Individual and Combined Effects of Nanoplastics and Cadmium on the Rhizosphere Bacterial Community of Sedum alfredii Hance
Abstract
1. Introduction
2. Materials and Methods
2.1. S. alfredii Hance
2.2. Soil
2.3. PS NPs
2.4. Experimental Design
2.5. Rhizosphere Soil Collection
2.6. Analysis of Soil Physical and Chemical Properties
2.7. High-Throughput Analysis of Soil Bacterial Community
2.8. Data Processing and Analysis
3. Results
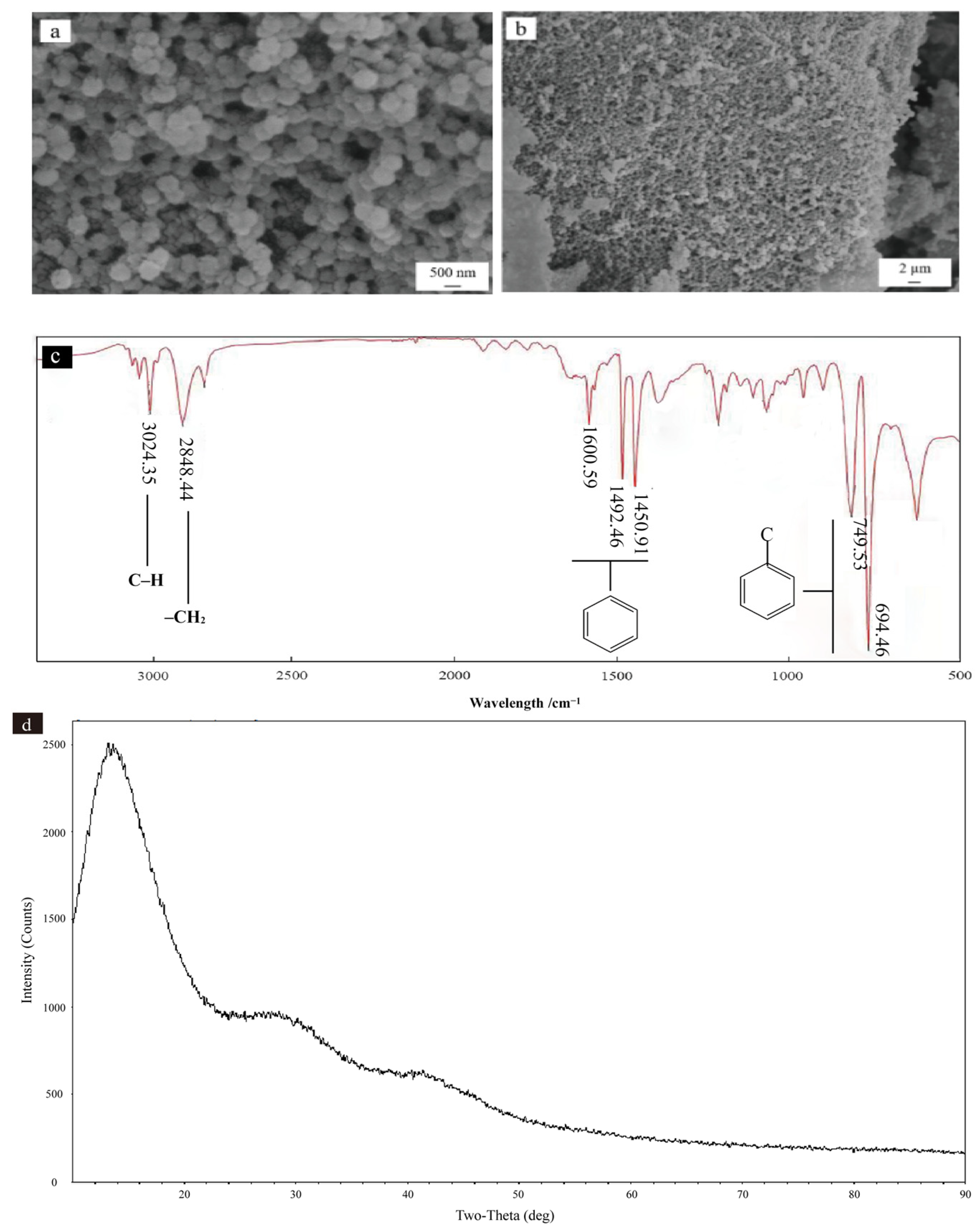
3.1. Analysis of Physicochemical Properties of PS NP
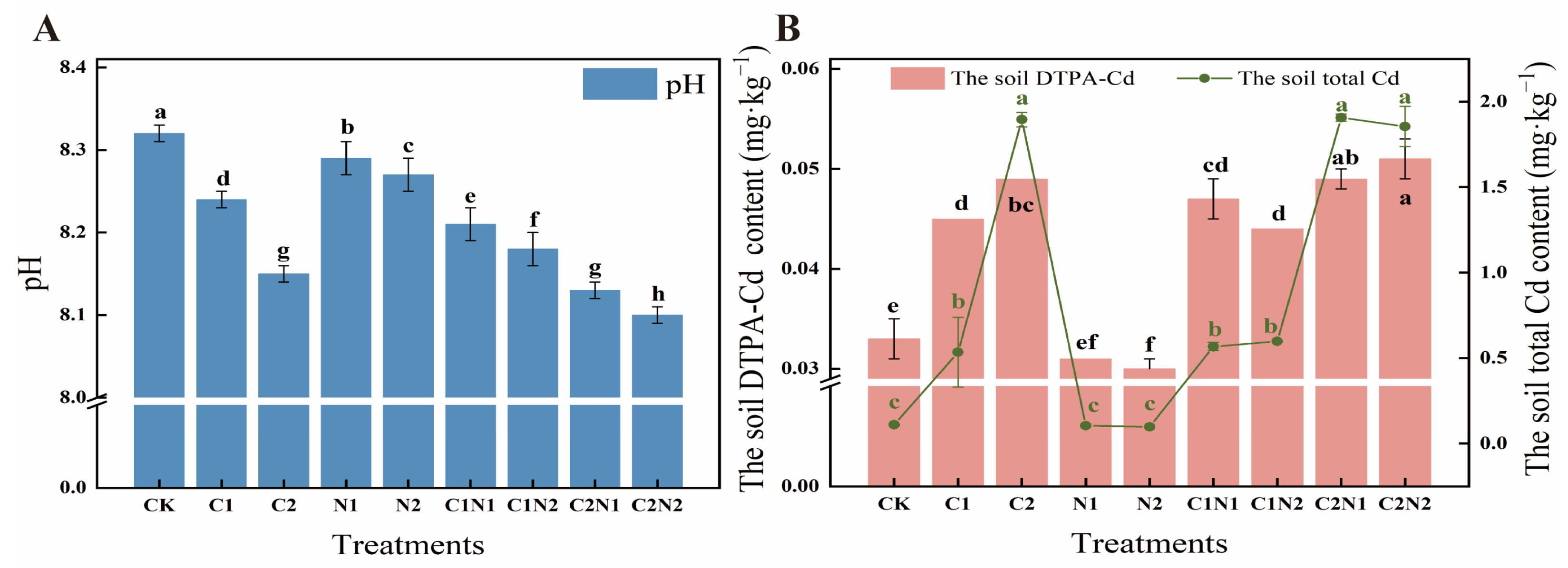
3.2. Individual and Combined Effects of PS NP and Cd on Soil Physicochemical Properties
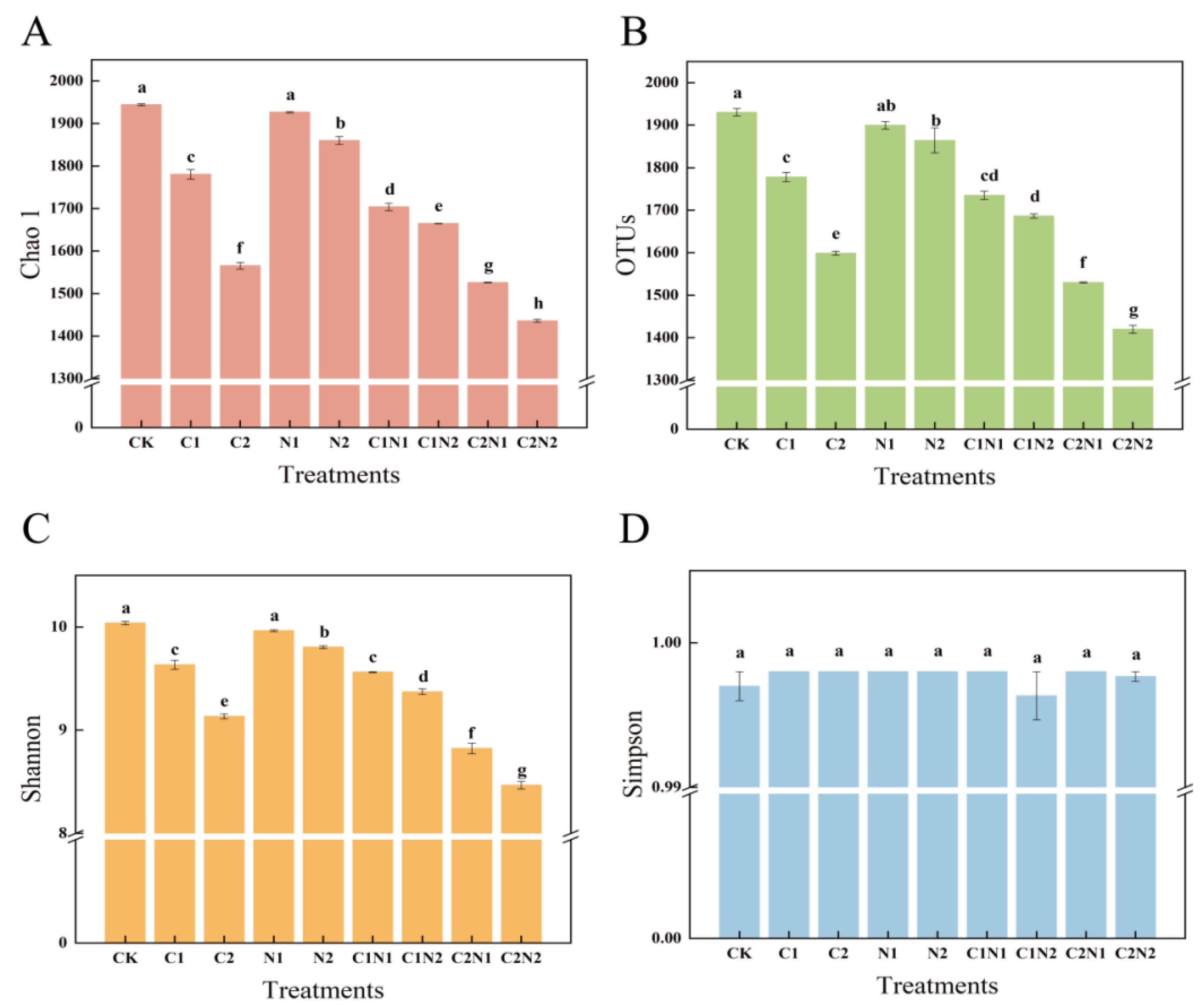
