Arbuscular Mycorrhizal Fungi: Boosting Crop Resilience to Environmental Stresses
Abstract
1. Introduction
2. Effects of AM Fungi on Plant Drought Tolerance
3. Effects of AM Fungi on Plant Salt Tolerance
4. Effects of AM Fungi on Temperature Stress
5. Effects of AM Fungi on Heavy Metal Stress
| Host Plants | AM Fungi Strains | Heavy Metal Stress Type | Responses Related to AM Fungi Inoculation | References |
|---|---|---|---|---|
| Bidens parviflora | Funneliformis mosseae | Lead (Pb) | Enhanced oxidative stress defense via increased activity of superoxide dismutase, catalase, ascorbate peroxidase, and glutathione reductase; improved chlorophyll concentration and photosynthesis efficiency; increased root Pb accumulation to protect aerial parts. | [176] |
| Broussonetia papyrifera | Rhizophagus irregularis | Cadmium (Cd) | Improved growth and photosynthesis; regulated ROS under low and medium Cd stress; enhanced AsA-GSH cycle under high Cd stress; modulated Cd chelation, soil pH, GRSP content, and phosphorus-related Cd dynamics; differential gene regulation for heavy metal transport. | [177] |
| Maize (Zea mays L.) | Glomus mosseae, Indigenous P2 fungal culture | Cadmium (Cd), Zinc (Zn), Copper (Cu), Lead (Pb), Manganese (Mn) | Experiment 1: Enhanced biomass, reduced Cd, Cu, Zn, and Mn concentrations, indicating protection against metal toxicity. Experiment 2: Varied responses; increased Cu in shoots and Zn in both treatments, increased Pb concentration in roots, no significant change in Cd. Root–shoot translocation of Cu and Zn increased. | [194] |
| Red Clover | Glomus mosseae | Zinc (Zn) | Enhanced Zn uptake at lower levels; reduced translocation to shoots at higher levels; increased P nutrition; hyphae directly absorbed and transferred Zn to roots. | [195] |
| Pteris vittata | Glomus mosseae, Gigaspora margarita | Arsenic (As) | Phytoremediation techniques are receiving more attention as decontaminating strategies. Increased As concentration in pinnae, higher P concentration, enhanced As translocation and plant growth. | [196] |
| Kenaf (Hibiscus cannabinus L.) | Rhizophagus aggreratus | Cadmium (Cd) | Improved nutrient transport efficiency and plant growth; increased cell wall polysaccharide content binding Cd in roots, reducing its transport to aerial parts; enhanced soil balcomycin content aiding in Cd chelation; upregulated expression of genes like Hc.GH3.1, Hc.ARK, and Hc.PHR1 enhancing Cd tolerance. | [213] |
| Rice (Oryza sativa) | Funneliformis mosseae (Fm), Rhizophagus intraradices (Ri) | Cadmium (Cd) | Decreased root and shoot Cd concentrations, especially with Ri. Altered expression of Cd transporters (Nramp5, HMA3) influencing Cd uptake. Ri treatment led to higher abundance of Actinobacteria, reducing soil Cd availability. | [214] |
| Sunflower (Helianthus annuus L.) | Funneliformis mosseae, Rhizophagus intraradices, Claroideoglomus etunicatum | Cadmium (Cd) | Increased growth, chlorophyll content, and cell membrane stability. Enhanced antioxidant enzyme activities, increased proline and total phenols, reduced lipid peroxidation and hydrogen peroxide production. Mitigated negative impacts on fatty acids and phosphatase activities under cadmium stress. | [215] |
| Medicago sativa | Glomus aggregatum, G. intraradices, G. elunicatum, G. versiforme | Cadmium (Cd) | Increased shoot and root biomass, especially in combination with biochar. Enhanced N, P, K, Ca uptake; reduced Cd concentration in plant tissues. | [216] |
6. Effects of AM Fungi on Waterlogging Stress
| Host Plants | AM Fungi Strains | Waterlogging Stress Condition | Responses Related to AM Fungi Inoculation | References |
|---|---|---|---|---|
| Dyera polyphylla | Glomus clarum, Gigaspora decipiens | Permanent and seasonal waterlogging | Enhanced tolerance to waterlogging stress; increased nitrogen and phosphorus content | [232] |
| Pterocarpus officinalis | Glomus intraradices | Permanent and seasonal waterlogging | Increased phosphorus uptake | [250] |
| Panicum hemitomon Schultes and Leersia hexandra Schwartz | Acaulospora trappei, Scutellospora heterogama, A. laevis, Glomus leptotichum, Glomus etunicatum and Glomus gerdemannii. | following rooting-zone flood regimes | Increased phosphorus uptake | [233] |
| Panicum hemitomon Schult L. and Typha latifolia L. | AM fungal assemblages—collected from different vegetation communities in a Florida wetland | flooded conditions | Improved some plant-growth and P-nutrition parameters at lower P levels relative to nonmycorrhizal controls, but generally conferred no benefit or was detrimental at higher P levels. | [234] |
| Typha latifolia | Not Specified(using fieldcollected soils were maintained for 12 weeks to increase the biomass of mycorrhizal fungi.) | Inundated soils at three levels of phosphorous availability conditions for 11 weeks. | Increased phosphorus and nitrogen uptake | [235] |
| Prunus persica Batsch | Not Specified | 3 days of flooding | Increased phosphorus, nitrogen uptake and root activity; inhibited ethanol | [236] |
| Aster tripolium | Glomus geosporum | tidal flooding conditions for 56 d | Improved osmotic regulation through accumulation of sugars and proline; enhanced oxidative stress defense | [239] |
| Poncirus trifoliata | Diversispora spurca | waterlogging | Increased superoxide dismutase and catalase activities in leaf and root under both NWL and WL, thereby, resulting in lower oxidative damage in terms of malondialdehide concentration. | [242] |
| Citrus junos | Diversispora spuraca | Waterlogging for 37 d | Significantly increased root catalase (CAT) activity in non-stressed seedlings and increased root soluble protein concentration and leaf CAT activity in waterlogged seedlings, thereby inducing lower oxidative damage. | [243] |
| Prunes persica (L.) Batsch | Funneliformis mosseae | Waterlogging for 12 d | Increased accumulation of proline; increase in P5CS activity and a decrease in δ-OAT and ProDH activity; enhanced chlorophyll concentration and photosynthesis efficiency | [240] |
7. Effects of AM Fungi on Plant Resistance to Biotic Stresses
| Host Plants | AM Fungi Strains | Biological Stress Type | Responses Related to AM Fungi Inoculation | References |
|---|---|---|---|---|
| Artichoke (Cynara scolymus L.) | Glomus vicosum | Verticillium wilt caused by Vertcillium dahliae | Increased activity of antioxidant enzymes: ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), and superoxide dismutase (SOD) | [255] |
| Banana (Musa acuminata ‘Cavendish’ cv. ‘Brail’) | Rhizophagus irregularis (Ri) | Fusarium wilt caused by Fusarium oysporum f. sp. cubense | Increased plant dry weights in stem, leaf, and overall; up-regulation of defense-related genes (POD, PAL, PYR, HBP-1b); enhanced expression of growth-related genes (IAA, GH3, SAUR, ARR8). | [256] |
| Pea (Pisum sativum) | Glomus intraradices, Glomus claroideum | Pea root-rot caused by Aphanomyces euteiches | Reduced disease incidence, especially with G. intraradices; enhanced mycorrhizal development and potential induction of tolerance against pea root-rot. | [257] |
| Rice (Oryza sativa, japonica subspecies) | Funneliformis mosseae, Rhizophagus irregularis | Blast disease caused by Magnaporthe oryzae | Enhanced root colonization, especially by R. irregularis; increased Pi content in leaves; improved growth, productivity, and blast resistance, varying by rice cultivar; significant increase in grain yield in field conditions | [259] |
| Eggplant | Glomus mosseae (Gm), Ggaspora gigantea (Gg) | Root-knot nematode (M. javanica) | Reduced root-knot nematode infestation; improved growth traits and fruit biochemical content; higher levels of mycorrhization (68.20%); outperformed single treatments in most traits | [261] |
| Medicago truncatula (‘Jemalong’, line A17) | Rhizophagus irregularis | Pea aphid (Acyrthosiphon pisum) | Increased preference by adult aphids for highly AM fungi-colonized plants; mixed age aphids showed reduced weight on low AM colonized plants, indicating possible priming by AM fungi; gene expression changes in roots related to gibberellin metabolism. | [271] |
| Ageratina adenophora | Claroideoglomus etunicatum, Septoglomus constrictum Claroideoglomus etunicatum, Septoglomus constrictum | Aphis gossypii | Enhanced growth (increased aboveground and root biomass) and resistance to A. gossypii, elevated polyphenol oxidase, jasmonic acid, and flavonoid levels, and reduced A. gossypii nymph survival and density, with C. etunicatum showing a greater effect than S. constrictum. | [272] |
| Plantago major and Poa annua | Rhizoglomus irregulare | Generalist aphid (Myzus persicae) | Slight increases in sucrose proportions and shifts in amino acid profiles in phloem exudates. Negative effects on aphid survival in P. major, but positive effects in P. annua on the next aphid generation. | [273] |
8. Effects of AM Fungi on Crop Yield
9. Application Potential of AM Fungi
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marvin, H.J.; Bouzembrak, Y. A system approach towards prediction of food safety hazards: Impact of climate and agrichemical use on the occurrence of food safety hazards. Agric. Syst. 2020, 178, 102760. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2009; pp. 153–188. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Yadav, S. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 21, 640–656. [Google Scholar] [CrossRef]
- Pandey, P.; Senthil-Kumar, M. Plant-pathogen interaction in the presence of abiotic stress: What do we know about plant responses? Plant Physiol. Rep. 2019, 24, 541–549. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Simon, L.; Bousquet, J.; Lévesque, R.C.; Lalonde, M. Origin and diversification of endomycorrhizal fungi and coincidence with vascular land plants. Nature 1993, 363, 67–69. [Google Scholar] [CrossRef]
- Fang, F.; Wang, C.; Wu, F.; Tang, M.; Doughty, R. Arbuscular mycorrhizal fungi mitigate nitrogen leaching under poplar seedlings. Forests 2020, 11, 325. [Google Scholar] [CrossRef]
- Kilpeläinen, J.; Barbero-López, A.; Adamczyk, B.; Aphalo, P.J.; Lehto, T. Morphological and ecophysiological root and leaf traits in ectomycorrhizal, arbuscular-mycorrhizal and non-mycorrhizal Alnus incana seedlings. Plant Soil 2019, 436, 283–297. [Google Scholar] [CrossRef]
- Adjoud, D.; Plenchette, C.; Halli-Hargas, R.; Lapeyrie, F. Response of 11 eucalyptus species to inoculation with three arbuscular mycorrhizal fungi. Mycorrhiza 1996, 6, 129–135. [Google Scholar] [CrossRef]
- Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002, 154, 275–304. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Matsuzaki, K.-i.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef]
- Li, C.; Chang, P.-P.; Ghebremariam, K.M.; Qin, L.; Liang, Y. Overexpression of tomato SpMPK3 gene in Arabidopsis enhances the osmotic tolerance. Biochem. Biophys. Res. Commun. 2014, 443, 357–362. [Google Scholar] [CrossRef]
- Bahadur, A.; Batool, A.; Nasir, F.; Jiang, S.; Mingsen, Q.; Zhang, Q.; Pan, J.; Liu, Y.; Feng, H. Mechanistic insights into arbuscular mycorrhizal fungi-mediated drought stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 4199. [Google Scholar] [CrossRef]
- Calvo-Polanco, M.; Sánchez-Castro, I.; Cantos, M.; García, J.L.; Azcón, R.; Ruiz-Lozano, J.M.; Beuzón, C.R.; Aroca, R. Effects of different arbuscular mycorrhizal fungal backgrounds and soils on olive plants growth and water relation properties under well-watered and drought conditions. Plant Cell Environ. 2016, 39, 2498–2514. [Google Scholar] [CrossRef]
- Ajeesh, R.; Kumar, V.; Santoshkumar, A. Harnessing Arbuscular Mycorrhizal Fungi (AMF) for quality seedling production. Res. J. Agric. For. Sci. 2015, 2320, 6063. Available online: https://www.researchgate.net/profile/Vikas-Kumar-147/publication/277774535_Harnessing_Arbuscular_Mycorrhizal_Fungi_AMF_for_Quality_Seedling_Production/links/5572fd8a08ae75215868f2fc/Harnessing-Arbuscular-Mycorrhizal-Fungi-AMF-for-Quality-Seedling-Production.pdf (accessed on 13 November 2024).
