Histological Dissection of Fusarium-Banana Interaction Using a GFP-Tagged Subtropical Race 4 Strain of Fusarium oxysporum f. sp. cubense on Banana Cultivars with Differing Levels of Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Fungal Strain and Inoculum Preparation
2.3. Inoculations and Growth Conditions
2.4. Symptoms Assessment and Reisolation
2.5. Investigation of Fungal Colonisation
2.5.1. Laser Scanning Microscopy
2.5.2. Scanning Electron Microscopy
3. Results
3.1. Symptoms Assessment and Reisolation
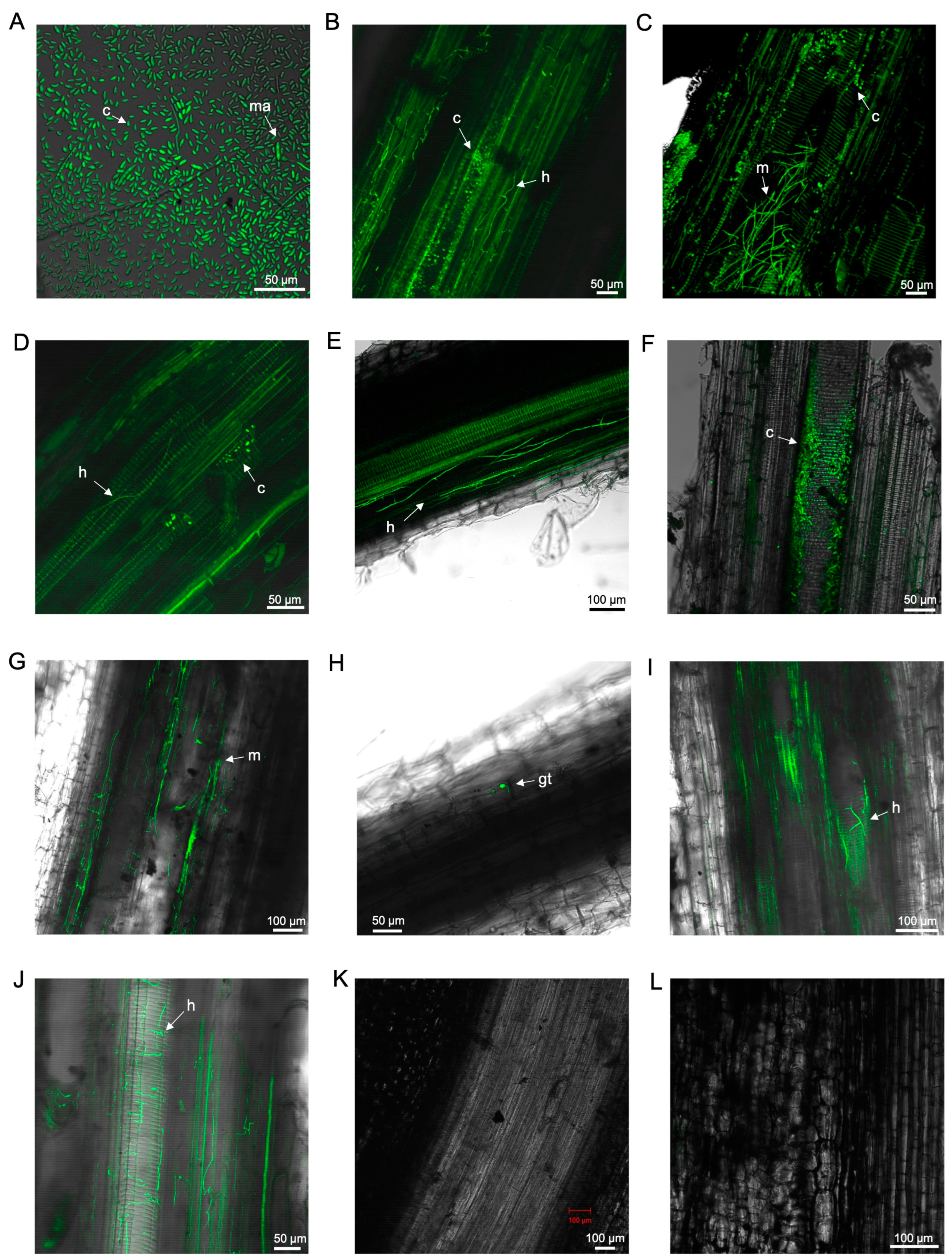
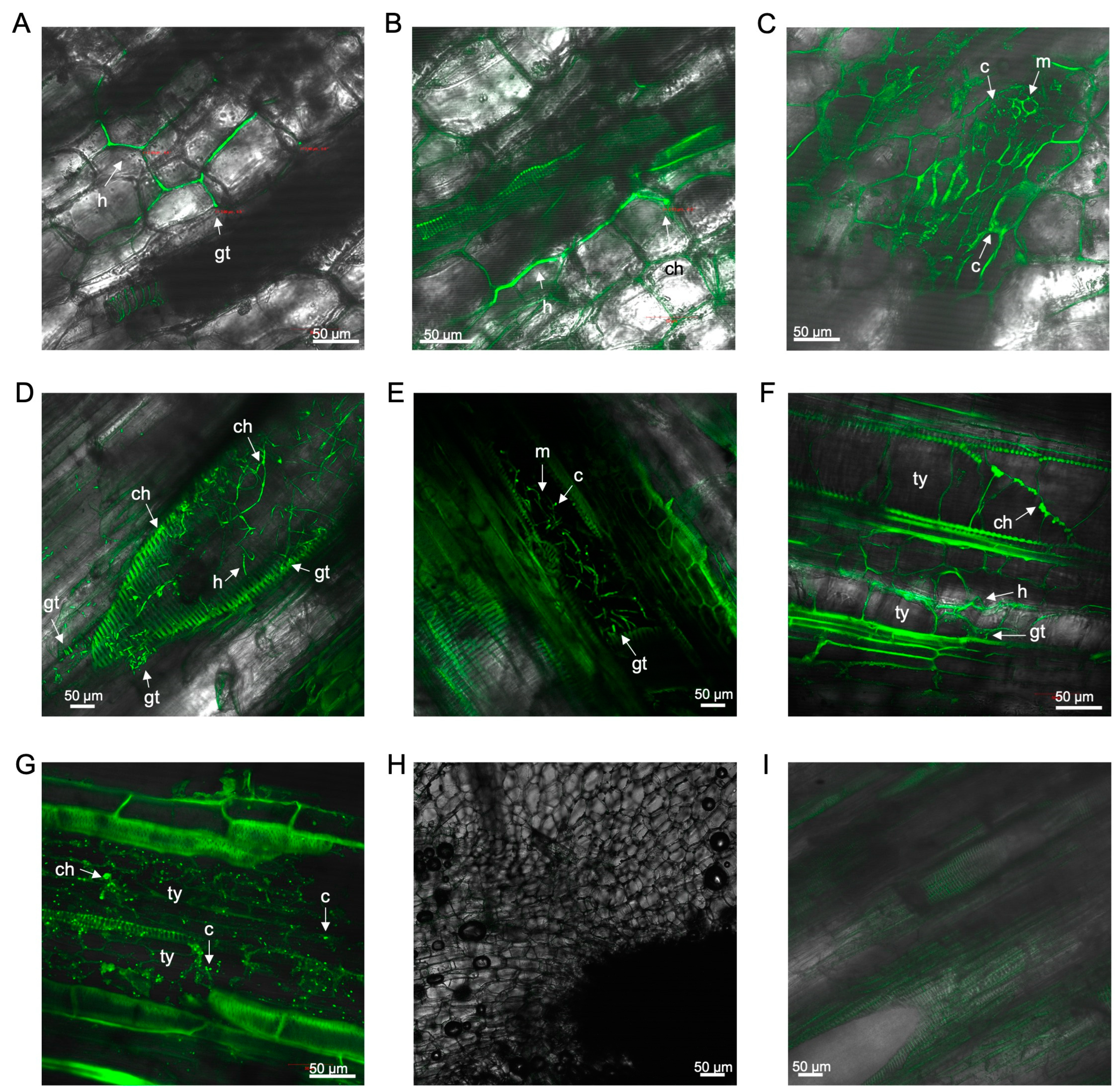
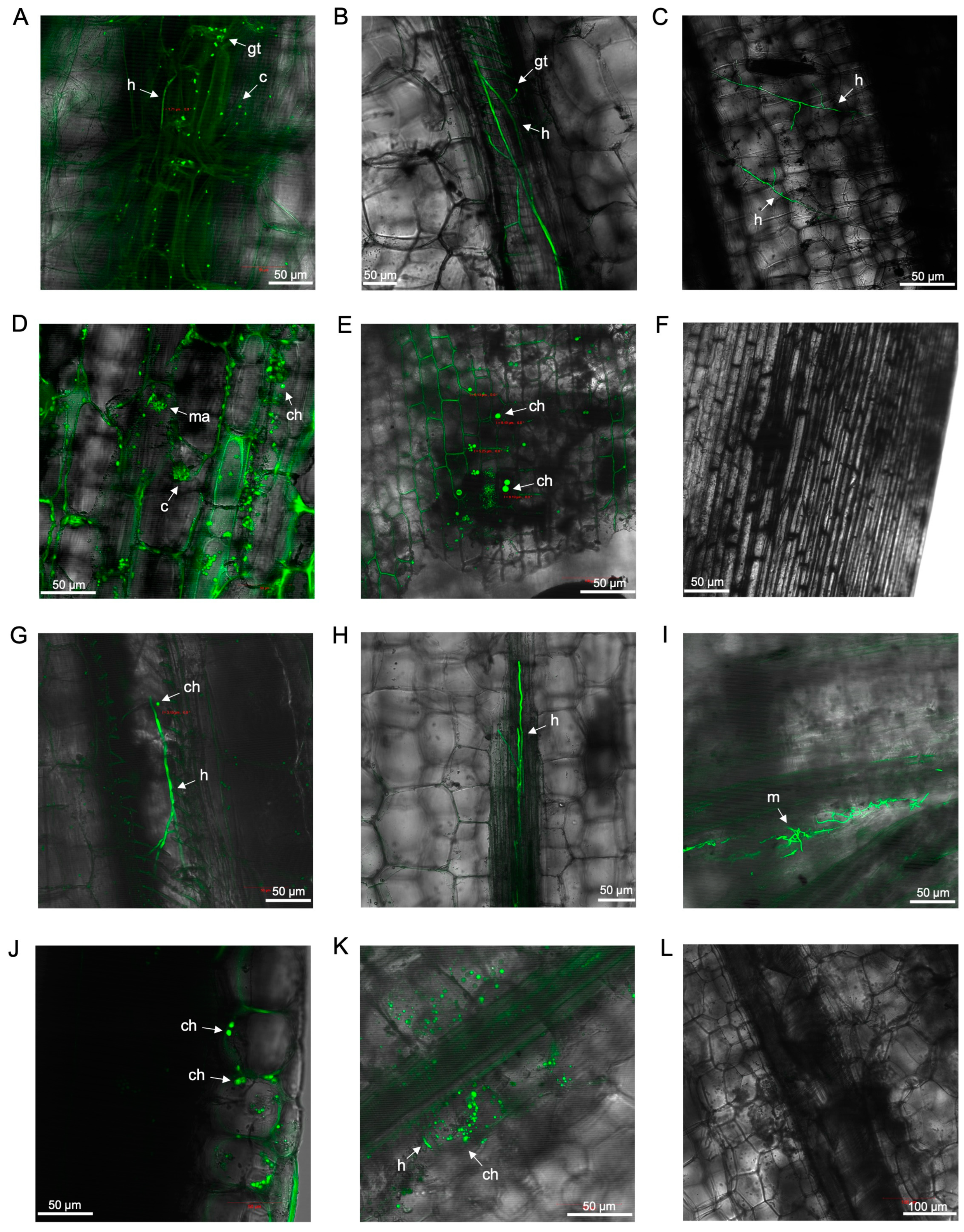
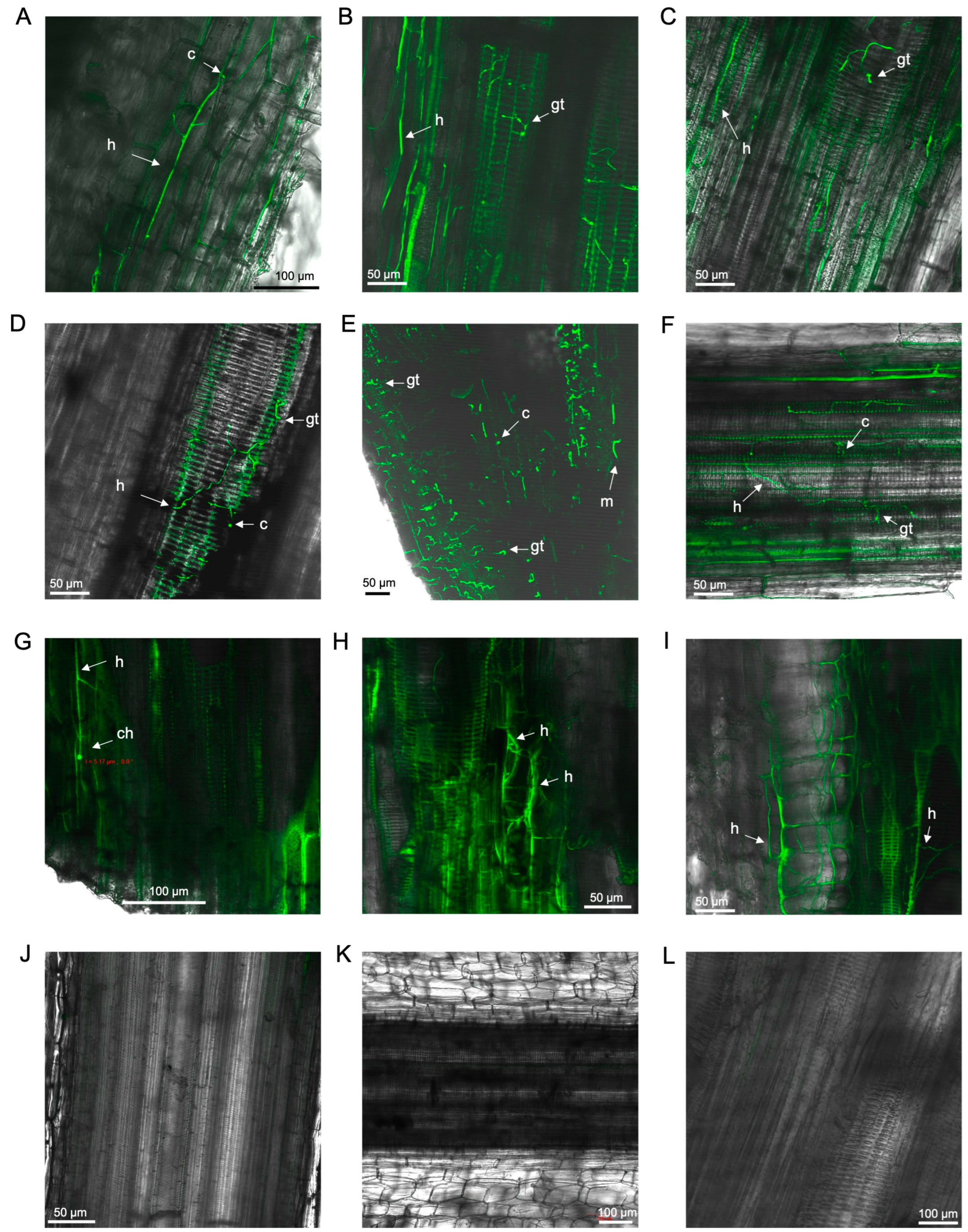
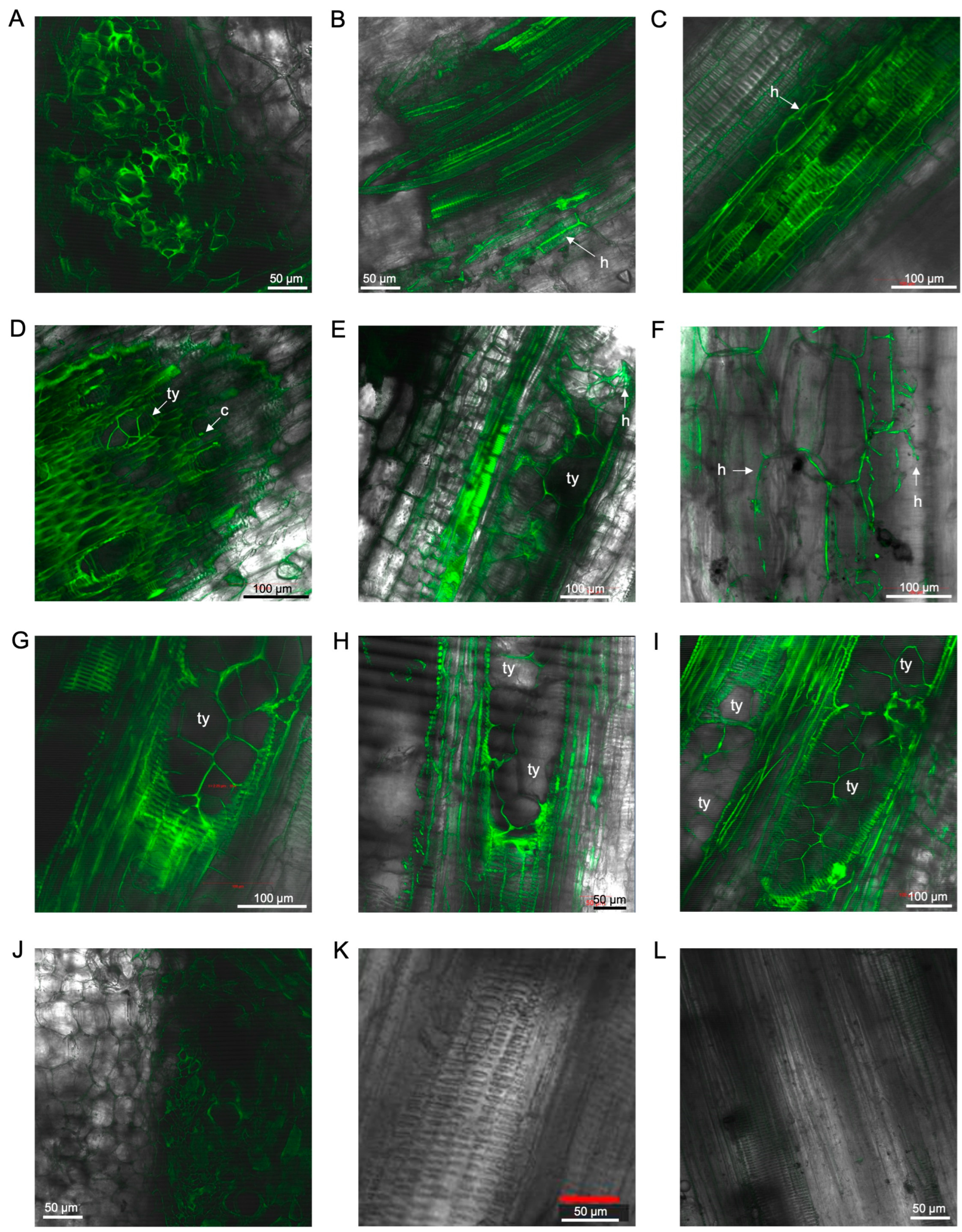
3.2. Laser Scanning Microscopy
3.2.1. Observations on Susceptible Cultivar FHIA02
3.2.2. Observations on Resistant Cultivar FHIA25
3.2.3. Observations on Cultivars Williams, GCTCV119 and Lady Finger
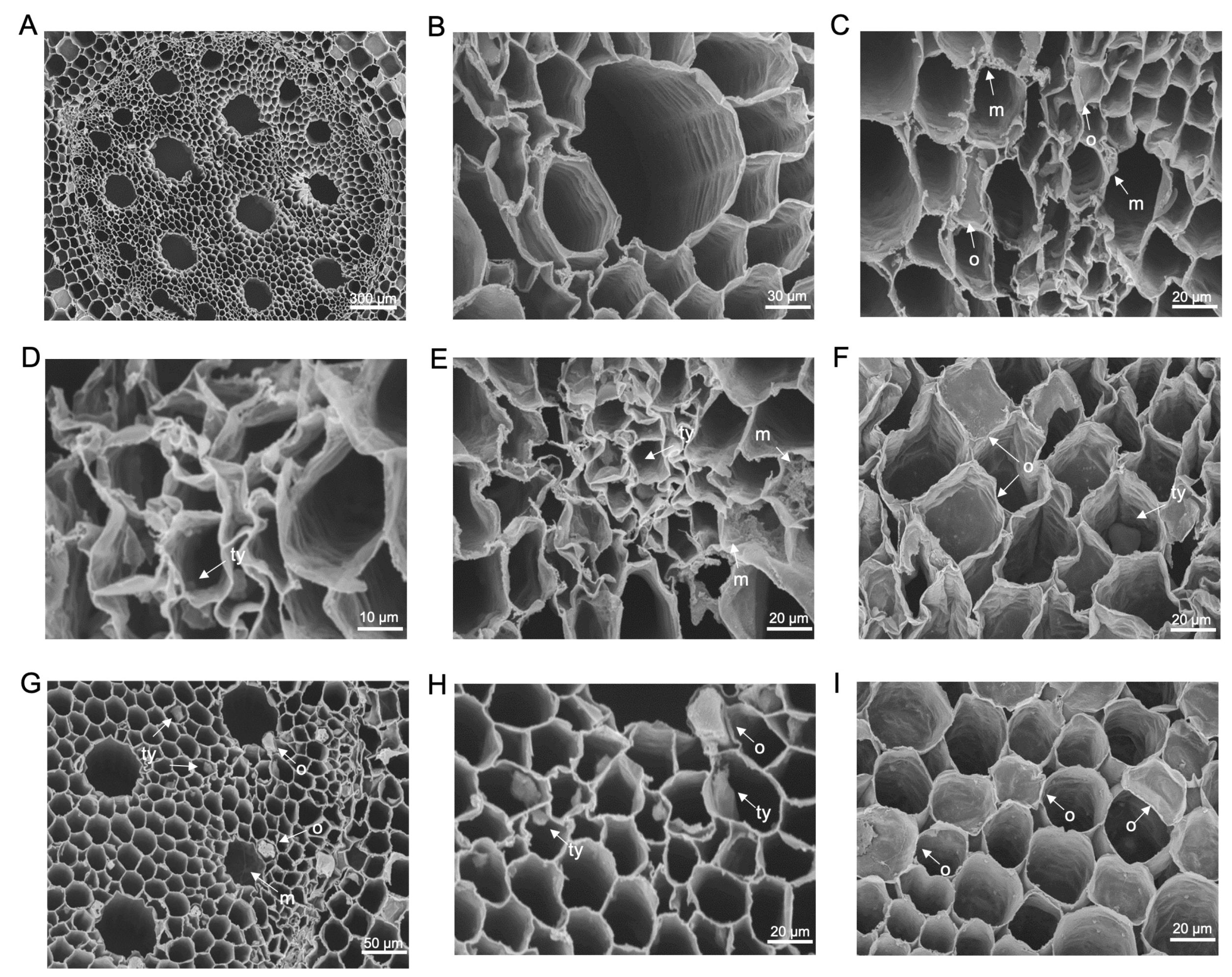
3.3. Scanning Electron Microscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. 2024. Available online: https://www.fao.org/statistics/en (accessed on 24 October 2024).
- Ortiz, R.; Ferris, R.; Vuylsteke, D. Banana and plantain breeding. In Bananas and Plantains; Springer: Berlin/Heidelberg, Germany, 1995; pp. 110–146. [Google Scholar]
- Maryani, N.; Lombard, L.; Poerba, Y.; Subandiyah, S.; Crous, P.; Kema, G. Phylogeny and genetic diversity of the banana Fusarium wilt pathogen Fusarium oxysporum f. sp. cubense in the Indonesian centre of origin. Stud. Mycol. 2019, 92, 155–194. [Google Scholar] [CrossRef]
