 | 18.3 ± 4.2 |
| Pb | 1.8 ± 0.6 | 1.7 ± 0.4 | 2.2 ± 0.3 | 22.0 ± 1.0 | |
| Cd | 1.2 ± 0.2 | 2.2 ± 0.3 | 0.0 ± 0.0 | 1.6 ± 0.1 | |
| Co, Hg, As, Se | n.d. * | n.d. | n.d. | n.d. | |
Appendix B



References
- Borowitzka, M.A. High-Value Products from Microalgae—Their Development and Commercialisation; Springer: Amsterdam, The Netherlands, 2013; Volume 25, pp. 743–756. [Google Scholar]
- Fabris, M.; Abbriano, R.M.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; McCauley, J.I.; Kuzhiuparambil, U.; Ray, P.; et al. Emerging Technologies in Algal Biotechnology: Toward the Establishment of a Sustainable, Algae-Based Bioeconomy. Front. Plant Sci. 2020, 11, 279. [Google Scholar] [CrossRef]
- Lage, S.; Gojkovic, Z.; Funk, C.; Gentili, G.F. Algal Biomass from Wastewater and Flue Gases as a Source of Bioenergy. Energies 2018, 11, 664. [Google Scholar] [CrossRef]
- Pittman, J.K.; Dean, A.P.; Osundeko, O. The Potential of Sustainable Algal Biofuel Production Using Wastewater Resources. Bioresour. Technol. 2011, 102, 17–25. [Google Scholar] [CrossRef]
- Razzak, S.A.; Hossain, M.M.; Lucky, R.A.; Bassi, A.S.; de Lasa, H. Integrated CO2 Capture, Wastewater Treatment and Biofuel Production by Microalgae Culturing—A Review. Renew. Sustain. Energy Rev. 2013, 27, 622–653. [Google Scholar] [CrossRef]
- Rodolfi, L.; Chini Zittelli, G.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for Oil: Strain Selection, Induction of Lipid Synthesis and Outdoor Mass Cultivation in a Low-Cost Photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Haver, L.V.; Nayar, S. Polyelectrolyte Flocculants in Harvesting Microalgal Biomass for Food and Feed Applications. Algal Res. 2017, 24, 167–180. [Google Scholar] [CrossRef]
- Milledge, J.J.; Heaven, S. A Review of the Harvesting of Micro-Algae for Biofuel Production. Rev. Environ. Sci. Bio/Technol. 2012, 12, 165–178. [Google Scholar] [CrossRef]
- Sanyano, N.; Chetpattananondh, P.; Chongkhong, S. Coagulation–Flocculation of Marine Chlorella Sp. Biodiesel Production. Bioresour. Technol. 2013, 147, 471–476. [Google Scholar] [CrossRef]
- Singh, G.; Patidar, S.K. Microalgae Harvesting Techniques: A Review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar] [CrossRef]
- Trovão, M.; Pereira, H.; Silva, J.; Páramo, J.; Quelhas, P.; Santos, T.; Silva, J.T.; Machado, A.; Gouveia, L.; Barreira, L.; et al. Growth Performance, Biochemical Composition and Sedimentation Velocity of Tetraselmis Sp. CTP4 under Different Salinities Using Low-Cost Lab- and Pilot-Scale Systems. Heliyon 2019, 5, e01553. [Google Scholar] [CrossRef]
- Salim, S.; Vermuë, M.H.; Wijffels, R.H. Ratio between Autoflocculating and Target Microalgae Affects the Energy-Efficient Harvesting by Bio-Flocculation. Bioresour. Technol. 2012, 118, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Tredici, M.R. Photobiology of Microalgae Mass Cultures: Understanding the Tools for the next Green Revolution. Biofuels 2010, 1, 143–162. [Google Scholar] [CrossRef]
- Li, S.; Hu, T.; Xu, Y.; Wang, J.; Chu, R.; Yin, Z.; Mo, F.; Zhu, L. A Review on Flocculation as an Efficient Method to Harvest Energy Microalgae: Mechanisms, Performances, Influencing Factors and Perspectives. Renew. Sustain. Energy Rev. 2020, 131, 110005. [Google Scholar] [CrossRef]
