Clinical and In Vitro Safety of Heyndrickxia coagulans AO 1167B: A Double-Blind, Placebo-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Provenance of H. coagulans AO1167B
2.2. Culture Conditions of Bacterial Strain
2.3. Phenotypic Characterization of the Organism
2.4. Genotypic Characterization of the Organism
2.5. Hemolytic Activity
2.6. Cytotoxicity in Vero Cells
2.7. Human Safety Assessment
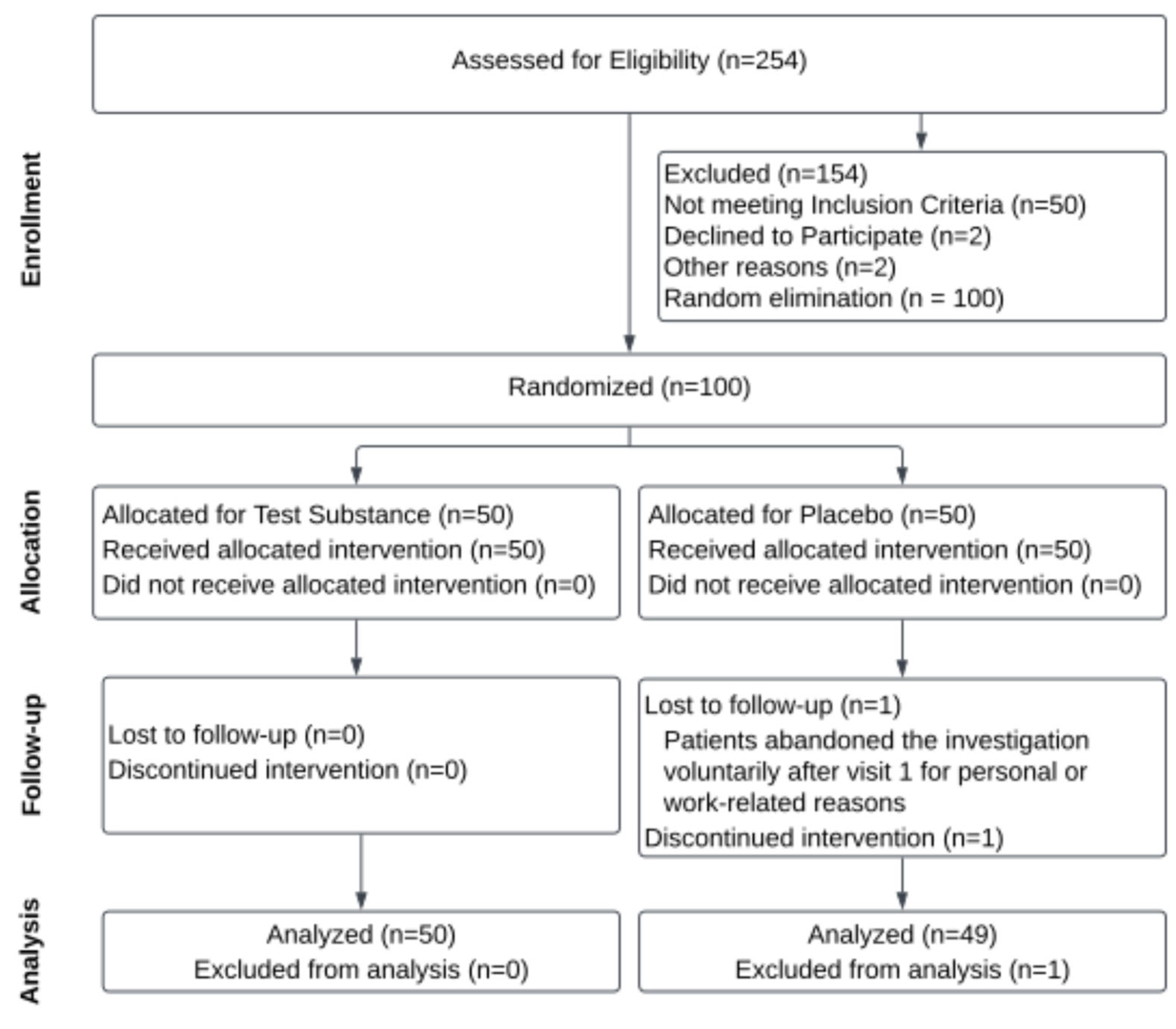
2.7.1. Study Design
2.7.2. Study Outcomes
2.7.3. Safety Assessment
2.7.4. Sample Collection, Processing, and Data Management
2.7.5. Clinical Determinations
2.7.6. Occurrence of Adverse Events Determination
2.7.7. Statistical Analyses
3. Results
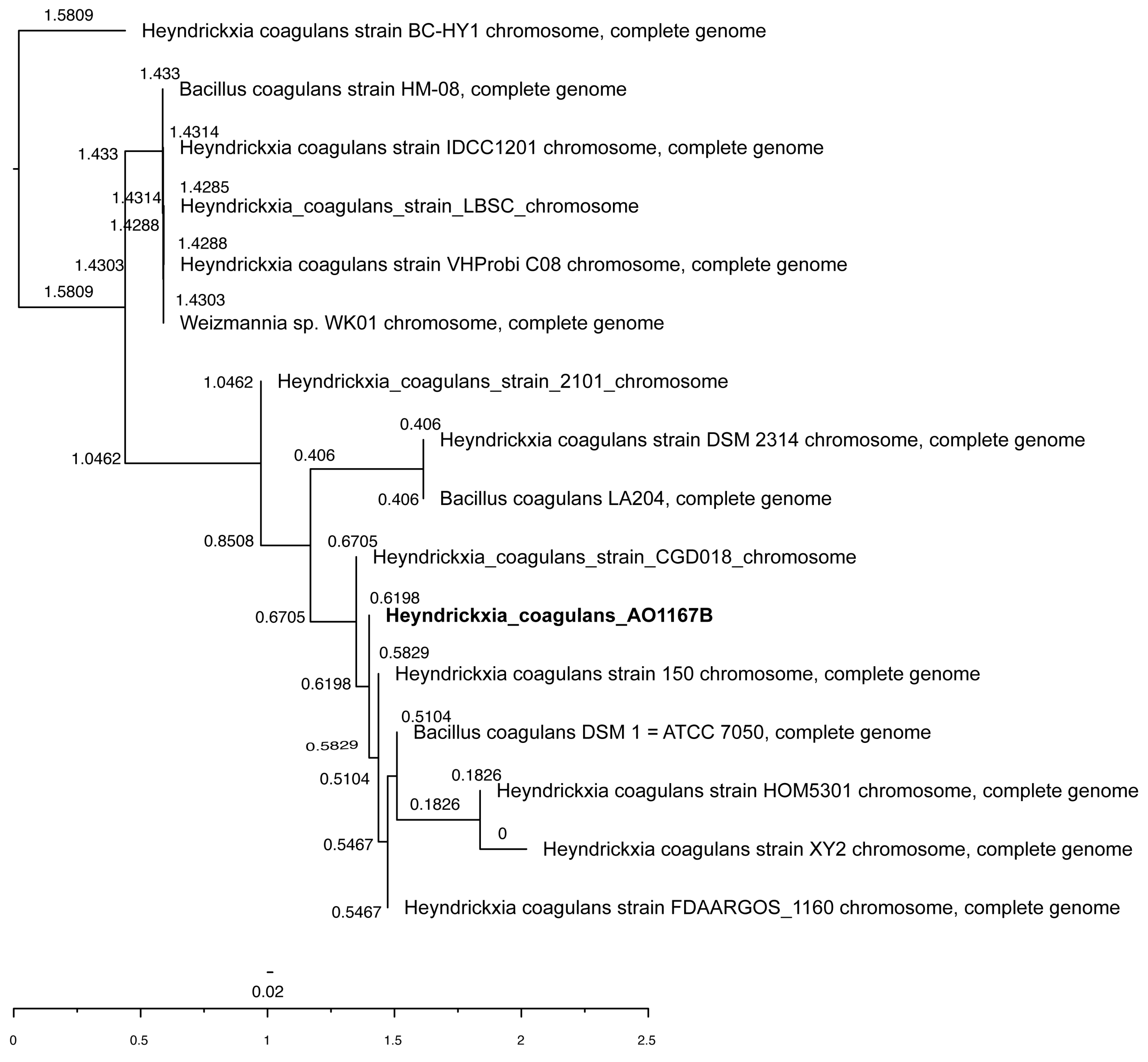
3.1. Species and Strain Identification
3.2. Traits Associated with Probiotic Properties
3.2.1. Phenotypic Traits
3.2.2. Genotypic Properties
3.3. Safety Assessment of H. coagulans AO1167B
3.3.1. Antimicrobial Resistance and Virulence Factors
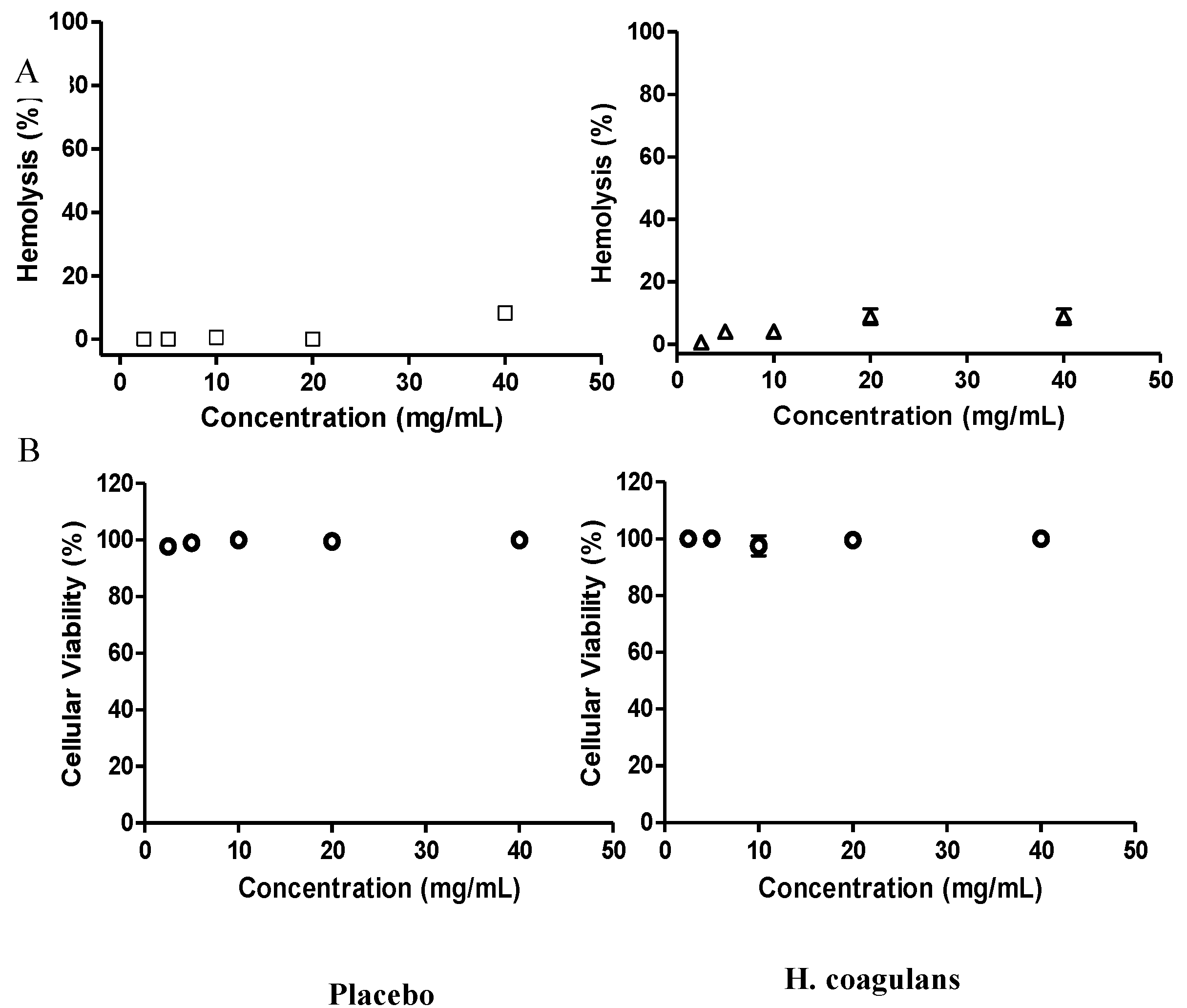
3.3.2. Hemolytic Activity and Cytotoxicity
3.3.3. Clinical and Hematological Determinations
3.3.4. Adverse Events
3.3.5. Health Questionnaire Analysis
4. Discussion
4.1. Species and Strain Identification
4.2. Traits of H. coagulans AO1167B Associated with Probiotic Properties
4.2.1. Phenotypic Traits
4.2.2. Genotypic Traits
4.3. Antimicrobial Resistance
4.4. Virulence Genes
4.5. Bioactive Amines
4.6. Clinical Study
4.6.1. Demographic and Safety Overview
4.6.2. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hammer, B.W. Bacteriological studies on the coagulation of evaporated milk. Iowa Agric. Exp. Stn. Res. Bull. 1915, 19, 119–131. [Google Scholar]
- Gupta, R.S.; Patel, S.; Saini, N.; Chen, S. Robust demarcation of 17 distinct Bacillus species clades, proposed as novel Bacillaceae genera, by phylogenomics and comparative genomic analyses: Description of Robertmurraya kyonggiensis sp. nov. and proposal for an emended genus Bacillus limiting it only to the members of the Subtilis and Cereus clades of species. Int. J. Syst. Evol. Microbiol. 2020, 70, 5753–5798. [Google Scholar] [PubMed]
- Narsing Rao, M.P.; Banerjee, A.; Liu, G.-H.; Thamchaipenet, A. Genome-based reclassification of Bacillus acidicola, Bacillus pervagus and the genera Heyndrickxia, Margalitia and Weizmannia. Int. J. Syst. Evol. Microbiol. 2023, 73, 005961. [Google Scholar] [CrossRef]
- Andreoletti, O.; Budka, H.; Buncic, S.; Colin, P.; Collins, J.D.; De Koeijer, A.; Griffin, J.; Havelaar, A.; Hope, J.; Klein, G.; et al. The maintenance of the list of QPS microorganisms intentionally added to food or feed—Scientific Opinion of the Panel on Biological Hazards. EFSA J. 2008, 6, 923. [Google Scholar] [CrossRef]
- Sanders, M.E.; Morelli, L.; Tompkins, T.A. Sporeformers as Human Probiotics: Bacillus, Sporolactobacillus, and Brevibacillus. Compr. Rev. Food Sci. Food Saf. 2003, 2, 101–110. [Google Scholar] [CrossRef]
- De Vecchi, E.; Drago, L. Lactobacillus sporogenes or Bacillus coagulans: Misidentification or mislabelling? Int. J. Probiotics Prebiotics 2006, 1, 3–10. [Google Scholar]
- Hun, L. Bacillus coagulans significantly improved abdominal pain and bloating in patients with IBS. Postgrad. Med. 2009, 121, 119–124. [Google Scholar] [CrossRef]
- Gupta, A.K.; Maity, C.J.M. Efficacy and safety of Bacillus coagulans LBSC in irritable bowel syndrome: A prospective, interventional, randomized, double-blind, placebo-controlled clinical study [CONSORT Compliant]. Medicine 2021, 100, e23641. [Google Scholar] [CrossRef]
- Baron, M. Original Research: A Patented Strain of Bacillus coagulans Increased Immune Response to Viral Challenge. Postgrad. Med. 2009, 121, 114–118. [Google Scholar] [CrossRef]
- Sadeghirashed, S.; Kazemi, F.; Taheri, S.; Ebrahimi, M.T.; Arasteh, J. A novel probiotic strain exerts therapeutic effects on mouse model of multiple sclerosis by altering the expression of inflammasome and IDO genes and modulation of T helper cytokine profile. Metab. Brain Dis. 2022, 37, 197–207. [Google Scholar] [CrossRef]
- Endres, J.R.; Clewell, A.; Jade, K.A.; Farber, T.; Hauswirth, J.; Schauss, A.G. Safety assessment of a proprietary preparation of a novel Probiotic, Bacillus coagulans, as a food ingredient. Food Chem. Toxicol. 2009, 47, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Sunhare, R.; Shyam Prasad, S.V.; Kodimule, S.; Prabu, R. Assessing the probiotic potential and safety of newly isolated Bacillus coagulans VHBAX-04: In vitro and in silico evaluations. Int. J. Food Sci. Technol. 2024, 59, 5355–5362. [Google Scholar] [CrossRef]