- Lombard, L.; Sandoval-Denis, M.; Lamprecht, S.; Crous, P.W. Epitypification of Fusarium oxysporum—Clearing the taxonomic chaos. Persoonia Mol. Phylogeny Evol. Fungi 2019, 43, 1–47. [Google Scholar] [CrossRef]
- Czislowski, E.; Fraser-Smith, S.; Zander, M.; O’Neill, W.T.; Meldrum, R.A.; Tran-Nguyen, L.T.T.; Batley, J.; Aitken, E.A.B. Investigation of the diversity of effector genes in the banana pathogen, Fusarium oxysporum f. sp. cubense, reveals evidence of horizontal gene transfer. Mol. Plant Pathol. 2018, 19, 1155–1171. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef] [PubMed]
- Ploetz, R.C. Fusarium wilt of banana is caused by several pathogens referred to as Fusarium oxysporum f. sp. cubense. Phytopathology 2006, 96, 653–656. [Google Scholar] [CrossRef]
- Gordon, T.R. Fusarium oxysporum and the Fusarium Wilt Syndrome. Annu. Rev. Phytopathol. 2017, 55, 23–39. [Google Scholar] [CrossRef]
- Stover, R.H. Fusarial Wilt (Panama Disease) of Bananas and Other Musa Species; Commonwealth Mycological Institute: Kew, UK, 1962; p. 117. [Google Scholar]
- Zhang, L.; Yuan, T.; Wang, Y.; Zhang, D.; Bai, T.; Xu, S.; Wang, Y.; Tang, W.; Zheng, S.J. Identification and evaluation of resistance to Fusarium oxysporum f. sp. cubense tropical race 4 in Musa acuminata Pahang. Euphytica 2018, 214, 106. [Google Scholar] [CrossRef]
