Enhanced Production of IL-10 in PCR-Positive Dogs Infected with E. canis and A. phagocytophilum Facilitate Specific Immune Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects and Blood Samples
2.2. Ethical Approval
2.3. Rapid Diagnostic Test
2.4. DNA Extraction
2.5. PCR Detection of Pathogens
2.6. ELISA Tests
2.7. Statistical Analysis
3. Results
3.1. Concentration of IL-10 in Seropositive Samples, Tested for Specific Antibodies with Rapid Diagnostic Test and ELISA
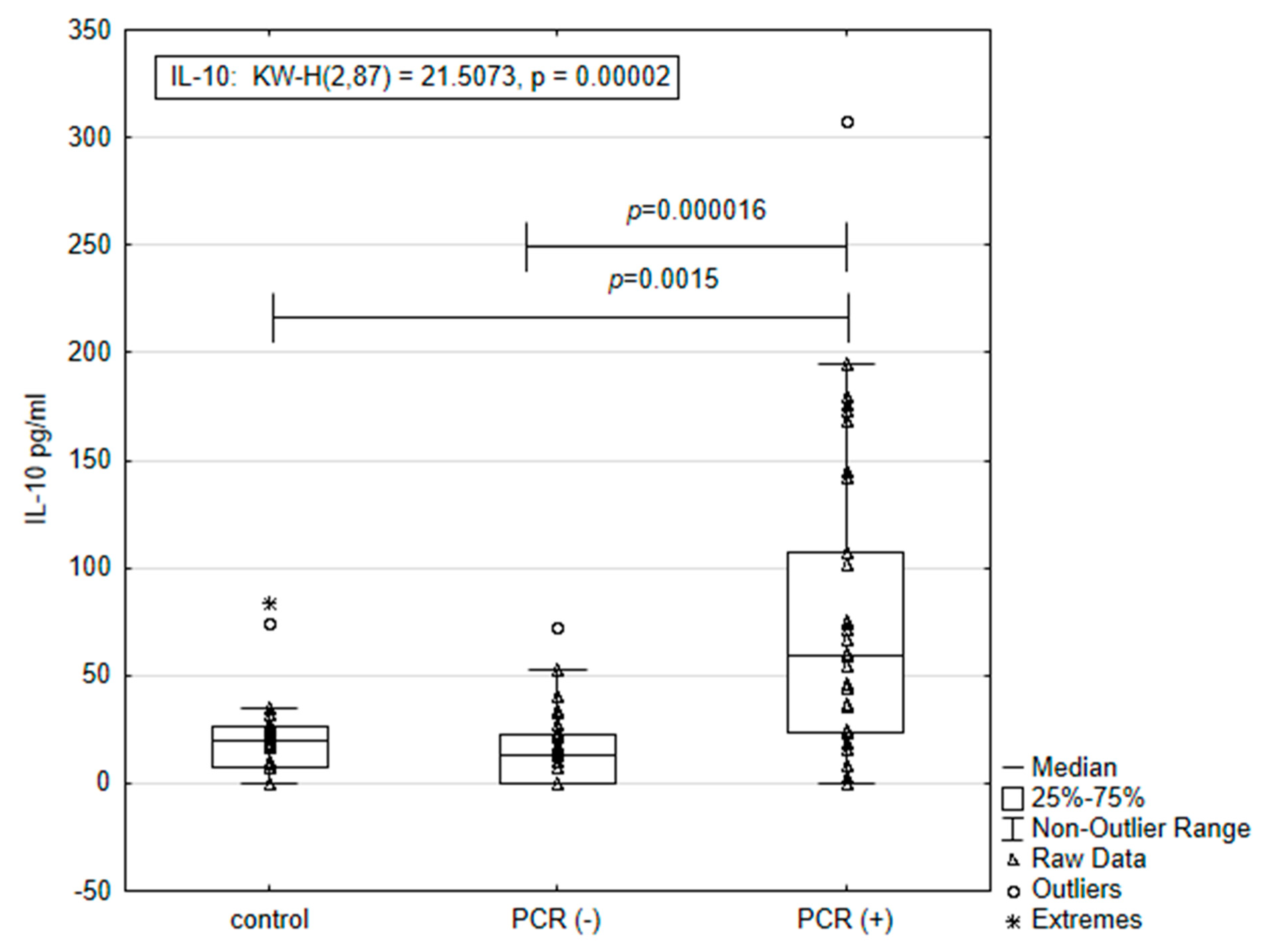
3.2. Concentration of IL-10 According to Results from PCR Tests for the Pathogens of Anaplasmataceae Family
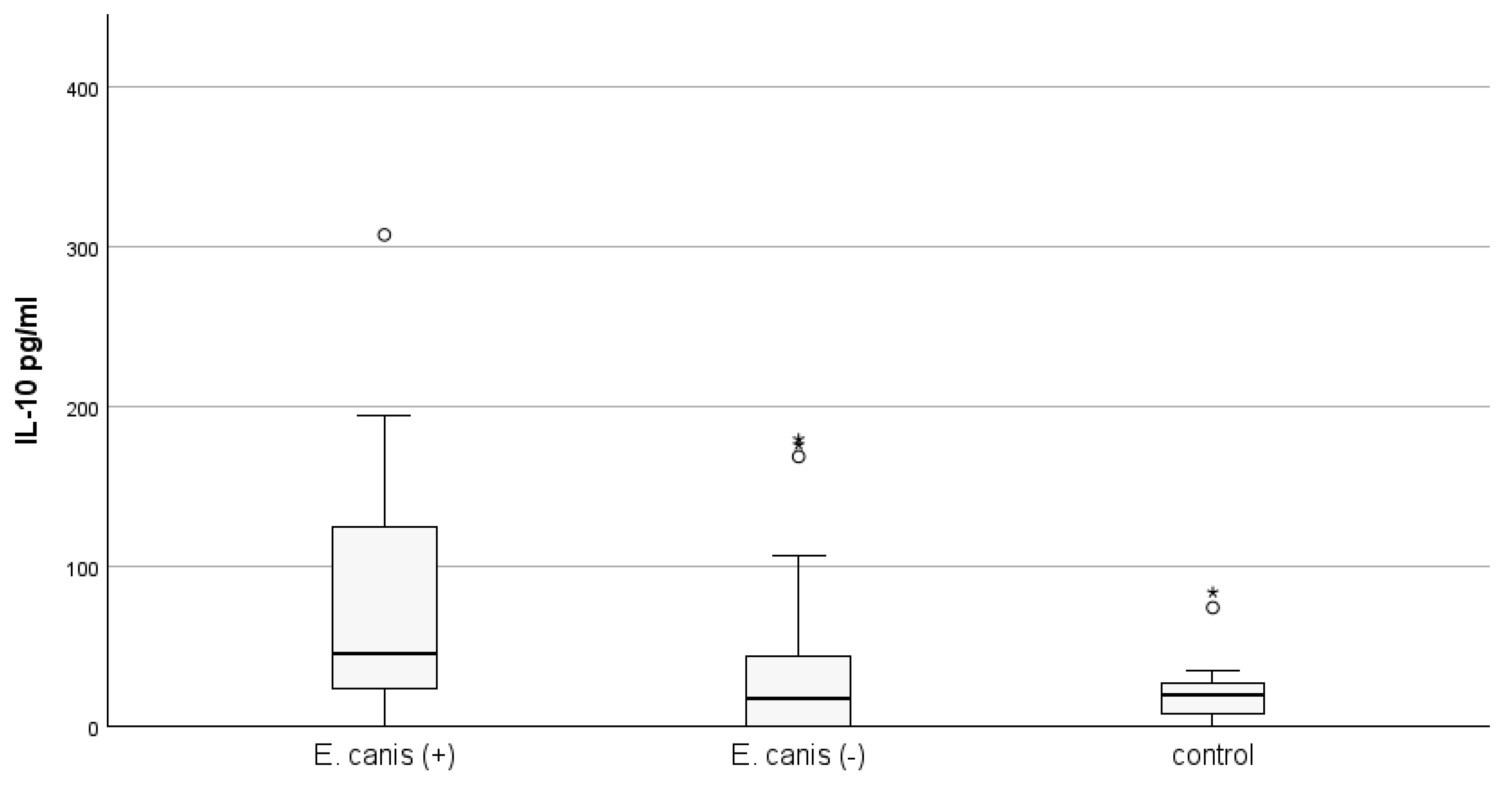
