Pulsed Electric Field Pretreatment Enhances the Enzyme Hydrolysis of Baker’s Yeast
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strain
2.2. Pulsed Electric Field (PEF) Treatment
2.3. Determination of Irreversible Electropermeabilization
2.4. Treatment with Alcalase
2.5. Preparation of the Cell Lysate
2.6. Analytical Methods
2.7. Determination of Cell Viability and Cell Cycle of Human Keratinocyte Cell Line HaCat
2.8. Statistics
3. Results and Discussion
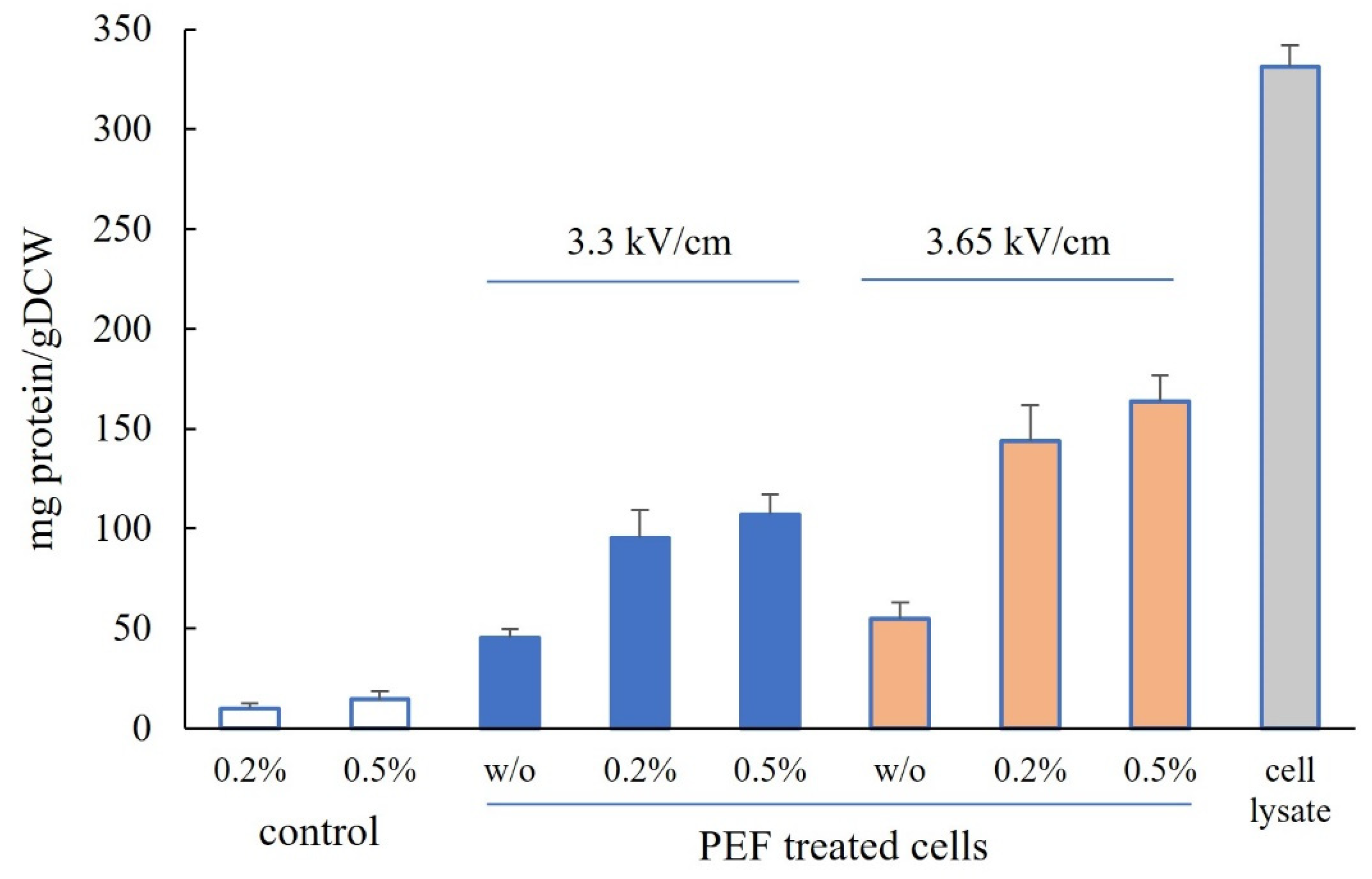
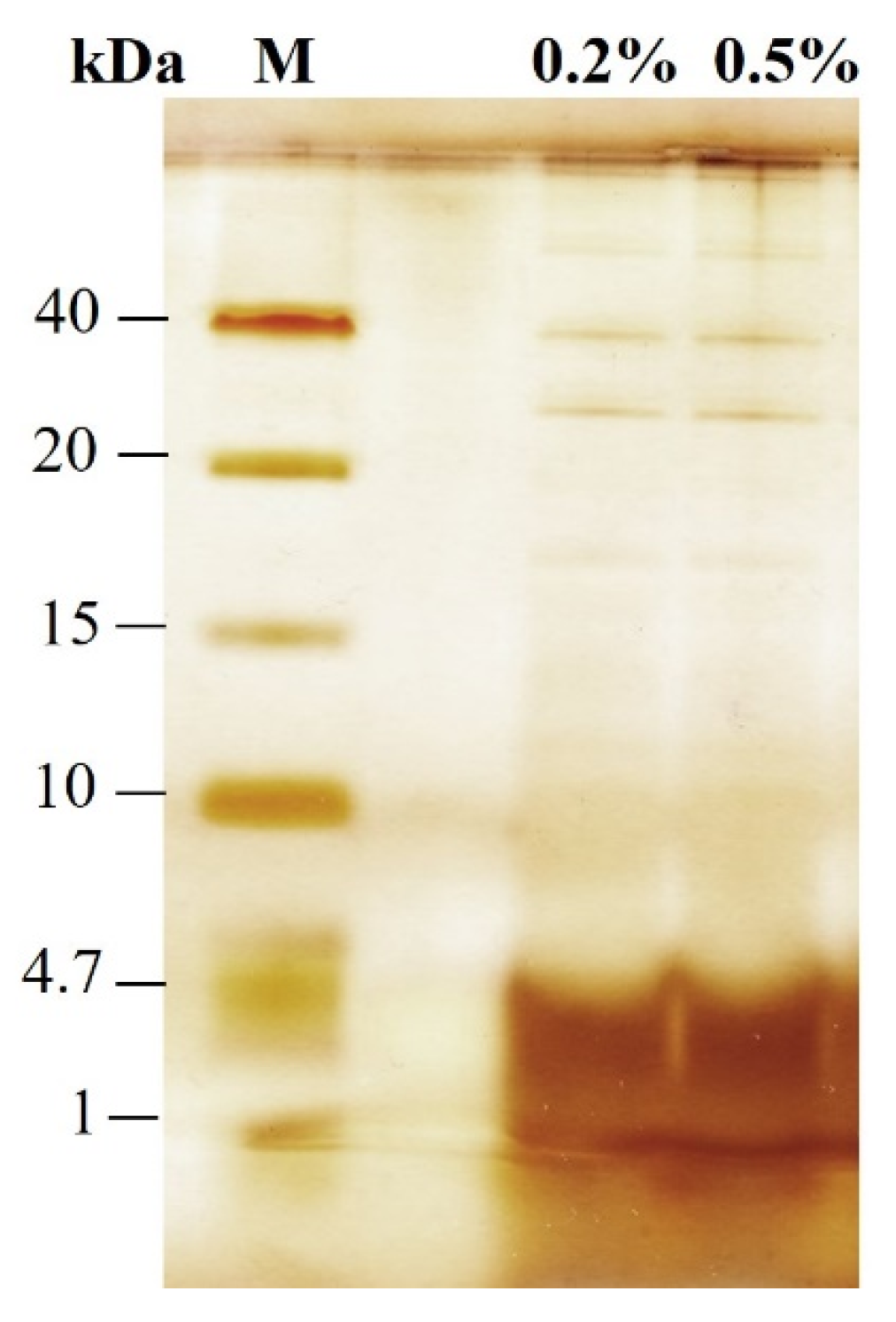
3.1. Protein Release
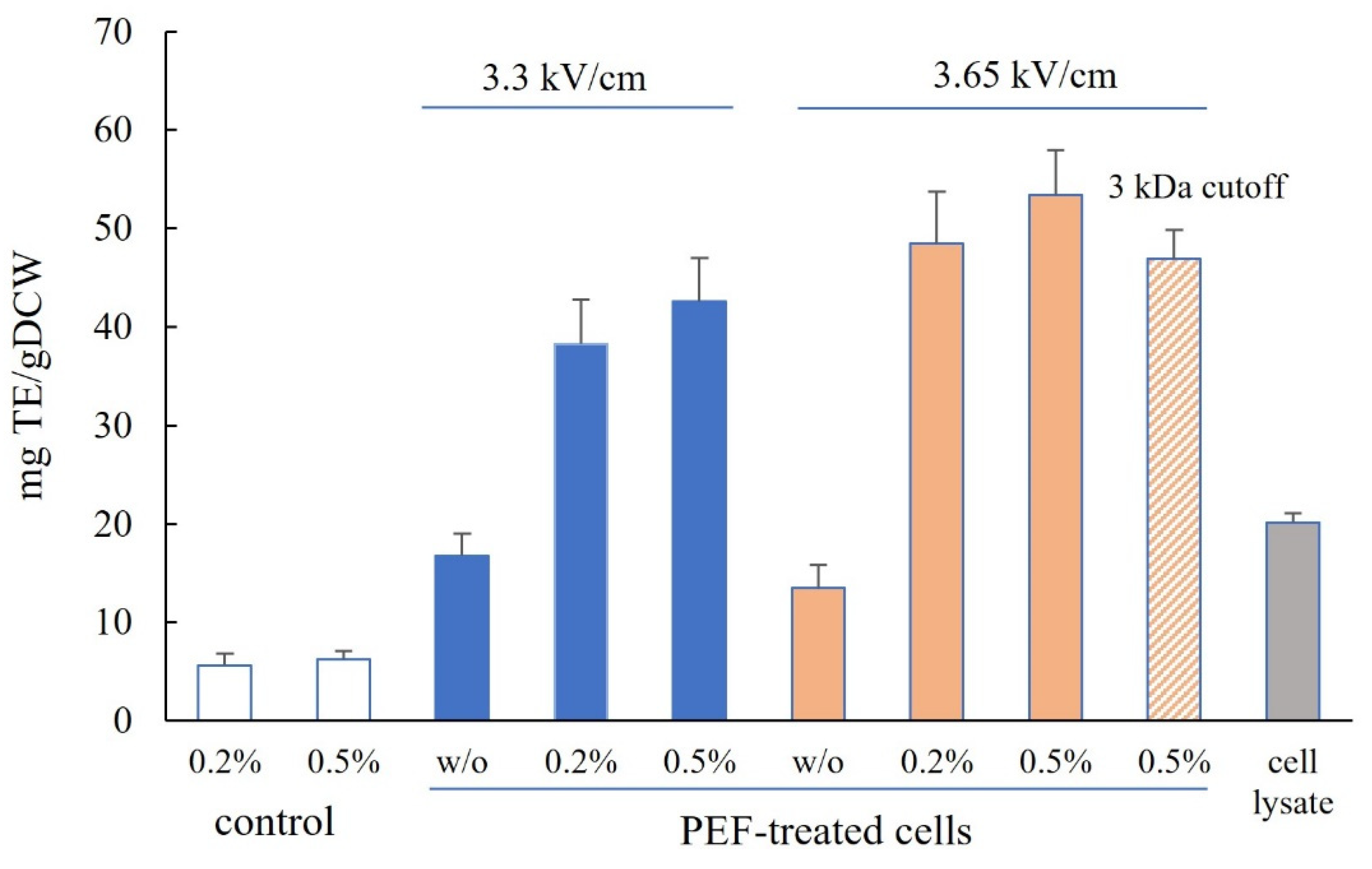
3.2. Release of Free α-Amino Nitrogen
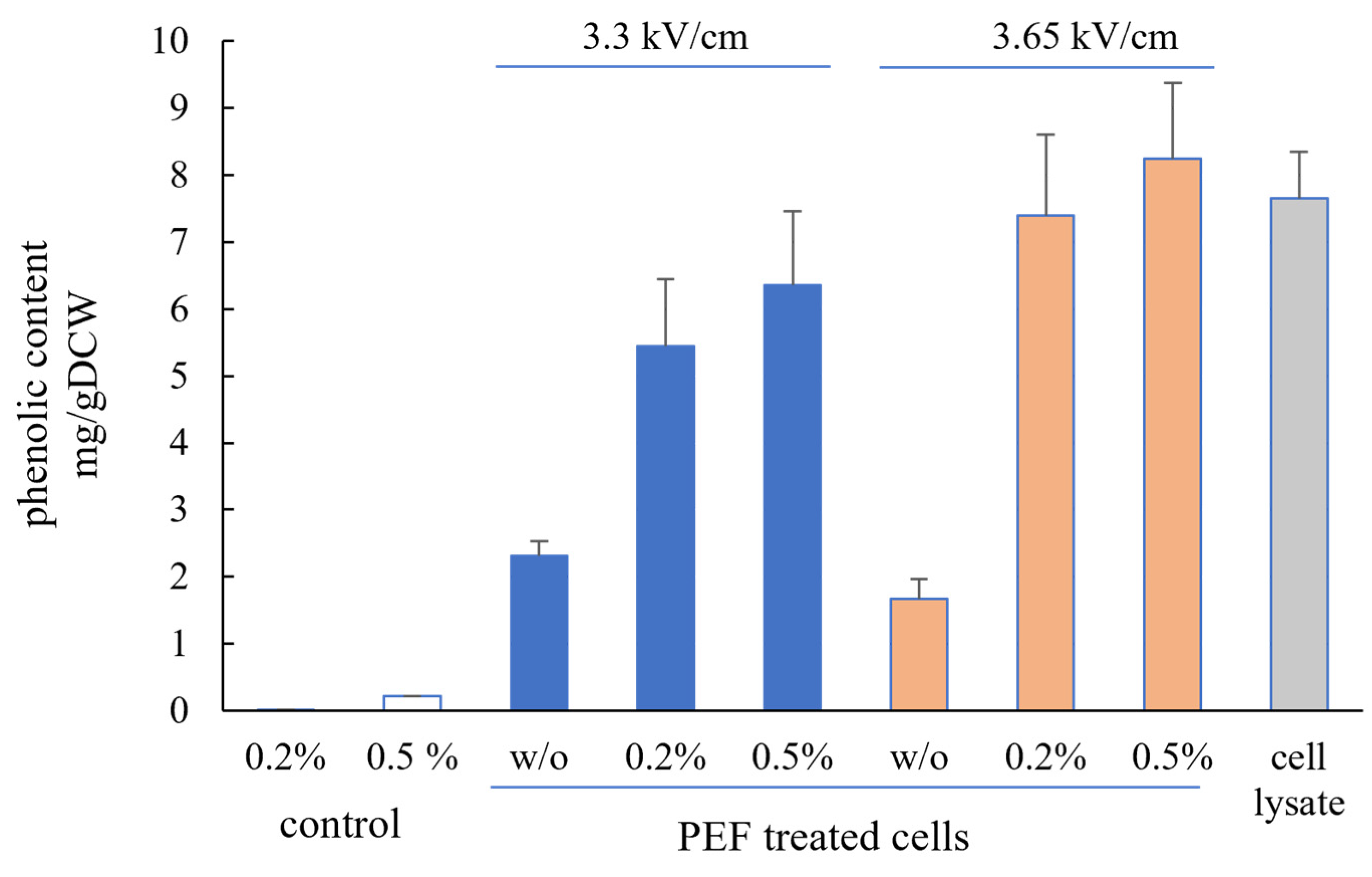
3.3. Antioxidant Activity and Phenolic Content
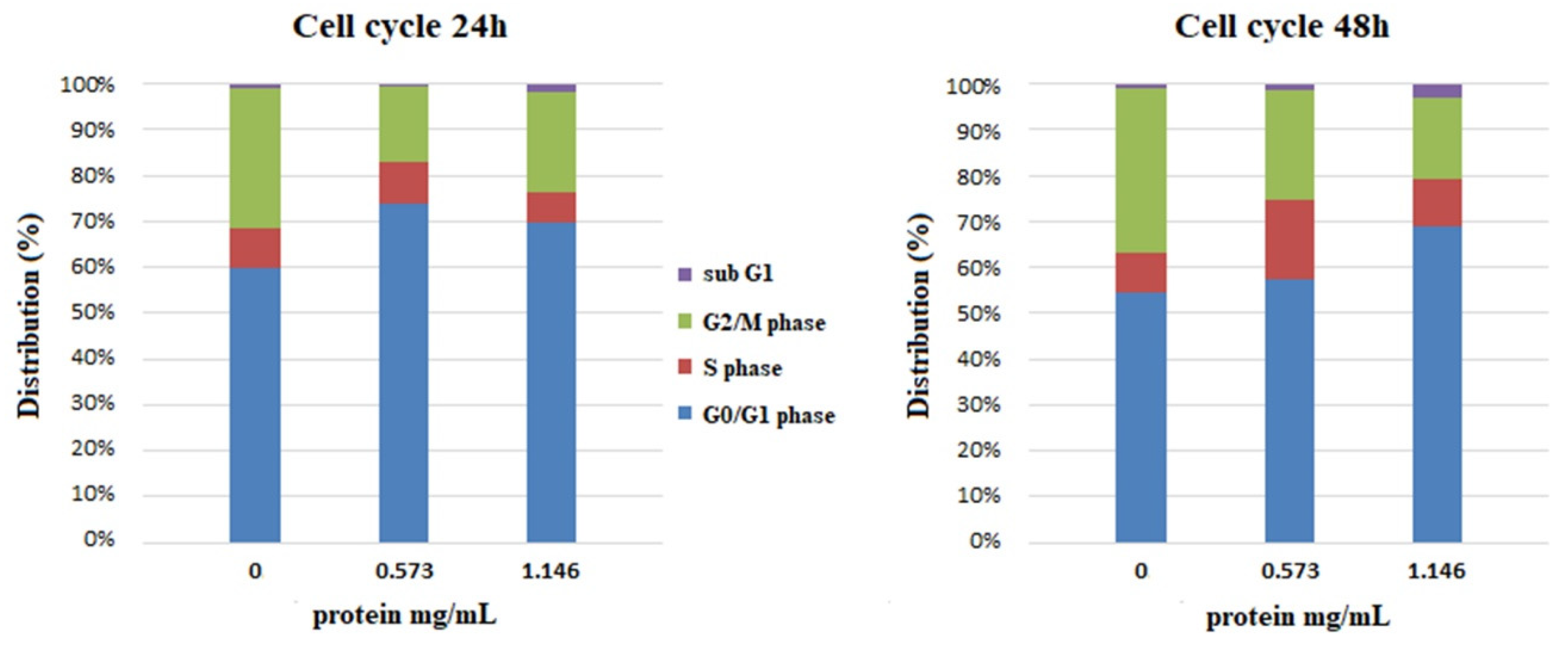
3.4. Effect of the Extracts on Cell Viability and Cell Cycle of the Human Keratinocytes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Halász, A.; Lásztity, R. Use of Yeast Biomass in Food Production; CRC Press: Boca Raton, FL, USA, 1991; pp. 127–135. [Google Scholar]
- Nandy, S.K.; Srivastava, R.K. A review on sustainable yeast biotechnological processes and applications. Microbiol. Res. 2018, 207, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Yuan, H.; Liu, M.; Liu, Q.; Zhang, S.; Liu, H.; Jiang, Y.; Huang, D.; Wang, T. Yeast Extract: Characteristics, Production, Applications and Future Perspectives. J. Microbiol. Biotechnol. 2023, 33, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Podpora, B.; Swiderski, F.; Sadowska, A.; Piotrowska, A.; Rakowska, R. Spent brewer’s yeast autolysates as a new and valuable component of functional food and dietary supplements. J. Food Process. Technol. 2015, 6, 1000526. [Google Scholar] [CrossRef]
- Takalloo, Z.; Nikkhah, M.; Nemati, R.; Jalilian, N.; Sajedi, R.H. Autolysis, plasmolysis and enzymatic hydrolysis of baker’s yeast (Saccharomyces cerevisiae): A comparative study. J. Food Process. Technol. 2020, 57, 1763–1773. [Google Scholar] [CrossRef]