- Shaikh, S.S.; Patel, S.; Malik, A.; Kumar, S.A. Efficacy and safety assessment of probiotic Bacillus coagulans (Heyndrickxia coagulans) BCP92 for treatment of diarrhea. Toxicol. Res. 2024, 13, tfae182. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.S.; Jhala, D.; Patel, A.; Chettiar, S.S.; Ghelani, A.; Malik, A.; Sengupta, P. In-silico analysis of probiotic attributes and safety assessment of probiotic strain Bacillus coagulans BCP92 for human application. Lett. Appl. Microbiol. 2024, 77, ovad145. [Google Scholar] [CrossRef]
- Zhang, Y.; Overbeck, T.J.; Skebba, V.L.P.; Gandhi, N.N. Genomic and Phenotypic Safety Assessment of Probiotic Bacillus coagulans Strain JBI-YZ6.3. Probiotics Antimicrob. Proteins 2024, 19, 1–11. [Google Scholar] [CrossRef]
- Panel, E.B.; Herman, L. Update of the list of qualified presumption of safety (QPS) recommended microorganisms intentionally added to food or feed as notified to EFSA. EFSA J. 2023, 21, 7747. [Google Scholar]
- Konuray, G.; Erginkaya, Z. Potential use of Bacillus coagulans in the food industry. Foods 2018, 7, 92. [Google Scholar] [CrossRef]
- Callaway, T.R.; Ricke, S.C. Direct-Fed Microbials and Prebiotics for Animals; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Lian, W.-C.; Hsiao, H.-C.; Chou, C.-C. Viability of microencapsulated bifidobacteria in simulated gastric juice and bile solution. Int. J. Food Microbiol. 2003, 86, 293–301. [Google Scholar] [CrossRef]
- García-Ruiz, A.; de Llano, D.G.; Esteban-Fernández, A.; Requena, T.; Bartolomé, B.; Moreno-Arribas, M.V. Assessment of probiotic properties in lactic acid bacteria isolated from wine. Food Microbiol. 2014, 44, 220–225. [Google Scholar] [CrossRef]
- Humphries, R.; Bobenchik, A.M.; Hindler, J.A.; Schuetz, A.N. Overview of Changes to the Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing, M100, 31st Edition. J. Clin. Microbiol. 2021, 59, e0021321. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [PubMed]
- Kolmogorov, M.; Yuan, J.; Lin, Y.; Pevzner, P.A. Assembly of long, error-prone reads using repeat graphs. Nat. Biotechnol. 2019, 37, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K. Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 2014, 9, e112963. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Carattoli, A.; Hasman, H. PlasmidFinder and in silico pMLST: Identification and typing of plasmid replicons in whole-genome sequencing (WGS). In Horizontal Gene Transfer; Methods in Molecular Biology Series; Humana: New York, NY, USA, 2020; pp. 285–294. [Google Scholar]
- McArthur, A.G.; Waglechner, N.; Nizam, F.; Yan, A.; Azad, M.A.; Baylay, A.J.; Bhullar, K.; Canova, M.J.; De Pascale, G.; Ejim, L. The comprehensive antibiotic resistance database. Antimicrob. Agents Chemother. 2013, 57, 3348–3357. [Google Scholar]
- Chen, L.; Yang, J.; Yu, J.; Yao, Z.; Sun, L.; Shen, Y.; Jin, Q. VFDB: A reference database for bacterial virulence factors. Nucleic Acids Res. 2005, 33, D325–D328. [Google Scholar] [CrossRef]
- Feddern, V.; Mazzuco, H.; Fonseca, F.; De Lima, G. A review on biogenic amines in food and feed: Toxicological aspects, impact on health and control measures. Anim. Prod. Sci. 2019, 59, 608–618. [Google Scholar] [CrossRef]
- Lefevre, M.; Racedo, S.M.; Denayrolles, M.; Ripert, G.; Desfougères, T.; Lobach, A.R.; Simon, R.; Pélerin, F.; Jüsten, P.; Urdaci, M.C. Safety assessment of Bacillus subtilis CU1 for use as a probiotic in humans. Regul. Toxicol. Pharmacol. 2017, 83, 54–65. [Google Scholar] [CrossRef]
- Soto, C.; Bergado, G.; Blanco, R.; Griñán, T.; Rodríguez, H.; Ros, U.; Pazos, F.; Lanio, M.E.; Hernández, A.M.; Álvarez, C. Sticholysin II-mediated cytotoxicity involves the activation of regulated intracellular responses that anticipates cell death. Biochimie 2018, 148, 18–35. [Google Scholar]
- Shrestha, B.; Dunn, L. The declaration of Helsinki on medical research involving human subjects: A review of seventh revision. J. Nepal Heal. Res. Counc. 2020, 17, 548–552. [Google Scholar]
- Christiansen, T.; Lauritsen, J. EpiData-Comprehensive Data Management and Basic Statistical Analysis System; EpiData Association: Odense, Denamark, 2010. [Google Scholar]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2012, 10, 28–55. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Liu, H. Establishment of a method for measuring total complement activity based on a hemolysis system using own red blood cells. J. Immunol. Methods 2016, 430, 21–27. [Google Scholar] [PubMed]
- Kovacik, A.; Tvrda, E.; Fulopova, D.; Cupka, P.; Kovacikova, E.; Zbynovska, K.; Massanyi, P. In vitro assessment of gentamicin cytotoxicity on the selected mammalian cell line (Vero cells). Adv. Res. Life Sci. 2017, 1, 111–116. [Google Scholar] [CrossRef]
- Cao, J.; Yu, Z.; Liu, W.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Probiotic characteristics of Bacillus coagulans and associated implications for human health and diseases. J. Funct. Foods 2020, 64, 103643. [Google Scholar] [CrossRef]