- Van Nuland, M.E.; Ware, I.M.; Schadt, C.W.; Yang, Z.; Bailey, J.K.; Schweitzer, J.A. Natural soil microbiome variation affects spring foliar phenology with consequences for plant productivity and climate-driven range shifts. New Phytol. 2021, 232, 762–775. [Google Scholar] [CrossRef]
- Xu, J.; Liu, S.; Song, S.; Guo, H.; Tang, J.; Yong, J.W.; Ma, Y.; Chen, X. Arbuscular mycorrhizal fungi influence decomposition and the associated soil microbial community under different soil phosphorus availability. Soil Biol. Biochem. 2018, 120, 181–190. [Google Scholar] [CrossRef]
- Paszkowski, U. A journey through signaling in arbuscular mycorrhizal symbioses. New Phytol. 2006, 172, 35–46. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yano, K. Nitrogen delivery to maize via mycorrhizal hyphae depends on the form of N supplied. Plant Cell Environ. 2005, 28, 1247–1254. [Google Scholar] [CrossRef]
- Jackson, L.E.; Burger, M.; Cavagnaro, T.R. Roots, nitrogen transformations, and ecosystem services. Annu. Rev. Plant Biol. 2008, 59, 341–363. [Google Scholar] [CrossRef]
- Bhale, U.; Bansode, S.; Singh, S. Multifactorial role of arbuscular mycorrhizae in agroecosystem. In Fungi and Their Role in Sustainable Development: Current Perspectives; Springer: Singapore, 2018; pp. 205–220. [Google Scholar] [CrossRef]
- Zhu, X.-C.; Song, F.-B.; Liu, S.-Q.; Liu, T.-D. Effects of arbuscular mycorrhizal fungus on photosynthesis and water status of maize under high temperature stress. Plant Soil 2011, 346, 189–199. [Google Scholar] [CrossRef]
- Zhu, X.; Cao, Q.; Sun, L.; Yang, X.; Yang, W.; Zhang, H. Stomatal conductance and morphology of arbuscular mycorrhizal wheat plants response to elevated CO2 and NaCl stress. Front. Plant Sci. 2018, 9, 1363. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-C.; Song, F.-B.; Xu, H.-W. Arbuscular mycorrhizae improves low temperature stress in maize via alterations in host water status and photosynthesis. Plant Soil 2010, 331, 129–137. [Google Scholar] [CrossRef]
- Gholamhoseini, M.; Ghalavand, A.; Dolatabadian, A.; Jamshidi, E.; Khodaei-Joghan, A. Effects of arbuscular mycorrhizal inoculation on growth, yield, nutrient uptake and irrigation water productivity of sunflowers grown under drought stress. Agric. Water Manag. 2013, 117, 106–114. [Google Scholar] [CrossRef]
- Boyer, L.R.; Brain, P.; Xu, X.-M.; Jeffries, P. Inoculation of drought-stressed strawberry with a mixed inoculum of two arbuscular mycorrhizal fungi: Effects on population dynamics of fungal species in roots and consequential plant tolerance to water deficiency. Mycorrhiza 2015, 25, 215–227. [Google Scholar] [CrossRef]
- Whipps, J.M. Prospects and limitations for mycorrhizas in biocontrol of root pathogens. Can. J. Bot. 2004, 82, 1198–1227. [Google Scholar] [CrossRef]
- Dickson, S. The Arum-Paris continuum of mycorrhizal symbioses. New Phytol. 2004, 163, 187–200. Available online: https://www.jstor.org/stable/1514440 (accessed on 13 November 2024). [CrossRef] [PubMed]
- Van Der Heijden, M.G.; Klironomos, J.N.; Ursic, M.; Moutoglis, P.; Streitwolf-Engel, R.; Boller, T.; Wiemken, A.; Sanders, I.R. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 1998, 396, 69–72. [Google Scholar] [CrossRef]
- Choi, J.; Summers, W.; Paszkowski, U. Mechanisms underlying establishment of arbuscular mycorrhizal symbioses. Annu. Rev. Phytopathol. 2018, 56, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Hayashi, H. Strigolactones: Chemical signals for fungal symbionts and parasitic weeds in plant roots. Ann. Bot. 2006, 97, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.J.; Buuren, M.L.v. A phosphate transporter from the mycorrhizal fungus Glomus versiforme. Nature 1995, 378, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef] [PubMed]
- Classen, A.T.; Sundqvist, M.K.; Henning, J.A.; Newman, G.S.; Moore, J.A.; Cregger, M.A.; Moorhead, L.C.; Patterson, C.M. Direct and indirect effects of climate change on soil microbial and soil microbial-plant interactions: What lies ahead? Ecosphere 2015, 6, 1–21. [Google Scholar] [CrossRef]
- Victorino, Í.M.M.; Berruti, A.; Orgiazzi, A.; Voyron, S.; Bianciotto, V.; Lumini, E. High-throughput DNA sequence-based analysis of AMF communities. In Arbuscular Mycorrhizal Fungi: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2020; pp. 99–116. [Google Scholar] [CrossRef]
- Frostegård, Å.; Tunlid, A.; Bååth, E. Use and misuse of PLFA measurements in soils. Soil Biol. Biochem. 2011, 43, 1621–1625. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, T.; Ye, J.; Liu, S.; Shibistova, O.; Wang, P.; Wang, J.; Li, Y.; Guggenberger, G.; Kuzyakov, Y. Initial utilization of rhizodeposits with rice growth in paddy soils: Rhizosphere and N fertilization effects. Geoderma 2019, 338, 30–39. [Google Scholar] [CrossRef]
- Allsup, C.M.; Lankau, R.A.; Paige, K.N. Herbivory and soil water availability induce changes in arbuscular mycorrhizal fungal abundance and composition. Microb. Ecol. 2022, 84, 141–152. [Google Scholar] [CrossRef]
- Zheng, J.; Cui, M.; Wang, C.; Wang, J.; Wang, S.; Sun, Z.; Ren, F.; Wan, S.; Han, S. Elevated CO2, warming, N addition, and increased precipitation affect different aspects of the arbuscular mycorrhizal fungal community. Sci. Total Environ. 2022, 806, 150522. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Taniguchi, T.; Shi, W.-Y.; Li, G.; Yamanaka, N.; Du, S. Land-use types and soil chemical properties influence soil microbial communities in the semiarid Loess Plateau region in China. Sci. Rep. 2017, 7, 45289. [Google Scholar] [CrossRef]
- Xue, P.-P.; Carrillo, Y.; Pino, V.; Minasny, B.; McBratney, A.B. Soil properties drive microbial community structure in a large scale transect in South Eastern Australia. Sci. Rep. 2018, 8, 11725. [Google Scholar] [CrossRef]
- Adenan, S.; Oja, J.; Alatalo, J.M.; Shraim, A.M.; Alsafran, M.; Tedersoo, L.; Zobel, M.; Ahmed, T. Diversity of arbuscular mycorrhizal fungi and its chemical drivers across dryland habitats. Mycorrhiza 2021, 31, 685–697. [Google Scholar] [CrossRef]
- Kaushal, M.; Wani, S.P. Rhizobacterial-plant interactions: Strategies ensuring plant growth promotion under drought and salinity stress. Agric. Ecosyst. Environ. 2016, 231, 68–78. [Google Scholar] [CrossRef]
- Liu, J.; Guo, C.; Chen, Z.-L.; He, J.-D.; Zou, Y.-N. Mycorrhizal inoculation modulates root morphology and root phytohormone responses in trifoliate orange under drought stress. Emir. J. Food Agric. 2016, 28, 251. [Google Scholar] [CrossRef]
- Von Mark, V.C. Root traits contributing to plant productivity under drought. Front. Media 2013, 4, 442. Available online: https://www.frontiersin.org/journals/plant-science (accessed on 13 November 2024).
- Pozo, M.J.; López-Ráez, J.A.; Azcón-Aguilar, C.; García-Garrido, J.M. Phytohormones as integrators of environmental signals in the regulation of mycorrhizal symbioses. New Phytol. 2015, 205, 1431–1436. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Xia, R.-X.; Zou, Y.-N.; Wang, G.-Y. Osmotic solute responses of mycorrhizal citrus (Poncirus trifoliata) seedlings to drought stress. Acta Physiol. Plant. 2007, 29, 543–549. [Google Scholar] [CrossRef]
- Azcón, R.; Gomez, M.; Tobar, R. Physiological and nutritional responses by Lactuca sativa L. to nitrogen sources and mycorrhizal fungi under drought conditions. Biol. Fertil. Soils 1996, 22, 156–161. [Google Scholar] [CrossRef]
- Yooyongwech, S.; Phaukinsang, N.; Cha-um, S.; Supaibulwatana, K. Arbuscular mycorrhiza improved growth performance in Macadamia tetraphylla L. grown under water deficit stress involves soluble sugar and proline accumulation. Plant Growth Regul. 2013, 69, 285–293. [Google Scholar] [CrossRef]
- Ruíz-Sánchez, M.; Armada, E.; Muñoz, Y.; de Salamone, I.E.G.; Aroca, R.; Ruíz-Lozano, J.M.; Azcón, R. Azospirillum and arbuscular mycorrhizal colonization enhance rice growth and physiological traits under well-watered and drought conditions. J. Plant Physiol. 2011, 168, 1031–1037. [Google Scholar] [CrossRef]
- Abdel-Salam, E.; Alatar, A.; El-Sheikh, M.A. Inoculation with arbuscular mycorrhizal fungi alleviates harmful effects of drought stress on damask rose. Saudi J. Biol. Sci. 2018, 25, 1772–1780. [Google Scholar] [CrossRef]
- Mo, Y.; Wang, Y.; Yang, R.; Zheng, J.; Liu, C.; Li, H.; Ma, J.; Zhang, Y.; Wei, C.; Zhang, X. Regulation of plant growth, photosynthesis, antioxidation and osmosis by an arbuscular mycorrhizal fungus in watermelon seedlings under well-watered and drought conditions. Front. Plant Sci. 2016, 7, 644. [Google Scholar] [CrossRef]
- Zhou, Q.; Ravnskov, S.; Jiang, D.; Wollenweber, B. Changes in carbon and nitrogen allocation, growth and grain yield induced by arbuscular mycorrhizal fungi in wheat (Triticum aestivum L.) subjected to a period of water deficit. Plant Growth Regul. 2015, 75, 751–760. [Google Scholar] [CrossRef]
- Han, Y.; Lou, X.; Zhang, W.; Xu, T.; Tang, M. Arbuscular mycorrhizal fungi enhanced drought resistance of Populus cathayana by regulating the 14-3-3 family protein genes. Microbiol. Spectr. 2022, 10, e02456-21. [Google Scholar] [CrossRef] [PubMed]
- Rani, B.; Madan, S.; Pooja, K.; Sharma, K.; Kumari, N.; Kumar, A. Mitigating the effect of drought stress on yield in wheat (Triticum aestivum) using arbuscular mycorrhiza fungi (Glomus mosseae). Indian J. Agric. Sci. 2018, 88, 95–100. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Xia, R.-X. Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J. Plant Physiol. 2006, 163, 417–425. [Google Scholar] [CrossRef]
- Zou, Y.-N.; Huang, Y.-M.; Wu, Q.-S.; He, X.-H. Mycorrhiza-induced lower oxidative burst is related with higher antioxidant enzyme activities, net H2O2 effluxes, and Ca2+ influxes in trifoliate orange roots under drought stress. Mycorrhiza 2015, 25, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Fiorilli, V.; Maghrebi, M.; Novero, M.; Votta, C.; Mazzarella, T.; Buffoni, B.; Astolfi, S.; Vigani, G. Arbuscular mycorrhizal symbiosis differentially affects the nutritional status of two durum wheat genotypes under drought conditions. Plants 2022, 11, 804. [Google Scholar] [CrossRef]
- Zhang, F.; Zou, Y.-N.; Wu, Q.-S. Quantitative estimation of water uptake by mycorrhizal extraradical hyphae in citrus under drought stress. Sci. Hortic. 2018, 229, 132–136. [Google Scholar] [CrossRef]
- Ji, L.; Tan, W.; Chen, X. Arbuscular mycorrhizal mycelial networks and glomalin-related soil protein increase soil aggregation in Calcaric Regosol under well-watered and drought stress conditions. Soil Tillage Res. 2019, 185, 1–8. [Google Scholar] [CrossRef]
- Singh, A.K.; Zhu, X.; Chen, C.; Wu, J.; Yang, B.; Zakari, S.; Jiang, X.J.; Singh, N.; Liu, W. The role of glomalin in mitigation of multiple soil degradation problems. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1604–1638. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Zhang, F.; Zhang, D.-J.; Srivastava, A.; Wu, Q.-S.; Zou, Y.-N. Mycorrhiza stimulates root-hair growth and IAA synthesis and transport in trifoliate orange under drought stress. Sci. Rep. 2018, 8, 1978. [Google Scholar] [CrossRef]
- Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytol. 2007, 173, 808–816. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Xiang, X.; He, J.-S.; Wang, C.; Cao, G.; Adams, J.; Chu, H. Composition of the soil fungal community is more sensitive to phosphorus than nitrogen addition in the alpine meadow on the Qinghai-Tibetan Plateau. Biol. Fertil. Soils 2016, 52, 1059–1072. [Google Scholar] [CrossRef]
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Cascone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiol. 2016, 171, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-F.; Wu, H.-H.; Zou, Y.-N.; Wu, Q.-S.; Kuča, K. Mycorrhizal response strategies of trifoliate orange under well-watered, salt stress, and waterlogging stress by regulating leaf aquaporin expression. Plant Physiol. Biochem. 2021, 162, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, J.M.; del Mar Alguacil, M.; Bárzana, G.; Vernieri, P.; Aroca, R. Exogenous ABA accentuates the differences in root hydraulic properties between mycorrhizal and non-mycorrhizal maize plants through regulation of PIP aquaporins. Plant Mol. Biol. 2009, 70, 565–579. [Google Scholar] [CrossRef]
- He, F.; Zhang, H.; Tang, M. Aquaporin gene expression and physiological responses of Robinia pseudoacacia L. to the mycorrhizal fungus Rhizophagus irregularis and drought stress. Mycorrhiza 2016, 26, 311–323. [Google Scholar] [CrossRef]
- Ouledali, S.; Ennajeh, M.; Zrig, A.; Gianinazzi, S.; Khemira, H. Estimating the contribution of arbuscular mycorrhizal fungi to drought tolerance of potted olive trees (Olea europaea). Acta Physiol. Plant. 2018, 40, 81. [Google Scholar] [CrossRef]
- Canali, S.; Roccuzzo, G.; Tittarelli, F.; Ciaccia, C.; Fiorella, S.; Intrigliolo, F. Organic Citrus: Soil fertility and plant nutrition management. In Advances in Citrus Nutrition; Springer: Berlin/Heidelberg, Germany, 2012; pp. 353–368. [Google Scholar] [CrossRef]
- Abbaspour, H.; Saeidi-Sar, S.; Afshari, H.; Abdel-Wahhab, M. Tolerance of mycorrhiza-infected pistachio (Pistacia vera L.) seedling to drought stress under glasshouse conditions. J. Plant Physiol. 2012, 169, 704–709. [Google Scholar] [CrossRef]
- Sato, T.; Hachiya, S.; Inamura, N.; Ezawa, T.; Cheng, W.; Tawaraya, K. Secretion of acid phosphatase from extraradical hyphae of the arbuscular mycorrhizal fungus Rhizophagus clarus is regulated in response to phosphate availability. Mycorrhiza 2019, 29, 599–605. [Google Scholar] [CrossRef]
- Cheng, H.-Q.; Zou, Y.-N.; Wu, Q.-S.; Kuča, K. Arbuscular mycorrhizal fungi alleviate drought stress in trifoliate orange by regulating H+-ATPase activity and gene expression. Front. Plant Sci. 2021, 12, 659694. [Google Scholar] [CrossRef]
- Hameed, A.; Wu, Q.-S.; Abd-Allah, E.F.; Hashem, A.; Kumar, A.; Lone, H.A.; Ahmad, P. Role of AM fungi in alleviating drought stress in plants. In Use of Microbes for the Alleviation of Soil Stresses: Volume 2: Alleviation of Soil Stress by PGPR and Mycorrhizal Fungi; Springer: Berlin/Heidelberg, Germany, 2014; pp. 55–75. [Google Scholar] [CrossRef]
- Boutasknit, A.; Baslam, M.; Ait-El-Mokhtar, M.; Anli, M.; Ben-Laouane, R.; Douira, A.; El Modafar, C.; Mitsui, T.; Wahbi, S.; Meddich, A. Arbuscular mycorrhizal fungi mediate drought tolerance and recovery in two contrasting carob (Ceratonia siliqua L.) ecotypes by regulating stomatal, water relations, and (in)organic adjustments. Plants 2020, 9, 80. [Google Scholar] [CrossRef]
- Saia, S.; Aissa, E.; Luziatelli, F.; Ruzzi, M.; Colla, G.; Ficca, A.G.; Cardarelli, M.; Rouphael, Y. Growth-promoting bacteria and arbuscular mycorrhizal fungi differentially benefit tomato and corn depending upon the supplied form of phosphorus. Mycorrhiza 2020, 30, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhang, H.; Fang, F.; Wu, N.; Zhang, Y.; Tang, M. Effects of nitrogen and exogenous Rhizophagus irregularis on the nutrient status, photosynthesis and leaf anatomy of Populus× canadensis 'Neva'. J. Plant Growth Regul. 2017, 36, 824–835. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, H.; Chen, H.; Tang, M. Arbuscular mycorrhizas influence Lycium barbarum tolerance of water stress in a hot environment. Mycorrhiza 2017, 27, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.-S.; Zou, Y.-N. Mycorrhizal influence on nutrient uptake of citrus exposed to drought stress. Philipp. Agric. Sci. 2009, 92, 33–38. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20093103693 (accessed on 13 November 2024).
