Effects of mscM Gene on Desiccation Resistance in Cronobacter sakazakii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Growth Conditions
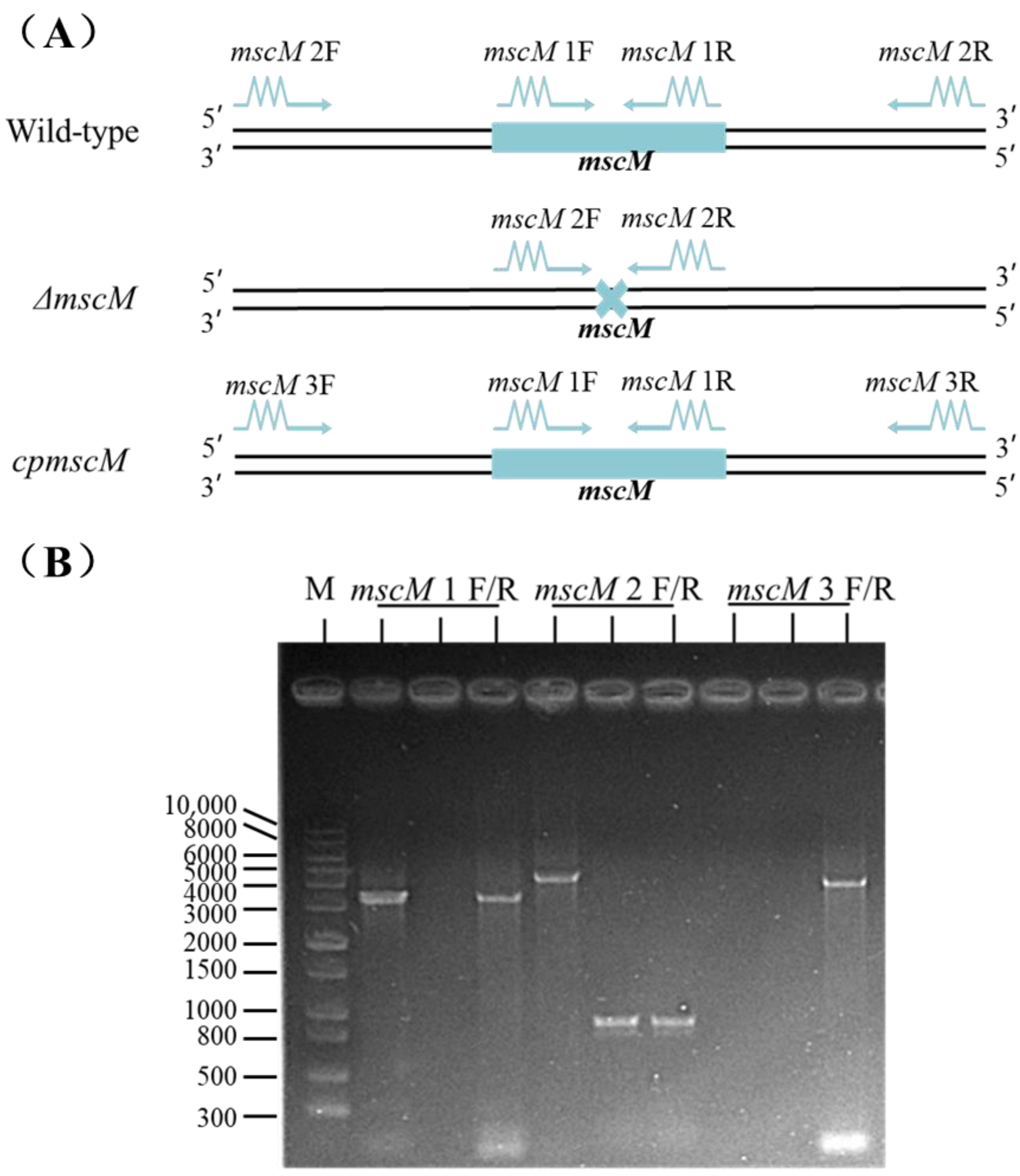
2.2. Deletion of the mscM Gene and Gene Complementation
2.3. Bacterial Growth Curves
2.4. Desiccation Resistance Evaluation
2.5. Determination of Intracellular K+ Contents
2.6. Determination of Total K+, Na+, Ca2+, and Mg2+ Contents
2.7. Permeability Assay
2.8. Bacterial Adhesion/Invasion