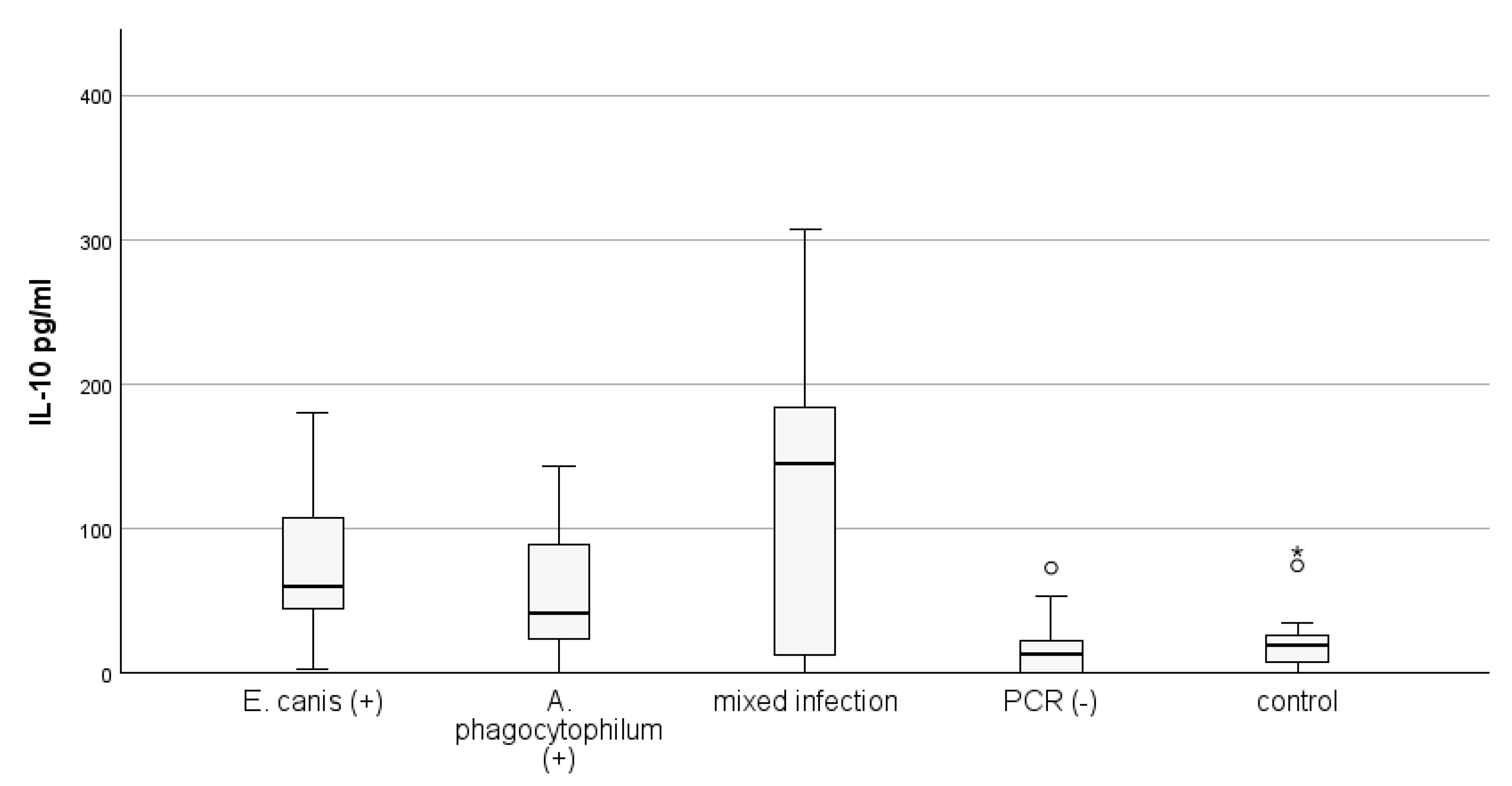
3.3. Concentration of IL-10 According to Results from Species-Specific PCR Tests for E. canis and A. phagocytophylum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guillemi, E.C.; Tomassone, L.; Farber, M.D. Tick-borne rickettsiales: Molecular tools for the study of an emergent group of pathogens. J. Microbiol. Methods 2015, 119, 87. [Google Scholar] [CrossRef] [PubMed]
- Pruneau, L.; Moumène, A.; Meyer, D.F.; Marcelino, I.; Lefrançois, T.; Vachiéry, N. Understanding Anaplasmataceae pathogenesis using “Omics” approaches. Front. Cell. Infect. Microbiol. 2014, 4, 86. [Google Scholar] [CrossRef]