- Wu, Q.-S.; Zou, Y.-N.; He, X.-H. Differences of hyphal and soil phosphatase activities in drought-stressed mycorrhizal trifoliate orange (Poncirus trifoliata) seedlings. Sci. Hortic. 2011, 129, 294–298. [Google Scholar] [CrossRef]
- Mathur, S.; Tomar, R.S.; Jajoo, A. Arbuscular mycorrhizal fungi (AMF) protects photosynthetic apparatus of wheat under drought stress. Photosynth. Res. 2019, 139, 227–238. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Xu, G.; Zhou, L.; Li, Y. Arbuscular mycorrhizal fungi improve the growth and drought tolerance of Zenia insignis seedlings under drought stress. New For. 2019, 50, 593–604. [Google Scholar] [CrossRef]
- Al-Arjani, A.-B.F.; Hashem, A.; Abd_Allah, E.F. Arbuscular mycorrhizal fungi modulates dynamics tolerance expression to mitigate drought stress in Ephedra foliata Boiss. Saudi J. Biol. Sci. 2020, 27, 380–394. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Su, Y.; Lei, Y.; Mustafa, N.S.A.; Ahmad, P.; Zhang, L. Improved drought tolerance by AMF inoculation in maize (Zea mays) involves physiological and biochemical implications. Plants 2019, 8, 579. [Google Scholar] [CrossRef]
- Grümberg, B.C.; Urcelay, C.; Shroeder, M.A.; Vargas-Gil, S.; Luna, C.M. The role of inoculum identity in drought stress mitigation by arbuscular mycorrhizal fungi in soybean. Biol. Fertil. Soils 2015, 51, 1–10. [Google Scholar] [CrossRef]
- Chen, W.; Meng, P.; Feng, H.; Wang, C. Effects of arbuscular mycorrhizal fungi on growth and physiological performance of Catalpa bungei C.A.Mey. under drought stress. Forests 2020, 11, 1117. [Google Scholar] [CrossRef]
- Alotaibi, M.O.; Ikram, M.; Alotaibi, N.M.; Hussain, G.S.; Ghoneim, A.M.; Younis, U.; Naz, N.; Danish, S. Examining the role of AMF-Biochar in the regulation of spinach growth attributes, nutrients concentrations, and antioxidant enzymes in mitigating drought stress. Plant Stress 2023, 10, 100205. [Google Scholar] [CrossRef]
- Chareesri, A.; De Deyn, G.B.; Sergeeva, L.; Polthanee, A.; Kuyper, T.W. Increased arbuscular mycorrhizal fungal colonization reduces yield loss of rice (Oryza sativa L.) under drought. Mycorrhiza 2020, 30, 315–328. [Google Scholar] [CrossRef]
- García, I.V.; Mendoza, R.E. Arbuscular mycorrhizal fungi and plant symbiosis in a saline-sodic soil. Mycorrhiza 2007, 17, 167–174. [Google Scholar] [CrossRef]
- Giri, B.; Kapoor, R.; Mukerji, K. Influence of arbuscular mycorrhizal fungi and salinity on growth, biomass, and mineral nutrition of Acacia auriculiformis. Biol. Fertil. Soils 2003, 38, 170–175. [Google Scholar] [CrossRef]
- Juniper, S.; Abbott, L. Soil salinity delays germination and limits growth of hyphae from propagules of arbuscular mycorrhizal fungi. Mycorrhiza 2006, 16, 371–379. [Google Scholar] [CrossRef]
- Giri, B.; Mukerji, K.G. Mycorrhizal inoculant alleviates salt stress in Sesbania aegyptiaca and Sesbania grandiflora under field conditions: Evidence for reduced sodium and improved magnesium uptake. Mycorrhiza 2004, 14, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Giri, B.; Kapoor, R.; Mukerji, K.G. Improved tolerance of Acacia nilotica to salt stress by arbuscular mycorrhiza, Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues. Microb. Ecol. 2007, 54, 753–760. [Google Scholar] [CrossRef]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Liu, S.; Guo, X.; Feng, G.; Maimaitiaili, B.; Fan, J.; He, X. Indigenous arbuscular mycorrhizal fungi can alleviate salt stress and promote growth of cotton and maize in saline fields. Plant Soil 2016, 398, 195–206. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Porcel, R.; Azcon, C.; Aroca, R. Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: New challenges in physiological and molecular studies. J. Exp. Bot. 2012, 63, 4033–4044. [Google Scholar] [CrossRef]
- Sannazzaro, A.I.; Echeverría, M.; Albertó, E.O.; Ruiz, O.A.; Menéndez, A.B. Modulation of polyamine balance in Lotus glaber by salinity and arbuscular mycorrhiza. Plant Physiol. Biochem. 2007, 45, 39–46. [Google Scholar] [CrossRef]
- Garg, N.; Pandey, R. High effectiveness of exotic arbuscular mycorrhizal fungi is reflected in improved rhizobial symbiosis and trehalose turnover in Cajanus cajan genotypes grown under salinity stress. Fungal Ecol. 2016, 21, 57–67. [Google Scholar] [CrossRef]
- Yano-Melo, A.M.; Saggin, O.J., Jr.; Maia, L.C. Tolerance of mycorrhized banana (Musa sp. cv. Pacovan) plantlets to saline stress. Agric. Ecosyst. Environ. 2003, 95, 343–348. [Google Scholar] [CrossRef]
- Rabie, G.H. Contribution of arbuscular mycorrhizal fungus to red kidney and wheat plants tolerance grown in heavy metal-polluted soil. Afr. J. Biotechnol. 2005, 4, 332–345. Available online: https://www.ajol.info/index.php/ajb/article/view/15103 (accessed on 13 November 2024).
- Cho, K.; Toler, H.; Lee, J.; Ownley, B.; Stutz, J.C.; Moore, J.L.; Augé, R.M. Mycorrhizal symbiosis and response of sorghum plants to combined drought and salinity stresses. J. Plant Physiol. 2006, 163, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Al-Karaki, G.N. Nursery inoculation of tomato with arbuscular mycorrhizal fungi and subsequent performance under irrigation with saline water. Sci. Hortic. 2006, 109, 1–7. [Google Scholar] [CrossRef]
- Sannazzaro, A.I.; Ruiz, O.A.; Albertó, E.O.; Menéndez, A.B. Alleviation of salt stress in Lotus glaber by Glomus intraradices. Plant Soil 2006, 285, 279–287. [Google Scholar] [CrossRef]
- Cantrell, I.C.; Linderman, R.G. Preinoculation of lettuce and onion with VA mycorrhizal fungi reduces deleterious effects of soil salinity. Plant Soil 2001, 233, 269–281. [Google Scholar] [CrossRef]
- Asghari, H.; Marschner, P.; Smith, S.; Smith, F. Growth response of Atriplex nummularia to inoculation with arbuscular mycorrhizal fungi at different salinity levels. Plant Soil 2005, 273, 245–256. [Google Scholar] [CrossRef]
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: Current understanding and new challenges. Front. Plant Sci. 2019, 10, 470. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Zou, Y.-N.; He, X.-H. Contributions of arbuscular mycorrhizal fungi to growth, photosynthesis, root morphology and ionic balance of citrus seedlings under salt stress. Acta Physiol. Plant. 2010, 32, 297–304. [Google Scholar] [CrossRef]
- Marschner, H.; Dell, B. Nutrient uptake in mycorrhizal symbiosis. Plant Soil 1994, 159, 89–102. [Google Scholar] [CrossRef]
- Feng, G.; Zhang, F.; Li, X.; Tian, C.; Tang, C.; Rengel, Z. Improved tolerance of maize plants to salt stress by arbuscular mycorrhiza is related to higher accumulation of soluble sugars in roots. Mycorrhiza 2002, 12, 185–190. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 2008, 18, 287–296. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Tullio, M.; Rivera, C.M.; Rea, E. Alleviation of salt stress by arbuscular mycorrhizal in zucchini plants grown at low and high phosphorus concentration. Biol. Fertil. Soils 2008, 44, 501–509. [Google Scholar] [CrossRef]
- Hajiboland, R.; Joudmand, A. The K/Na replacement and function of antioxidant defence system in sugar beet (Beta vulgaris L.) cultivars. Acta Agric. Scand. B Soil Plant Sci. 2009, 59, 246–259. [Google Scholar] [CrossRef]
- Marschner, H.; Kuiper, P.; Kylin, A. Genotypic differences in the response of sugar beet plants to replacement of potassium by sodium. Physiol. Plant. 1981, 51, 239–244. [Google Scholar] [CrossRef]
- Hajiboland, R.; Joudmand, A.; Fotouhi, K. Mild salinity improves sugar beet (Beta vulgaris L.) quality. Acta Agric. Scand. B Soil Plant Sci. 2009, 59, 295–305. [Google Scholar] [CrossRef]
- Russell, J.E. Soil Conditions and Plant Growth; Daya Books: Delhi, India, 2002. [Google Scholar]
- Barea, J.M.; Azcón, R.; Azcón-Aguilar, C. Interactions between mycorrhizal fungi and bacteria to improve plant nutrient cycling and soil structure. In Microorganisms in Soils: Roles in Genesis and Functions; Varma, A., Buscot, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 195–212. [Google Scholar] [CrossRef]
- Dasgan, H.Y.; Aktas, H.; Abak, K.; Cakmak, I. Determination of screening techniques to salinity tolerance in tomatoes and investigation of genotype responses. Plant Sci. 2002, 163, 695–703. [Google Scholar] [CrossRef]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Hanway, J. Soil analysis methods as used in the Iowa State College Soil Testing Laboratory. Iowa Agric. 1952, 57, 1. [Google Scholar] [CrossRef]
- Ojala, J.; Jarrell, W.; Menge, J.; Johnson, E. Influence of mycorrhizal fungi on the mineral nutrition and yield of onion in saline soil. Agron. J. 1983, 75, 255–259. [Google Scholar] [CrossRef]
- Mohammad, M.J.; Malkawi, H.I.; Shibli, R. Effects of arbuscular mycorrhizal fungi and phosphorus fertilization on growth and nutrient uptake of barley grown on soils with different levels of salts. J. Plant Nutr. 2003, 26, 125–137. [Google Scholar] [CrossRef]
- Al-Karaki, G.N.; Clark, R.B. Growth, mineral acquisition, and water use by mycorrhizal wheat grown under water stress. J. Plant Nutr. 1998, 21, 263–276. [Google Scholar] [CrossRef]
- Porcel, R.; Aroca, R.; Azcon, R.; Ruiz-Lozano, J.M. Regulation of cation transporter genes by the arbuscular mycorrhizal symbiosis in rice plants subjected to salinity suggests improved salt tolerance due to reduced Na+ root-to-shoot distribution. Mycorrhiza 2016, 26, 673–684. [Google Scholar] [CrossRef]
- Zandavalli, R.B.; Dillenburg, L.R.; de Souza, P.V.D. Growth responses of Araucaria angustifolia (Araucariaceae) to inoculation with the mycorrhizal fungus Glomus clarum. Appl. Soil Ecol. 2004, 25, 245–255. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 2003, 13, 309–317. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Zhang, X.; Tang, M. Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through improved photosynthesis, water status, and K+/Na+ homeostasis. Front. Plant Sci. 2017, 8, 1739. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, J.; Cui, L.; Tang, Z.; Ci, D.; Zou, X.; Zhang, X.; Yu, X.; Wang, Y.; Si, T. The multifaceted roles of arbuscular mycorrhizal fungi in peanut responses to salt, drought, and cold stress. BMC Plant Biol. 2023, 23, 36. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, F.; Zheng, X.; Pan, H.; Wen, Y.; Song, F. Effects of AMF compound inoculants on growth, ion homeostasis, and salt tolerance-related gene expression in Oryza sativa L. under salt treatments. Rice 2023, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Gill, S.; Ramzan, M.; Ahmad, M.Z.; Danish, S.; Huang, P.; Al Obaid, S.; Alharbi, S.A. Uncovering the impact of AM fungi on wheat nutrient uptake, ion homeostasis, oxidative stress, and antioxidant defense under salinity stress. Sci. Rep. 2023, 13, 8249. [Google Scholar] [CrossRef] [PubMed]
- Diao, F.; Dang, Z.; Xu, J.; Ding, S.; Hao, B.; Zhang, Z.; Zhang, J.; Wang, L.; Guo, W. Effect of arbuscular mycorrhizal symbiosis on ion homeostasis and salt tolerance-related gene expression in halophyte Suaeda salsa under salt treatments. Microbiol. Res. 2021, 245, 126688. [Google Scholar] [CrossRef]
- Diagne, N.; Ndour, M.; Djighaly, P.I.; Ngom, D.; Ngom, M.C.N.; Ndong, G.; Svistoonoff, S.; Cherif-Silini, H. Effect of plant growth promoting rhizobacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) on salt stress tolerance of Casuarina obesa (Miq.). Front. Sustain. Food Syst. 2020, 4, 601004. [Google Scholar] [CrossRef]
- Evelin, H.; Giri, B.; Kapoor, R. Ultrastructural evidence for AMF mediated salt stress mitigation in Trigonella foenum-graecum. Mycorrhiza 2013, 23, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Al-Karaki, G.N.; Hammad, R. Mycorrhizal influence on fruit yield and mineral content of tomato grown under salt stress. J. Plant Nutr. 2001, 24, 1311–1323. [Google Scholar] [CrossRef]
- Balliu, A.; Sallaku, G.; Rewald, B. AMF inoculation enhances growth and improves the nutrient uptake rates of transplanted, salt-stressed tomato seedlings. Sustainability 2015, 7, 15967–15981. [Google Scholar] [CrossRef]
- Parihar, M.; Rakshit, A.; Rana, K.; Tiwari, G.; Jatav, S.S. The effect of arbuscular mycorrhizal fungi inoculation in mitigating salt stress of pea (Pisum sativum L.). Commun. Soil Sci. Plant Anal. 2020, 51, 1545–1559. [Google Scholar] [CrossRef]
- Janah, I.; Meddich, A.; Elhasnaoui, A.; Khayat, S.; Anli, M.; Boutasknit, A.; Aissam, S.; Loutfi, K. Arbuscular mycorrhizal fungi mitigates salt stress toxicity in Stevia rebaudiana Bertoni through the modulation of physiological and biochemical responses. J. Soil Sci. Plant Nutr. 2023, 23, 152–162. [Google Scholar] [CrossRef]