2.9. Hydrophobicity Assay
2.10. Biofilm Formation
2.11. Statistical Analysis
3. Results
3.1. Construction of the mscM Mutant Strain
3.2. Effects of MscM on the Bacterial Growth Curves
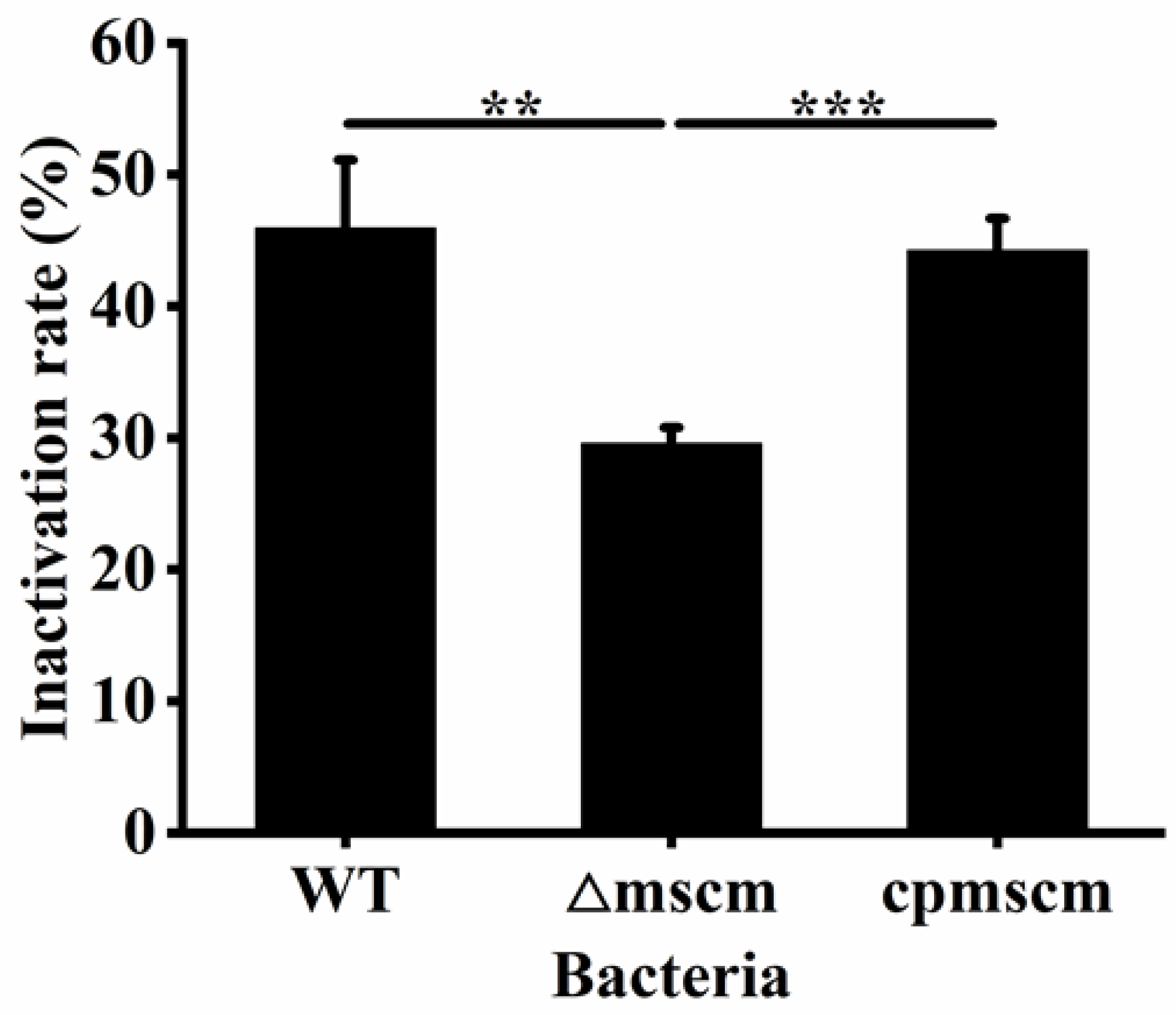
3.3. Effects of MscM on the Desiccation Resistance of C. sakazakii
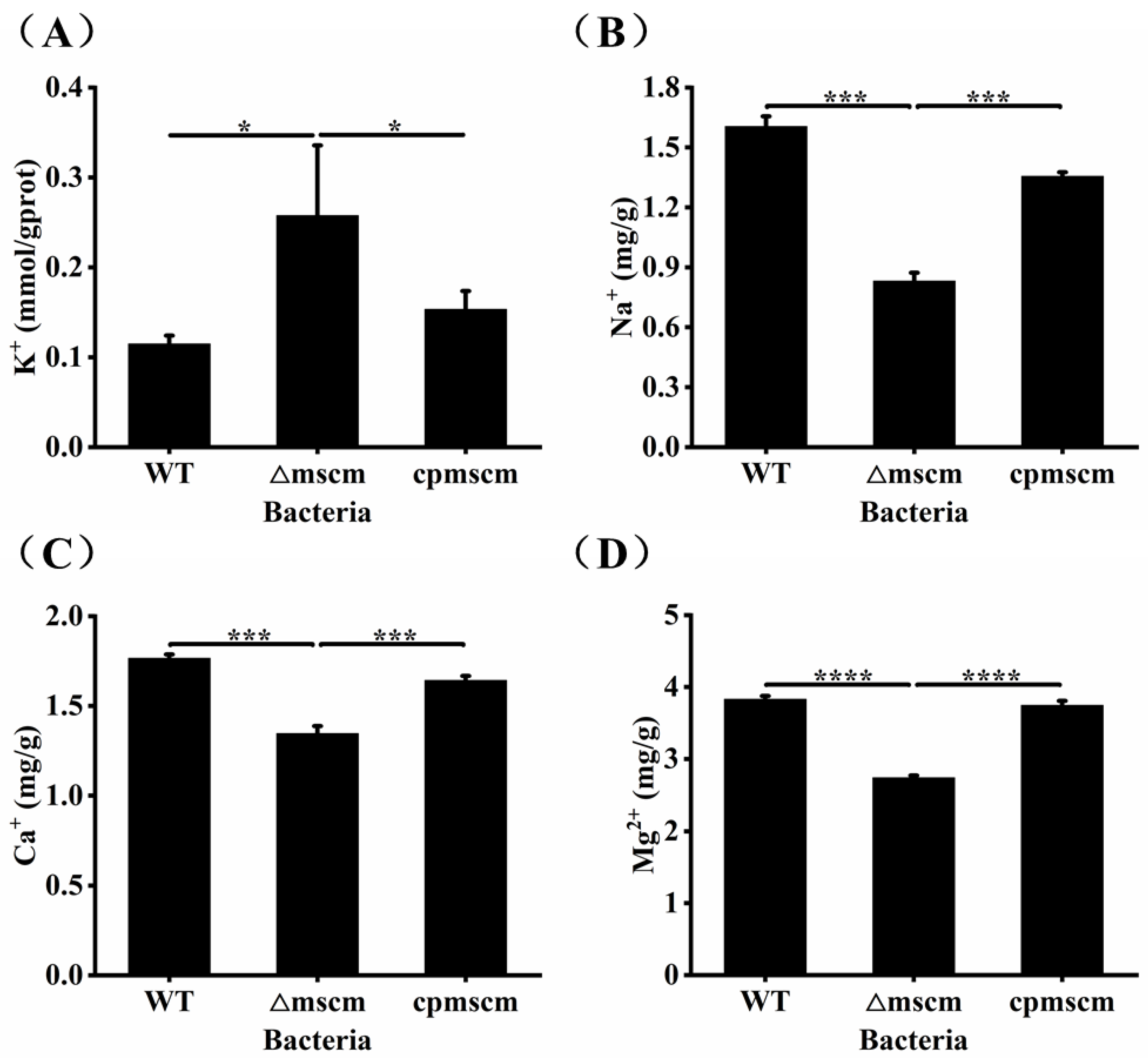
3.4. The Different Ion Contents in WT, ΔmscM and cpmscM Strains
3.5. Detection of the Outer Membrane Permeability
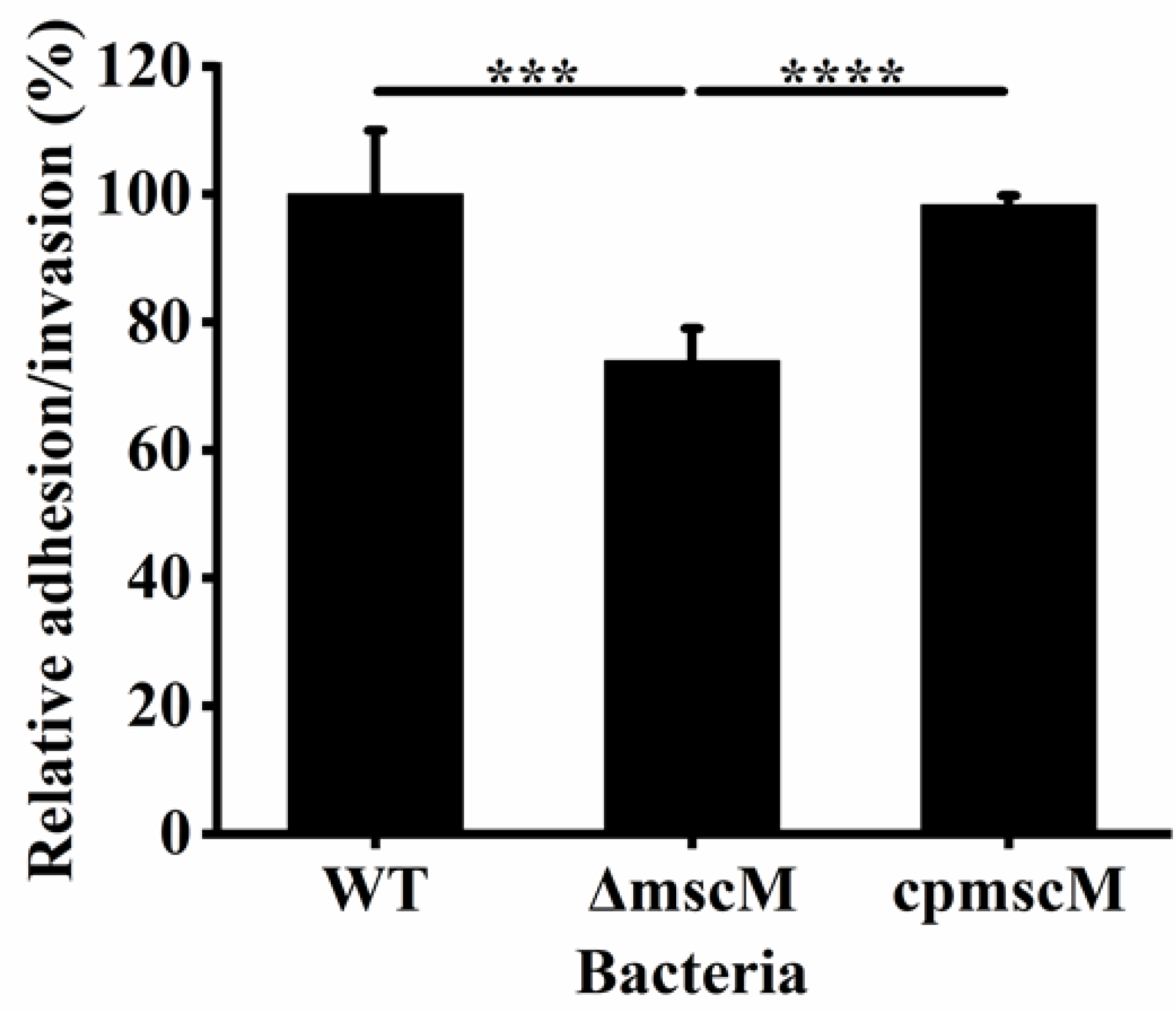