- Dahmani, M.; Davoust, B.; Sambou, M.; Bassene, H.; Scandola, P.; Ameur, T.; Raoult, D.; Fenollar, F.; Mediannikov, O. Molecular investigation and phylogeny of species of the Anaplasmataceae infecting animals and ticks in Senegal. Parasit. Vectors 2019, 12, 495. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, W.L.; Allen, K.E.; McQuiston, J.H.; Breitschwerdt, E.B.; Little, S.E. The increasing recognition of rickettsial pathogens in dogs and people. Trends Parasitol. 2010, 26, 205. [Google Scholar] [CrossRef]
- Pantchev, N.; Schnyder, M.; Vrhovec, M.G.; Schaper, R.; Tsachev, I. Current surveys of the seroprevalence of Borrelia burgdorferi, Ehrlichia canis, Anaplasma phagocytophilum, Leishmania infantum, Babesia canis, Angiostrongylus vasorum and Dirofilaria immitis in dogs in Bulgaria. Parasitol. Res. 2015, 114, 117. [Google Scholar] [CrossRef]
- Nair, A.D.; Cheng, C.; Ganta, C.K.; Sanderson, M.W.; Alleman, A.R.; Munderloh, U.G.; Ganta, R.R. Comparative Experimental Infection Study in Dogs with Ehrlichia canis, E. chaffeensis, Anaplasma platys and A. phagocytophilum. PLoS ONE 2016, 11, e0148239. [Google Scholar] [CrossRef]
- ESCCAP. Guideline 5: Control of Vector-Borne Diseases in Dogs and Cats, 4th ed.; ESCCAP: Malvern, UK, 2023; Available online: https://www.esccap.org/uploads/docs/5y4xn3fr_0775_ESCCAP_Guideline_GL5_20221228_1p (accessed on 2 September 2024).