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C.M. Harvesting Techniques Applied to Microalgae: A Review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef]
- Labeeuw, L.; Commault, A.S.; Kuzhiumparambil, U.; Emmerton, B.; Nguyen, L.N.; Nghiem, L.D.; Ralph, P.J. A Comprehensive Analysis of an Effective Flocculation Method for High Quality Microalgal Biomass Harvesting. Sci. Total Environ. 2021, 752, 141708. [Google Scholar] [CrossRef] [PubMed]
- Okoro, V.; Azimov, U.; Munoz, J.; Hernandez, H.H.; Phan, A.N. Microalgae Cultivation and Harvesting: Growth Performance and Use of Flocculants—A Review. Renew. Sustain. Energy Rev. 2019, 115, 109364. [Google Scholar] [CrossRef]
- Vandamme, D.; Foubert, I.; Meesschaert, B.; Muylaert, K. Flocculation of Microalgae Using Cationic Starch. J. Appl. Phycol. 2010, 22, 525–530. [Google Scholar] [CrossRef]
- de Souza Leite, L.; Hoffmann, M.T.; Daniel, L.A. Coagulation and Dissolved Air Flotation as a Harvesting Method for Microalgae Cultivated in Wastewater. J. Water Process Eng. 2019, 32, 100947. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Mat Yasin, N.H.; Derek, C.J.C.; Lim, J.K. Optimization of Microalgae Coagulation Process Using Chitosan. Chem. Eng. J. 2011, 173, 879–882. [Google Scholar] [CrossRef]
- ‘t Lam, G.P.; Vermuë, M.H.; Olivieri, G.; van den Broek, L.A.M.; Barbosa, M.J.; Eppink, M.H.M.; Wijffels, R.H.; Kleinegris, D.M.M. Cationic Polymers for Successful Flocculation of Marine Microalgae. Bioresour. Technol. 2014, 169, 804–807. [Google Scholar] [CrossRef]
- Bilanovic, D.; Shelef, G.; Sukenik, A. Flocculation of Microalgae with Cationic Polymers—Effects of Medium Salinity. Biomass 1988, 17, 65–76. [Google Scholar] [CrossRef]
- Knuckey, R.M.; Brown, M.R.; Robert, R.; Frampton, D.M.F. Production of Microalgal Concentrates by Flocculation and Their Assessment as Aquaculture Feeds. Aquac. Eng. 2006, 35, 300–313. [Google Scholar] [CrossRef]
- Udom, I.; Zaribaf, B.H.; Halfhide, T.; Gillie, B.; Dalrymple, O.; Zhang, Q.; Ergas, S.J. Harvesting Microalgae Grown on Wastewater. Bioresour. Technol. 2013, 139, 101–106. [Google Scholar] [CrossRef]
- Xu, Y.; Purton, S.; Baganz, F. Chitosan Flocculation to Aid the Harvesting of the Microalga Chlorella Sorokiniana. Bioresour. Technol. 2013, 129, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.P.; Nguyen, L.N.; Emmerton, B.; Wang, Q.; Ralph, P.J.; Nghiem, L.D. Factors Governing Microalgae Harvesting Efficiency by Flocculation Using Cationic Polymers. Bioresour. Technol. 2021, 340, 125669. [Google Scholar] [CrossRef] [PubMed]
- Spain, O.; Funk, C. A Step towards More Eco-Friendly and Efficient Microalgal Harvesting: Inducing Flocculation in the Non-Naturally Flocculating Strain Chlorella Vulgaris (13-1) without Chemical Additives. Algal Res. 2024, 79, 103450. [Google Scholar] [CrossRef]
- Oh, H.-M.; Lee, S.J.; Park, M.-H.; Kim, H.-S.; Kim, H.-C.; Yoon, J.-H.; Kwon, G.-S.; Yoon, B.-D. Harvesting of Chlorella Vulgaris Using a Bioflocculant from Paenibacillus Sp. AM49. Biotechnol. Lett. 2001, 23, 1229–1234. [Google Scholar] [CrossRef]
- Salim, S.; Bosma, R.; Vermuë, M.H.; Wijffels, R.H. Harvesting of Microalgae by Bio-Flocculation. J. Appl. Phycol. 2011, 23, 849–855. [Google Scholar] [CrossRef]