- Hashem, A.; Alqarawi, A.A.; Radhakrishnan, R.; Al-Arjani, A.-B.F.; Aldehaish, H.A.; Egamberdieva, D.; Abd_Allah, E.F. Arbuscular mycorrhizal fungi regulate the oxidative system, hormones and ionic equilibrium to trigger salt stress tolerance in Cucumis sativus L. Saudi J. Biol. Sci. 2018, 25, 1102–1114. [Google Scholar] [CrossRef]
- Peng, Z.; Zulfiqar, T.; Yang, H.; Wang, M.; Zhang, F. Effect of arbuscular mycorrhizal fungi (AMF) on photosynthetic characteristics of cotton seedlings under saline-alkali stress. Sci. Rep. 2024, 14, 8633. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, X.; Zhukova, A.; Tang, Z.; Weng, Y.; Li, Z.; Yang, Y. Arbuscular mycorrhizal fungi (AMF) species and abundance exhibit different effects on saline-alkaline tolerance in Leymus chinensis. J. Plant Interact. 2020, 15, 266–279. [Google Scholar] [CrossRef]
- Farghaly, F.A.; Nafady, N.A.; Abdel-Wahab, D.A. The efficiency of arbuscular mycorrhiza in increasing tolerance of Triticum aestivum L. to alkaline stress. BMC Plant Biol. 2022, 22, 490. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Gong, X.; Zhang, X.; Zhang, W.; Sun, J.; Chen, B. Effects of arbuscular mycorrhizal fungi on photosynthesis, ion balance of tomato plants under saline-alkali soil condition. J. Plant Nutr. 2020, 43, 682–698. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, J.; Yang, F.; Tao, S.; Yan, X.; Zhou, Z.; Zhang, Y. Arbuscular mycorrhizal fungi improve the growth and performance of the seedlings of Leymus chinensis under alkali and drought stresses. PeerJ 2022, 10, e12890. [Google Scholar] [CrossRef]
- Liu, H.; Tang, H.; Ni, X.; Zhang, Y.; Wang, Y. Interactive effects of Epichloë endophytes and arbuscular mycorrhizal fungi on saline-alkali stress tolerance in tall fescue. Front. Microbiol. 2022, 13, 855890. [Google Scholar] [CrossRef]
- Zheng, X.; Li, A.; Nie, R.; Wu, C.; Ji, X.; Tang, J.; Zhang, J. Differential strategies of two arbuscular mycorrhizal fungi varieties in the protection of Lycium ruthenicum under saline–alkaline stress. J. Fungi 2024, 10, 554. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, W.; Wang, Y.; Zhang, L.; Huang, S.; Lin, J. Metabolomics analysis reveals the alkali tolerance mechanism in Puccinellia tenuiflora plants inoculated with arbuscular mycorrhizal fungi. Microorganisms 2020, 8, 327. [Google Scholar] [CrossRef]
- Bastow, R.; Mylne, J.S.; Lister, C.; Lippman, Z.; Martienssen, R.A.; Dean, C. Vernalization requires epigenetic silencing of FLC by histone methylation. Nature 2004, 427, 164–167. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Yang, S. Molecular regulation of plant responses to environmental temperatures. Mol. Plant 2020, 13, 544–564. [Google Scholar] [CrossRef]
- Devi, T.S.; Gupta, S.; Kapoor, R. Arbuscular mycorrhizal fungi in alleviation of cold stress in plants. In Advancing Frontiers in Mycology & Mycotechnology: Basic and Applied Aspects of Fungi; Springer: Berlin/Heidelberg, Germany, 2019; pp. 435–455. [Google Scholar] [CrossRef]
- Ma, J.; Janoušková, M.; Li, Y.; Yu, X.; Yan, Y.; Zou, Z.; He, C. Impact of arbuscular mycorrhizal fungi (AMF) on cucumber growth and phosphorus uptake under cold stress. Funct. Plant Biol. 2015, 42, 1158–1167. [Google Scholar] [CrossRef]
- Ma, J.; Janoušková, M.; Ye, L.; Bai, L.; Dong, R.; Yan, Y.; Yu, X.; Zou, Z.; Li, Y.; He, C. Role of arbuscular mycorrhiza in alleviating the effect of cold on the photosynthesis of cucumber seedlings. Photosynthetica 2019, 57, 86–95. [Google Scholar] [CrossRef]
- Pasbani, B.; Salimi, A.; Aliasgharzad, N.; Hajiboland, R. Colonization with arbuscular mycorrhizal fungi mitigates cold stress through improvement of antioxidant defense and accumulation of protecting molecules in eggplants. Sci. Hortic. 2020, 272, 109575. [Google Scholar] [CrossRef]
- Chu, X.T.; Fu, J.J.; Sun, Y.F.; Xu, Y.M.; Miao, Y.J.; Xu, Y.F.; Hu, T.M. Effect of arbuscular mycorrhizal fungi inoculation on cold stress-induced oxidative damage in leaves of Elymus nutans Griseb. South Afr. J. Bot. 2016, 104, 21–29. [Google Scholar] [CrossRef]
- Reva, M.; Cano, C.; Herrera, M.-A.; Bago, A. Arbuscular mycorrhizal inoculation enhances endurance to severe heat stress in three horticultural crops. HortScience 2021, 56, 396–406. [Google Scholar] [CrossRef]
- Cabral, C.; Ravnskov, S.; Tringovska, I.; Wollenweber, B. Arbuscular mycorrhizal fungi modify nutrient allocation and composition in wheat (Triticum aestivum L.) subjected to heat-stress. Plant Soil 2016, 408, 385–399. [Google Scholar] [CrossRef]
- Maya, M.A.; Matsubara, Y.-i. Influence of arbuscular mycorrhiza on the growth and antioxidative activity in cyclamen under heat stress. Mycorrhiza 2013, 23, 381–390. [Google Scholar] [CrossRef]
- Wei, H.; He, W.; Kuang, Y.; Wang, Z.; Wang, Y.; Hu, W.; Tang, M.; Chen, H. Arbuscular mycorrhizal symbiosis and melatonin synergistically suppress heat-induced leaf senescence involves in abscisic acid, gibberellin, and cytokinin-mediated pathways in perennial ryegrass. Environ. Exp. Bot. 2023, 213, 105436. [Google Scholar] [CrossRef]
- Yeasmin, R.; Bonser, S.P.; Motoki, S.; Nishihara, E. Arbuscular mycorrhiza influences growth and nutrient uptake of asparagus (Asparagus officinalis L.) under heat stress. HortScience 2019, 54, 846–850. [Google Scholar] [CrossRef]
- Duc, N.H.; Csintalan, Z.; Posta, K. Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants. Plant Physiol. Biochem. 2018, 132, 297–307. [Google Scholar] [CrossRef]
- Shirani Bidabadi, S.; Mehralian, M. Arbuscular mycorrhizal fungi inoculation to enhance chilling stress tolerance of watermelon. Gesunde Pflanz. 2020, 72, 171–179. [Google Scholar] [CrossRef]
- Umar, A.; Mwaheb, M.A.; Ameen, F.; Almomani, F.; Dufossé, L.; Gancarz, M. Role of ectomycorrhizal colonization in enhancement of nutrients for survival of plants collected from mountainous cold stress areas. BMC Microbiol. 2024, 24, 304. [Google Scholar] [CrossRef]
- Li, W.; Wu, H.; Hua, J.; Zhu, C.; Guo, S. Arbuscular mycorrhizal fungi enhanced resistance to low-temperature weak-light stress in snapdragon (Antirrhinum majus L.) through physiological and transcriptomic responses. Front. Plant Sci. 2024, 15, 1330032. [Google Scholar] [CrossRef]
- Ye, D.; Zhou, X.; Liu, X.; Wang, W.; Bian, J.; He, Z. Application of AMF alleviates growth and physiological characteristics of Impatiens walleriana under sub-low temperature. Horticulturae 2024, 10, 856. [Google Scholar] [CrossRef]
- Ndeko, A.B.; Founoune-Mboup, H.; Kane, A.; Cournac, L. Arbuscular mycorrhizal fungi alleviate the negative effect of temperature stress in millet lines with contrasting soil aggregation potential. Gesunde Pflanz. 2022, 74, 53–67. [Google Scholar] [CrossRef]
- Caradonia, F.; Francia, E.; Morcia, C.; Ghizzoni, R.; Moulin, L.; Terzi, V.; Ronga, D. Arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria avoid processing tomato leaf damage during chilling stress. Agronomy 2019, 9, 299. [Google Scholar] [CrossRef]
- Mathur, S.; Sharma, M.P.; Jajoo, A. Improved photosynthetic efficacy of maize (Zea mays) plants with arbuscular mycorrhizal fungi (AMF) under high temperature stress. J. Photochem. Photobiol. B Biol. 2018, 180, 149–154. [Google Scholar] [CrossRef]
- Mathur, S.; Jajoo, A. Arbuscular mycorrhizal fungi protect maize plants from high temperature stress by regulating photosystem II heterogeneity. Ind. Crops. Prod. 2020, 143, 111934. [Google Scholar] [CrossRef]
- Liu, X.-R.; Rong, Z.-Y.; Tian, X.; Hashem, A.; Abd_Allah, E.F.; Zou, Y.-N.; Wu, Q.-S. Mycorrhizal fungal effects on plant growth, osmolytes, and CsHsp70s and CsPIPs expression in leaves of cucumber under a short-term heat stress. Plants 2023, 12, 2917. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Ma, T.; Guo, S.; Liu, R.; Li, M. Leaf anatomy, photosynthesis and chlorophyll fluorescence of lettuce as influenced by arbuscular mycorrhizal fungi under high temperature stress. Sci. Hortic. 2021, 280, 109933. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Farooq, M.; Hussain, S.; Maqsood, M.; Hussain, M.; Ishfaq, M.; Ahmad, M.; Anjum, M.Z. Lead toxicity in plants: Impacts and remediation. J. Environ. Manag. 2019, 250, 109557. [Google Scholar] [CrossRef]
- Li, X.-L.; George, E.; Marschner, H. Extension of the phosphorus depletion zone in VA-mycorrhizal white clover in a calcareous soil. Plant Soil 1991, 136, 41–48. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Fang, Y.; Wang, Q.; Cao, H.; Yang, G.; Deng, L.; Wang, Y.; Zhou, Y.; Anastopoulos, I. Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: A critical review. J. Hazard. Mater. 2021, 402, 123919. [Google Scholar] [CrossRef]
- Gao, X.; Guo, H.; Zhang, Q.; Guo, H.; Zhang, L.; Zhang, C.; Gou, Z.; Liu, Y.; Wei, J.; Chen, A. Arbuscular mycorrhizal fungi (AMF) enhanced the growth, yield, fiber quality and phosphorus regulation in upland cotton (Gossypium hirsutum L.). Sci. Rep. 2020, 10, 2084. [Google Scholar] [CrossRef]
- Ferrol, N.; Tamayo, E.; Vargas, P. The heavy metal paradox in arbuscular mycorrhizas: From mechanisms to biotechnological applications. J. Exp. Bot. 2016, 67, 6253–6265. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, B.; Xu, J.; Li, Z.; Tang, Z.; Wu, X. Heavy metal domestication enhances beneficial effects of arbuscular mycorrhizal fungi on lead (Pb) phytoremediation efficiency of Bidens parviflora through improving plant growth and root Pb accumulation. Environ. Sci. Pollut. Res. 2022, 29, 32988–33001. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Z.; Ren, Y.; Jiang, Z.; Chen, H.; Hu, W.; Tang, M. The alleviation mechanisms of cadmium toxicity in Broussonetia papyrifera by arbuscular mycorrhizal symbiosis varied with different levels of cadmium stress. J. Hazard. Mater. 2023, 459, 132076. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Y.; Ghosh, A.; Song, Y.; Chen, H.; Tang, M. Assessment of arbuscular mycorrhizal fungi status and heavy metal accumulation characteristics of tree species in a lead–zinc mine area: Potential applications for phytoremediation. Environ. Sci. Pollut. Res. 2015, 22, 13179–13193. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, H.; Song, Y.; Yang, Y.; Chen, H.; Tang, M. Subcellular compartmentalization and chemical forms of lead participate in lead tolerance of Robinia pseudoacacia L. with Funneliformis mosseae. Front. Plant Sci. 2017, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Meharg, A.A. The mechanistic basis of interactions between mycorrhizal associations and toxic metal cations. Mycol. Res. 2003, 107, 1253–1265. [Google Scholar] [CrossRef]
- Pawlowska, T.E.; Charvat, I. Influence of edaphic and environmental factors on arbuscular mycorrhizae. In Arbuscular Mycorrhizae: Interactions in Plants, Rhizosphere and Soils; Science Publishers, Inc.: Enfield, UK, 2002; pp. 105–134. [Google Scholar]
- de Andrade, S.A.; da Silveira, A.P. Mycorrhiza influence on maize development under Cd stress and P supply. Braz. J. Plant Physiol. 2008, 20, 39–50. [Google Scholar] [CrossRef]
- Joner, E.J.; Briones, R.; Leyval, C. Metal-binding capacity of arbuscular mycorrhizal mycelium. Plant Soil 2000, 226, 227–234. [Google Scholar] [CrossRef]
- Bi, Y.; Li, X.; Christie, P. Influence of early stages of arbuscular mycorrhiza on uptake of zinc and phosphorus by red clover from a low-phosphorus soil amended with zinc and phosphorus. Chemosphere 2003, 50, 831–837. [Google Scholar] [CrossRef]
- Christie, P.; Li, X.; Chen, B. Arbuscular mycorrhiza can depress translocation of zinc to shoots of host plants in soils moderately polluted with zinc. Plant Soil 2004, 261, 209–217. [Google Scholar] [CrossRef]
- Zhu, J.-K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Gonzalez-Chavez, M.; Carrillo-Gonzalez, R.; Wright, S.; Nichols, K. The role of glomalin, a protein produced by arbuscular mycorrhizal fungi, in sequestering potentially toxic elements. Environ. Pollut. 2004, 130, 317–323. [Google Scholar] [CrossRef]
- Khan, A.G. Mycorrhizoremediation—An enhanced form of phytoremediation. J. Zhejiang Univ. Sci. B 2006, 7, 503–514. [Google Scholar] [CrossRef]
- Al-Garni, S.M.S. Increased heavy metal tolerance of cowpea plants by dual inoculation of an arbuscular mycorrhizal fungi and nitrogen-fixer Rhizobium bacterium. Afr. J. Biotechnol. 2006, 5, 133–142. Available online: https://tspace.library.utoronto.ca/bitstream/1807/6651/1/jb06020.pdf (accessed on 13 November 2024).