- Chomel, B. Tick-borne infections in dogs—An emerging infectious threat. Vet. Parasitol. 2011, 179, 294. [Google Scholar] [CrossRef]
- Carrade, D.; Foley, J.E.; Borjesson, D.L.; Sykes, J.E. Canine granulocytic anaplasmosis: A review. J. Vet. Med. 2009, 23, 1129. [Google Scholar] [CrossRef]
- Ismail, N.; Bloch, K.C.; McBride, J.W. Human ehrlichiosis and anaplasmosis. Clin. Lab. Med. 2010, 30, 261. [Google Scholar] [CrossRef]
- Sainz, Á.; Roura, X.; Miró, G.; Estrada-Peña, A.; Kohn, B.; Harrus, S.; Solano-Gallego, L. Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasit. Vectors 2015, 8, 7. [Google Scholar] [CrossRef]
- Brubaker, S.W.; Bonham, K.S.; Zanoni, I.; Kagan, J.C. Innate Immune Pattern Recognition: A Cell Biological Perspective. In Annual Review of Immunology; Littman, D.R., Yokoyama, W.M., Eds.; Annual Reviews: Palo Alto, CA, USA, 2015; Volume 33, p. 257. [Google Scholar]
- Kim, Y.K.; Shin, J.S.; Nahm, M.H. NOD-like receptors in infection, immunity, and diseases. Yonsei Med. J. 2016, 57, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Thai, V.; McCabe, A.; Jones, M.; MacNamara, K.C. Type I Interferons Promote Severe Disease in a Mouse Model of Lethal Ehrlichiosis. Infect. Immun. 2014, 82, 4. [Google Scholar] [CrossRef] [PubMed]
- Winslow, G.M.; Bitsaktsis, C. Immunity to the ehrlichiae: New tools and recent developments. Curr. Opin. Infect. Dis. 2005, 18, 217. [Google Scholar] [CrossRef] [PubMed]
- Birkner, K.; Steiner, B.; Rinkler, C.; Kern, Y.; Aichele, P.; Bogdan, C.; von Loewenich, F.D. The elimination of Anaplasma phagocytophilum requires CD4+ T cells but is independent of Th1 cytokines and a wide spectrum of effector mechanisms. Eur. J. Immunol. 2008, 38, 3395. [Google Scholar] [CrossRef] [PubMed]
- El Hamiani Khatat, S.; Daminet, S.; Duchateau, L.; Elhachim, L.; Kachani, M.; Sahibi, H. Epidemiological and Clinicopathological Features of Anaplasma phagocytophilum Infection in Dogs: A Systematic Review. Front. Vet. Sci. 2021, 8, 686644. [Google Scholar] [CrossRef]
- Ismail, N.; Sharma, A.; Soong, L.; Walker, D.H. Review: Protective Immunity and Immunopathology of Ehrlichiosis. Zoonoses 2022, 2, e979. [Google Scholar] [CrossRef]
- Ismail, N.; Stevenson, H.L.; Walker, D.H. Role of tumor necrosis factor alpha (TNF-alpha) and interleukin-10 in the pathogenesis of severe murine monocytotropic ehrlichiosis: Increased resistance of TNF receptor p55- and p75-deficient mice to fatal ehrlichial infection. Infect Immun. 2006, 74, 1846. [Google Scholar] [CrossRef]
- Liu, J.; Eberts, M.; Bewsey, H.; O’Connor, T.P.; Chandrashekar, R.; Breitschwerdt, E.B. Sensitivity and specificity levels of two rapid assays for antibodies to Anaplasma spp. in dogs. J. Vet. Diagn. Investig. 2018, 30, 290–293. [Google Scholar] [CrossRef]
- Marumoto, K.; Joncour, G.; Lamanda, P.; Inokuma, H.; Brouqui, P. Detection of Anaplasma phagocytophilum and Ehrlichia sp. HF strains in Ixodes ricinus ticks in Brittany, France. Clin. Microbiol. Infect. 2007, 3, 3388. [Google Scholar]
- Stanilov, I.; Blazhev, A.; Miteva, L. Anaplasma and Ehrlichia Species in Ixodidae Ticks Collected from Two Regions of Bulgaria. Microorganisms 2023, 11, 594. [Google Scholar] [CrossRef]