- Papazi, A.; Makridis, P.; Divanach, P. Harvesting Chlorella Minutissima Using Cell Coagulants. J. Appl. Phycol. 2010, 22, 349–355. [Google Scholar] [CrossRef]
- Besson, A.; Formosa-Dague, C.; Guiraud, P. Flocculation-Flotation Harvesting Mechanism of Dunaliella Salina: From Nanoscale Interpretation to Industrial Optimization. Water Res. 2019, 155, 352–361. [Google Scholar] [CrossRef]
- Şirin, S.; Trobajo, R.; Ibanez, C.; Salvadó, J. Harvesting the Microalgae Phaeodactylum Tricornutum with Polyaluminum Chloride, Aluminium Sulphate, Chitosan and Alkalinity-Induced Flocculation. J. Appl. Phycol. 2012, 24, 1067–1080. [Google Scholar] [CrossRef]
- Beach, E.S.; Eckelman, M.J.; Cui, Z.; Brentner, L.; Zimmerman, J.B. Preferential Technological and Life Cycle Environmental Performance of Chitosan Flocculation for Harvesting of the Green Algae Neochloris Oleoabundans. Bioresour. Technol. 2012, 121, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Cui, Y.; Yuan, W. Flocculation Optimization of Microalga Nannochloropsis Oculata. Appl. Biochem. Biotechnol. 2013, 169, 2049–2063. [Google Scholar] [CrossRef]
- Koley, S.; Prasad, S.; Bagchi, S.K.; Mallick, N. Development of a Harvesting Technique for Large-Scale Microalgal Harvesting for Biodiesel Production. RSC Adv. 2017, 7, 7227–7237. [Google Scholar] [CrossRef]
- Matter, I.; Darwesh, O.; El-baz, F. Using the Natural Polymer Chitosan in Harvesting Scenedesmus Species under Different Concentrations and Cultural pH Values. Int. J. Pharm. Bio. Sci. 2016, 7, 254–260. [Google Scholar] [CrossRef]
- Kim, D.-G.; Oh, H.-M.; Park, Y.-H.; Kim, H.-S.; Lee, H.-G.; Ahn, C.-Y. Optimization of Flocculation Conditions for Botryococcus Braunii Using Response Surface Methodology. J. Appl. Phycol. 2013, 25, 875–882. [Google Scholar] [CrossRef]
- Wang, L.; Liang, W.; Yu, J.; Liang, Z.; Ruan, L.; Zhang, Y. Flocculation of Microcystis Aeruginosa Using Modified Larch Tannin. Environ. Sci. Technol. 2013, 47, 5771–5777. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Zhang, R.; Li, S.; Xia, W.; Ma, J. Removal of Microcystis Aeruginosa and Its Extracellular Organic Matters by Using Covalently Bonded Coagulant: An Alternative Choice in Enhanced Coagulation for Algae-Polluted Water Treatment. J. Clean. Prod. 2023, 419, 138337. [Google Scholar] [CrossRef]
- Gojkovic, Z.; Guidi, F.; Bustamante, B.; Venuleo, M.; de Assunçao, P.A.C.J.; Portillo, E. Scaling-Up and Semi-Continuous Cultivation of Locally Isolated Marine Microalgae Tetraselmis Striata in the Subtropical Island of Gran Canaria (Canary Islands, Spain). Processes 2021, 9, 1326. [Google Scholar] [CrossRef]
- Guillard, R.R.; Ryther, J.H. Studies of Marine Planktonic Diatoms. I. Cyclotella Nana Hustedt, and Detonula Confervacea (Cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Nieves, M.; Voltolina, D.; Piña, P. Growth and Biomass Production of Tetraselmis Suecica and Dunaliella Tertiolecta in a Standard Medium Added with Three Products of Zeolitic Nature. Aquac. Eng. 2005, 32, 403–410. [Google Scholar] [CrossRef]
- Kholssi, R.; Marks, E.; Miñón, J.; Montero, O.; Debdoubi, A.; Rad, C. Biofertilizing Effect of Chlorella Sorokiniana Suspensions on Wheat Growth. J. Plant Growth Regul. 2018, 38, 644–649. [Google Scholar] [CrossRef]