- Warman, N.M.; Aitken, E.A.B. The Movement of Fusarium oxysporum f.sp. cubense (Sub-Tropical Race 4) in susceptible cultivars of banana. Front. Plant Sci. 2018, 9, 1748. [Google Scholar] [CrossRef] [PubMed]
- Pegg, K.G.; Coates, L.M.; O’Neill, W.T.; Turner, D.W. The epidemiology of Fusarium wilt of banana. Front. Plant Sci. 2019, 10, 1395. [Google Scholar] [CrossRef]
- Bai, T.; Qin, M.; Li, X.; Fan, H.; Xu, S.; Zeng, L.; Zheng, S.J. An additional threat to ‘Cavendish’ banana growers and traders: The infection of banana peduncles by Fusarium oxysporum f. sp. cubense Tropical Race 4 (Foc TR4). Plant Health Prog. 2020, 21, 312–316. [Google Scholar] [CrossRef]
- Ploetz, R.C. Management of Fusarium wilt of banana: A review with special reference to tropical race 4. Crop Prot. 2015, 73, 7–15. [Google Scholar] [CrossRef]
- Hwang, S.C.; Ko, W.H. Cavendish banana cultivars resistant to Fusarium wilt acquired through somaclonal variation in Taiwan. Plant Dis. 2004, 88, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Buddenhagen, I. Understanding strain diversity in Fusarium oxysporum f. sp. cubense and history of introduction of ‘Tropical Race 4’ to better manage banana production. Acta Hortic. 2009, 828, 193–204. [Google Scholar] [CrossRef]
- Mostert, D.; Molina, A.B.; Daniells, J.; Fourie, G.; Hermanto, C.; Chao, C.P.; Fabregar, E.; Sinohin, V.G.; Masdek, N.; Thangavelu, R.; et al. The distribution and host range of the banana Fusarium wilt fungus, Fusarium oxysporum f. sp. cubense, in Asia. PLoS ONE 2017, 12, e0181630. [Google Scholar] [CrossRef]