3.3. Individual and Combined Effects of PS NP and Cd on Rhizosphere Bacterial Community Diversity
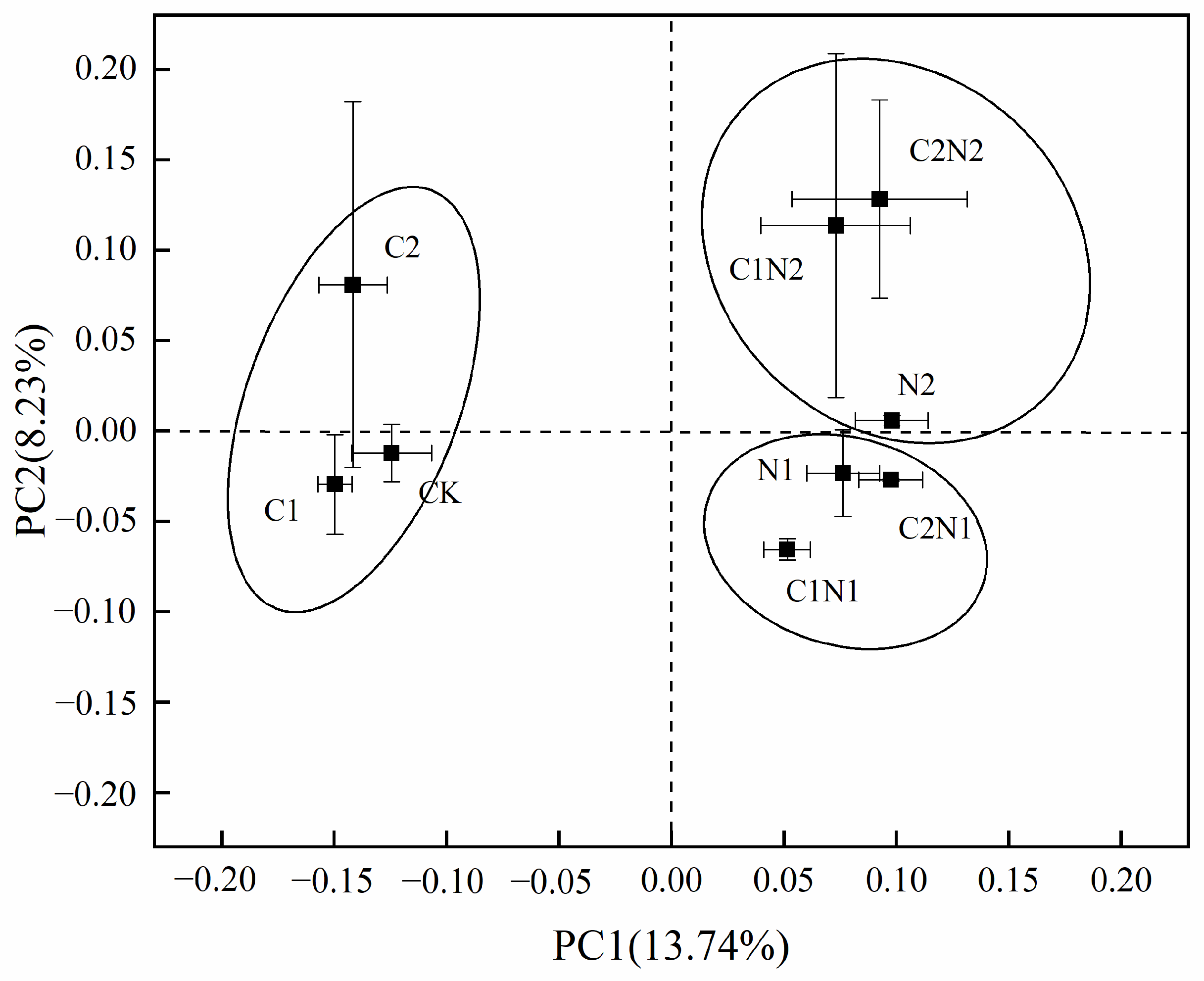
3.4. Individual and Combined Effects of PS NP and Cd on Rhizosphere Bacterial Community Structure
3.5. Individual and Combined Effects of PS NP and Cd on Rhizosphere Bacterial Community Composition
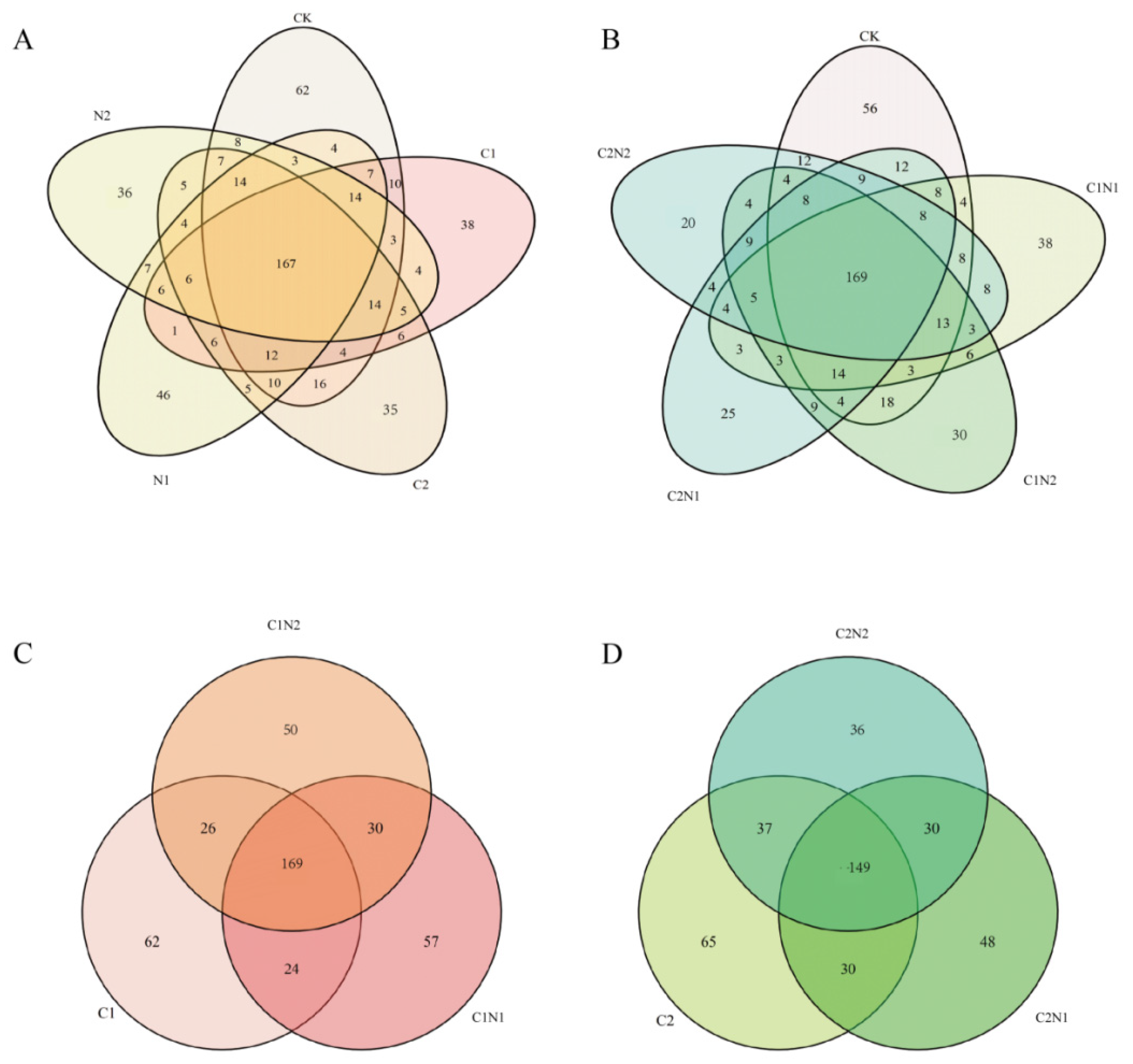
3.5.1. Analysis of Common and Unique Species
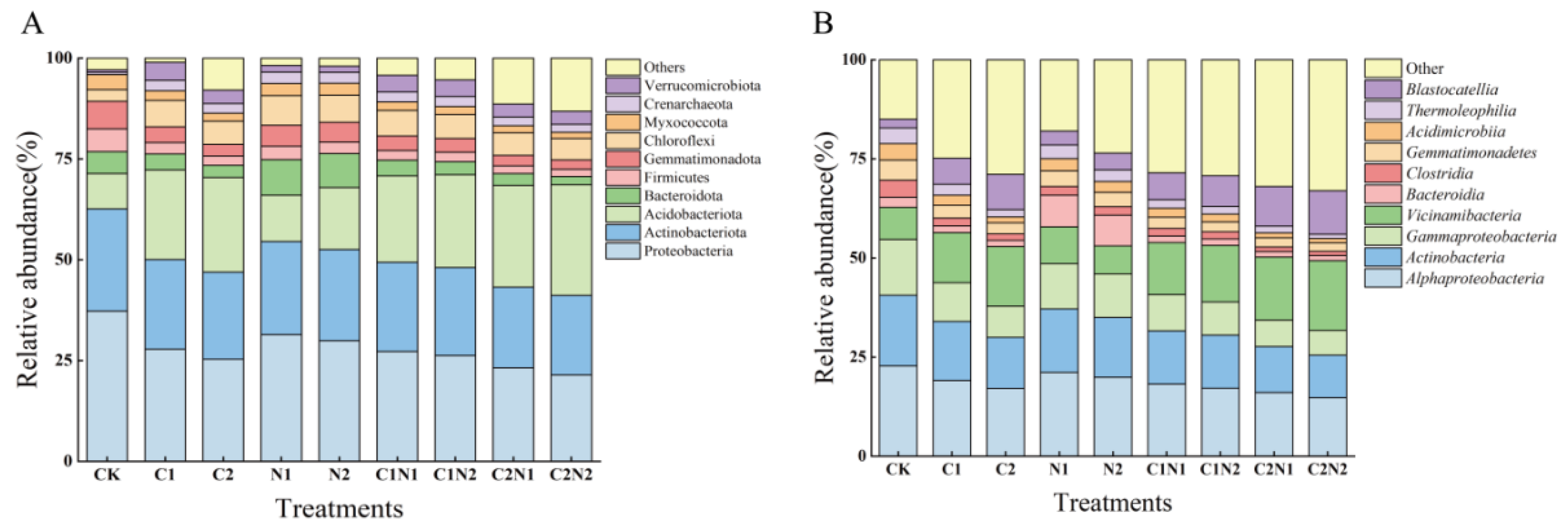
3.5.2. Individual and Combined Effects of PS NP and Cd on the Phylum-Level Composition of the Soil Bacterial Community
3.5.3. Individual and Combined Effects of PS NP and Cd on the Class-Level Composition of the Soil Bacterial Community
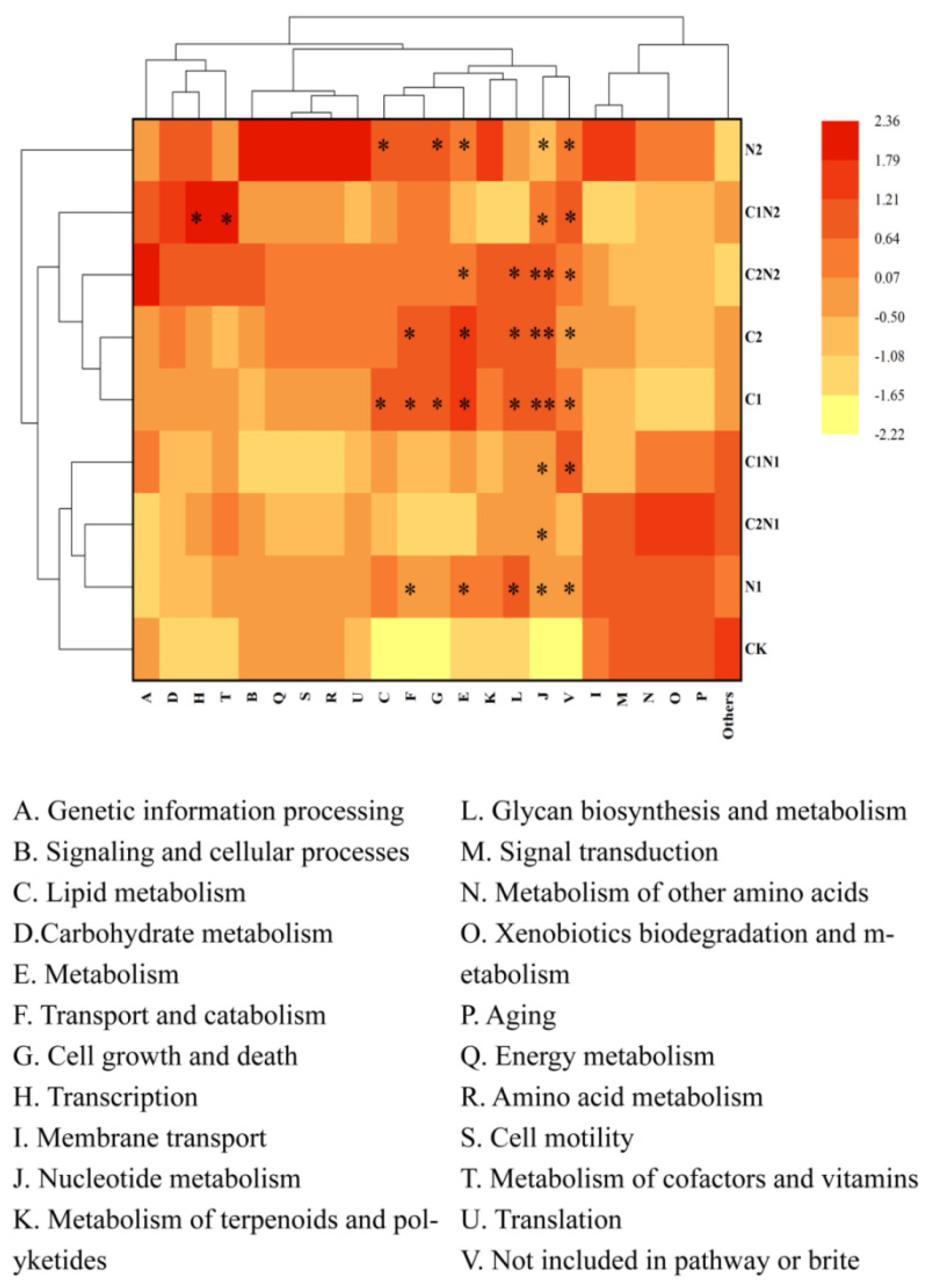