- Gojkovic, Z.; Shchukarev, A.; Ramstedt, M.; Funk, C. Cryogenic X-Ray Photoelectron Spectroscopy Determines Surface Composition of Algal Cells and Gives Insights into Their Spontaneous Sedimentation. Algal Res. 2020, 47, 101836. [Google Scholar] [CrossRef]
- Guidi, F.; Gojkovic, Z.; Venuleo, M.; Assunçao, P.A.C.J.; Portillo, E. Long-Term Cultivation of a Native Arthrospira Platensis (Spirulina) Strain in Pozo Izquierdo (Gran Canaria, Spain): Technical Evidence for a Viable Production of Food-Grade Biomass. Processes 2021, 9, 1333. [Google Scholar] [CrossRef]
- Doan, T.T.Y.; Sivaloganathan, B.; Obbard, J.P. Screening of Marine Microalgae for Biodiesel Feedstock. Biomass Bioenergy 2011, 35, 2534–2544. [Google Scholar] [CrossRef]
- Gojkovic, Z.; Vilchez, C.; Torronteras, R.; Vigara, J.; Gomez-Jacinto, V.; Janzer, N.; Gomez-Ariza, J.-L.; Marova, I.; Garbayo, I. Effect of Selenate on Viability and Selenomethionine Accumulation of Chlorella Sorokiniana Grown in Batch Culture. Sci. World J. 2014, 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.P.; Nguyen, L.N.; Lesage, G.; Nghiem, L.D. Synergistic Effect of Dual Flocculation between Inorganic Salts and Chitosan on Harvesting Microalgae Chlorella Vulgaris. Environ. Technol. Innov. 2020, 17, 100622. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.; Ryan, P. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1. [Google Scholar]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products Towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae Biorefinery: High Value Products Perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.Y.Y.; Khoo, K.S.; Chew, K.W.; Tao, Y.; Ho, S.-H.; Show, P.L. Potential Utilization of Bioproducts from Microalgae for the Quality Enhancement of Natural Products. Bioresour. Technol. 2020, 304, 122997. [Google Scholar] [CrossRef] [PubMed]
- Fasaei, F.; Bitter, J.H.; Slegers, P.M.; Boxtel, A.J.B. van Techno-Economic Evaluation of Microalgae Harvesting and Dewatering Systems. Algal Res. 2018, 31, 347–362. [Google Scholar] [CrossRef]
- Yang, Z.; Hou, J.; Miao, L. Harvesting Freshwater Microalgae with Natural Polymer Flocculants. Algal Res. 2021, 57, 102358. [Google Scholar] [CrossRef]
- Mathimani, T.; Mallick, N. A Comprehensive Review on Harvesting of Microalgae for Biodiesel—Key Challenges and Future Directions. Renew. Sustain. Energy Rev. 2018, 91, 1103–1120. [Google Scholar] [CrossRef]
- Vergini, S.; Aravantinou, A.F.; Manariotis, I.D. Harvesting of Freshwater and Marine Microalgae by Common Flocculants and Magnetic Microparticles. J. Appl. Phycol. 2016, 28, 1041–1049. [Google Scholar] [CrossRef]
- Branyikova, I.; Prochazkova, G.; Potocar, T.; Jezkova, Z.; Branyik, T. Harvesting of Microalgae by Flocculation. Fermentation 2018, 4, 93. [Google Scholar] [CrossRef]
- Chatsungnoen, T.; Chisti, Y. Harvesting Microalgae by Flocculation–Sedimentation. Algal Res. 2016, 13, 271–283. [Google Scholar] [CrossRef]
- Wu, X.; Ge, X.; Wang, D.; Tang, H. Distinct Coagulation Mechanism and Model between Alum and High Al13-PACl. Colloids Surf. A Physicochem. Eng. Asp. 2007, 305, 89–96. [Google Scholar] [CrossRef]