3.6. Effects of MscM on Bacterial Adhesion/Invasion
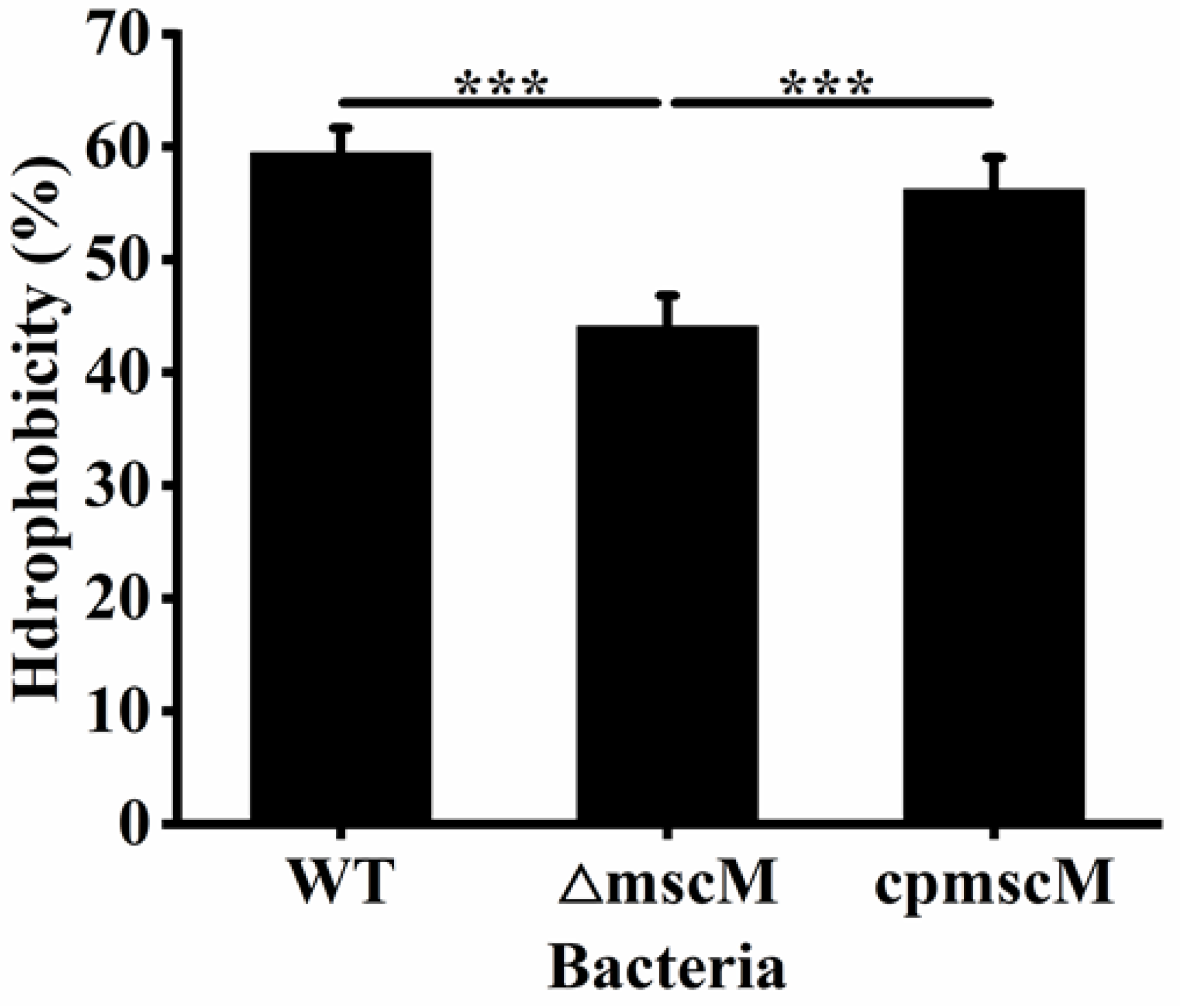
3.7. Effects of MscM on the Surface Hydrophobicity
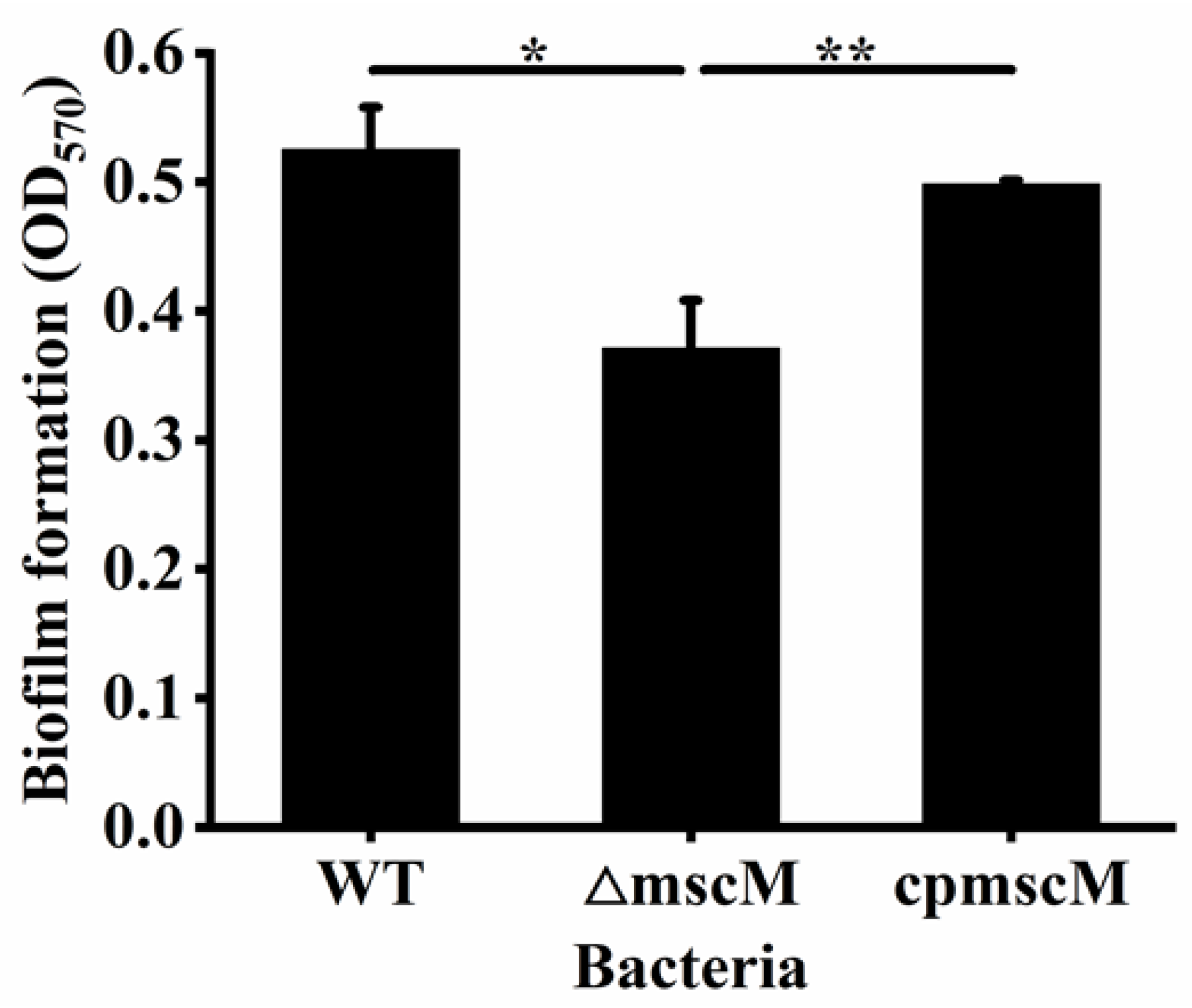
3.8. Effects of MscM on the Biofilm Formation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jin, T.; Pang, L.; Yue, T.; Niu, L.; Li, T.; Liang, Y.; Zhang, Y.; Yan, C.; Yang, B.; Zhang, C.; et al. The role of DsbA and PepP genes in the environmental tolerance and virulence factors of Cronobacter