- Nazari, M.; Lim, S.Y.; Watanabe, M.; Sharma, R.S.; Cheng, N.A.; Watanabe, M. Molecular detection of Ehrlichia canis in dogs in Malaysia. PLoS Negl. Trop. Dis. 2013, 7, e1982. [Google Scholar] [CrossRef] [PubMed]
- Matei, I.A.; Kalmár, Z.; Magdaş, C.; Magdaş, V.; Toriay, H.; Dumitrache, M.O.; Ionica, A.M.; D’Amico, G.; Sándor, A.D.; Marcutan, D.I.; et al. Anaplasma phagocytophilum in questing Ixodes ricinus ticks from Romania. Ticks Tick Borne Dis. 2015, 6, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, S.P.; Paludo, G.R.; Silva, J.N.; Honório-França, A.C.; França, E.L. Hemorheological Evaluation and Cytokine Production in Dogs Naturally Infected with Anaplasmataceae. In Parasitology and Microbiology Research, 1st ed.; Pacheco, G.A.B., Ed.; IntechOpen: London, UK, 2020; pp. 1–25. [Google Scholar]
- Faria, J.L.; Munhoz, T.D.; João, C.F.; Vargas-Hernández, G.; André, M.R.; Pereira, W.A.; Machado, R.Z.; Tinucci-Costa, M. Ehrlichia canis (Jaboticabal strain) induces the expression of TNF-α in leukocytes and splenocytes of experimentally infected dogs. Rev. Bras. Parasitol. Vet. 2011, 20, 71. [Google Scholar] [CrossRef] [PubMed]
- Scorpio, D.G.; von Loewenich, F.D.; Goebel, H.; Bogdan, C.; Dumler, J.S. Innate immune response to Anaplasma phagocytophilum contributes to hepatic injury. Clin. Vaccine Immunol. 2006, 13, 806. [Google Scholar] [CrossRef] [PubMed]
- Yager, E.; Bitsaktsis, C.; Nandi, B.; McBride, J.W.; Winslow, G. Essential role for humoral immunity during Ehrlichia infection in immunocompetent mice. Infect Immun. 2005, 73, 8009. [Google Scholar] [CrossRef]
- Day, M.J. The immunopathology of canine vector-borne diseases. Parasites Vectors 2011, 4, 48. [Google Scholar] [CrossRef]
- Torina, A.; Villari, S.; Blanda, V.; Vullo, S.; La Manna, M.P.; Shekarkar Azgomi, M.; Di Liberto, D.; de la Fuente, J.; Sireci, G. Innate Immune Response to Tick-Borne Pathogens: Cellular and Molecular Mechanisms Induced in the Hosts. Int. J. Mol. Sci. 2020, 21, 5437. [Google Scholar] [CrossRef]
- MacNamara, K.C.; Racine, R.; Chatterjee, M.; Borjesson, D.; Winslow, G.M. Diminished Hematopoietic Activity Associated with Alterations in Innate and Adaptive Immunity in a Mouse Model of Human Monocytic Ehrlichiosis. Infect. Immun. 2009, 77, 4061. [Google Scholar] [CrossRef]
- Naimi, W.A.; Gumpf, J.J.; Green, R.S.; Izac, J.R.; Zellner, M.P.; Conrad, D.H.; Marconi, R.T.; Martin, R.K.; Carlyon, J.A. Immunization against Anaplasma phagocytophilum adhesin binding domains confers protection against infection in the mouse model. Infect. Immun. 2020, 88, e00106-20. [Google Scholar] [CrossRef]
- Hess, P.R.; English, R.V.; Hegarty, B.C.; Brown, G.D.; Breitschwerdt, E.B. Experimental Ehrlichia canis infection in the dog does not cause immunosuppression. Vet. Immunol. Immunopathol. 2006, 109, 117. [Google Scholar] [CrossRef]