- Tóth, A.G.; Csabai, I.; Judge, M.F.; Maróti, G.; Becsei, Á.; Spisák, S.; Solymosi, N. Mobile antimicrobial resistance genes in probiotics. Antibiotics 2021, 10, 1287. [Google Scholar] [CrossRef]
- Zhang, S.; Li, P.; Lee, S.; Wang, Y.; Tan, C.; Shang, N. Weizmannia coagulans: An ideal probiotic for gut health. Food Sci. Hum. Wellness 2024, 13, 16–26. [Google Scholar]
- Liang, J.; Li, C.; Chen, Z.; Guo, F.; Dou, J.; Wang, T.; Xu, Z.S. Progress of research and application of Heyndrickxia coagulans (Bacillus coagulans) as probiotic bacteria. Front. Cell. Infect. Microbiol. 2024, 14, 1415790. [Google Scholar]
- Aulitto, M.; Martinez-Alvarez, L.; Fiorentino, G.; Limauro, D.; Peng, X.; Contursi, P. A comparative analysis of Weizmannia coagulans genomes unravels the genetic potential for biotechnological applications. Int. J. Mol. Sci. 2022, 23, 3135. [Google Scholar] [CrossRef]
- Altun, G.K.; Erginkaya, Z. Identification and characterization of Bacillus coagulans strains for probiotic activity and safety. LWT 2021, 151, 112233. [Google Scholar]
- Afzaal, M.; Saeed, F.; Saeed, M.; Azam, M.; Hussain, S.; Mohamed, A.A.; Alamri, M.S.; Anjum, F.M. Survival and stability of free and encapsulated probiotic bacteria under simulated gastrointestinal and thermal conditions. Int. J. Food Prop. 2020, 23, 1899–1912. [Google Scholar] [CrossRef]
- Ayyash, M.M.; Abdalla, A.K.; AlKalbani, N.S.; Baig, M.A.; Turner, M.S.; Liu, S.-Q.; Shah, N.P. Invited review: Characterization of new probiotics from dairy and nondairy products—Insights into acid tolerance, bile metabolism and tolerance, and adhesion capability. J. Dairy Sci. 2021, 104, 8363–8379. [Google Scholar] [CrossRef]
- da Silva, T.F.; Glória, R.d.A.; Americo, M.F.; Freitas, A.d.S.; de Jesus, L.C.L.; Barroso, F.A.L.; Laguna, J.G.; Coelho-Rocha, N.D.; Tavares, L.M.; Le Loir, Y. Unlocking the potential of probiotics: A comprehensive review on research, production, and regulation of probiotics. Probiotics Antimicrob. Proteins 2024, 16, 1687–1723. [Google Scholar] [CrossRef] [PubMed]
- Fijan, S.; Fijan, T.; Connil, N. Overview of probiotic strains of Weizmannia coagulans, previously known as Bacillus coagulans, as food supplements and their use in human health. Appl. Microbiol. 2023, 3, 935–947. [Google Scholar] [CrossRef]
- Kapse, N.; Engineer, A.; Gowdaman, V.; Wagh, S.; Dhakephalkar, P. Functional annotation of the genome unravels probiotic potential of Bacillus coagulans HS243. Genomics 2019, 111, 921–929. [Google Scholar] [CrossRef]
- Wu, Y.-P.; Liu, D.-M.; Zhao, S.; Huang, Y.-Y.; Yu, J.-J.; Zhou, Q.-Y. Assessing the safety and probiotic characteristics of Bacillus coagulans 13002 based on complete genome and phenotype analysis. LWT 2022, 155, 112847. [Google Scholar] [CrossRef]
- Begley, M.; Hill, C.; Gahan, C.G. Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 2006, 72, 1729–1738. [Google Scholar] [CrossRef]
- Yu, Y.; Zong, M.; Lao, L.; Wen, J.; Pan, D.; Wu, Z. Adhesion properties of cell surface proteins in Lactobacillus strains in the GIT environment. Food Funct. 2022, 13, 3098–3109. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Średnicka, P.; Juszczuk-Kubiak, E.; Wójcicki, M.; Akimowicz, M.; Roszko, M. Probiotics as a biological detoxification tool of food chemical contamination: A review. Food Chem. Toxicol. 2021, 153, 112306. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- García, G.; Soto, J.; Díaz, A.; Barreto, J.; Soto, C.; Pérez, A.B.; Boffill, S.; Cano, R.D.J. Randomized Clinical Trials Demonstrate the Safety Assessment of Alkalihalobacillus clausii AO1125 for Use as a Probiotic in Humans. Microorganisms 2024, 12, 2299. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, I.M.; Frederich, M.; Bagwe, R.; Lannig, G.; Sukhotin, A.A. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 2012, 79, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Foley, M.H.; O’Flaherty, S.; Barrangou, R.; Theriot, C.M. Bile salt hydrolases: Gatekeepers of bile acid metabolism and host-microbiome crosstalk in the gastrointestinal tract. PLoS Pathog. 2019, 15, e1007581. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Antimicrobial and anti-adhesive properties of biosurfactant produced by lactobacilli isolates, biofilm formation and aggregation ability. J. Gen. Appl. Microbiol. 2013, 59, 425–436. [Google Scholar] [CrossRef]
- Deng, Z.; Luo, X.M.; Liu, J.; Wang, H. Quorum sensing, biofilm, and intestinal mucosal barrier: Involvement the role of probiotic. Front. Cell. Infect. Microbiol. 2020, 10, 538077. [Google Scholar] [CrossRef]
- Aziz, K.; Gilbert, J.A.; Zaidi, A.H. Genomic and Phenotypic Insight into the Probiotic Potential of Lactic Acid Bacterial spp. Associated with the Human Gut Mucosa. Probiotics Antimicrob. Proteins 2023, 1–29. [Google Scholar] [CrossRef]