- Alves, E.M.; Souza, J.F.; Oliva Neto, P. Advances in yeast autolysis technology—A faster and safer new bioprocess. Braz. J. Food Technol. 2021, 21, e2020249. [Google Scholar] [CrossRef]
- Xie, J.; Cui, C.; Ren, J.; Zhao, M.; Zhao, L.; Wang, W. High solid concentrations facilitate enzymatic hydrolysis of yeast cells. Food Bioprod. Process. 2017, 103, 114–121. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Q.; Wu, X.; Algharib, S.A.; Gong, F.; Hu, J.; Luo, W.; Zhou, M.; Pan, Y.; Yan, Y.; et al. Structure, preparation, modification, and bioactivities of β-glucan and mannan from yeast cell wall: A review. Int. J. Biol. Macromol. 2021, 173, 445–456. [Google Scholar] [CrossRef]
- Vieira, E.F.; Carvalho, J.; Pinto, E.; Cunha, S.; Almeida, A.A.; Ferreira, I.M.P.L.V.O. Nutritive value, antioxidant activity and phenolic compounds profile of brewer’s spent yeast extract. J. Food Compos. Anal. 2016, 52, 44–51. [Google Scholar] [CrossRef]
- Li, Y.; Wei, G.; Chen, J. Glutathione: A review on biotechnological production. Appl. Microbiol. Biotechnol. 2004, 66, 233–241. [Google Scholar] [CrossRef]
- Marson, G.V.; de Castro, R.J.S.; da Costa Machado, M.T.; Zandonadi, F.S.; Barros, H.D.F.Q.; Maróstica Júnior, M.R.; Sussulini, A.; Hubinger, M.D. Proteolytic enzymes positively modulated the physicochemical and antioxidant properties of spent yeast protein hydrolysates. Process Biochem. 2020, 91, 34–45. [Google Scholar] [CrossRef]
- Mirzaei, M.; Shavandi, A.; Mirdamadi, S.; Soleymanzadeh, N.; Motahari, P.; Mirdamadi, N.; Moser, M.; Subra, G.; Alimoradi, H.; Goriely, S. Bioactive peptides from yeast: A comparative review on production methods, bioactivity, structure-function relationship, and stability. Trends Food Sci. Technol. 2021, 118, 297–315. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Ferreira, C.; Pereira, J.O.; Pintado, M.E.; Carvalho, A.P. Spent brewer’s yeast (Saccharomyces cerevisiae) as a potential source of bioactive peptides: An overview. Int. J. Biol. Macromol. 2022, 208, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Mirdamadi, S.; Ehsani, M.R.; Aminlari, M.; Hosseini, E. Purification and identification of antioxidant and ACE-inhibitory peptide from Saccharomyces cerevisiae protein hydrolysate. J. Funct. Foods 2015, 19, 259–268. [Google Scholar] [CrossRef]
- Vukašinović-Milić, T.V.; Rakin, M.; Šiler-Marinković, S. Utilization of baker’s yeast (Saccharomyces cerevisiae) for the production of yeast extract: Effects of different enzymatic treatments on solid, protein and carbohydrate recovery. J. Serb. Chem. Soc. 2007, 72, 451–457. [Google Scholar] [CrossRef]
- Marson, G.V.; Machado, M.T.C.; de Castro, R.J.S.; Hubinger, M.D. Sequential hydrolysis of spent brewer’s yeast improved its physico-chemical characteristics and antioxidant properties: A strategy to transform waste into added-value biomolecules. Process Biochem. 2019, 84, 91–102. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Morellon-Sterling, R.; Siar, E.H.; Tavano, O.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Use of Alcalase in the production of bioactive peptides: A review. Int. J. Biol. Macromol. 2020, 165, 2143–2196. [Google Scholar] [CrossRef] [PubMed]
- Paiva dos Santos, K.; Mellinger-Silva, C.; Iraidy Santa Brígida, A.; Rocha Barros Gonçalves, L. Modifying Alcalase Activity and Stability by Immobilization onto Chitosan Aiming at the Production of Bioactive Peptides by Hydrolysis of Tilapia Skin Gelatin. Process Biochem. 2020, 97, 27–36. [Google Scholar] [CrossRef]
- Conway, J.; Gaudreau, H.; Champagne, C.P. The effect of the addition of proteases and glucanases during yeast autolysis on the production and properties of yeast extracts. Can. J. Microbiol. 2001, 47, 515–521. [Google Scholar] [CrossRef]