3.6. Functional Analysis of Rhizosphere Bacterial Community Exposed to Individual and Combined PS NP and Cd Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rillig, M.C.; Kim, S.W.; Zhu, Y.G. The soil plastisphere. Nat. Rev. Microbiol. 2024, 22, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Singh, K.; Singh, B. Microplastics in soil: Impacts and microbial diversity and degradation. Pedosphere 2022, 32, 49–60. [Google Scholar] [CrossRef]
- Li, L.; Luo, Y.; Li, R.; Zhou, Q.; Peijnenburg, W.J.G.M.; Yin, N.; Yang, J.; Tu, C.; Zhang, Y. Effective uptake of submicrometre plastics by crop plants via a crack-entry mode. Nat. Sustain. 2020, 3, 929–937. [Google Scholar] [CrossRef]
- Yuan, J.H.; Ma, J.; Sun, Y.R.; Zhou, T.; Zhao, Y.C.; Yu, F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020, 715, 136968. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.Y.; Chen, P.R.; Liu, B.; Wang, Q.Y.; Zhang, R.N. Current situation research on microplastic pollution in terrestrial soil ecosystems. J. Shandong Jianzhu Univ. 2021, 36, 75–82. [Google Scholar] [CrossRef]
- Niu, J.R.; Jian, M.F.; Rao, J.X.; Zhang, J.Y.; Yang, W.J.; Ding, H.J. Effects of exposure to polyethylene microplastics in a simulated soil environment on the ecophysiology of Viola philippica. Chin. J. Appl. Environ. Biol. 2022, 28, 1190–1198. [Google Scholar] [CrossRef]
- Qiu, C.C.; Li, G.X.; Li, Q.S.; Yan, C.Z. Effects of polystyrene nanoplastics (PS-NPs) on the physiology Allium sativum L. Environ. Sci. 2022, 43, 4387–4393. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Helmberger, M.S.; Tiemann, L.K.; Grieshop, M.J. Towards an ecology of soil microplastics. Functional. Ecol. 2020, 34, 550–560. [Google Scholar] [CrossRef]
- Ma, Y.N.; Huang, A.N.; Cao, S.Q.; Sun, F.F.; Wang, L.H.; Guo, H.Y.; Ji, R. Effects of nanoplastics and microplastics on toxicity, bioaccumulation, and environmental fate of phenanthrene in fresh water. Environ. Pollut. 2016, 219, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Ecology and Environment of the People’s Republic of China. Bulletin of the National Soil Pollution Survey; Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2014.