- Patlevič, P.; Vašková, J.; Švorc, P., Jr.; Vaško, L.; Švorc, P. Reactive oxygen species and antioxidant defense in human gastrointestinal diseases. Integr. Med. Res. 2016, 5, 250–258. [Google Scholar] [CrossRef]
- Rodriguez Ayala, F.; Bartolini, M.; Grau, R. The stress-responsive alternative sigma factor SigB of Bacillus subtilis and its relatives: An old friend with new functions. Front. Microbiol. 2020, 11, 1761. [Google Scholar] [CrossRef]
- Adhikary, S.; Saha, J.; Dutta, P.; Pal, A. Bacterial Homeostasis and Tolerance to Potentially Toxic Metals and Metalloids through Diverse Transporters: Metal-Specific Insights. Geomicrobiol. J. 2024, 41, 496–518. [Google Scholar] [CrossRef]
- Lee, H.; Song, J.; Lee, B.; Cha, J.; Lee, H. Food carbohydrates in the gut: Structural diversity, microbial utilization, and analytical strategies. Food Sci. Biotechnol. 2024, 33, 2123–2140. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, J.; McEwan, A.G.; Kappler, U. Bacterial acetate metabolism and its influence on human epithelia. Emerg. Top. Life Sci. 2023, 8, 1–13. [Google Scholar]
- Li, S.; Li, N.; Wang, C.; Zhao, Y.; Cao, J.; Li, X.; Zhang, Z.; Li, Y.; Yang, X.; Wang, X. Gut microbiota and immune modulatory properties of human breast Milk streptococcus salivarius and S. parasanguinis strains. Front. Nutr. 2022, 9, 798403. [Google Scholar] [CrossRef] [PubMed]
- Colin, R.; Ni, B.; Laganenka, L.; Sourjik, V. Multiple functions of flagellar motility and chemotaxis in bacterial physiology. FEMS Microbiol. Rev. 2021, 45, fuab038. [Google Scholar] [CrossRef]
- Panel, E.F. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA J. 2018, 16, 5206–5230. [Google Scholar]
- Haranahalli Nataraj, B.; Behare, P.V.; Yadav, H.; Srivastava, A.K. Emerging pre-clinical safety assessments for potential probiotic strains: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 8155–8183. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 2001, 14, 227. [Google Scholar] [CrossRef]
- Henderson, P.J.; Maher, C.; Elbourne, L.D.; Eijkelkamp, B.A.; Paulsen, I.T.; Hassan, K.A. Physiological functions of bacterial “multidrug” efflux pumps. Chem. Rev. 2021, 121, 5417–5478. [Google Scholar] [CrossRef]
- Pradhan, D.; Mallappa, R.H.; Grover, S. Comprehensive approaches for assessing the safety of probiotic bacteria. Food Control 2020, 108, 106872. [Google Scholar] [CrossRef]
- Smith, W.P.; Wucher, B.R.; Nadell, C.D.; Foster, K.R. Bacterial defences: Mechanisms, evolution and antimicrobial resistance. Nat. Rev. Microbiol. 2023, 21, 519–534. [Google Scholar] [CrossRef]
- Spiro, S. Regulation of denitrification. In Metalloenzymes in Denitrification: Applications and Environmental Impacts; The Royal Society of Chemistry: London, UK, 2016; pp. 312–330. [Google Scholar]
- Fassarella, M.; Blaak, E.E.; Penders, J.; Nauta, A.; Smidt, H.; Zoetendal, E.G. Gut microbiome stability and resilience: Elucidating the response to perturbations in order to modulate gut health. Gut 2021, 70, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Bazaka, K.; Crawford, R.J.; Nazarenko, E.L.; Ivanova, E.P. Bacterial extracellular polysaccharides. In Bacterial Adhesion: Chemistry, Biology and Physics; Advances in Experimental Medicine and Biology Series; Springer: Dordrecht, The Netherlands, 2011; pp. 213–226. [Google Scholar]
- Lebeer, S.; Claes, I.; Tytgat, H.L.; Verhoeven, T.L.; Marien, E.; von Ossowski, I.; Reunanen, J.; Palva, A.; de Vos, W.M.; De Keersmaecker, S.C. Functional analysis of Lactobacillus rhamnosus GG pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl. Environ. Microbiol. 2012, 78, 185–193. [Google Scholar] [CrossRef]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.M.; Krüger, A.; Tauqeer Alam, M. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. 2015, 90, 927–963. [Google Scholar] [CrossRef] [PubMed]
- Palmnäs-Bédard, M.S.A.; Costabile, G.; Vetrani, C.; Åberg, S.; Hjalmarsson, Y.; Dicksved, J.; Riccardi, G.; Landberg, R. The human gut microbiota and glucose metabolism: A scoping review of key bacteria and the potential role of SCFAs. Am. J. Clin. Nutr. 2022, 116, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Prior, J.L.; Prior, R.G.; Hitchen, P.G.; Diaper, H.; Griffin, K.F.; Morris, H.R.; Dell, A.; Titball, R.W. Characterization of the O antigen gene cluster and structural analysis of the O antigen of Francisella tularensis subsp. tularensis. J. Med. Microbiol. 2003, 52, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Valvano, M.A. Undecaprenyl phosphate recycling comes out of age. Mol. Microbiol. 2008, 67, 232–235. [Google Scholar] [CrossRef]
- Soumya, M.; Nampoothiri, K.M. An overview of functional genomics and relevance of glycosyltransferases in exopolysaccharide production by lactic acid bacteria. Int. J. Biol. Macromol. 2021, 184, 1014–1025. [Google Scholar] [CrossRef]