- Liu, D.; Ding, L.; Sun, J.; Boussetta, N.; Vorobiev, E. Yeast cell disruption strategies for recovery of intracellular bio-active compounds—A review. Innov. Food Sci. Emerg. Technol. 2016, 36, 181–192. [Google Scholar] [CrossRef]
- Klis, F.M.; Boorsma, A.; de Groot, P.W. Cell wall construction in Saccharomyces cerevisiae. Yeast 2006, 23, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Jacob, F.F.; Striegel, L.; Rychlik, M.; Hutzler, M.; Methner, F.-J. Yeast extract production using yeast from beer manufacture: Influence of industrially applicable disruption methods on selected groups with biotechnological relevance. Eur. Food Res. Technol. 2019, 245, 1169–1182. [Google Scholar] [CrossRef]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Golberg, A.; Sack, M.; Teissie, J.; Pataro, G.; Pliquett, U.; Saulis, G.; Stefan, T.; Miklavcic, D.; Vorobiev, E.; Frey, W. Energy-efficient biomass processing with pulsed electric fields for bioeconomy and sustainable development. Biotechnol. Biofuels 2016, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.C.; Chizmadzhev, Y.A. Theory of electroporation: A review. Bioelectrochem. Bioenerg. 1996, 41, 135. [Google Scholar] [CrossRef]
- Teissie, J.; Golzio, M.; Rols, M.-P. Mechanisms of cell membrane electropermeabilization: A minireview of our present (lack of?) knowledge. Biochim. Biophys. Acta 2005, 1724, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Goettel, M.; Eing, C.; Gusbeth, C.; Straessner, R.; Frey, W. Pulsed electric field assisted extraction of intracellular valuables from microalgae. Algal Res. 2013, 2, 401–408. [Google Scholar] [CrossRef]
- Liu, D.; Lebovka, N.I.; Vorobiev, E. Impact of electric pulse treatment on selective extraction of intracellular compounds from Saccharomyces cerevisiae yeasts. Food Bioprocess Technol. 2013, 6, 576–584. [Google Scholar] [CrossRef]