- Kwon, H.; Lu, M.; Lee, E.Y.; Lee, J. Harvesting of Microalgae Using Flocculation Combined with Dissolved Air Flotation. Biotechnol. Bioprocess. Eng. 2014, 19, 143–149. [Google Scholar] [CrossRef]
- Ferriols, V.M.E.; Aguilar, R. Efficiency of Various Flocculants in Harvesting the Green Microalgae Tetraselmis Tetrahele (Chlorodendrophyceae: Chlorodendraceae). AACL Bioflux 2012, 5, 265–273. [Google Scholar]
- Gorin, K.V.; Sergeeva, Y.E.; Butylin, V.V.; Komova, A.V.; Pojidaev, V.M.; Badranova, G.U.; Shapovalova, A.A.; Konova, I.A.; Gotovtsev, P.M. Methods Coagulation/Flocculation and Flocculation with Ballast Agent for Effective Harvesting of Microalgae. Bioresour. Technol. 2015, 193, 178–184. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Shobana, S.; Bakonyi, P.; Nemestóthy, N.; Xia, A.; Banu, J.R.; Kumar, G. A Review on Chemical Mechanism of Microalgae Flocculation via Polymers. Biotechnol. Rep. 2019, 21, e00302. [Google Scholar] [CrossRef] [PubMed]
- Matter, I.A.; Bui, V.K.H.; Jung, M.; Seo, J.Y.; Kim, Y.-E.; Lee, Y.-C.; Oh, Y.-K. Flocculation Harvesting Techniques for Microalgae: A Review. Appl. Sci. 2019, 9, 3069. [Google Scholar] [CrossRef]
- Demir, I.; Besson, A.; Guiraud, P.; Formosa-Dague, C. Towards a Better Understanding of Microalgae Natural Flocculation Mechanisms to Enhance Flotation Harvesting Efficiency. Water Sci. Technol. 2020, 82, 1009–1024. [Google Scholar] [CrossRef] [PubMed]
- Lama, S.; Muylaert, K.; Karki, T.B.; Foubert, I.; Henderson, R.K.; Vandamme, D. Flocculation Properties of Several Microalgae and a Cyanobacterium Species during Ferric Chloride, Chitosan and Alkaline Flocculation. Bioresour. Technol. 2016, 220, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, Z.; Hiltunen, E. Microalgae Chlorella Vulgaris Biomass Harvesting by Natural Flocculant: Effects on Biomass Sedimentation, Spent Medium Recycling and Lipid Extraction. Biotechnol. Biofuels 2018, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Blockx, J.; Verfaillie, A.; Thielemans, W.; Muylaert, K. Unravelling the Mechanism of Chitosan-Driven Flocculation of Microalgae in Seawater as a Function of pH. ACS Sustain. Chem. Eng. 2018, 6, 11273–11279. [Google Scholar] [CrossRef]
- Farid, M.S.; Shariati, A.; Badakhshan, A.; Anvaripour, B. Using Nano-Chitosan for Harvesting Microalga Nannochloropsis Sp. Bioresour. Technol. 2013, 131, 555–559. [Google Scholar] [CrossRef]
- Kan, C.; Huang, C.; Pan, J.R. Time Requirement for Rapid-Mixing in Coagulation. Colloids Surf. A Physicochem. Eng. Asp. 2002, 203, 1–9. [Google Scholar] [CrossRef]
- Li, T.; Hu, J.; Zhu, L. Self-Flocculation as an Efficient Method to Harvest Microalgae: A Mini-Review. Water 2021, 13, 2585. [Google Scholar] [CrossRef]
- Ndikubwimana, T.; Zeng, X.; Murwanashyaka, T.; Manirafasha, E.; He, N.; Shao, W.; Lu, Y. Harvesting of Freshwater Microalgae with Microbial Bioflocculant: A Pilot-Scale Study. Biotechnol. Biofuels 2016, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rawat, I.; Kumar, R.R.; Mutanda, T.; Bux, F. Dual Role of Microalgae: Phycoremediation of Domestic Wastewater and Biomass Production for Sustainable Biofuels Production. Appl. Energy 2011, 88, 3411–3424. [Google Scholar] [CrossRef]