- Xiao, L.; Yang, Y.; Han, S.; Rui, X.; Ma, K.; Zhang, C.; Wang, G.; Li, W. Effects of genes required for exopolysaccharides biosynthesis in Lacticaseibacillus paracasei S-NB on cell surface characteristics and probiotic properties. Int. J. Biol. Macromol. 2023, 224, 292–305. [Google Scholar] [CrossRef]
- Mazhar, S.; Simon, A.; Khokhlova, E.; Colom, J.; Leeuwendaal, N.; Deaton, J.; Rea, K. In vitro safety and functional characterization of the novel Bacillus coagulans strain CGI314. Front. Microbiol. 2024, 14, 1302480. [Google Scholar] [CrossRef]
- Pessione, E.; Cirrincione, S. Bioactive molecules released in food by lactic acid bacteria: Encrypted peptides and biogenic amines. Front. Microbiol. 2016, 7, 876. [Google Scholar] [CrossRef]
- Alvarez, M.A.; Moreno-Arribas, M.V. The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci. Technol. 2014, 39, 146–155. [Google Scholar] [CrossRef]
- Santos, M.S. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.J. Biosynthesis of polyamines in eukaryotes, archaea, and bacteria. In Polyamines: A Universal Molecular Nexus for Growth, Survival, and Specialized Metabolism; Springer: Tokyo, Japan, 2015; pp. 3–14. [Google Scholar]
- Lucas, P. Ornithine and lysine decarboxylation in bacteria. In Handbook of Microbial Metabolism of Amino Acids; CAB International: Wallingford, UK, 2017; pp. 116–127. [Google Scholar]
- Kalman, D.S.; Schwartz, H.I.; Alvarez, P.; Feldman, S.; Pezzullo, J.C.; Krieger, D.R. A prospective, randomized, double-blind, placebo-controlled parallel-group dual site trial to evaluate the effects of a Bacillus coagulans-based product on functional intestinal gas symptoms. BMC Gastroenterol. 2009, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Maity, C.; Gupta, A.K. A prospective, interventional, randomized, double-blind, placebo-controlled clinical study to evaluate the efficacy and safety of Bacillus coagulans LBSC in the treatment of acute diarrhea with abdominal discomfort. Eur. J. Clin. Pharmacol. 2019, 75, 21–31. [Google Scholar] [CrossRef]
- Li, Z.; Guan, Z.; Bai, N.; Yan, Y.; Niu, Z.; Xu, J.; Gao, W.; Chen, W. Bacillus coagulans TBC169 probiotics for the recovery of intestinal function after gynecological laparoscopic surgery: A randomized, placebo-controlled trial. Int. J. Clin. Pharm. 2022, 44, 1287–1295. [Google Scholar] [CrossRef]
- Bang, W.Y.; Ban, O.-H.; Lee, B.S.; Oh, S.; Park, C.; Park, M.-K.; Jung, S.K.; Yang, J.; Jung, Y.H. Genomic-, phenotypic-, and toxicity-based safety assessment and probiotic potency of Bacillus coagulans IDCC 1201 isolated from green malt. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab026. [Google Scholar] [CrossRef]
- Saroj, D.B.; Gupta, A.K. Genome based safety assessment for Bacillus coagulans strain LBSC (DSM 17654) for probiotic application. Int. J. Food Microbiol. 2020, 318, 108523. [Google Scholar] [CrossRef]
- Cabarkapa, D.; Whetstone, J.M.; Patterson, A.M.; Mosier, E.M.; Cabarkapa, D.V.; Fry, A.C. Relationship between Health-Related Physical Fitness Parameters and Functional Movement Screening Scores Acquired from a Three-Dimensional Markerless Motion Capture System. Int. J. Environ. Res. Public Health 2022, 19, 4551. [Google Scholar] [CrossRef]
- Lai, C.W.; Jadhav, S.; Njei, B.; Ye, A.; Wactawski-Wende, J.; Mumford, S.L.; Schisterman, E.F.; Rotman, Y. Rhythmic Fluctuations in Levels of Liver Enzymes During Menstrual Cycles of Healthy Women and Effects of Body Weight. Clin. Gastroenterol. Hepatol. 2020, 18, 2055–2063. [Google Scholar] [CrossRef]
- Sotoudegan, F.; Daniali, M.; Hassani, S.; Nikfar, S.; Abdollahi, M. Reappraisal of probiotics’ safety in human. Food Chem. Toxicol. 2019, 129, 22–29. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Natarajan, S.; Sivakumar, A.; Pande, A.; Majeed, S.; Ali, F. A Double-Blind, Placebo-Controlled, Parallel StudyEvaluating the Safety of Bacillus coagulans MTCC 5856 in Healthy Individuals. J. Clin. Toxicol. 2016, 6, 283. [Google Scholar] [CrossRef]
- Cutting, S.M.; Ricca, E. Bacterial Spore-Formers: Friends and Foes; Blackwell Publishing Ltd.: Oxford, UK, 2014; Volume 358, pp. 107–109. [Google Scholar]
| Gene | Enzyme | Product |
|---|---|---|
| hdcA | Histidine decarboxylase | Histamine |
| tdcA | Tyrosine decarboxylase | Tyramine |
| speC | Ornithine decarboxylase | Putrescine |
| ldc | Lysine decarboxylase | Cadaverine |
| aguA | Agmatine ureohydrolase | Agmatine |
| aguD | Agmatine deiminase antiporter | Agmatine |
| speE | Spermidine synthetase | Spermidine |
| speF | Spermine synthetase | Spermine |
| speG | Spermidine synthetase | Spermidine |
| tyrDC | Tyrosine decarboxylase | Phenylethylamine |
| tph | Tryptophan hydroxylase | Serotonin |