- Liang, W.; Wu, Z.B.; Cheng, S.P.; Zhou, Q.H.; Hu, H.Y. Roles of substrate microorganisms and urease activities in wastewater purification in a constructed wetland system. Ecol. Eng. 2003, 21, 191–195. [Google Scholar] [CrossRef]
- Pan, J.; Yu, L. Effects of Cd or/and Pb on soil enzyme activities and microbial community structure. Ecol. Eng. 2011, 37, 1889–1894. [Google Scholar] [CrossRef]
- Lu, M.; Xu, K.; Chen, J. Effect of pyrene and cadmium on microbial activity and community structure in soil. Chemosphere 2013, 91, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Y.; Zhang, X.Q.; Zhang, S.Q.; Zhang, S.W.; Sun, Y.H. Interactions of microplastics and cadmium on plant growth and arbuscular mycorrhizal fungal communities in an agricultural soil. Chemosphere 2020, 254, 126791. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Huang, S.; Zhang, H.; Tong, Y.; Wen, D.; Xia, X.; Wang, H.; Luo, Y.; Barceló, D. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 2020, 707, 135634. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Ossowicki, A.; Yang, X.; Lwanga, E.H.; Dini-Andreote, F.; Geissen, V.; Garbeva, P. Effects of plastic mulch film residues on wheat rhizosphere and soil properties. J. Hazard. Mater. 2020, 387, 121711. [Google Scholar] [CrossRef]
- Yang, W.W.; Cheng, P.; Adams, C.A.; Zhang, S.W.; Sun, Y.H.; Yu, H.W.; Wang, F.Y. Effects of microplastics on plant growth and arbuscular mycorrhizal fungal communities in a soil spiked with ZnO nanoparticles. Soil Biol. Biochem. 2021, 155, 108179. [Google Scholar] [CrossRef]
- Bläsing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef]
- Arthur, E.L.; Rice, P.J.; Rice, P.J.; Anderson, T.A.; Baladi, S.M.; Henderson, K.L.D.; Coats, J.R. Phytoremediation—An overview. Crit. Rev. Plant Sci. 2005, 24, 109–122. [Google Scholar] [CrossRef]
- Wang, P.; Chao, D. Phytoremediation of heavy metal contamination and related molecular mechanisms in plants. Chin. J. Biotechnol. 2020, 36, 426–435. (In Chinese) [Google Scholar] [CrossRef]
- Kaur, H.; Kumar, A.; Bindra, S.; Sharma, A. Phytoremediation: An emerging green technology for dissipation of PAHs from soil. J. Geochem. Explor. 2024, 259, 107426. [Google Scholar] [CrossRef]
- Rozman, U.; Blažič, A.; Kalčíková, G. Phytoremediation: A promising approach to remove microplastics from the aquatic environment. Environ. Pollut. 2023, 338, 122690. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.E.; Long, X.; Ni, W.; Fu, C. Sedum alfredii H: A new Zn hyperaccumulating plant first found in China. Chin. Sci. Bull. 2002, 47, 1634–1637. [Google Scholar] [CrossRef]
- Yang, X.E.; Long, X.X.; Ye, H.B.; He, Z.L.; Calvert, D.V.; Stoffella, P.J. Cadmium tolerance and hyperaccumulation in a new Zn-hyperaccumulating plant species (Sedum alfredii Hance). Plant Soil 2004, 259, 181–189. [Google Scholar] [CrossRef]
- Tian, S.K.; Lu, L.L.; Yang, X.O.; Webb, S.M.; Du, Y.H.; Brown, P.H. Spatial imaging and speciation of lead in the accumulator plant Sedum alfredii by microscopically focused synchrotron X-ray investigation. Environ. Sci. Technol. 2010, 44, 5920–5926. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.D.; Wang, K.; Liu, T.; Wang, H.X.; Lin, Z.; Qian, J.; Lu, L.L.; Tian, S.K. Unique rhizosphere micro-characteristics facilitate phytoextraction of multiple metals in soil by the hyperaccumulating plant Sedum alfredii. Environ. Sci. Technol. 2017, 51, 5675–5684. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Liu, X.; Rahman, S.U.; Rehman, A.; Zhao, C.; Li, X.; Yucheng, B.; Hui, N. Responses of microbial communities in rhizocompartments of king grass to phytoremediation of cadmium-contaminated soil. Sci. Total Environ. 2023, 904, 167226. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Lin, H.; Li, B.; Dong, Y.B.; Yin, T.T. Responses of microbial communities and metabolic activities in the rhizosphere during phytoremediation of Cd-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 202, 110958. [Google Scholar] [CrossRef]
- Ning, R.Y.; Liu, N.; Cheng, H.Y.; Chen, Y.Y.; Zhang, H.B.; Luo, Y.; Wang, X.J. Effects of microplastics, cadmium and their combination on the growth and cadmium accumulation of hyperaccumulators. Acta Sci. Circumstantiae 2022, 42, 415–425. [Google Scholar] [CrossRef]
- Fuller, S.; Gautam, A. A Procedure for Measuring Microplastics using Pressurized Fluid Extraction. Environ. Sci. Technol. 2016, 50, 5774–5780. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of Microplastics on the Soil Biophysical Environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Gu, Y.J.; Cao, Z.H.; Bian, X.J.; Lan, T.; Sang, J.R.; Jiang, L.; Wang, Y.; Qian, H.; Chen, Y.C.; et al. Endogenous cGMP-dependent protein kinase reverses EGF-induced MAPK/ERK signal transduction through phosphorylation of VASP at Ser239. Oncol. Lett. 2012, 4, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.L.; Yang, X.M.; Pelaez, A.M.; Huerta Lwanga, E.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and micro- plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Z.; Yang Liang Li, J.; Fu, D.D.; Hu, W.W.; Fan, Z.Q.; Peng, L.C. Single and combined effects of microplastics and cadmium on the germination characteristics of rice seeds. J. Agro-Environ. Sci. 2021, 40, 44–53. [Google Scholar] [CrossRef]
- Wang, L.; Cui, X.F.; Cheng, H.G.; Chen, F.; Wang, J.T.; Zhao, X.Y.; Lin, C.Y.; Pu, X. A review of soil cadmium contamination in China including a health risk assessment. Environ. Sci. Pollut. Res. 2015, 22, 16441–16452. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Ecology and Environment of the People’s Republic of China. Environmental Quality Standards for Soil; Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2018.