- Show, K.-Y.; Lee, D.-J. Chapter 5—Algal Biomass Harvesting. In Biofuels from Algae; Pandey, A., Lee, D.-J., Chisti, Y., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 85–110. ISBN 978-0-444-59558-4. [Google Scholar]
- Tan, J.S.; Lee, S.Y.; Chew, K.W.; Lam, M.K.; Lim, J.W.; Ho, S.H.; Show, P. LA Review on Microalgae Cultivation and Harvesting, and Their Biomass Extraction Processing Using Ionic Liquids. Bioengineered 2020, 11, 116–129. [Google Scholar] [CrossRef]
- Dassey, A.J.; Theegala, C.S. Harvesting Economics and Strategies Using Centrifugation for Cost Effective Separation of Microalgae Cells for Biodiesel Applications. Bioresour. Technol. 2013, 128, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Japar, A.S.; Takriff, M.S.; Mohd Yasin, N.H.; Mahmod, S.S. Optimization of Chlorella Biomass Harvesting by Flocculation and Its Potential for Biofuel Production. J. Appl. Phycol. 2021, 33, 1621–1629. [Google Scholar] [CrossRef]
- Elcik, H.; Karadag, D.; Kara, A.I.; Cakmakci, M. Microalgae Biomass Harvesting Using Chitosan Flocculant: Optimization of Operating Parameters by Response Surface Methodology. Separations 2023, 10, 507. [Google Scholar] [CrossRef]
- Aguilar, F.; Autrup, H.; Barlow, S.; Castle, L.; Crebelli, R.; Dekant, W.; Engel, K.-H.; Gontard, N.; Gott, D.; Grilli, S.; et al. Safety of Aluminium from Dietary Intake. EFSA J. 2008, 754, 1–34. [Google Scholar]
- Bresson, J. Scientific Opinion on Dietary Reference Values for Iron. EFSA J. 2015, 13, 4254. [Google Scholar] [CrossRef]
- EC. Commission Regulation Commission Regulation (EC) No 1334/2003 of 25 July 2003 Amending the Conditions for Authorisation of a Number of Additives in Feedingstuffs Belonging to the Group of Trace Elements 2003. Off. J. Eur. Union. 2003, 1334, 11–15. [Google Scholar]
- Liang, S.; Kang, Y.; Zeng, L.; Qin, Y.; Wang, L.; Zhang, Q.; Luo, J. How Chlorella Sorokiniana and Its High Tolerance to Pb Might Be a Potential Pb Biosorbent. Pol. J. Environ. Stud. 2017, 26, 1139–1146. [Google Scholar] [CrossRef]
- Politaeva, N.; Smyatskaya, Y.; Al Afif, R.; Pfeifer, C.; Mukhametova, L. Development of Full-Cycle Utilization of Chlorella Sorokiniana Microalgae Biomass for Environmental and Food Purposes. Energies 2020, 13, 2648. [Google Scholar] [CrossRef]
- Ghannam, H.; Talab, A.; Dolganova, N.; Hussein, A.; Abdelmaguid, N. Characterization of Chitosan Extracted from Different Crustacean Shell Wastes. J. Appl. Sci. 2016, 16, 454–461. [Google Scholar] [CrossRef]
- EU. European Commission Regulation COMMISSION REGULATION (EU) No 1275/2013 of 6 December 2013 Amending Annex I to Directive 2002/32/EC of the European Parliament and of the Council as Regards Maximum Levels for Arsenic, Cadmium, Lead, Nitrites, Volatile Mustard Oil and Harmful Botanical Impurities 2013. Off. J. Eur. Union. 2013, 1275, 86–92. [Google Scholar]
- Kim, D.-Y.; Lee, K.; Lee, J.; Lee, Y.-H.; Han, J.-I.; Park, J.-Y.; Oh, Y.-K. Acidified-Flocculation Process for Harvesting of Microalgae: Coagulant Reutilization and Metal-Free-Microalgae Recovery. Bioresour. Technol. 2017, 239, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, L.; Zhang, H.; Li, C.; Zhang, X.; Hu, Q. A Novel Low Cost Microalgal Harvesting Technique with Coagulant Recovery and Recycling. Bioresour. Technol. 2018, 266, 343–348. [Google Scholar] [CrossRef] [PubMed]