| aadc | Aromatic amino acid decarboxylase | Serotonin |
| tbh | Tyramine β-hydroxylase | Octopamine |
| tam | Tryptamine synthase | Tryptamine |
| sufI | Copper-containing nitrite reductase | Nitric oxide |
| nasD | Nitrite reductase | Nitric oxide |
| Demographic Variables | H. coagulans AO1167B Cohort (n = 50) | Placebo Cohort (n = 49) | p Value | |||
|---|---|---|---|---|---|---|
| Number | % | Number | % | |||
| Sex | Female | 31 | 62 | 31 | 63.3 | 0.896 a |
| Male | 19 | 38 | 18 | 36.7 | ||
| Age (years) Median ± SD | 42.82 ± 16.3 | 44.43 ± 14.74 | 1.0 b | |||
| BMI Median ± SD | 23.20 ± 3.93 | 23.63 ± 4.17 | 0.764 a | |||
| Gene | Encoded Protein |
|---|---|
| Acid tolerance | |
| atpA | ATP synthase subunit alpha |
| atpB | ATP synthase subunit beta |
| ldh1 | L-lactate dehydrogenase 1 |
| ldhD | D-lactate dehydrogenase |
| pgi | Glucose-6-phosphate isomerase |
| groL | 60 kDa chaperonin |
| cspB | Cold shock protein |
| teaD | TRAP-T-associated universal stress protein |
| ald | Alanine dehydrogenase |
| gabD | Succinate-semialdehyde dehydrogenase |
| fdhD | formate dehydrogenase |
| pgi | Glucose-6-phosphate isomerase |
| atpB | ATP synthase subunit B |
| Acid/bile tolerance | |
| arcD1 | Arginine/ornithine antiporter ArcD1 |
| iPGM | bisphosphoglycerate-independent phosphoglycerate mutase |
| argF | Ornithine carbamoyltransferase |
| gpmI | 2,3-bisphosphoglycerate-independent phosphoglycerate mutase |
| argR | Arginine repressor |
| Adhesion and aggregation | |
| eno | Enolase |
| mntH | Manganese transferase/Divalent metal cation transporter |
| ywgD | Tyrosine-protein kinase |
| tpiA | Triosephosphate isomerase |
| ugtP | Glucosyltransferase |
| tuf | Elongation factor Tu |
| spaCBA | Pilin |
| Antioxidant defense | |
| trxA | Thioredoxin |
| katE | Catalase |
| Detoxification | |
| arsC | Arsenate reductase |
| bsh | Bile salt hydrolase |
| cadA | Cadmium-transporting ATPase |
| hsp18 | 18 kDa heat shock protein |
| Bile tolerance | |
| nagB | Glucosamine-6-phosphate deaminase |
| pyrG | CTP (cytidine triphosphate synthetase) synthase |
| nagB | Glucosamine-6-phosphate deaminase |
| bshB | Bile salt hydrolase |
| Biofilm formation/Adhesion/Chemotaxis | |
| luxS | Lyase |
| cheA | Chemotaxis protein CheA |
| srfA | Surfactin synthetase |
| hag | Flagellin |
| motB | Motility protein |
| manX | mannose-specific EIIAB component |
| Carbohydrate metabolism | |
| galT | UDP-glucose--hexose-1-phosphate uridylyl transferase |
| nagA | Glucosamine-6-phosphate deaminase |
| bgaB | Beta-galactosidase |
| agaA | alpha galactosidase |
| bbmA | intracellular maltogenic amylase |
| gntR | Xylose utilization operon |
| xylA | Xylose isomerase |
| galE | UDP-glucose 4-epimerase |
| Cell wall remodeling | |
| pbp | Penicillin-binding protein |
| murA | UDP-N-acetylglucosamine enol pyruvyl transferase |
| Immune modulation | |
| hemA | Glutamyl-tRNA reductase |
| groEL | Chaperonin GroEL |
| magl | Monoacylglycerol lipase |
| ctkA | Serine/threonine protein kinase |
| Metabolism | |
| feoB | Ferrous ion transporter |
| ldh | L-lactate dehydrogenase |
| lacA | Beta-galactosidase |
| glnA | Glutamine synthetase |
| UreA | Urease |
| methH | Methionine synthase |
| phoB | Phosphate regulon protein |
| pstS | Phosphate-binding protein |
| clpP | Protease ClpP |
| purA | Adenylosuccinate synthetase |
| SCFA (acetate) production | |
| ackA | Acetate kinase |
| pta | Phosphotransacetylase |
| Stress tolerance/Response | |
| phoPR | Two-component response regulator |
| sigH | Sigma factor H |
| sigB | Sigma factor B |
| clpL | Stress response protein |
| msrA | Methionine sulfoxide reductase |
| acoA | Oxidoreductase |
| yvbW | Amino acid permease |
| emrY | Multidrug resistance protein |
| TeaD | Universal stress protein |
| Vitamin biosynthesis | |
| cobA | Adenosylcobalamin synthase |
| fadA | 3-ketoacyl-CoA thiolase @ Acetyl-CoA acetyltransferase |
| fadD_1 | Long-chain-fatty-acid-CoA ligase |
| bioB | Biotin synthase |
| hpt | Hypoxanthine-guanine phosphoribosyltransferase |
| dfrA | Dihydrofolate reductase |
| thyA2 | Thymidylate synthase |
| serA | D-3-phosphoglycerate dehydrogenase |
| dagK | Diacylglycerol kinase |
| thieE | Thiamine phosphate synthase |
| Antimicrobial | M100 Susceptible Breakpoint for Staphylococcus aureus | Staphylococcus aureus ATCC 29213 | H. coagulans AO1167B | ||
|---|---|---|---|---|---|
| Zone Diameter (mm) 1 | Interpretation | Zone Diameter (mm) | Interpretation | ||
| Ampicillin | ≥29 | 41.8 | S 2 | 45.3 | S |
| Chloramphenicol | ≥19 | 24.5 | S | 33.2 | S |
| Clindamycin | NA | 29 | S | 44.1 | S |
| Erythromycin | ≥15 | 28.4 | S | 41.5 | S |
| Gentamicin | ≥18 | 24.2 | S | 36.2 | S |
| Kanamycin | NA | 24 | S | 36.2 | S |
| Streptomycin | ≥18 | 17.1 | S | 25.4 | NA 3 |