- Ganeva, V.; Galutzov, B.; Teissié, J. High yield electroextraction of proteins from yeast by a flow process. Anal. Biochem. 2003, 315, 77–84. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed electric field-assisted extraction of valuable compounds from microorganisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 530–552. [Google Scholar] [CrossRef]
- Zhang, R.; Lebovka, N.; Marchal, L.; Vorobiev, E.; Grimi, N. Pulsed electric energy and ultrasonication assisted green solvent extraction of bio-molecules from different microalgal species. Innov. Food Sci. Emerg. Technol. 2020, 62, 102358. [Google Scholar] [CrossRef]
- Silve, A.; Papachristou, I.; Wüstner, R.; Sträßner, R.; Schirmer, M.; Leber, K.; Guo, B.; Interrante, L.; Posten, C.; Frey, W. Extraction of lipids from wet microalga Auxenochlorella protothecoides using pulsed electric field treatment and ethanol-hexane blends. Algal Res. 2018, 29, 212–222. [Google Scholar] [CrossRef]
- Gorte, O.; Nazarova, N.; Papachristou, I.; Wüschner, R.; Leber, K.; Syldatk, C.; Ochsenreither, K.; Frey, W.; Silve, A. Pulsed electric field treatment promotes lipid extraction from oleaginous yeast Saitzyma podzolica DSM 27192. Front. Bioeng. Biotechnol. 2020, 8, 575379. [Google Scholar] [CrossRef] [PubMed]
- Ganeva, V.; Kranz, A. Selective extraction of recombinant membrane proteins from Hansenula polymorpha by pulsed electric field and lytic enzyme pretreatment. Microb. Cell Fact. 2023, 2, 251. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, M.; Lin, S.; Liu, J.; Yang, Y.; Jin, Y. Optimization of Extraction Parameters for Protein from Beer Waste Brewing Yeast Treated by Pulsed Electric Fields (PEF). Afr. J. Microbiol. Res. 2012, 6, 4739–4746. [Google Scholar] [CrossRef]
- Ganeva, V.; Galutzov, B.; Angelova, B.; Suckow, M. Electroinduced Extraction of Human Ferritin Heavy Chain Expressed in Hansenula polymorpha. Appl. Biochem. Biotechnol. 2018, 184, 1286–1307. [Google Scholar] [CrossRef] [PubMed]