- Gaunt, S.D.; Beall, M.J.; Stillman, B.A.; Lorentzen, L.; Diniz, P.P.V.P.; Chandrashekar, R.; Breitschwerdt, E.B. Experimental infection and co-infection of dogs with Anaplasma platys and Ehrlichia canis: Hematologic, serologic and molecular findings. Parasit. Vectors 2010, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Scorpio, D.G.; Dumler, J.S.; Barat, N.C.; Cook, J.A.; Barat, C.E.; Stillman, B.A.; DeBisceglie, K.C.; Beall, M.J.; Chandrashekar, R. Comparative strain analysis of Anaplasma phagocytophilum infection and clinical outcomes in a canine model of granulocytic anaplasmosis. Vector Borne Zoonotic Dis. 2011, 11, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Dumler, J.S.; Barat, N.C.; Barat, C.E.; Bakken, J.S. Human granulocytic anaplasmosis and macrophage activation. Clin. Infect. Dis. 2007, 45, 199. [Google Scholar] [CrossRef] [PubMed]
- Asawapattanakul, T.; Pintapagung, T.; Piratae, S.; Juntautsa, S.; Chancharoen, P. Erythrocyte sedimentation rate, C-reactive protein, and interleukin-6 as inflammatory biomarkers in dogs naturally infected with Ehrlichia canis. Vet. World 2021, 14, 2325. [Google Scholar] [CrossRef]
- Johns, J.L.; MacNamara, K.C.; Walker, N.J.; Winslow, G.M.; Borjesson, D.L. Infection with Anaplasma phagocytophilum Induces Multilineage Alterations in Hematopoietic Progenitor Cells and Peripheral Blood Cells. Infect. Immun. 2009, 77, 4070. [Google Scholar] [CrossRef]
- Habib, S.; El Andaloussi, A.; Hisham, A.; Ismail, N. NK Cell-Mediated Regulation of Protective Memory Responses against Intracellular Ehrlichial Pathogens. PLoS ONE 2016, 11, e0153223. [Google Scholar] [CrossRef]
- Castro, M.B.D.; Szabó, M.P.J.; Aquino, L.P.C.T.D.; Dagnoni, A.S.; Alessi, A.C.; Costa, M.T.; Nakaghi, A.C.H.; Santi, M.D.; Calchi, A.C.; André, M.R.; et al. Immunophenotypical and pathological changes in dogs experimentally infected with Ehrlichia canis. Braz. J. Vet. Parasitol. 2022, 31, e021621. [Google Scholar] [CrossRef]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef]
- Peñaloza, H.F.; Schultz, B.M.; Nieto, P.A.; Salazar, G.A.; Suazo, I.; Gonzalez, P.A.; Riedel, C.A.; Alvarez-Lobos, M.M.; Kalergis, A.M.; Bueno, S.M. Opposing roles of IL-10 in acute bacterial infection. Cytokine Growth Factor Rev. 2016, 32, 17. [Google Scholar] [CrossRef]
- Kumar, R.; Ng, S.; Engwerda, C. The Role of IL-10 in Malaria: A Double Edged Sword. Front. Immunol. 2019, 10, 229. [Google Scholar] [CrossRef]
- Lima, A.L.; Santos, G.J.L.; Roatt, B.M.; Reis, A.B.; Freitas, J.C.C.; Nunes-Pinheiro, D.C.S. Serum TNF-α and IL-10 in Ehrlichia spp. naturally infected dogs. Act. Sci. Vet. 2015, 43, 1322. [Google Scholar]
- do Carmo, G.M.; Crivellenti, L.Z.; Bottari, N.B.; Machado, G.; Borin-Crivellenti, S.; Moresco, R.N.; Duarte, T.; Duarte, M.; Tinucci-Costa, M.; Morsch, V.M.; et al. Butyrylcholinesterase as a marker of inflammation and liver injury in the acute and subclinical phases of canine ehrlichiosis. Comp. Immunol. Microbiol. Infect. Dis. 2015, 43, 16. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Aldana, D.K.; Gutiérrez-Grajales, E.J.; Osorio-Navia, D.; Chacón-Peña, M.; Trejos-Mendoza, A.E.; Pérez-Vargas, S.; Valencia-Mejía, L.; Marín-Arboleda, L.F.; Martínez-Hidalgo, J.P.; Reina-Mora, M.A.; et al. Haematological Alterations Associated with Selected Vector-Borne Infections and Exposure in Dogs from Pereira, Risaralda, Colombia. Animals 2022, 12, 3460. [Google Scholar] [CrossRef] [PubMed]