| Tetracycline | ≥23 | 31.9 | S | 45.8 | S |
| Vancomycin | ≥21 | 18.7 | S | 36.3 | NA |
| Gene | Length (bp) | Resistance Mechanism | AMR Gene Function | % Identity of Matching Region |
|---|---|---|---|---|
| vanH gene in vanO cluster | 990 | glycopeptide | glycopeptide resistance gene cluster | 31.78 |
| vanY gene in vanM cluster | 795 | glycopeptide | glycopeptide resistance gene cluster | 35.63 |
| vanT gene in vanG cluster | 1152 | glycopeptide | glycopeptide resistance gene cluster | 34.38 |
| vanW gene in vanI cluster | 1824 | glycopeptide | glycopeptide resistance gene cluster | 32.00 |
| qacG | 744 | efflux | small multidrug resistance protein that facilitates the efflux of QACs 1 | 44.4 |
| qacJ | 744 | efflux | small multidrug resistance protein that facilitates the efflux of QACs | 46.46 |
| acrB | 3165 | multidrug | resistance-nodulation-cell division (RND) efflux pump | 80.41 |
| Clinical Chemistry and Haematology | Normal Range | H. coagulans Cohort (n = 50) | Placebo Cohort (n = 49) | p Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Day 60 | Baseline | Day 60 | p1 | p2 | p3 | p4 | ||
| Creatinine | 47.6–113.4 μmol/L | 78.23 ± 18.09 | 75.53 ± 17.22 | 73.10 ± 21.59 | 76.167 ± 17.84 | NS | NS | 0.032 | NS |
| Urea | <8.3 mmol/L | 4.27 ± 1.05 | 4.28 ± 1.01 | 4.02 ± 1.16 | 4.47 ± 1.12 | NS | NS | NS | 0.012 |
| ALAT | <45 U/L | 14.98 ± 6.55 | 13.73 ± 5.78 | 19.44 ± 9.45 | 16.26 ± 6.71 | 0.008 | 0.047 | NS | 0.002 |
| ASAT | 40 U/L | 13.74 ± 4.76 | 22.83 ± 6.18 | 18.13 ± 4.78 | 13.35 ± 5.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| GGT | <50 U/L | 21.70 ± 16.82 | 19.64 ± 14.51 | 22.57± 16.62 | 24.80 ± 20.21 | NS | NS | NS | 0.026 |
| Total Protein | 60–80 g/L | 70.84 ± 4.03 | 71.07 ± 3.53 | 70.59 ± 6.22 | 70.77 ± 4.00 | NS | NS | NS | NS |
| Albumin | 35–52 g/L | 44.45 ± 3.40 | 39.92 ± 2.19 | 44.78 ± 3.38 | 44.65 ± 2.89 | NS | <0.01 | <0.01 | NS |
| Glycemia | 4.2–6.1 μmol/L | 4.70 ± 0.46 | 4.61 ± 0.60 | 4.74 ± 0.55 | 4.69 ± 0.46 | NS | NS | NS | NS |
| Cholesterol | 2.81–5.2 mmol/L | 4.38 ± 1.15 | 4.47 ± 0.92 | 4.95 ± 1.24 | 4.49 ± 1.11 | 0.020 | NS | NS | <0.01 |
| Triglycerides | 0.46–1.8mmol/L | 1.131 ± 0.74 | 1.60 ± 0.52 | 1.29 ±0.87 | 1.33 ± 0.69 | NS | 0.031 | 0.001 | NS |
| Total bilirubin | <17 mmol/L | 8.05 ± 4.47 | 9.37 ± 3.21 | 9.32 ± 5.33 | 7.62 ± 4.49 | NS | 0.028 | 0.01 | 0.002 |
| Direct bilirubin | <5.1 mmol/L | 3.02 ± 1.23 | 2.95 ± 1.13 | 4.03 ± 4.59 | 2.95 ± 1.24 | NS | NS | NS | 0.001 |
| WBC | (4.5–11) × 109/μL | 6.37± 2.00 | 6.31± 2.35 | 6.26 ± 1.62 | 6.52 ± 1.96 | NS | NS | NS | NS |
| RBC | (F = 4.2–5.4/M = 4.7–6.1) cels/μL | 4.56 ± 0.38 | 4.55 ± 0.38 | 4.51 ± 0.40 | 4.56 ± 0.39 | NS | NS | NS | NS |
| HBG | (F = 12.3–15.3/M = 14.0–17.5) g/dL | 132.74 ± 13.65 | 132.88 ± 13.31 | 131.53 ± 15.45 | 132.51 ± 13.71 | NS | NS | NS | NS |
| HTC | (F = 36–45/M = 42–50) % | 0.42 ± 0.03 | 0.42 ± 0.03 | 0.40 ± 0.04 | 0.42 ± 0.03 | NS | NS | NS | 0.001 |
| MVC | 80–96.1% | 92.57± 5.24 | 91.49 ± 5.31 | 90.79 ± 6.15 | 92.30 ± 5.53 | NS | NS | 0.003 | <0.01 |
| MCH | 33.4–35.5 g/dL | 29.11 ± 2.05 | 29.24 ± 1.96 | 29.15 ± 2.36 | 29.02 ± 2.13 | 0.046 | 0.013 | <0.01 | NS |
| PLT | (172–450) × 103/mL | 257.70 ± 70.08 | 267.06 ± 66.51 | 261.88 ± 52.73 | 259.67 ± 62.52 | NS | NS | NS | NS |
| RDWCV | (11–14) % | 1.05 ± 1.22 | 12.96 ± 1.08 | 12.82 ± 1.90 | 13.12 ± 1.22 | NS | NS | 0.017 | 0.014 |
| MPV | (F: 12–16/M: 14–17.4) g/dL | 10.45 ± 0.90 | 10.66 ± 0.88 | 10.34 ± 0.90 | 10.35 ± 0.80 | NS | NS | 0.040 | NS |
| Neutrophil | 1.42–6.34 × 109/L | 3.60± 1.55 | 3.65 ± 1.90 | 3.36 ± 1.16 | 3.66 ± 1.56 | NS | NS | NS | NS |
| lymphocytes | 0.71–4.53 × 109/L | 2.02 ± 0.64 | 1.91 ± 0.63 | 2.12 ± 0.63 | 2.10 ± 0.66 | NS | NS | NS | NS |
| Monocytes | 0.14–0.72 × 109/L | 0.52 ± 0.17 | 0.55 ± 0.23 | 0.52 ± 0.13 | 0.52 ± 0.16 | NS | NS | NS | NS |
| Eosinophils | 0–0.54 × 109/L | 0.19 ± 0.16 | 0.19 ± 0.19 | 0.20 ± 0.17 | 0.19 ± 0.13 | NS | NS | NS | NS |
| Basophils | 0–0.18 × 109/L | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | NS | NS | NS | NS |
| Bioimpedance Variables | Baseline | Day 60 | Baseline | Day 60 | p1 | p2 | p3 | p4 | |
| Weight | 68.49 ± 12.09 | 66.45 ± 12.60 | 66.64 ± 13.72 | 66.43 ± 13.57 | NS | ||||
| BMI | 24.07 ± 3.65 | 23.13 ± 3.99 | 23.63 ± 4.17 | 23.57 ± 4.15 | NS | ||||
| Adverse Event | H. coagulans Cohort (n = 50) | Placebo Cohort (n = 49) | ||||
|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Mild | Moderate | Severe | |
| Abdominal/GI discomfort | 2 (4.0) | 0 | 0 | 0 | ||
| acne rosacea | 0 | 0 | 0 | 0 | 0 | 0 |
| Anxiety Depression | 0 | 0 | 0 | 0 | 0 | 0 |
| Joint pain | 0 | 0 | 0 | 0 | 0 | 0 |
| Bronchitis | 0 | 0 | 0 | 0 | 0 | 0 |
| Excision of birthmarks | 0 | 0 | 0 | 0 | 0 | 0 |