- Ganeva, V.; Angelova, B.; Galutzov, B.; Goltsev, V.; Zhiponova, M. Extraction of proteins and other intracellular bioactive compounds from baker’s yeast by pulsed electric field treatment. Front. Bioeng. Biotechnol. 2020, 8, 552335. [Google Scholar] [CrossRef]
- Berzosa, A.; Delso, C.; Sanz, J.; Sánchez-Gimeno, C.; Raso, J. Sequential extraction of compounds of interest from yeast biomass assisted by pulsed electric fields. Front. Bioeng. Biotechnol. 2023, 11, 1197710. [Google Scholar] [CrossRef]
- Ganeva, V.; Galutzov, B.; Teissie, J. Evidence that pulsed electric field treatment enhances the cell wall porosity of yeast cells. Appl. Biochem. Biotechnol. 2014, 172, 1540–1552. [Google Scholar] [CrossRef]
- Ganeva, V.; Galutzov, B.; Teissie, J. Electroinduced Release of Invertase from Saccharomyces cerevisiae. Biotechnol. Lett. 2002, 24, 1853–1856. [Google Scholar] [CrossRef]
- Akaberi, S.; Gusbeth, C.; Silve, A.; Senthilnathan, D.S.; Navarro-López, E.; Molina-Grima, E.; Müller, G.; Frey, W. Effect of pulsed electric field treatment on enzymatic hydrolysis of proteins of Scenedesmus almeriensis. Algal Res. 2019, 43, 101656. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Lamoolphak, W.; Goto, M.; Sasaki, M.; Suphantharika, M.; Muangnapoh, C.; Prommuag, C.; Shotipruk, A. Hydrothermal decomposition of yeast cells for production of proteins and amino acids. J. Hazard. Mater. 2006, 137, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Mauro, S. Total antioxidant capacity of plant foods, beverages, and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Schägger, H. Tricine–SDS-PAGE. Nat. Protoc. 2006, 1, 16–22. [Google Scholar] [CrossRef]
- Nesterenko, M.V.; Tilley, M.; Upton, S.J. A simple modification of Blum’s silver stain method allows for 30-minute detection of proteins in polyacrylamide gels. J. Biochem. Biophys. Method. 1994, 28, 239–242. [Google Scholar] [CrossRef]