- Tonin, C.; Vandenkoornhuyse, P.; Joner, E.; Straczek, J.; Leyval, C. Assessment of arbuscular mycorrhizal fungi diversity in the rhizosphere of Viola calaminaria and effect of these fungi on heavy metal uptake by clover. Mycorrhiza 2001, 10, 161–168. [Google Scholar] [CrossRef]
- Liao, J.; Lin, X.; Cao, Z.; Shi, Y.; Wong, M.H. Interactions between arbuscular mycorrhizae and heavy metals under sand culture experiment. Chemosphere 2003, 50, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, L.; Richards, A.; Rimmer, D. Effects of mycorrhizal colonisation on Thymus polytrichus from heavy-metal-contaminated sites in northern England. Mycorrhiza 2004, 14, 47–54. [Google Scholar] [CrossRef]
- Citterio, S.; Prato, N.; Fumagalli, P.; Aina, R.; Massa, N.; Santagostino, A.; Sgorbati, S.; Berta, G. The arbuscular mycorrhizal fungus Glomus mosseae induces growth and metal accumulation changes in Cannabis sativa L. Chemosphere 2005, 59, 21–29. [Google Scholar] [CrossRef]
- Weissenhorn, I.; Leyval, C.; Belgy, G.; Berthelin, J. Arbuscular mycorrhizal contribution to heavy metal uptake by maize (Zea mays L.) in pot culture with contaminated soil. Mycorrhiza 1995, 5, 245–251. [Google Scholar] [CrossRef]
- Chen, B.; Li, X.; Tao, H.; Christie, P.; Wong, M.H. The role of arbuscular mycorrhiza in zinc uptake by red clover growing in a calcareous soil spiked with various quantities of zinc. Chemosphere 2003, 50, 839–846. [Google Scholar] [CrossRef]
- Trotta, A.; Falaschi, P.; Cornara, L.; Minganti, V.; Fusconi, A.; Drava, G.; Berta, G. Arbuscular mycorrhizae increase the arsenic translocation factor in the As hyperaccumulating fern Pteris vittata L. Chemosphere 2006, 65, 74–81. [Google Scholar] [CrossRef]
- Wu, Z.; McGrouther, K.; Huang, J.; Wu, P.; Wu, W.; Wang, H. Decomposition and the contribution of glomalin-related soil protein (GRSP) in heavy metal sequestration: Field experiment. Soil Biol. Biochem. 2014, 68, 283–290. [Google Scholar] [CrossRef]
- Vodnik, D.; Grčman, H.; Maček, I.; Van Elteren, J.; Kovačevič, M. The contribution of glomalin-related soil protein to Pb and Zn sequestration in polluted soil. Sci. Total Environ. 2008, 392, 130–136. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Sun, Y.; Wu, Z.; Li, T.; Hu, Y.; Su, D.; Lv, J.; Li, G.; Zhang, Z. Transformation and immobilization of chromium by arbuscular mycorrhizal fungi as revealed by SEM–EDS, TEM–EDS, and XAFS. Environ. Sci. Technol. 2015, 49, 14036–14047. [Google Scholar] [CrossRef]
- Feng, Z.; Ren, H.; Song, H.; Zou, Y.; Vosatka, M.; Huang, S.; Lu, H.; Cheng, F. Arbuscular mycorrhizal fungi induced changes of Pb migration and bacterial community in both the rhizosphere and non-rhizosphere soils of Paspalum notatum. Water Air Soil Pollut. 2023, 234, 156. [Google Scholar] [CrossRef]
- Dueck, T.A.; Visser, P.; Ernst, W.; Schat, H. Vesicular-arbuscular mycorrhizae decrease zinc-toxicity to grasses growing in zinc-polluted soil. Soil Biol. Biochem. 1986, 18, 331–333. [Google Scholar] [CrossRef]
- Turnau, K.; Kottke, I.; Oberwinkler, F. Element localization in mycorrhizal roots of Pteridium aquilinum(L.) Kuhn collected from experimental plots treated with cadmium dust. New Phytol. 1993, 123, 313–324. [Google Scholar] [CrossRef]
- González-Guerrero, M.; Benabdellah, K.; Valderas, A.; Azcón-Aguilar, C.; Ferrol, N. GintABC1 encodes a putative ABC transporter of the MRP subfamily induced by Cu, Cd, and oxidative stress in Glomus intraradices. Mycorrhiza 2010, 20, 137–146. [Google Scholar] [CrossRef] [PubMed]
- González-Guerrero, M.; Cano, C.; Azcón-Aguilar, C.; Ferrol, N. GintMT1 encodes a functional metallothionein in Glomus intraradices that responds to oxidative stress. Mycorrhiza 2007, 17, 327–335. [Google Scholar] [CrossRef]
- Li, X.; Christie, P. Changes in soil solution Zn and pH and uptake of Zn by arbuscular mycorrhizal red clover in Zn-contaminated soil. Chemosphere 2001, 42, 201–207. [Google Scholar] [CrossRef]
- Pauwels, R.; Jansa, J.; Püschel, D.; Müller, A.; Graefe, J.; Kolb, S.; Bitterlich, M. Root growth and presence of Rhizophagus irregularis distinctly alter substrate hydraulic properties in a model system with Medicago truncatula. Plant Soil 2020, 457, 131–151. [Google Scholar] [CrossRef]
- Hildebrandt, U.; Regvar, M.; Bothe, H. Arbuscular mycorrhiza and heavy metal tolerance. Phytochemistry 2007, 68, 139–146. [Google Scholar] [CrossRef]
- Zhan, F.; Li, B.; Jiang, M.; Yue, X.; He, Y.; Xia, Y.; Wang, Y. Arbuscular mycorrhizal fungi enhance antioxidant defense in the leaves and the retention of heavy metals in the roots of maize. Environ. Sci. Pollut. Res. 2018, 25, 24338–24347. [Google Scholar] [CrossRef]
- Aloui, A.; Recorbet, G.; Gollotte, A.; Robert, F.; Valot, B.; Gianinazzi-Pearson, V.; Aschi-Smiti, S.; Dumas-Gaudot, E. On the mechanisms of cadmium stress alleviation in Medicago truncatula by arbuscular mycorrhizal symbiosis: A root proteomic study. Proteomics 2009, 9, 420–433. [Google Scholar] [CrossRef]
- Park, J.; Song, W.Y.; Ko, D.; Eom, Y.; Hansen, T.H.; Schiller, M.; Lee, T.G.; Martinoia, E.; Lee, Y. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J. 2012, 69, 278–288. [Google Scholar] [CrossRef]
- Cailliatte, R.; Lapeyre, B.; Briat, J.-F.; Mari, S.; Curie, C. The NRAMP6 metal transporter contributes to cadmium toxicity. Biochem. J. 2009, 422, 217–228. [Google Scholar] [CrossRef]
- Stommel, M.; Mann, P.; Franken, P. EST-library construction using spore RNA of the arbuscular mycorrhizal fungus Gigaspora rosea. Mycorrhiza 2001, 10, 281–285. [Google Scholar] [CrossRef]
- Pan, J.; Cao, S.; Xu, G.; Rehman, M.; Li, X.; Luo, D.; Wang, C.; Fang, W.; Xiao, H.; Liao, C.; et al. Comprehensive analysis reveals the underlying mechanism of arbuscular mycorrhizal fungi in kenaf cadmium stress alleviation. Chemosphere 2023, 314, 137566. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Wu, L.; Luo, N.; Mo, C.H.; Wong, M.H.; Li, H. Arbuscular mycorrhizal fungi and the associated bacterial community influence the uptake of cadmium in rice. Geoderma 2019, 337, 749–757. [Google Scholar] [CrossRef]
- Ef, A.; Abeer, H.; Alqarawi, A.; Hend, A.A. Alleviation of adverse impact of cadmium stress in sunflower (Helianthus annuus L.) by arbuscular mycorrhizal fungi. Pak. J. Bot. 2015, 47, 785–795. [Google Scholar]
- Zhang, F.; Liu, M.; Li, Y.; Che, Y.; Xiao, Y. Effects of arbuscular mycorrhizal fungi, biochar and cadmium on the yield and element uptake of Medicago sativa. Sci. Total Environ. 2019, 655, 1150–1158. [Google Scholar] [CrossRef]
- Kozlowski, T. Responses of woody plants to flooding. In Flooding Plant Growth; Academic Press: London, UK, 1984; pp. 129–163. [Google Scholar]
- Tanaka, K.; Masumori, M.; Yamanoshita, T.; Tange, T. Morphological and anatomical changes of Melaleuca cajuputi under submergence. Trees 2011, 25, 695–704. [Google Scholar] [CrossRef]
- Cannell, M.G.R. Growth Control in Woody Plants; Academic Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Elzenga, J.T.M.; van Veen, H. Waterlogging and plant nutrient uptake. In Waterlogging Signalling and Tolerance in Plants; Mancuso, S., Shabala, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 23–35. [Google Scholar] [CrossRef]
- Ashraf, M.A. Waterlogging stress in plants: A review. Afr. J. Agric. Res. 2012, 7, 1976–1981. [Google Scholar] [CrossRef]
- Yin, D.; Zhang, Z.; Luo, H. Anatomical responses to waterlogging in Chrysanthemum zawadskii. Sci. Hortic. 2012, 146, 86–91. [Google Scholar] [CrossRef]
- Smith, J.E. Mycorrhizal Symbiosis (Third Edition). Soil Sci. Soc. Am. J. 2009, 73, 694. Available online: https://books.google.com.tw/books?hl=zh-CN&lr=&id=qLciOJaG0C4C&oi=fnd&pg=PP1&dq=Smith,+J.E.+Mycorrhizal+Symbiosis+(Third+Edition).+Soil+Science+Society+of+America+Journal+2009,+73,+694-694.&ots=zsuRjSVDsK&sig=b5VTl4L2WbMnBqUfoGXv_U39M7M&redir_esc=y#v=onepage&q&f=false (accessed on 13 November 2024).
- Wang, B.; Qiu, Y.L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef]
- Clayton, J.S.; Bagyaraj, D.J. Vesicular-arbuscular mycorrhizas in submerged aquatic plants of New Zealand. Aquat. Bot. 1984, 19, 251–262. [Google Scholar] [CrossRef]
- Tanner, C.C.; Clayton, J.S. Vesicular arbuscular mycorrhiza studies with a submerged aquatic plant. Trans. Br. Mycol. Soc. 1985, 85, 683–688. [Google Scholar] [CrossRef]
- Beck-Nielsen, D.; Vindbæk Madsen, T. Occurrence of vesicular–arbuscular mycorrhiza in aquatic macrophytes from lakes and streams. Aquat. Bot. 2001, 71, 141–148. [Google Scholar] [CrossRef]
- Šraj-Kržič, N.; Pongrac, P.; Klemenc, M.; Kladnik, A.; Regvar, M.; Gaberščik, A. Mycorrhizal colonisation in plants from intermittent aquatic habitats. Aquat. Bot. 2006, 85, 331–336. [Google Scholar] [CrossRef]
- de Marins, J.F.; Carrenho, R.; Thomaz, S.M. Occurrence and coexistence of arbuscular mycorrhizal fungi and dark septate fungi in aquatic macrophytes in a tropical river–floodplain system. Aquat. Bot. 2009, 91, 13–19. [Google Scholar] [CrossRef]
- Wolfe, B.E.; Mummey, D.L.; Rillig, M.C.; Klironomos, J.N. Small-scale spatial heterogeneity of arbuscular mycorrhizal fungal abundance and community composition in a wetland plant community. Mycorrhiza 2007, 17, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Tuheteru, F.D.; Wu, Q.-S. Arbuscular mycorrhizal fungi and tolerance of waterlogging stress in plants. In Arbuscular Mycorrhizas and Stress Tolerance of Plants; Wu, Q.-S., Ed.; Springer: Singapore, 2017; pp. 43–66. [Google Scholar] [CrossRef]
- Graham, L.L.B.; Turjaman, M.; Page, S.E. Shorea balangeran and Dyera polyphylla (syn. Dyera lowii) as tropical peat swamp forest restoration transplant species: Effects of mycorrhizae and level of disturbance. Wetl. Ecol. Manag. 2013, 21, 307–321. [Google Scholar] [CrossRef]
- Miller, S.P.; Sharitz, R.R. Manipulation of flooding and arbuscular mycorrhiza formation influences growth and nutrition of two semiaquatic grass species. Funct. Ecol. 2000, 14, 738–748. [Google Scholar] [CrossRef]
- Ipsilantis, I.; Sylvia, D.M. Interactions of assemblages of mycorrhizal fungi with two Florida wetland plants. Appl. Soil Ecol. 2007, 35, 261–271. [Google Scholar] [CrossRef]
- Dunham, R.M.; Ray, A.M.; Inouye, R.S. Growth, physiology, and chemistry of mycorrhizal and nonmycorrhizal Typha latifolia seedlings. Wetlands 2003, 23, 890–896. [Google Scholar] [CrossRef]
- Rutto, K.L.; Mizutani, F.; Kadoya, K. Effect of root-zone flooding on mycorrhizal and non-mycorrhizal peach (Prunus persica Batsch) seedlings. Sci. Hortic. 2002, 94, 285–295. [Google Scholar] [CrossRef]
- Cecilia, J.; Bagyaraj, D.J. Selection of efficient vesicular-arbuscular mycorrhizal fungi for wetland rice—A preliminary screen. Mycorrhiza 1994, 4, 265–268. [Google Scholar] [CrossRef]
- Solaiman, M.Z.; Hirata, H. Effectiveness of arbuscular mycorrhizal colonization at nursery-stage on growth and nutrition in wetland rice (Oryza sativa L.) after transplanting under different soil fertility and water regimes. Soil Sci. Plant Nutr. 1996, 42, 561–571. [Google Scholar] [CrossRef]
- Neto, D.; Carvalho, L.M.; Cruz, C.; Martins-Loução, M.A. How do mycorrhizas affect C and N relationships in flooded Aster tripolium plants? Plant Soil 2006, 279, 51–63. [Google Scholar] [CrossRef]
- Tuo, X.-Q.; Li, S.; Wu, Q.-S.; Zou, Y.-N. Alleviation of waterlogged stress in peach seedlings inoculated with Funneliformis mosseae: Changes in chlorophyll and proline metabolism. Sci. Hortic. 2015, 197, 130–134. [Google Scholar] [CrossRef]
- Osundina, M.A. Nodulation and growth of mycorrhizal Casuarina equisetifolia J.R. and G. First in response to flooding. Biol. Fertil. Soils 1997, 26, 95–99. [Google Scholar] [CrossRef]
- Zou, Y.N.; Srivastava, A.K.; Wu, Q.S.; Huang, Y.M. Increased tolerance of trifoliate orange (Poncirus trifoliata) seedlings to waterlogging after inoculation with arbuscular mycorrhizal fungi. J. Anim. Plant Sci. 2014, 24, 1415–1420. Available online: https://www.researchgate.net/publication/287789094_Increased_tolerance_of_trifoliate_orange_Poncirus_Trifoliata_seedlings_to_waterlogging_after_inoculation_with_arbuscular_mycorrhizal_fungi (accessed on 13 November 2024).