| Bruises after a fall | 0 | 0 | 0 | 0 | 0 | 0 |
| Crotid stenosis | 0 | 0 | 0 | 0 | 0 | 0 |
| Cataract Surgery | 0 | 0 | 0 | 0 | 0 | 0 |
| Chondrocalcinosis | 0 | 0 | 0 | 0 | 0 | 0 |
| Colonoscopy and fibroscopy | 0 | 0 | 0 | 0 | 0 | 0 |
| Cystitis | 0 | 0 | 0 | 0 | 0 | 0 |
| Dental pain | 0 | 0 | 0 | 0 | 0 | 0 |
| Diarrhoea | 3 (6.0) | 0 | 0 | 2 (2.0) | 0 | 0 |
| Dizziness and nausea | 0 | 0 | 0 | 0 | 0 | 0 |
| Oedema | 0 | 0 | 0 | 0 | 1 (1.0) | 0 |
| Gases | 5 (10.0) | 0 | 0 | 0 | 0 | 0 |
| General aches | 0 | 0 | 0 | 0 | 0 | 0 |
| Genital herpes | 0 | 0 | 0 | 0 | 0 | 0 |
| Headache | 1 (2.0) | 0 | 0 | 0 | 0 | 0 |
| Haemorrhoids | 0 | 0 | 0 | 0 | 0 | 0 |
| Infection | 0 | 0 | 0 | 0 | 0 | 0 |
| Inflamed prostate | 0 | 0 | 0 | 0 | 0 | 0 |
| Migraine | 0 | 0 | 0 | 0 | 0 | 0 |
| Mouth ulcer | 0 | 0 | 0 | 0 | 0 | 0 |
| Muscle discomfort | 0 | 0 | 0 | 0 | 0 | 0 |
| Nasal obstruction | 0 | 0 | 0 | 0 | 0 | 0 |
| Orthopaedic pain | 0 | 0 | 0 | 0 | 0 | 0 |
| Pain following capsule consumption | 0 | 0 | 0 | 0 | 0 | 0 |
| Palpitations | 0 | 0 | 0 | 0 | 0 | 0 |
| Radio-infiltration (shoulder) | 0 | 0 | 0 | 0 | 0 | 0 |
| Rhinitis | 0 | 0 | 0 | 0 | 0 | 0 |
| Sore throat | 0 | 0 | 0 | 0 | 0 | 0 |
| Tracheitis | 0 | 0 | 0 | 0 | 0 | 0 |
| Trouble sleeping (insomnia) | 0 | 0 | 0 | 0 | 0 | 0 |
| Vaginal dryness | 0 | 0 | 0 | 0 | 0 | 0 |
| Vagal seizures during or after taking a blood sample. | 0 | 0 | 0 | 0 | 0 | 0 |
| vitamin D deficiency | 0 | 0 | 0 | 0 | 0 | 0 |
| Others (constipation) | 2 (4.0) | 0 | 0 | 0 | 0 | 0 |
| Question | Associated Answer | Placebo (n = 49) | Cases (n = 50) | Kappa Test/p Value | |
|---|---|---|---|---|---|
| Before/After | Before/After | Placebo | Cases | ||
| 1-general, would you say your health is: | Excellent | 5/6 | 4/8 | 0.94 p = 0.000 | 0.78 p = 0.000 |
| Very Good | 21/21 | 32/28 | |||
| Good | 22/21 | 14/14 | |||
| Fair | 2/2 | 0/0 | |||
| 6-During the past 4 weeks, to what extent has your physical health or emotional problems interfered with your normal social activities with family, friends, neighbors, or groups? | Not at all | 14/36 | 39/40 | 0.95 p = 0.000 | 0.82 p = 0.000 |
| Slightly | 14/11 | 11/10 | |||
| Moderately | 18/1 | 0/0 | |||
| Quite a bit | 1/0 | 0/0 | |||
| Extremely | 1/0 | 0/0 | |||
| 7-How much bodily pain have you had during the past 4 weeks? | None | 14/18 | 19/22 | 0.82 p = 0.000 | 0.77 p = 0.000 |
| Very mild | 14/14 | 15/16 | |||
| mild | 18/14 | 13/9 | |||
| moderate | 1/1 | 3/3 | |||
| severe | 1/1 | 0/0 | |||
| 11a-I seem to get sick a little easier than other people | Definitely true | 0/0 | 0/0 | 0.96 p = 0.000 | 0.92 p = 0.000 |
| Mostly true | 3/3 | 0/0 | |||
| Don’t know | 2/2 | 5/4 | |||
| Mostly False | 10/9 | 12/12 | |||
| Definitely False | 33/34 | 32/33 | |||
| 11b-I am as healthy as anybody I know | Definitely true | 19/19 | 17/17 | 0.97 p = 0.000 | 0.97 p = 0.000 |
| Mostly true | 16/15 | 25/26 | |||
| Don’t know | 6/6 | 5/4 | |||
| Mostly False | 4/4 | 1/1 | |||
| Definitely False | 3/4 | 1/1 | |||
| 11c-I expect my health to get worse | Definitely true | 1/1 | 0/0 | 0.96 p = 0.000 | 0.91 p = 0.000 |
| Mostly true | 0/0 | 0/1 | |||
| Don’t know | 19/18 | 7/6 | |||
| Mostly False | 5/5 | 6/7 | |||
| Definitely False | 23/24 | 36/35 | |||
| 11d-My health is excellent | Definitely true | 9/10 | 9/8 | 0.93 p = 0.000 | 0.92 p = 0.000 |
| Mostly true | 27/25 | 31/33 | |||
| Don’t know | 6/6 | 8/7 | |||
| Mostly False | 3/3 | 1/1 | |||
| Definitely False | 3/4 | 0/0 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, G.; Soto, J.; Díaz, A.; Barreto, J.; Soto, C.; Pérez, A.B.; Boffill, S.; Gutiérrez, Á.; Cano, R.d.J. Clinical and In Vitro Safety of Heyndrickxia coagulans AO 1167B: A Double-Blind, Placebo-Controlled Trial. Microorganisms 2024, 12, 2584. https://doi.org/10.3390/microorganisms12122584
García G, Soto J, Díaz A, Barreto J, Soto C, Pérez AB, Boffill S, Gutiérrez Á, Cano RdJ. Clinical and In Vitro Safety of Heyndrickxia coagulans AO 1167B: A Double-Blind, Placebo-Controlled Trial. Microorganisms. 2024; 12(12):2584. https://doi.org/10.3390/microorganisms12122584
Chicago/Turabian StyleGarcía, Gissel, Josanne Soto, Antonio Díaz, Jesús Barreto, Carmen Soto, Ana Beatriz Pérez, Suselys Boffill, Ángela Gutiérrez, and Raúl de Jesús Cano. 2024. "Clinical and In Vitro Safety of Heyndrickxia coagulans AO 1167B: A Double-Blind, Placebo-Controlled Trial" Microorganisms 12, no. 12: 2584. https://doi.org/10.3390/microorganisms12122584
APA StyleGarcía, G., Soto, J., Díaz, A., Barreto, J., Soto, C., Pérez, A. B., Boffill, S., Gutiérrez, Á., & Cano, R. d. J. (2024). Clinical and In Vitro Safety of Heyndrickxia coagulans AO 1167B: A Double-Blind, Placebo-Controlled Trial. Microorganisms, 12(12), 2584. https://doi.org/10.3390/microorganisms12122584