- Munhoz, T.; Vargas, J.; Teixeira, L.; Staver, C.; Dita, M. Fusarium Tropical Race 4 in Latin America and the Caribbean: Status and global research advances towards disease management. Front. Plant Sci. 2024, 15, 1397617. [Google Scholar] [CrossRef] [PubMed]
- Ploetz, R.C. Fusarium wilt of banana. Phytopathology 2015, 105, 1512–1521. [Google Scholar] [CrossRef]
- O’Neill, W.T.; Henderson, J.; Pattemore, J.A.; O’Dwyer, C.; Perry, S.; Beasley, D.R.; Tan, Y.P.; Smyth, A.; Goosem, C.; Thomson, K.; et al. Detection of Fusarium oxysporum f. sp. cubense tropical race 4 strain in northern Queensland. Australas. Plant Dis. Notes 2016, 11, 33. [Google Scholar] [CrossRef]
- Dita, M.; Barquero, M.; Heck, D.; Mizubuti, E.S.G.; Staver, C.P. Fusarium wilt of banana: Current knowledge on epidemiology and research needs toward sustainable disease management. Front. Plant Sci. 2018, 9, 1468. [Google Scholar] [CrossRef]
- Dale, J.; James, A.; Paul, J.Y.; Khanna, H.; Smith, M.; Peraza-Echeverria, S.; Garcia-Bastidas, F.; Kema, G.; Waterhouse, P.; Mengersen, K.; et al. Transgenic Cavendish bananas with resistance to Fusarium wilt tropical race 4. Nat. Commun. 2017, 8, 1496. [Google Scholar] [CrossRef]