| Element | Microalgae | Untreated (Control) | AlCl3 Treated | FeCl3 Treated | Chitosan Treated |
|---|---|---|---|---|---|
| Al (ppm) | T. striata | 524.7 ± 17.8 b | 14,442.0 ± 2870.9 a,c,d | 562.7 ± 63.8 b | 673.0 ± 53.1 b |
| D. tertiolecta | 182.3 ± 21.7 b | 34,335.7 ± 5041.1 a,c,d | 528.0 ± 43.3 b | 96.3 ± 6.7 b | |
| C. sorokiniana | 164.7 ± 21.4 b | 40,429.3 ± 585.7 a,c,d | 202.7 ± 11.8 b | 256.0 ± 10.1 b | |
| Fe (ppm) | T. striata | 1219.7 ± 33.3 c | 1282.0 ± 162.8 c | 40,838.3 ± 2280.9 a,b,d | 1953.0 ± 68.8 c |
| D. tertiolecta | 456.0 ± 47.1 c | 605.3 ± 41.0 c | 53,237.0 ± 4847.4 a,b,d | 766.3 ± 100.3 c | |
| C. sorokiniana | 1250.3 ± 75.1 c | 1368.3 ± 119.8 c | 59,462.0 ± 2339.3 a,b,d | 1515.3 ± 67.0 c | |
| Ash (%) | T. striata | 18.8 ± 0.2 b,c | 23.1 ± 0.7 a,c,d | 27.3 ± 0.3 a,b,d | 19.0 ± 0.2 b,c |
| D. tertiolecta | 11.9 ± 0.1 b,c | 26.4 ± 0.9 a,c,d | 35.4 ± 4.3 a,b,d | 18.9 ± 2.4 b,c | |
| C. sorokiniana | 7.2 ± 0.1 b,c | 20.6 ± 0.1 a,d | 29.4 ± 0.4 a,d | 8.6 ± 2.9 b,c | |
| Water content (%) | T. striata | 70.3 ± 1.3 b,c,d | 81.3 ± 0.5 a,d | 80.6 ± 0.1 a | 78.2 ± 0.3 a,b |
| D. tertiolecta | 75.6 ± 0.2 b,c,d | 82.4 ± 0.5 a | 82.0 ± 0.9 a | 82.8 ± 1.6 a | |
| C. sorokiniana | 76.8 ± 0.1 b,c,d | 85.9 ± 0.5 a,d | 87.1 ± 0.4 a,d | 89.2 ± 0.7 a,b,c |
| Microalgae | Single-Step Centrifugation (EUR/m3) | Flocculation (EUR/m3) | ||
|---|---|---|---|---|
| AlCl3 | FeCl3 | Chitosan | ||
| T. striata | 1.17 | 0.12 | 0.16 | 3.15 |
| D. tertiolecta | 1.17 | 0.14 | 0.24 | 7.86 |
| C. sorokiniana | 1.17 | 0.14 | 0.24 | 7.82 |
| Estimated flocculant price (EUR/kg) | - | 0.53 | 0.81 | 76.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueira Garcia, L.; Gojkovic, Z.; Venuleo, M.; Guidi, F.; Portillo, E. The Use of Chemical Flocculants and Chitosan as a Pre-Concentration Step in the Harvesting Process of Three Native Microalgae Species from the Canary Islands Cultivated Outdoors at the Pilot Scale. Microorganisms 2024, 12, 2583. https://doi.org/10.3390/microorganisms12122583
Figueira Garcia L, Gojkovic Z, Venuleo M, Guidi F, Portillo E. The Use of Chemical Flocculants and Chitosan as a Pre-Concentration Step in the Harvesting Process of Three Native Microalgae Species from the Canary Islands Cultivated Outdoors at the Pilot Scale. Microorganisms. 2024; 12(12):2583. https://doi.org/10.3390/microorganisms12122583
Chicago/Turabian StyleFigueira Garcia, Laura, Zivan Gojkovic, Marianna Venuleo, Flavio Guidi, and Eduardo Portillo. 2024. "The Use of Chemical Flocculants and Chitosan as a Pre-Concentration Step in the Harvesting Process of Three Native Microalgae Species from the Canary Islands Cultivated Outdoors at the Pilot Scale" Microorganisms 12, no. 12: 2583. https://doi.org/10.3390/microorganisms12122583
APA StyleFigueira Garcia, L., Gojkovic, Z., Venuleo, M., Guidi, F., & Portillo, E. (2024). The Use of Chemical Flocculants and Chitosan as a Pre-Concentration Step in the Harvesting Process of Three Native Microalgae Species from the Canary Islands Cultivated Outdoors at the Pilot Scale. Microorganisms, 12(12), 2583. https://doi.org/10.3390/microorganisms12122583