- Wu, Q.-S.; Zou, Y.-N.; Huang, Y.-M. The arbuscular mycorrhizal fungus Diversispora spurca ameliorates effects of waterlogging on growth, root system architecture and antioxidant enzyme activities of citrus seedlings. Fungal Ecol. 2013, 6, 37–43. [Google Scholar] [CrossRef]
- Rozema, J.; Arp, W.; Van Diggelen, J.; Van Esbroek, M.; Broekman, R.; Punte, H. Occurrence and ecological significance of vesicular arbuscular mycorrhiza in the salt marsh environment. Acta Bot. Neerl. 1986, 35, 457–467. [Google Scholar] [CrossRef]
- Calvo-Polanco, M.; Molina, S.; Zamarreño, A.M.; García-Mina, J.M.; Aroca, R. The symbiosis with the arbuscular mycorrhizal fungus Rhizophagus irregularis drives root water transport in flooded tomato plants. Plant Cell Physiol. 2014, 55, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Stevens, K.J.; Wall, C.B.; Janssen, J.A. Effects of arbuscular mycorrhizal fungi on seedling growth and development of two wetland plants, Bidens frondosa L., and Eclipta prostrata (L.) L., grown under three levels of water availability. Mycorrhiza 2011, 21, 279–288. [Google Scholar] [CrossRef]
- Cooke, J.C.; Lefor, M.W. Comparison of vesicular-arbuscular mycorrhizae in plants from disturbed and adjacent undisturbed regions of a coastal salt marsh in Clinton, Connecticut, USA. Environ. Manag. 1990, 14, 131–137. [Google Scholar] [CrossRef]
- Sengupta, A.; Chaudhuri, S. Vesicular arbuscular mycorrhiza (VAM) in pioneer salt marsh plants of the Ganges river delta in West Bengal (India). Plant Soil 1990, 122, 111–113. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Q.; Koide, R.T.; Peng, Z.; Zhou, J.; Gu, X.; Gao, W.; Yu, M. Arbuscular mycorrhizal fungal mediation of plant-plant interactions in a marshland plant community. Sci. World J. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fougnies, L.; Renciot, S.; Muller, F.; Plenchette, C.; Prin, Y.; de Faria, S.M.; Bouvet, J.M.; Sylla, S.N.; Dreyfus, B.; Bâ, A.M. Arbuscular mycorrhizal colonization and nodulation improve flooding tolerance in Pterocarpus officinalis Jacq. seedlings. Mycorrhiza 2007, 17, 159–166. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Lozowicka, B. Biochemical compounds and stress markers in lettuce upon exposure to pathogenic Botrytis cinerea and fungicides inhibiting oxidative phosphorylation. Planta 2022, 255, 61. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Łuniewski, S.; Kaczyński, P.; Łozowicka, B. The influence of humic acids and nitrophenols on metabolic compounds and pesticide behavior in wheat under biotic stress. Agronomy 2023, 13, 1378. [Google Scholar] [CrossRef]
- Abarca, C.; Fernandez Bidondo, L.; Bompadre, J.; Velázquez, M.S. Arbuscular mycorrhizal fungi in tomato tolerance to pathogens and nematodes: A comprehensive review. Sci. Hortic. 2024, 329, 112969. [Google Scholar] [CrossRef]
- Wang, W.; Shi, J.; Xie, Q.; Jiang, Y.; Yu, N.; Wang, E. Nutrient exchange and regulation in arbuscular mycorrhizal symbiosis. Mol. Plant 2017, 10, 1147–1158. [Google Scholar] [CrossRef]
- Villani, A.; Tommasi, F.; Paciolla, C. The arbuscular mycorrhizal fungus Glomus viscosum improves the tolerance to verticillium wilt in artichoke by modulating the antioxidant defense systems. Cells 2021, 10, 1944. [Google Scholar] [CrossRef]
- Lin, P.; Zhang, M.; Wang, M.; Li, Y.; Liu, J.; Chen, Y. Inoculation with arbuscular mycorrhizal fungus modulates defense-related genes expression in banana seedlings susceptible to wilt disease. Plant Signal. Behav. 2021, 16, 1884782. [Google Scholar] [CrossRef]
- Thygesen, K.; Larsen, J.; Bødker, L. Arbuscular mycorrhizal fungi reduce development of pea root-rot caused by Aphanomyces euteiches using oospores as pathogen inoculum. Eur. J. Plant Pathol. 2004, 110, 411–419. [Google Scholar] [CrossRef]
- Allen, M.F. Mycorrhizal fungi: Highways for water and nutrients in arid soils. Vadose Zone J. 2007, 6, 291–297. [Google Scholar] [CrossRef]
- Campo, S.; Martín-Cardoso, H.; Olivé, M.; Pla, E.; Catala-Forner, M.; Martínez-Eixarch, M.; San Segundo, B. Effect of root colonization by arbuscular mycorrhizal fungi on growth, productivity and blast resistance in rice. Rice 2020, 13, 42. [Google Scholar] [CrossRef]
- Nanjundappa, A.; Bagyaraj, D.J.; Saxena, A.K.; Kumar, M.; Chakdar, H. Interaction between arbuscular mycorrhizal fungi and Bacillus spp. in soil enhancing growth of crop plants. Fungal Biol. Biotechnol. 2019, 6, 23. [Google Scholar] [CrossRef]
- Sharma, M.; Saini, I.; Kaushik, P.; Aldawsari, M.M.; Balawi, T.A.; Alam, P. Mycorrhizal fungi and Pseudomonas fluorescens application reduces root-knot nematode (Meloidogyne javanica) infestation in eggplant. Saudi J. Biol. Sci. 2021, 28, 3685–3691. [Google Scholar] [CrossRef]
- Abad, P.; Favery, B.; Rosso, M.-N.; Castagnone-Sereno, P. Root-knot nematode parasitism and host response: Molecular basis of a sophisticated interaction. Mol. Plant Pathol. 2003, 4, 217–224. [Google Scholar] [CrossRef]
- Li, Y.-D.; Ding, T.-T.; Duan, T.-Y. Effect of AM fungi on alfalfa responses to aphid stress. Acta Prataculturae Sin. 2020, 29, 155. [Google Scholar] [CrossRef]
- Jin, Z.B.; Xie, L.; Wang, Y.S.; Kong, Y.; Liu, F.; Zhu, Z.J. Cultivation of tomato mycorrhizal seedlings in different substrates and their resistance to southern root-knot nematode. Mycosystema 2021, 40, 121–128. [Google Scholar] [CrossRef]
- Vos, C.; Claerhout, S.; Mkandawire, R.; Panis, B.; De Waele, D.; Elsen, A. Arbuscular mycorrhizal fungi reduce root-knot nematode penetration through altered root exudation of their host. Plant Soil 2012, 354, 335–345. [Google Scholar] [CrossRef]
- Vallejos-Torres, G.; Espinoza, E.; Marín-Díaz, J.; Solis, R.; Arévalo, L.A. The role of arbuscular mycorrhizal fungi against root-knot nematode infections in coffee plants. J. Soil Sci. Plant Nutr. 2021, 21, 364–373. [Google Scholar] [CrossRef]
- Bernardo, V.F.; Garita, S.A.; Arango, M.C.; Ripodas, J.I.; Saparrat, M.C.N.; Ruscitti, M.F. Arbuscular mycorrhizal fungi against the false root-knot nematode activity in Capsicum annuum: Physiological responses in plants. Biocontrol Sci. Technol. 2021, 31, 119–131. [Google Scholar] [CrossRef]
- Selvaraj, T.; Padmanabhan, C.; Jeong, Y.-J.; Kim, H. Occurrence of vesicular-arbuscular mycorrhizal (VAM) fungi and their effect on plant growth in endangered vegetations. J. Microbiol. Biotechnol. 2004, 14, 885–890. Available online: https://koreascience.kr/article/JAKO200411922627015.page (accessed on 13 November 2024).
- Lingua, G.; D'Agostino, G.; Massa, N.; Antosiano, M.; Berta, G. Mycorrhiza-induced differential response to a yellows disease in tomato. Mycorrhiza 2002, 12, 191–198. [Google Scholar] [CrossRef]
- Tahiri-Alaoui, A.; Antoniw, J. Cloning of genes associated with the colonization of tomato roots by the arbuscular mycorrhizal fungus Glomus mosseae. Agronomie 1996, 16, 699–707. Available online: https://hal.science/hal-00885769v1 (accessed on 13 November 2024). [CrossRef]
- Maurya, A.K.; Kelly, M.P.; Mahaney, S.M.; Gomez, S.K. Arbuscular mycorrhizal symbiosis alters plant gene expression and aphid weight in a tripartite interaction. J. Plant Interact. 2018, 13, 294–305. [Google Scholar] [CrossRef]
- Du, E.; Chen, Y.; Li, Y.; Zhang, F.; Sun, Z.; Hao, R.; Gui, F. Effect of arbuscular mycorrhizal fungi on the responses of Ageratina adenophora to Aphis gossypii herbivory. Front. Plant Sci. 2022, 13, 1015947. [Google Scholar] [CrossRef]
- Stallmann, J.; Schweiger, R. Effects of arbuscular mycorrhiza on primary metabolites in phloem exudates of Plantago major and Poa annua and on a generalist aphid. Int. J. Mol. Sci. 2021, 22, 13086. [Google Scholar] [CrossRef]
- Lehmann, A.; Rillig, M.C. Arbuscular mycorrhizal contribution to copper, manganese and iron nutrient concentrations in crops–A meta-analysis. Soil Biol. Biochem. 2015, 81, 147–158. [Google Scholar] [CrossRef]
- Karagiannidis, N.; Hadjisavva-Zinoviadi, S. The mycorrhizal fungus Glomus mosseae enhances growth, yield and chemical composition of a durum wheat variety in 10 different soils. Nutr. Cycl. Agroecosyst. 1998, 52, 1–7. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Chaoxing, H. Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci. Hortic. 2011, 127, 228–233. [Google Scholar] [CrossRef]
- Cely, M.V.T.; de Oliveira, A.G.; de Freitas, V.F.; de Luca, M.B.; Barazetti, A.R.; dos Santos, I.M.O.; Gionco, B.; Garcia, G.V.; Prete, C.E.C.; Andrade, G. Inoculant of arbuscular mycorrhizal fungi (Rhizophagus clarus) increase yield of soybean and cotton under field conditions. Front. Microbiol. 2016, 7, 720. [Google Scholar] [CrossRef]
- Daei, G.; Ardekani, M.R.; Rejali, F.; Teimuri, S.; Miransari, M. Alleviation of salinity stress on wheat yield, yield components, and nutrient uptake using arbuscular mycorrhizal fungi under field conditions. J. Plant Physiol. 2009, 166, 617–625. [Google Scholar] [CrossRef]
- Li, H.; Ye, Z.H.; Chan, W.F.; Chen, X.W.; Wu, F.Y.; Wu, S.C.; Wong, M.H. Can arbuscular mycorrhizal fungi improve grain yield, As uptake and tolerance of rice grown under aerobic conditions? Environ. Pollut. 2011, 159, 2537–2545. [Google Scholar] [CrossRef]
- Habibzadeh, Y.; Pirzad, A.; Zardashti, M.R.; Jalilian, J.; Eini, O. Effects of arbuscular mycorrhizal fungi on seed and protein yield under water-deficit stress in mung bean. Agron. J. 2013, 105, 79–84. [Google Scholar] [CrossRef]
- Kakabouki, I.; Stavropoulos, P.; Roussis, I.; Mavroeidis, A.; Bilalis, D. Contribution of arbuscular mycorrhizal fungi (AMF) in improving the growth and yield performances of flax (Linum usitatissimum L.) to salinity stress. Agronomy 2023, 13, 2416. [Google Scholar] [CrossRef]
- Erman, M.; Demir, S.; Ocak, E.; Tüfenkçi, Ş.; Oğuz, F.; Akköprü, A. Effects of Rhizobium, arbuscular mycorrhiza and whey applications on some properties in chickpea (Cicer arietinum L.) under irrigated and rainfed conditions 1—Yield, yield components, nodulation and AMF colonization. Field Crops Res. 2011, 122, 14–24. [Google Scholar] [CrossRef]
- Yilmaz, A.; Karik, Ü. AMF and PGPR enhance yield and secondary metabolite profile of basil (Ocimum basilicum L.). Ind. Crops Prod. 2022, 176, 114327. [Google Scholar] [CrossRef]
- Cozzolino, V.; Di Meo, V.; Piccolo, A. Impact of arbuscular mycorrhizal fungi applications on maize production and soil phosphorus availability. J. Geochem. Explor. 2013, 129, 40–44. [Google Scholar] [CrossRef]
- Séry, D.J.-M.; Kouadjo, Z.G.C.; Voko, B.R.R.; Zézé, A. Selecting native arbuscular mycorrhizal fungi to promote cassava growth and increase yield under field conditions. Front. Microbiol. 2016, 7, 2063. [Google Scholar] [CrossRef]
- Navarro, J.M.; Morte, A. Arbuscular mycorrhizal fungi as biofertilizers to increase the plant quality of sour-orange seedlings. Agronomy 2024, 14, 230. [Google Scholar] [CrossRef]
- Li, M.-Y.; Wang, W.; Mo, F.; Ren, A.-T.; Wang, Z.-Y.; Zhu, Y.; Xiong, Y.-C. Seven-year long-term inoculation with Funneliformis mosseae increases maize yield and soil carbon storage evidenced by in situ 13C-labeling in a dryland. Sci. Total Environ. 2024, 944, 173975. [Google Scholar] [CrossRef]
- Addisu, E. Arbuscular mycorrhizal fungi (AMF) in optimizing nutrient bioavailability and reducing agrochemicals for maintaining sustainable agroecosystems. In Arbuscular Mycorrhizal Fungi in Agriculture; Rodrigo Nogueira de, S., Ed.; IntechOpen: Rijeka, Croatia, 2022; p. Ch.9. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, P.; Cao, F.; Liu, X.; Ji, M.; Xie, M. Effects of AMF on maize yield and soil microbial community in sandy and saline soils. Plants 2024, 13, 2056. [Google Scholar] [CrossRef]
- Duan, H.-X.; Luo, C.-L.; Li, J.-Y.; Wang, B.-Z.; Naseer, M.; Xiong, Y.-C. Improvement of wheat productivity and soil quality by arbuscular mycorrhizal fungi is density- and moisture-dependent. Agron. Sustain. Dev. 2021, 41, 3. [Google Scholar] [CrossRef]
- Bhantana, P.; Rana, M.S.; Sun, X.-C.; Moussa, M.G.; Saleem, M.H.; Syaifudin, M.; Shah, A.; Poudel, A.; Pun, A.B.; Bhat, M.A.; et al. Arbuscular mycorrhizal fungi and its major role in plant growth, zinc nutrition, phosphorous regulation and phytoremediation. Symbiosis 2021, 84, 19–37. [Google Scholar] [CrossRef]
- Douds, D.D.; Pfeffer, P.E.; Shachar-Hill, Y. Carbon partitioning, cost, and metabolism of arbuscular mycorrhizas. In Arbuscular Mycorrhizas: Physiology and Function; Kapulnik, Y., Douds, D.D., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 107–129. Available online: https://link.springer.com/chapter/10.1007/978-94-017-0776-3_6 (accessed on 13 November 2024).