- Chen, A.; Sun, J.; Martin, G.; Gray, L.-A.; Hřibová, E.; Christelová, P.; Yahiaoui, N.; Rounsley, S.; Lyons, R.; Batley, J.; et al. Identification of a major QTL-controlling resistance to the Subtropical Race 4 of Fusarium oxysporum f. sp. cubense in Musa acuminata ssp. malaccensis. Pathogens 2023, 12, 289. [Google Scholar] [CrossRef]
- Chen, A.; Sun, J.; Viljoen, A.; Mostert, D.; Xie, Y.; Mangila, L.; Bothma, S.; Lyons, R.; Hřibová, E.; Christelová, P.; et al. Genetic mapping, candidate gene identification and marker validation for host plant resistance to the race 4 of Fusarium oxysporum f. sp. cubense using Musa acuminata ssp. malaccensis. Pathogens 2023, 12, 820. [Google Scholar] [CrossRef]
- Rebouças, T.A.; de Jesus Rocha, A.; Cerqueira, T.S.; Adorno, P.R.; Barreto, R.Q.; dos Santos Ferreira, M.; Lino, L.S.M.; de Oliveira Amorim, V.B.; dos Santos-Serejo, J.A.; Haddad, F.; et al. Pre-selection of banana somaclones resistant to Fusarium oxysporum f. sp. cubense, subtropical race 4. Crop Prot. 2021, 147, 105692. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Sun, J.; Matthews, A.; Armas-Egas, L.; Chen, N.; Hamill, S.; Mintoff, S.; Tran-Nguyen, L.T.; Batley, J.; Aitken, E.A. Assessing variations in host resistance to Fusarium oxysporum f. sp. cubense race 4 in Musa species, with a focus on the subtropical race 4. Front. Microbiol. 2019, 10, 1062. [Google Scholar] [CrossRef]
- Mintoff, S.J.; Nguyen, T.V.; Kelly, C.; Cullen, S.; Hearnden, M.; Williams, R.; Daniells, J.W.; Tran-Nguyen, L.T. Banana cultivar field screening for resistance to Fusarium oxysporum f. sp. cubense tropical race 4 in the Northern Territory. J. Fungi 2021, 7, 627. [Google Scholar] [CrossRef]