- Li, E.; Mira de Orduña, R.A. A Rapid Method for the Determination of Microbial Biomass by Dry Weight Using a Moisture Analyser with an Infrared Heating Source and an Analytical Balance. Lett. Appl. Microbiol. 2010, 50, 283–288. [Google Scholar] [CrossRef]
- Veleva, R.; Moskova-Doumanova, V.; Todorova, M.; Trendafilova, A.; Doumanov, J.; Topouzova-Hristova, T. Cytotoxicity of Flavonoid Glycosides, Flavonoids, and Phenolic Acids from Inula oculus-christi on Mammalian Cell Lines. J. BioSci. Biotechnol. 2017, 5, 219–224. [Google Scholar]
- Trendafilova, A.; Staleva, P.; Petkova, Z.; Ivanova, V.; Evstatieva, Y.; Nikolova, D.; Rasheva, I.; Atanasov, N.; Topouzova-Hristova, T.; Veleva, R.; et al. Phytochemical Profile, Antioxidant Potential, Antimicrobial Activity, and Cytotoxicity of Dry Extract from Rosa damascena Mill. Molecules 2023, 28, 7666. [Google Scholar] [CrossRef]
- Mao, Y.; Chen, L.; Zhang, L.; Bian, Y.; Meng, C. Synergistic Hydrolysis of Soy Proteins Using Immobilized Proteases: Assessing Peptide Profiles. Foods 2023, 12, 4115. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, G.; Stefanou, N.; Andreou, V.; Taoukis, P. Effect of Pulsed Electric Fields on the Production of Yeast Extract by Autolysis. Innov. Food Sci. Emerg. Technol. 2018, 48, 287–295. [Google Scholar] [CrossRef]
- Kasper, J.R.; Andrews, E.C.; Park, C. Product Inhibition in Native-State Proteolysis. PLoS ONE 2014, 9, e111416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Olsen, K.; Grossi, A.; Otte, J. Effect of Pretreatment on Enzymatic Hydrolysis of Bovine Collagen and Formation of ACE-Inhibitory Peptides. Food Chem. 2013, 141, 2343–2354. [Google Scholar] [CrossRef] [PubMed]
- Lourenço da Costa, E.; da Rocha Gontijo, J.A.; Netto, F.M. Effect of Heat and Enzymatic Treatment on the Antihypertensive Activity of Whey Protein Hydrolysates. Int. Dairy J. 2007, 17, 632–640. [Google Scholar] [CrossRef]
- Keskin Ulug, S.; Jahandideh, F.; Wu, J. Novel Technologies for the Production of Bioactive Peptides. Trends Food Sci. Technol. 2021, 108, 27–39. [Google Scholar] [CrossRef]