- Harris, D.; Pacovsky, R.; Paul, E. Carbon economy of soybean–Rhizobium–Glomus associations. New Phytol. 1985, 101, 427–440. [Google Scholar] [CrossRef]
- Wang, G.; Coleman, D.; Freckman, D.; Dyer, M.; McNaughton, S.; Agra, M.; Goeschl, J. Carbon partitioning patterns of mycorrhizal versus non-mycorrhizal plants: Real-time dynamic measurements using 11CO2. New Phytol. 1989, 112, 489–493. [Google Scholar] [CrossRef]
- Bhandari, K.B.; Acosta-Martínez, V.; Pérez-Guzmán, L.; West, C.P. Soil health within transitions from irrigation to limited irrigation and dryland management. Agric. Environ. Lett. 2022, 7, e20077. [Google Scholar] [CrossRef]
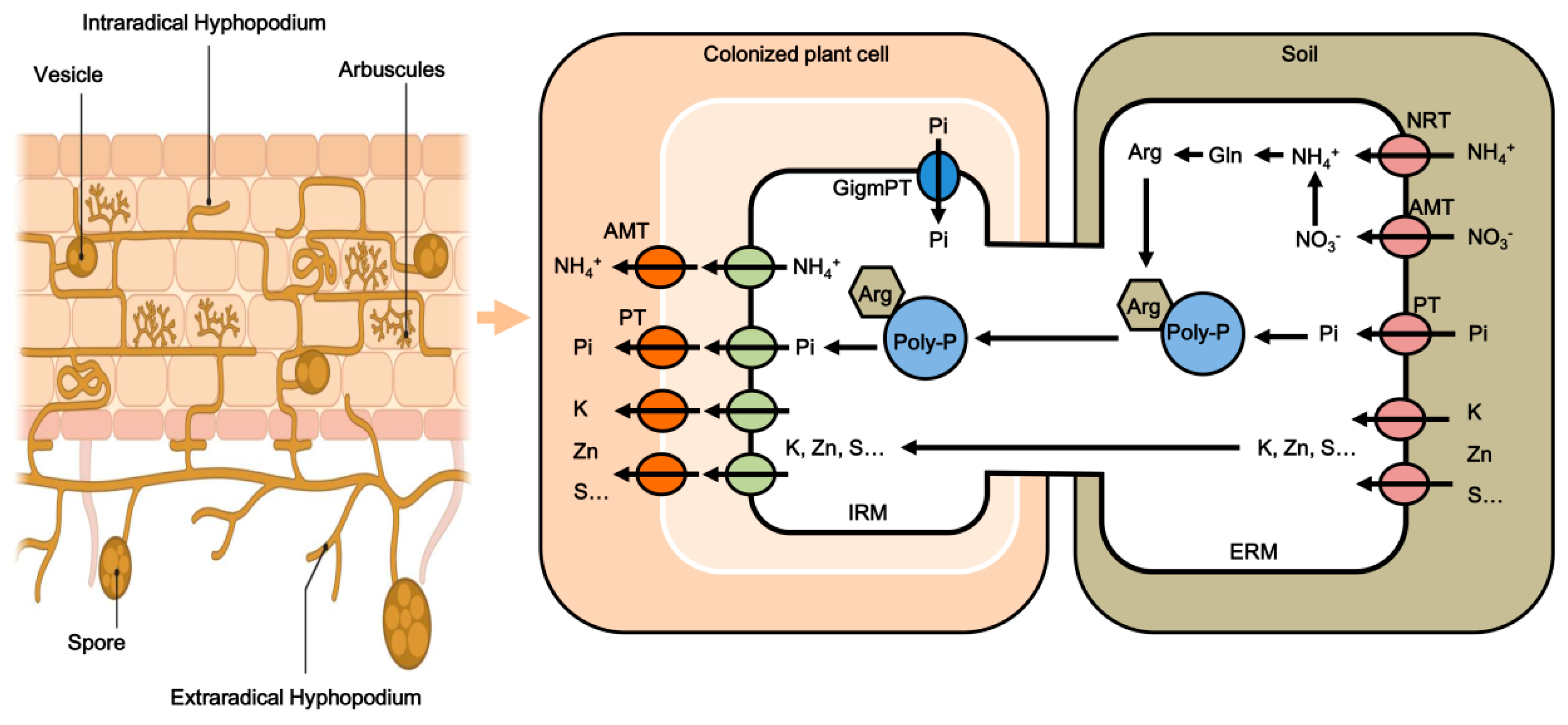

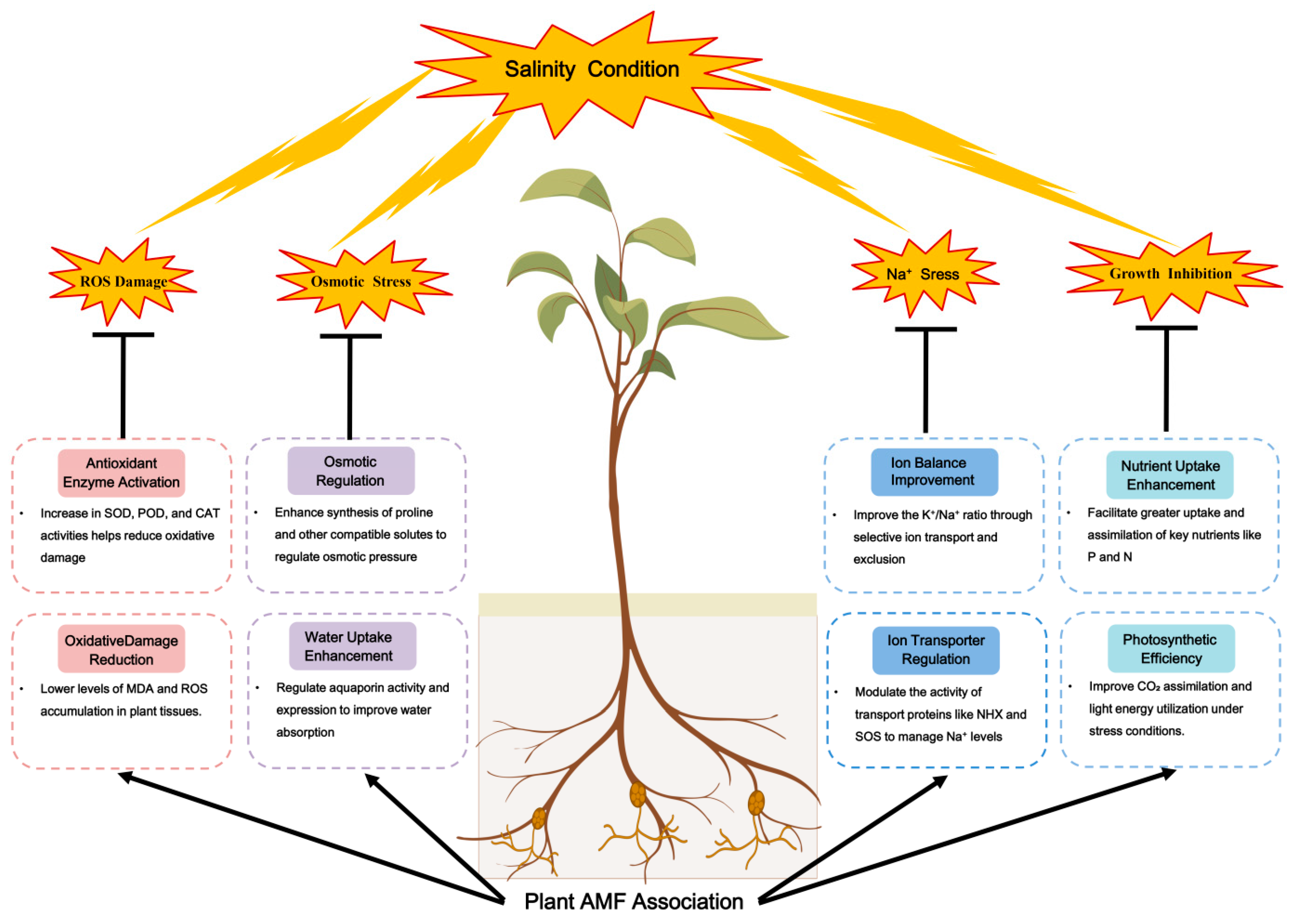
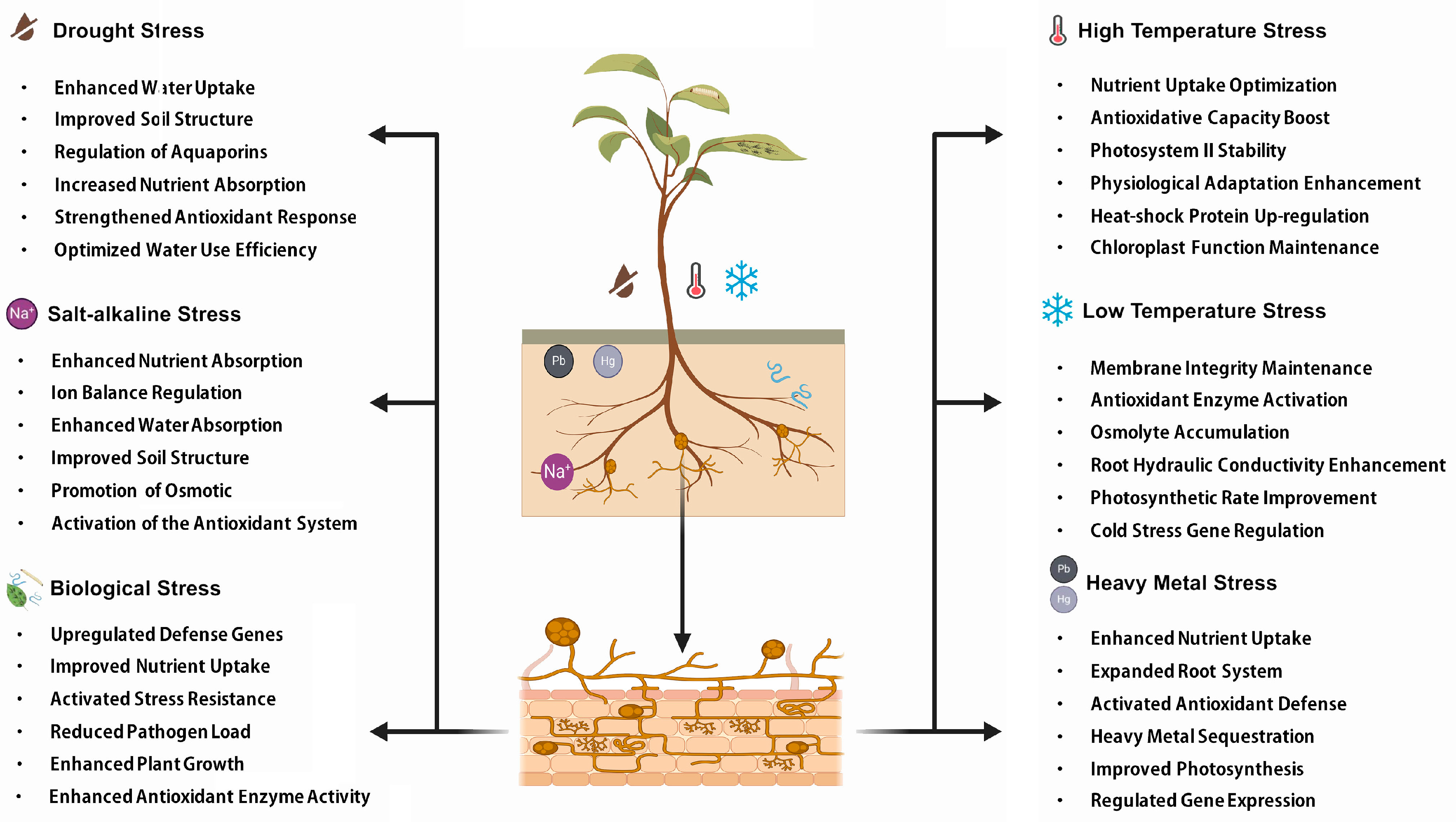
| Host Plants | AM Fungi Strains | Drought Stress Condition | Responses Related to AM Fungi Inoculation | References |
|---|---|---|---|---|
| Triticum aestivum; | Funneliformis mosseae, F. geosporum | Natural drought condition, with relative soil water content at 22% of control. | Increased relative water content (RWC) in leaves and soil, mitigated structural and functional damage to PSII and PSI under drought stress, and enhanced photochemical efficiency. | [83] |
| Zenia insignis | Funneliformis mosseae, Rhizoglomus intraradices, sispora versiformis | Drought stress treatment involved irrigation every 7–10 days to maintain soil moisture at 35–45% of field capacity over 4 months. | Enhanced plant biomass and antioxidant capacity, plant biomass, P uptake, and osmolytes (like soluble sugars and proline). | [84] |
| Ephedra foliata Boiss | Claroideoglomus etunicatum, Rhizophagus intraradices, Funneliformis mosseae | Drought stress induced by regulating Jensen’s nutrient solution supply, withholding water. | Increased plant biomass and antioxidant capacity, enhanced N metabolism, P uptake, osmolytes (like proline and glucose), improved nutrient absorption (K, Mg, Ca), higher phytohormone levels (IAA, IBA, GA, ABA), and enhanced P metabolism. | [85] |
| Zea mays L. | Glomus versiforme | Natural drought condition. | Enhanced plant biomass and antioxidant capacity, improved chlorophyll and carotenoid content, increased mineral uptake and assimilation, up-regulation of the antioxidant system, and elevated levels of compatible solutes (like proline, sugars, and free amino acids) under moderate and severe drought conditions. | [86] |
| Soybean (Glycine max L.) | Septoglomus constrictum, Glomus sp. and Glomus aggregatum | Soil allowed to dry to 7% volumetric moisture over 7 days, with daily water resupply; mycorrhizal plants harvested 60 days post-planting. | Enhanced plant biomass and antioxidant capacity, improved water content and nutrient concentrations (P and N), and maintained levels of osmotic metabolites (like soluble sugars and proline) under drought stress. | [87] |
| Catalpa bungei C.A.Mey. | Rhizophagus intraradices | moderate drought (50%), and severe drought (30%) of field capacity. | Enhanced plant growth and antioxidant capacity, increased photosynthetic efficiency and nutrient absorption (N, P), improved soil structure (GRSP, macro-aggregates). Reduced root/shoot ratio and modulated hormone levels (higher IAA, GA3; lower ABA, ZT), alleviating oxidative stress under drought conditions. | [88] |
| Spinacia oleracea L. | Commercial inoculum (Clonex® Root Maximizer) | Drought stress (DS) maintained at 30% of field capacity; no drought stress (NDS) at normal field capacity levels. | Enhanced growth parameters (shoot and root weight, length), increased photosynthetic activity (higher chlorophyll content, photosynthetic rate, stomatal conductance), and improved nutrient content (N, P, K). | [89] |
| Oryza sativa L. | Funneliformis mosseae, F. geosporus, Claroideoglomus claroideum, Glomus microaggregatum, Rhizophagus irregularis | Initial 42 days well-watered; drought initiated at 42 days with cycles of drying and rewetting, reaching soil water potential down to −80 kPa; recovery phase post −90 DAP. | Improved nutrient uptake (especially P), increased stomatal conductance and chlorophyll fluorescence, and modulated hormone levels (higher IAA, varied ABA) under drought stress. Reduced grain yield loss and maintained shoot and root biomass. | [90] |
| Host Plants | AM Fungi Strains | Responses Related to AM Fungi Inoculation | References |
|---|---|---|---|
| Casuarina obesa (Miq.) | Rhizophagus fasciculatus, Rhizophagus aggregatum | Enhanced survival rate, plant height, and biomass; increased chlorophyll and proline accumulation. | [133] |
| Trigonella foenum-graecum L. | Glomus intraradices | Reduced cellular and ultrastructural damage under salt stress, lower lipid peroxidation and electrolyte leakage, increased osmolytes (glycinebetaine, sugars), polyamines, and α-tocopherol enhancing ionic balance and stress tolerance. | [134] |
| Tomato (Lycopersicon esculentum Mill.) ‘Pello’ (salt-tolerant) and ‘Marriha’ (salt-sensitive) | Glomus mosseae | Enhanced fruit yield, shoot dry matter, and mineral content (P, K, Zn, Cu, Fe), while reducing Na+ concentration in tomatoes, leading to greater salt stress tolerance and higher root colonization. | [135] |
| Tomato (Solanum lycopersicum L.) | Glomus sp. mixture | Enhanced nutrient uptake and root system dry matter, maintaining higher growth rates under moderate-to-severe salt stress compared to non-inoculated plants. | [136] |
| Pisum sativum L. | Funneliformis mosseae and R. intraradices | Enhanced nutrient uptake, osmolyte accumulation, and reduced electrolyte leakage, leading to improved biomass production, chlorophyll synthesis, yield, and growth in pea under salinity stress. The consortium of R. fasciculatum and Gigaspora sp. was particularly effective. | [137] |
| Stevia rebaudiana Bertoni | Rhizophagus intraradices, consortium | Notable improvements in growth, physiological responses, and antioxidant enzyme activities in Stevia rebaudiana, reducing the negative impacts of salt stress. The AM fungi consortium demonstrated greater efficacy than Rhizophagus irregularis in enhancing plant resilience to salinity. | [138] |
| Cucumis sativus L. | Claroideoglomus etunicatum, Rhizophagus intraradices, Funneliformis mosseae | Mitigated salt stress in cucumbers by enhancing biomass, pigment synthesis, and antioxidant enzyme activities; increased ascorbic acid content and accumulation of phenols and proline helped neutralize superoxide radicals, while increased levels of jasmonic acid, salicylic acid, and essential minerals were observed alongside a reduced uptake of Na+. | [139] |
| Cotton (Xinluzao 45) | Funneliformis mosseae, Rhizophagus irregularis, Claroideoglomus etunicatum | Improved photosynthesis, increased CO2 concentration, transpiration, and energy use efficiency, significantly enhancing cotton growth, plant height, and root length under saline–alkali stress. Funneliformis mosseae showed the most significant improvement in growth and photosynthetic activity. | [140] |
| Leymus chinensis | Funneliformis mosseae, Rhizophagus intraradices, Diversispora versiformis, Acaulospora scrobiculata | Improved stress tolerance by enhancing growth, nutrient absorption, ion balance, and photosynthesis, particularly with Funneliformis mosseae, Rhizophagus intraradices, and mixtures showing greater benefits under high stress. | [141] |
| Triticum aestivum L. | Acaulospora laevis, Funneliformis geosporum, Funneliformis mosseae, Cetraspora armeniaca | Mitigated alkalinity stress in wheat by improving germination, biomass, photosynthetic pigments, and nutrient uptake (K, N, P). It also reduced lipid peroxidation and enhanced the activity of stress-related enzymes like catalase and peroxidase, contributing to better overall productivity and crop yield. | [142] |
| Processing Tomato (Lycopersicon esculentum Mill.) | Mixed fungi including Glomus clarum and Glomus intraradices | Improved growth, enhanced nutrient absorption (increased N uptake and reduced Na+ uptake), and optimized physiological processes under saline–alkali stress. This led to increased concentrations of soluble solids, vitamin C, soluble sugars, and lycopene in fruits, improved ion ratios (K+/Na+, Ca2+/Na+, Mg2+/Na+) in leaves and stems, and protected photosynthetic organs. AM fungi also boosted the chlorophyll content, photosynthetic rate, stomatal conductance, and transpiration rate, while optimizing the microbial community in the rhizosphere. | [143] |
| Leymus chinensis | BGC HEB02 | Mitigated growth inhibition under combined alkali and drought stresses by enhancing osmotic adjustment, improving ionic balance, and counteracting ion toxicity and oxidative damage. | [144] |
| Lolium arundinaceum | Funneliformis mosseae, Claroideoglomus etunicatum | The interaction between Epichloë endophytes and AM fungi significantly enhanced tall fescue’s resistance to saline–alkali stress by increasing biomass, nutrient uptake (organic carbon, total N, P), and K+ accumulation, while reducing Na+ concentrations. | [145] |
| Lycium ruthenicum | Funneliformis mosseae, Rhizophagus intraradices | Significantly enhanced growth and saline–alkaline resilience in black wolfberry, improving chlorophyll b and P absorption, reducing reactive oxygen species, and increasing abscisic acid accumulation, aiding in better ion management and stress response. | [146] |