- Zuo, C.; Deng, G.; Li, B.; Huo, H.; Li, C.; Hu, C.; Kuang, R.; Yang, Q.; Dong, T.; Sheng, O.; et al. Germplasm screening of Musa spp. for resistance to Fusarium oxysporum f. sp. cubense tropical race 4 (Foc TR4). Eur. J. Plant Pathol. 2018, 151, 723–734. [Google Scholar] [CrossRef]
- Zhan, N.; Kuang, M.; He, W.; Deng, G.; Liu, S.; Li, C.; Roux, N.; Dita, M.; Yi, G.; Sheng, O. Evaluation of resistance of banana genotypes with AAB genome to Fusarium wilt Tropical Race 4 in China. J. Fungi 2022, 8, 1274. [Google Scholar] [CrossRef]
- Molina, A.; Razo, S.; Sinohin, V.G.; Herradura, L. DA-BAR Terminal Report: Mitigating Banana Fusarium Wilt Tropical Race 4 Through a Farmer-Participatory Approach of Developing Disease Management Strategies; Bioversity International: Rome, Italy, 2018. [Google Scholar] [CrossRef]
- Viljoen, A.; Mostert, D.; Chiconela, T.; Beukes, I.; Fraser, C.; Dwyer, J.; Murray, H.; Amisse, J.; Matabuana, E.L.; Tazan, G.; et al. Occurrence and spread of the banana fungus Fusarium oxysporum f. sp. cubense TR4 in Mozambique. S. Afr. J. Sci. 2020, 116, 1–11. [Google Scholar] [CrossRef]
- Daly, A.; Walduck, G. Fusarium wilt of Bananas (Panama Disease):(Fusarium oxysporum f. sp. cubense); Agnote—Northern Territory of Australia; No.I51; Northern Territory Government: Darwin, NT, Australia, 2006; pp. 1–5.