- Shi, R.H.; Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press Co., Ltd.: Beijing, China, 1981. [Google Scholar]
- Park, S.Y.; Kim, C.G. A comparative study on the distribution behavior of microplastics through FT-IR analysis on different land uses in agricultural soils. Environ. Res. 2022, 215, 114404. [Google Scholar] [CrossRef] [PubMed]
- Wang, S. Study on the Adsorption Mechanism of Cadmium by Microplastics and Its Effect on the Growth of Ryegrass; Yangzhou University: Yangzhou, China, 2024. [Google Scholar] [CrossRef]
- Zhang, M.J.; Zhao, Y.R.; Qin, X.; Jia, W.Q.; Chai, L.W.; Huang, M.K.; Huang, Y. Microplastics from mulching film is a distinct habitat for bacteria in farmland soil. Sci. Total Environ. 2019, 688, 470–478. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Zeng, Y.P.; Chen, Y.; Liu, H.S.; Yan, X.Y.; Pu, S.Y. A new quantitative insight: Interaction of polyethylene microplastics with soil-microbiome-crop. J. Hazard. Mater. 2023, 460, 132302. [Google Scholar] [CrossRef]
- Schlichting, E.; Blume, H.P. Soil Formation; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Li, C.H.; Sun, H.R.; Shi, Y.L.; Zhao, Z.X.; Zhang, Z.; Zhao, P.; Gao, Q.Y.; Zhang, X.; Chen, B.; Li, Y.T.; et al. Polyethylene and poly (butyleneadipate-co-terephthalate)-based biodegradable microplastics modulate the bioavailability and speciation of Cd and As in soil: Insights into transformation mechanisms. J. Hazard. Mater. 2023, 445, 130638. [Google Scholar] [CrossRef]
- Hou, J.H. Effects of Polyethylene Microplastics on Soil Aggregate Properties and Microbial Diversity; Lanzhou Jiaotong University: Lanzhou, China, 2020. [Google Scholar] [CrossRef]
- Kim, K.R.; Owens, G.; Kwon, S.I. Influence of Indian mustard (Brassica juncea) on rhizosphere soil solution chemistry in long-term contaminated soils: A rhizobox study. Environ. Sci. Technol. 2010, 19, 11496–11506. [Google Scholar] [CrossRef]
- Wang, F.L.; Wang, X.X.; Song, N.N. Polyethylene microplastics increase cadmium uptake in lettuce (Lactuca sativa L.) by altering the soil microenvironment. Sci. Total Environ. 2021, 784, 147133. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Zhao, L.; Zhao, L.; Cheng, Z.; Yao, Y.; Yuan, C.; Wang, L.; Sun, H. LDPE microplastics affect soil microbial communities and nitrogen cycling. Sci. Total Environ. 2021, 773, 145640. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.Y.; Wang, Q.L.; Sun, Y.H.; Zhang, S.W.; Wang, F.Y. Microplastics change soil properties, heavy metal availability and bacterial community in a Pb-Zn-contaminated soil. J. Hazard. Mater. 2022, 424, 127364. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Adebayo, A.; Jia, J.; Xing, Y.; Deng, S.; Guo, L.; Liang, Y.; Zhang, D. Impacts of heavy metals and soil properties at a Nigerian e-waste site on soil microbial community. J. Hazard. Mater. 2019, 362, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Qaswar, M.; Yiren, L.; Jing, H.; Kaillou, L.; Mudasir, M.; Zhenzhen, L.; Hongqian, H.; Xianjin, L.; Jianhua, J.; Ahmed, W.; et al. Soil nutrients and heavy metal availability under long-term combined application of swine manure and synthetic fertilizers in acidic paddy soil. J. Soils Sediments 2020, 20, 2093–2106. [Google Scholar] [CrossRef]
- Wang, F.Y.; Zhang, X.Q.; Zhang, S.Q.; Zhang, S.W.; Sun, Y.H. Effects of co-contamination of microplastics and Cd on plant growth and Cd accumulation. Toxicol. Sci. 2020, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Han, B.; Sun, Y.; Wang, F. Microplastics influence the adsorption and desorption characteristics of Cd in an agricultural soil. J. Hazard. Mater. 2020, 388, 121775. [Google Scholar] [CrossRef]
- Li, M.; Wu, D.; Wu, D.; Guo, H.; Han, S. Influence of polyethylene-microplastic on environmental behaviors of metals in soil. Environ. Pollut. 2021, 28, 28329–28336. [Google Scholar] [CrossRef]
- Huang, F.; Hu, J.; Chen, L.; Wang, Z.; Sun, S.; Zhang, W.; Jiang, H.; Luo, Y.; Wang, L.; Zeng, Y.; et al. Microplastics may increase the environmental risks of Cd via promoting Cd uptake by plants: A meta-analysis. J. Hazard. Mater. 2023, 448, 130887. [Google Scholar] [CrossRef]
- Cultivated Land Quality Monitoring and Protection Center, Ministry of Agriculture and Rural Affairs. Classification of Indicators of Major Soil Properties; Cultivated Land Quality Monitoring and Protection Center, Ministry of Agriculture and Rural Affairs: Beijing, China, 2024. [Google Scholar]
- Duan, L.Y.; Zhang, Y.; Ren, X.M.; Li, Y.Y.; Zhang, Y.J.; Zhang, H.; Han, H.; Chen, Z.J. Effects of combined pollution of microplastics and cadmium on microbial community structure and function of pennisetum hydridum rhizosphere soil. Environ. Sci. 2023, 44, 6973–6981. [Google Scholar] [CrossRef]
- Bakker, D.P.; Klijnstra, J.W.; Busscher, H.J.; Vander, M.H.C. The effect of dissolved organic carbon on bacterial adhesion to conditioning films adsorbed on glass from natural seawater collected during different seasons. Biofouling 2003, 19, 391–397. [Google Scholar] [CrossRef] [PubMed]
- McCormick, A.; Hoellein, T.J.; Mason, S.A.; Schluep, J.; Kelly, J.J. Microplastic is an abundant and distinct microbial habitat in an urban river. Environ. Sci. Technol. 2014, 48, 11863–11871. [Google Scholar] [CrossRef] [PubMed]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt, J.M. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ. Sci. Technol. Lett. 2017, 4, 258–267. [Google Scholar] [CrossRef]