- Han, Z.; Cai, M.; Cheng, J.H.; Sun, D.W. Effects of Electric Fields and Electromagnetic Wave on Food Protein Structure and Functionality: A Review. Trends Food Sci. Technol. 2018, 75, 1–9. [Google Scholar] [CrossRef]
- Taha, A.; Casanova, F.; Šimonis, P.; Stankevič, V.; Gomaa, M.A.E.; Stirkė, A. Pulsed Electric Field: Fundamentals and Effects on the Structural and Techno-Functional Properties of Dairy and Plant Proteins. Foods 2022, 11, 1556. [Google Scholar] [CrossRef]
- Dumitrascu, L.; Lanciu (Dorofte), A.; Aprodu, I. A preliminary study on using ultrasounds for the valorization of spent brewer’s yeast. Ann. Univ. Dunarea Jos Galati Fascicle VI Food Technol. 2022, 46, 141–153. [Google Scholar] [CrossRef]
- Romero García, J.M.; Acién Fernández, F.G.; Fernández Sevilla, J.M. Development of a process for the production of l-amino acids concentrates from microalgae by enzymatic hydrolysis. Bioresour. Technol. 2012, 112, 164–170. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veleva, R.; Ganeva, V.; Zhiponova, M. Pulsed Electric Field Pretreatment Enhances the Enzyme Hydrolysis of Baker’s Yeast. Microorganisms 2024, 12, 2470. https://doi.org/10.3390/microorganisms12122470
Veleva R, Ganeva V, Zhiponova M. Pulsed Electric Field Pretreatment Enhances the Enzyme Hydrolysis of Baker’s Yeast. Microorganisms. 2024; 12(12):2470. https://doi.org/10.3390/microorganisms12122470
Chicago/Turabian StyleVeleva, Ralitsa, Valentina Ganeva, and Miroslava Zhiponova. 2024. "Pulsed Electric Field Pretreatment Enhances the Enzyme Hydrolysis of Baker’s Yeast" Microorganisms 12, no. 12: 2470. https://doi.org/10.3390/microorganisms12122470
APA StyleVeleva, R., Ganeva, V., & Zhiponova, M. (2024). Pulsed Electric Field Pretreatment Enhances the Enzyme Hydrolysis of Baker’s Yeast. Microorganisms, 12(12), 2470. https://doi.org/10.3390/microorganisms12122470