- Smith, M.; Langdon, P.; Pegg, K.; Daniells, J. Growth, yield and Fusarium wilt resistance of six FHIA tetraploid bananas (Musa spp.) grown in the Australian subtropics. Sci. Hortic. 2014, 170, 176–181. [Google Scholar] [CrossRef]
- Sanya, L.N.; Sseguya, H.; Kyazze, F.B.; Diiro, G.M.; Nakazi, F. The role of variety attributes in the uptake of new hybrid bananas among smallholder rural farmers in central Uganda. Agric. Food Secur. 2020, 9, 1. [Google Scholar] [CrossRef]
- Bani, M.; Pérez-De-Luque, A.; Rubiales, D.; Rispail, N. Physical and chemical barriers in root tissues contribute to quantitative resistance to Fusarium oxysporum f. sp. pisi in pea. Front. Plant Sci. 2018, 9, 199. [Google Scholar] [CrossRef]
- Ouellette, G.; Baayen, R.; Simard, M.; Rioux, D. Ultrastructural and cytochemical study of colonization of xylem vessel elements of susceptible and resistant Dianthus caryophyllus by Fusarium oxysporum f. sp. dianthi. Can. J. Bot. 1999, 77, 644–663. [Google Scholar] [CrossRef]
- De Micco, V.; Balzano, A.; Wheeler, E.A.; Baas, P. Tyloses and gums: A review of structure, function and occurrence of vessel occlusions. IAWA J. 2016, 37, 186–205. [Google Scholar] [CrossRef]
- VanderMolen, G.E.; Beckman, C.H.; Rodehorst, E. The ultrastructure of tylose formation in resistant banana following inoculation with Fusarium oxysporum f. sp. cubense. Physiol. Mol. Plant Pathol. 1987, 31, 185–200. [Google Scholar] [CrossRef]
- Li, C.; Chen, S.; Zuo, C.; Sun, Q.; Ye, Q.; Yi, G.; Huang, B. The use of GFP-transformed isolates to study infection of banana with Fusarium oxysporum f. sp. cubense race 4. Eur. J. Plant Pathol. 2011, 131, 327–340. [Google Scholar] [CrossRef]
- Xiao, R.F.; Zhu, Y.J.; Li, Y.D.; Liu, B. Studies on vascular infection of Fusarium oxysporum f. sp. cubense race 4 in banana by field survey and green fluorescent protein reporter. Int. J. Plant Pathol. 2013, 2, 44–51. [Google Scholar] [CrossRef]
- Anderson, J.; Aitken, E. Effect of in planta treatment of ‘Cavendish’ Banana with herbicides and fungicides on the colonisation and sporulation by Fusarium oxysporum f. sp. cubense Subtropical Race 4. J. Fungi 2021, 7, 184. [Google Scholar] [CrossRef]
- Ratnayake, K.; Joyce, D.C.; Webb, R.I. A convenient sample preparation protocol for scanning electron microscope examination of xylem-occluding bacterial biofilm on cut flowers and foliage. Sci. Hortic. 2012, 140, 12–18. [Google Scholar] [CrossRef]
- Rocha, A.d.J.; Soares, J.M.d.S.; Nascimento, F.d.S.; Rocha, A.d.S.; Amorim, V.B.O.d.; Ramos, A.P.d.S.; Ferreira, C.F.; Haddad, F.; Amorim, E.P. Molecular, histological and histochemical responses of banana cultivars challenged with Fusarium oxysporum f. sp. cubense with different levels of virulence. Plants 2022, 11, 2339. [Google Scholar] [CrossRef]
- Kashyap, A.; Planas-Marquès, M.; Capellades, M.; Valls, M.; Coll, N.S. Blocking intruders: Inducible physico-chemical barriers against plant vascular wilt pathogens. J. Exp. Bot. 2020, 72, 184–198. [Google Scholar] [CrossRef]
- Chen, A.; Morrison, S.; Gregson, A.; Le, D.P.; Urquhart, A.S.; Smith, L.J.; Aitken, E.A.; Gardiner, D.M. Fluorescently Tagged Verticillium dahliae to understand the infection process on cotton (Gossypium hirsutum) and weed plant species. Pathogens 2024, 13, 442. [Google Scholar] [CrossRef]
- Vallad, G.; Subbarao, K. Colonization of resistant and susceptible lettuce cultivars by a green fluorescent protein-tagged isolate of Verticillium dahliae. Phytopathology 2008, 98, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Lagopodi, A.L.; Ram, A.F.; Lamers, G.E.; Punt, P.J.; Van den Hondel, C.A.; Lugtenberg, B.J.; Bloemberg, G.V. Novel aspects of tomato root colonization and infection by Fusarium oxysporum f. sp. radicis-lycopersici revealed by confocal laser scanning microscopic analysis using the green fluorescent protein as a marker. Mol. Plant Microbe Interact. 2002, 15, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ling, X.; Liu, T.; Chen, T.; Yang, Y.; Yao, S.; Zhang, B. Microscopic observations of strawberry plant colonization by a GFP-labelled strain of Fusarium oxysporum f. sp. fragariae. Can. J. Plant Pathol. 2014, 36, 501–508. [Google Scholar] [CrossRef]