| Puccinellia tenuiflora | Rhizophagus intraradices | Increased biomass and altered metabolic responses under alkali stress, enhancing levels of amino acids, organic acids, flavonoids, sterols, and plant hormones (salicylic acid, abscisic acid), which improved osmotic adjustment, cell membrane stability, and stress resilience. | [147] |
| Host Plants | AM Fungi Strains | Extreme Temperature Stress Type | Responses Related to AM Fungi Inoculation | References |
|---|---|---|---|---|
| Cucumber (Cucumis sativus L. cv. Zhongnong No. 26) | Rhizophagus irregularis (isolate PH5) | Exposure to cold-stress conditions at 15/10 °C (day/night) for a period of 14 days | Countered the negative effects of cold stress by enhancing chlorophyll content, net photosynthetic rate, and photochemical quenching. Reduced non-photochemical quenching and moderated the increase in sugar contents, indicating improved photosynthetic efficiency and carbon sink strength. | [152] |
| Watermelon (Citrullus lanatus) cv. “Crimson Sweet” and “Charleston Gray” | Glomus intraradices | Subjected to chilling treatment in chambers maintained at 4 ± 0.5 °C for durations of 12 and 36 h | Significantly enhanced root and shoot dry mass, improved chlorophyll content and photosynthesis efficiency, and reduced oxidative stress markers such as H2O2 and MDA. Decreased electrolyte leakage and increased peroxidase activity, enhancing chilling resistance. | [161] |
| Kobresia filicina, K. myosuroides, Polygonum viviparum, Alnus nitida, Betula pendula, Pinus sylvestris, Trifolium repens | Cenococcum geophilum | Exposed to extreme cold conditions at +5 °C, −10 °C, −20 °C, −40 °C, and −50 °C, down to −125 °C. | Improved cold stress tolerance by enhancing root and shoot biomass, chlorophyll content, and photosynthetic efficiency. Significantly decreased oxidative stress markers like H2O2 and MDA, and moderated electrolyte leakage. Demonstrated exceptional resilience in K. myosuroides. | [162] |
| Snapdragon (Antirrhinum majus L.) ‘Red and White’ | Funneliformis mosseae and Glomus versiforme | Cold-stress conditions of 14/4 °C (day/night) sustained for 7 days. | Enhanced resistance to low-temperature and weak-light stress through physiological and transcriptomic responses. | [163] |
| Impatiens walleriana ‘Super Elf (Rose red)’ | Funneliformis mosseae, Glomus versiforme | Sub-low temperature treatment set at 12 °C/8 °C (day/night). | Improved plant growth and enhanced photosynthetic efficiency under sub-low temperature stress. Increased Fv′/Fm′, Y(II), and qP, while reducing NPQ, ROS (O2− and H2O2) accumulation, and cell membrane lipid peroxidation damage, indicating enhanced cold tolerance. | [164] |
| Four pearl millet lines | Rhizophagus aggregatus and Funneliformis mosseae | Heat-stress conditions of 37/32 °C (day/night) over a span of 60 days. | Improved plant growth and physiological responses under temperature stress. Increased chlorophyll concentration, root and shoot dry weight, especially under high temperature conditions, and enhanced soil aggregation. Funneliformis mosseae was more effective in promoting root colonization. | [165] |
| Processing Tomato (Genotypes: ‘Everton’, ‘Pearson’, ‘H3402’) | Funneliformis mosseae, Paraburkholderia graminis C4D1M | Chilling treatment executed at 1 °C for 24 h. | Reduced electrolytic leakage and improved efficiency of photosystem II after chilling stress. Enhanced seedling regrowth and photosystem II efficiency in a consortium with P. graminis. Specific improvement in modern genotypes under consortium treatment. | [166] |
| Zea mays | Rhizophagus intraradices, Funneliformis mosseae, and F. geosporum | High temperature stress conditions at a stable 44 °C ± 0.2 °C. | Enhanced photosynthetic activity, increased chlorophyll content, and improved overall plant growth under high temperature (44 °C). This included better quantum efficiency of PSII, higher net photosynthesis rate, and greater morphological development (leaf width, plant height, cob number). | [167] |
| Tomato (Solanum lycopersicum L.), Pepper (Capsicum annuum L.), Cucumber (Cucumis sativus L.) | Rhizophagus irregularis, commercial inoculant MYCOGEL (Agrocode Biosciences LTD, Almeria, Spain) | Severe heat stress with temperatures escalating to a peak of 45.6 °C. | Significantly improved the endurance, vigor, productivity, and fruit quality under severe heat stress by applying an ultra-pure in vitro-produced AM fungi concentrate directly to the roots at transplanting, simulating drip irrigation. | [155] |
| Maize (Zea mays L. cv. Navjot) | A mixed culture of AM fungi, primarily consisting of various Funneliformis species, was used | Recorded extreme summer conditions in May 2018, with maximum daily temperatures ranging between 43 and 44 °C in Indore (22°44′ N). | Enhanced PSII heterogeneity by facilitating the conversion of inactive β and γ centers to active α centers, and QB non-reducing centers to reducing centers, improving photosynthetic efficiency and stress resilience under high temperature stress. | [168] |
| Cucumis sativus L. | Diversispora versiformis | Heat-stressed environment characterized by 38 °C/30 °C (day/night) for a short-term (80 h) treatment. | Improved growth parameters (plant height, stem diameter, biomass), chlorophyll index, and osmolyte levels (sucrose, fructose, glucose, betaine, proline) under short-term heat stress. Up-regulated PIPs and Hsp70 gene expressions, enhancing heat tolerance. | [169] |
| Lettuce (Lactuca sativa L., cv. Shuangzi) | Funneliformis mosseae | High temperature stress condition at 35 °C. | Enhanced resilience to high temperature (35 °C) by improving chloroplast ultrastructure and photosynthetic efficiency. Increased chlorophyll a and b contents, net photosynthetic rate, and transpiration rate. Better maintenance of photosynthetic performance index and fluorescence parameters, suggesting protection against heat-induced PSII damage and improved energy fluxes. | [170] |
| Host Plants | AM Fungi Strains | Growth Condition | Responses Related to AM Fungi Inoculation | References |
|---|---|---|---|---|
| Glycine max L. and Gossypium hirsutum L. | Rhizophagus clarus | Field conditions | AM fungi inoculation increased around 20% of root colonization in both soybean and cotton; increased P and nitrogen content in plants, leading to higher yield. | [277] |
| Wheat genotypes of Roshan, Kavir and a mutated line of Tabasi | Glomus etunicatum, G. mosseae, G. intraradices | Nutrient uptake under field saline conditions | Glomus etunicatum > G. mosseae > G. intraradices. Enhanced wheat dry weight and grain yield; improved phosphorus uptake. | [278] |
| Lycopersicon esculentum L. cv. Zhongzha105 | Glomus mosseae | Laboratory simulated salt stress | AM fungi alleviated salt-induced reduction in root colonization, growth, chlorophyll content, and fruit yield of tomato plants. | [276] |
| Oryza sativa L. | G. geosporum, G. intraradices | Arsenic-contaminated soil | Significant effects on grain As concentration, grain yield, and grain P uptake; enhancement with suitable AM fungi. | [279] |
| Pterocarpus officinalis (Jacq.) | Glomus intraradices, Bradyrhizobium sp. | Flooding condition | Significant increases in yield, root colonization, and shoot phosphorus content. | [250] |
| Cicer arietinum L. | Glomus intraradices Shench&Shimith | Rain-fed conditions | Enhanced yield, root colonization, and phosphorus content in seed and shoot; effective in combined applications. | [282] |
| Ocimum basilicum L. | AM fungi (PGPR, AM fungi, and PGPR + AM fungi) | Bolu ecological conditions | Improved essential oil yield and composition; superior results compared to control in yield parameters. | [283] |
| Zea mays L. | Glomus intraradices | Field conditions | Improved productivity and growth comparable to conventional treatments; enhanced phosphorus availability. | [284] |
| Linum usitatissimum L. | Claroideoglomus etunicatum, Funneliformis mosseae, Glomus aggregatum | Irrigation water salinity | Increased chlorophyll content, nitrogen and phosphorus uptake, seed and stem fiber yield under salt stress conditions. | [281] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, W.; He, Q.; Guo, H.; Zhang, W.; Ma, L.; Li, J.; Wen, D. Arbuscular Mycorrhizal Fungi: Boosting Crop Resilience to Environmental Stresses. Microorganisms 2024, 12, 2448. https://doi.org/10.3390/microorganisms12122448
Nie W, He Q, Guo H, Zhang W, Ma L, Li J, Wen D. Arbuscular Mycorrhizal Fungi: Boosting Crop Resilience to Environmental Stresses. Microorganisms. 2024; 12(12):2448. https://doi.org/10.3390/microorganisms12122448
Chicago/Turabian StyleNie, Wenjing, Qinghai He, Hongen Guo, Wenjun Zhang, Lan Ma, Junlin Li, and Dan Wen. 2024. "Arbuscular Mycorrhizal Fungi: Boosting Crop Resilience to Environmental Stresses" Microorganisms 12, no. 12: 2448. https://doi.org/10.3390/microorganisms12122448
APA StyleNie, W., He, Q., Guo, H., Zhang, W., Ma, L., Li, J., & Wen, D. (2024). Arbuscular Mycorrhizal Fungi: Boosting Crop Resilience to Environmental Stresses. Microorganisms, 12(12), 2448. https://doi.org/10.3390/microorganisms12122448