- Ravnik, U.; Tozon, N.; Smrdel, K.S.; Zupanc, T.A. Anaplasmosis in dogs: The relation of haematological, biochemical and clinical alterations to antibody titre and PCR confirmed infection. Vet. Microbiol. 2011, 149, 172. [Google Scholar] [CrossRef] [PubMed]
- Villaescusa, A.; Tesouro, M.A.; García-Sancho, M.; Ayllón, T.; Rodríguez-Franco, F.; Sainz, A. Evaluation of peripheral blood lymphocyte subsets in family-owned dogs naturally infected by Ehrlichia canis. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 391. [Google Scholar] [CrossRef]
- Guthmiller, J.J.; Graham, A.C.; Zander, R.A.; Pope, R.L.; Butler, N.S. Cutting Edge: IL-10 Is Essential for the Generation of Germinal Center B Cell Responses and Anti-Plasmodium Humoral Immunity. J. Immunol. 2017, 198, 617. [Google Scholar] [CrossRef]
- Cardoso, S.P.; Honorio-França, A.C.; França, D.C.H.; Silva, L.P.S.; Fagundes-Triches, D.L.G.; Neves, M.C.B.; Cotrim, A.C.M.; Almeida, A.D.B.P.F.; França, E.L.; Sousa, V.R.F. Effects of Doxycycline Treatment on Hematological Parameters, Viscosity, and Cytokines in Canine Monocytic Ehrlichiosis. Biology 2023, 12, 1137. [Google Scholar] [CrossRef]

| Cases (n = 65) | SNAP Test | IgG Antibodies by ELISA | ||
|---|---|---|---|---|
| n (%) | IL-10 pg/mL mean ± SE | n (%) | IL-10 pg/mL mean ± SE | |
| Positive for E. canis only | 32 (49.2) | 51.25 ± 10.94 | 27 (41.5) | 49.52 ± 11.55 |
| Positive for A. phagocytophilum only | 21 (32.3) | 28.34 ± 8.32 | 14 (21.5) | 15.35 ± 3.73 |
| Positive for E. canis and A. phagocytophilum | 12 (18.5) | 66.62 ± 25.11 | 17 (26.2) | 67.14 ± 19.87 |
| All positive for E. canis | 44 (67.7) | 55.44 ± 10.38 | 44 (67.7) | 56.33 ± 10.39 |
| All positive for A. phagocytophilum | 33 (50.8) | 42.26 ± 10.81 | 31 (47.7) | 43.75 ± 11.85 |
| Cases with clinical symptoms negative for both pathogens | 0 | - | 7 (10.8) | 48.70 ± 21.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanilov, I.; Gospodinova, K.; Petrov, V.; Miteva, L.; Tsachev, I.; Stanilova, S. Enhanced Production of IL-10 in PCR-Positive Dogs Infected with E. canis and A. phagocytophilum Facilitate Specific Immune Responses. Microorganisms 2024, 12, 2516. https://doi.org/10.3390/microorganisms12122516
Stanilov I, Gospodinova K, Petrov V, Miteva L, Tsachev I, Stanilova S. Enhanced Production of IL-10 in PCR-Positive Dogs Infected with E. canis and A. phagocytophilum Facilitate Specific Immune Responses. Microorganisms. 2024; 12(12):2516. https://doi.org/10.3390/microorganisms12122516
Chicago/Turabian StyleStanilov, Iskren, Krasimira Gospodinova, Vladimir Petrov, Lyuba Miteva, Ilia Tsachev, and Spaska Stanilova. 2024. "Enhanced Production of IL-10 in PCR-Positive Dogs Infected with E. canis and A. phagocytophilum Facilitate Specific Immune Responses" Microorganisms 12, no. 12: 2516. https://doi.org/10.3390/microorganisms12122516
APA StyleStanilov, I., Gospodinova, K., Petrov, V., Miteva, L., Tsachev, I., & Stanilova, S. (2024). Enhanced Production of IL-10 in PCR-Positive Dogs Infected with E. canis and A. phagocytophilum Facilitate Specific Immune Responses. Microorganisms, 12(12), 2516. https://doi.org/10.3390/microorganisms12122516