- Pérez-Donoso, A.G.; Greve, L.C.; Walton, J.H.; Shackel, K.A.; Labavitch, J.M. Xylella fastidiosa infection and ethylene exposure result in xylem and water movement disruption in grapevine shoots. Plant Physiol. 2007, 143, 1024–1036. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Rost, T.L.; Matthews, M.A. Pruning-induced tylose development in stems of current-year shoots of Vitis vinifera (Vitaceae). Am. J. Bot. 2006, 93, 1567–1576. [Google Scholar] [CrossRef]
- Taylor, A.M.; Gartner, B.L.; Morrell, J.J. Heartwood formation and natural durability—A review. Wood Fiber Sci. 2002, 34, 587–611. [Google Scholar]
- Dute, R.R.; Duncan, K.M.; Duke, B. Tyloses in abscission scars of loblolly pine. IAWA J. 1999, 20, 67–74. [Google Scholar] [CrossRef]
- Shi, J.; Mueller, W.; Beckman, C. Vessel occlusion and secretory activities of vessel contact cells in resistant or susceptible cotton plants infected with Fusarium oxysporum f. sp. vasinfectum. Physiol. Mol. Plant Pathol. 1992, 40, 133–147. [Google Scholar] [CrossRef]
- Zhou, G.-D.; He, P.; Tian, L.; Xu, S.; Yang, B.; Liu, L.; Wang, Y.; Bai, T.; Li, X.; Li, S.; et al. Disentangling the resistant mechanism of Fusarium wilt TR4 interactions with different cultivars and its elicitor application. Front. Plant Sci. 2023, 14, 1145837. [Google Scholar] [CrossRef]
- De Ascensao, A.R.; Dubery, I.A. Panama disease: Cell wall reinforcement in banana roots in response to elicitors from Fusarium oxysporum f. sp. cubense Race Four. Phytopathology 2000, 90, 1173–1180. [Google Scholar] [CrossRef]
- Beckman, C.; Elgersma, D.; MacHardy, W. The localization of fusarial infections in the vascular tissue of single-dominant-gene resistant tomatoes. Phytopathology 1972, 62, 1256–1260. [Google Scholar] [CrossRef]
- Baayen, R.; Van Eijk, C.; Elgersma, D. Histology of roots of resistant and susceptible carnation cultivars from soil infested with Fusarium oxysporum f. sp. dianthi. Neth. J. Plant Pathol. 1989, 95, 3–13. [Google Scholar] [CrossRef]
- Tessier, B.; Mueller, W.; Morgham, A. Histopathology and ultrastructure of vascular responses in peas resistant or susceptible to Fusarium oxysporum f. sp. pisi. Phytopathology 1990, 80, 756–764. [Google Scholar] [CrossRef]
- Pereira, A.C.; Cruz, M.F.A.; Paula Júnior, T.J.; Rodrigues, F.A.; Carneiro, J.E.S.; Vieira, R.F.; Carneiro, P.C.S. Infection process of Fusarium oxysporum f. sp. phaseoli on resistant, intermediate and susceptible bean cultivars. Trop. Plant Pathol. 2013, 38, 323–328. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, A.; Chou, T.-Y.; Chen, Y.; Fallatah, S.M.A.; Anderson, J.; Sun, J.; Cosgrove, H.; Gao, S.; Ferguson, B.J.; Soper, A.; et al. Histological Dissection of Fusarium-Banana Interaction Using a GFP-Tagged Subtropical Race 4 Strain of Fusarium oxysporum f. sp. cubense on Banana Cultivars with Differing Levels of Resistance. Microorganisms 2024, 12, 2472. https://doi.org/10.3390/microorganisms12122472
Chen A, Chou T-Y, Chen Y, Fallatah SMA, Anderson J, Sun J, Cosgrove H, Gao S, Ferguson BJ, Soper A, et al. Histological Dissection of Fusarium-Banana Interaction Using a GFP-Tagged Subtropical Race 4 Strain of Fusarium oxysporum f. sp. cubense on Banana Cultivars with Differing Levels of Resistance. Microorganisms. 2024; 12(12):2472. https://doi.org/10.3390/microorganisms12122472
Chicago/Turabian StyleChen, Andrew, Ting-Yan Chou, Yi Chen, Sumayyah M. A. Fallatah, Jay Anderson, Jiaman Sun, Harry Cosgrove, Siyuan Gao, Brett J. Ferguson, Amelie Soper, and et al. 2024. "Histological Dissection of Fusarium-Banana Interaction Using a GFP-Tagged Subtropical Race 4 Strain of Fusarium oxysporum f. sp. cubense on Banana Cultivars with Differing Levels of Resistance" Microorganisms 12, no. 12: 2472. https://doi.org/10.3390/microorganisms12122472
APA StyleChen, A., Chou, T.-Y., Chen, Y., Fallatah, S. M. A., Anderson, J., Sun, J., Cosgrove, H., Gao, S., Ferguson, B. J., Soper, A., Gardiner, D. M., & Aitken, E. A. B. (2024). Histological Dissection of Fusarium-Banana Interaction Using a GFP-Tagged Subtropical Race 4 Strain of Fusarium oxysporum f. sp. cubense on Banana Cultivars with Differing Levels of Resistance. Microorganisms, 12(12), 2472. https://doi.org/10.3390/microorganisms12122472