- Ganesh, K.A.; Anjana, K.; Hinduja, M.; Sujitha, K.; Dharani, G. Review on plastic wastes in marine environment–biodegradation and biotechnological solutions. Mar. Pollut. Bull. 2020, 150, 110733. [Google Scholar] [CrossRef]
- De Tender, C.A.; Devriese, L.I.; Haegeman, A.; Maes, S.; Ruttink, T.; Dawyndt, P. Bacterial community profiling of plastic litter in the belgian part of the north sea. Environ. Sci. Technol. 2015, 49, 9629–9638. [Google Scholar] [CrossRef]
- Oberbeckmann, S.; Loeder, M.G.J.; Gerdts, G.; Osborn, A.M. Spatial and seasonal variation in diversity and structure of microbial biofilms on marine plastics in Northern European waters. FEMS Microbiol. Ecol. 2014, 90, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Timothy, J.H.; Meagan, W.; Olga, L.; Jamie, C. Abundance and environmental drivers of anthropogenic litter on 5 Lake Michigan beaches: A study facilitated by citizen science data collection. J. Great Lakes Res. 2015, 41, 78–86. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, Y.; Lin, X.; Zhang, H.; Zeng, J.; Hou, J.; Yang, Y.; Yao, T.; Knight, R.; Chu, H. Geographic distance and pH drive bacterial distribution in alkaline lake sediments across Tibetan Plateau. Environ. Microbiol 2012, 14, 2457–2466. [Google Scholar] [CrossRef]
- Li, W.L.; Wang, Z.C.; Yang, W.H.; Zhang, B.W.; Li, W.P. Effects of microplastics on bacterial community composition and diversity in sediments. Environ. Sci. 2022, 43, 2606–2613. [Google Scholar] [CrossRef]
- Hu, Z.E.; Xiao, M.L.; Ding, J.N.; Ji, J.H.; Chen, J.P.; Ge, T.D.; Lu, S.B. Response characteristics of soil microbial community under long-term film mulching. Environ. Sci. 2022, 43, 4745–4754. [Google Scholar] [CrossRef]
- Jiang, J.; Long, Y.C.; Hu, J.; Wang, L.Y. Characteristics of microplastics and potential pathogenic microorganisms in the soil surrounding a rural garbage dump. China Environ. Sci. 2023, 43, 3592–3603. [Google Scholar] [CrossRef]
- Ding, F.; Lai, J.L.; Ji, X.H.; Luo, X.G. Effects of polyethylene microplastics on the microbial community structure of maize rhizosphere soil. Chin. J. Eco-Agric. 2021, 29, 970–978. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Wang, H.; Liu, C.K.; He, B. Exploration of Cd pollution status of farmland soil and remediation technologies. Mod. Agric. Sci. Technol. 2022, 174–177. [Google Scholar] [CrossRef]
- Li, C.; Cui, Q.; Li, Y.; Zhang, K.; Lu, X.; Zhang, Y. Effect of LDPE and biodegradable PBAT primary microplastics on bacterial community after four months of soil incubation. J. Hazard. Mater. 2022, 429, 128353. [Google Scholar] [CrossRef]
- Zhang, M.; Jia, H.; Weng, Y.X.; Li, Z.T. Effects of polylactide/polybutylene adipate-co-terephthalate on bacterial community structure of soil and isolation of degrading bacteria. Microbiol. China 2020, 47, 420–430. [Google Scholar] [CrossRef]
- Drzewiecka, D. Significance and Roles of Proteus spp. Bacteria in Natural Environments. Microb. Ecol. 2016, 72, 741–758. [Google Scholar] [CrossRef]
- Liu, S.S.; Fu, J.P.; Cai, X.D.; Zhou, J.M.; Dang, Z.; Zhu, R.L. Effect of heavy metals pollution on ecological characteristics of soil microbes: A review. Ecol. Environ. Sci. 2018, 27, 1173–1178. [Google Scholar] [CrossRef]
- Liu, Y.L.; Yao, J.F.; Ding, S.D.; Wu, D.; Zhang, P.X.; Tie, B.Q.; Luo, S. Effects of inoculation with the cadmium-resistant bacterium Delftia sp. B9 on soil Cd stabilization and reduction of Cd bioaccumulation in rice. J. Agric. Resour. Environ. 2023, 40, 1339–1348. [Google Scholar] [CrossRef]
- Li, Y.Y. The Absorption of Heavy Metals and Bacterial Community Changes in the Rhizosphere; Anhui Agricultural University: Hefei, China, 2018. [Google Scholar]
- Ji, R.M.; Ma, Q.Y.; Ji, R. Plastisphere: The vector effects of microplastics on microbial communities. Environ. Prot. 2020, 48, 19–27. [Google Scholar] [CrossRef]
- Liu, S.S.; Cao, J.F.; Chen, X.Y.; Liang, Q.T. Effects of microplastics-polycyclic aromatic hydrocarbons on microbial community structure and function in agricultural soil. Guangdong Agric. Sci. 2022, 49, 64–72. [Google Scholar] [CrossRef]
| Treatments | Cd (mg·kg−1) | PS NPs (mg·kg−1) |
|---|---|---|
| CK | 0 | 0 |
| C1 | 0.6 | 0 |
| C2 | 4 | 0 |
| N1 | 0 | 100 |
| N2 | 0 | 1000 |
| C1N1 | 0.6 | 100 |
| C1N2 | 0.6 | 1000 |
| C2N1 | 4 | 100 |
| C2N2 | 4 | 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Cheng, H.; Li, Y.; Ning, R.; Lv, Y.; Wang, Q.; Zhang, H.; Liu, N. Individual and Combined Effects of Nanoplastics and Cadmium on the Rhizosphere Bacterial Community of Sedum alfredii Hance. Microorganisms 2024, 12, 2471. https://doi.org/10.3390/microorganisms12122471
Wang Y, Cheng H, Li Y, Ning R, Lv Y, Wang Q, Zhang H, Liu N. Individual and Combined Effects of Nanoplastics and Cadmium on the Rhizosphere Bacterial Community of Sedum alfredii Hance. Microorganisms. 2024; 12(12):2471. https://doi.org/10.3390/microorganisms12122471
Chicago/Turabian StyleWang, Yixiu, Hongyan Cheng, Yuenan Li, Ruiyan Ning, Yonghui Lv, Qing Wang, Haibo Zhang, and Na Liu. 2024. "Individual and Combined Effects of Nanoplastics and Cadmium on the Rhizosphere Bacterial Community of Sedum alfredii Hance" Microorganisms 12, no. 12: 2471. https://doi.org/10.3390/microorganisms12122471
APA StyleWang, Y., Cheng, H., Li, Y., Ning, R., Lv, Y., Wang, Q., Zhang, H., & Liu, N. (2024). Individual and Combined Effects of Nanoplastics and Cadmium on the Rhizosphere Bacterial Community of Sedum alfredii Hance. Microorganisms, 12(12), 2471. https://doi.org/10.3390/microorganisms12122471