sakazakii. Food. Res. Int. 2024, 190, 114555. [Google Scholar] [CrossRef] [PubMed]
- Ekundayo, T.C.; Ijabadeniyi, O.A. Global and regional prevalence of Cronobacter sakazakii in powdered milk and flour. Sci. Rep. 2024, 14, 6865. [Google Scholar] [CrossRef]
- Johler, S.; Stephan, R.; Hartmann, I.; Kuehner, K.A.; Lehner, A. Genes involved in yellow pigmentation of Cronobacter sakazakii ES5 and influence of pigmentation on persistence and growth under environmental stress. Appl. Environ. Microbiol. 2010, 76, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Du, X.J.; Wang, X.Y.; Dong, X.; Li, P.; Wang, S. Characterization of the desiccation tolerance of Cronobacter sakazakii strains. Front. Microbiol. 2018, 9, 2867. [Google Scholar] [CrossRef]
- Wang, L.; Forsythe, S.J.; Yang, X.; Fu, S.; Man, C.; Jiang, Y. Invited review: Stress resistance of Cronobacter spp. affecting control of its growth during food production. J. Dairy. Sci. 2021, 104, 11348–11367. [Google Scholar] [CrossRef]
- Hu, S.; Yu, Y.G.; Wu, X.W.; Xia, X.Z.; Xiao, X.L.; Wu, H. Comparative proteomic analysis of Cronobacter sakazakii by iTRAQ provides insights into response to desiccation. Food. Res. Int. 2017, 100 Pt 1, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Srikumar, S.; Cao, Y.; Yan, Q.; Van Hoorde, K.; Nguyen, S.; Cooney, S.; Gopinath, G.R.; Tall, B.D.; Sivasankaran, S.K.; Lehner, A.; et al. RNA Sequencing-based transcriptional overview of xerotolerance in Cronobacter sakazakii SP291. Appl. Environ. Microbiol. 2019, 85, e01993-18. [Google Scholar] [CrossRef]
- Feeney, A.; Sleator, R.D. An in silico analysis of osmotolerance in the emerging gastrointestinal pathogen Cronobacter sakazakii. Bioeng. Bugs. 2011, 2, 260–270. [Google Scholar] [CrossRef]
- Gralla, J.D.; Vargas, D.R. Potassium glutamate as a transcriptional inhibitor during bacterial osmoregulation. EMBO J. 2006, 25, 1515–1521. [Google Scholar] [CrossRef]
- Roe, A.J.; O’Byrne, C.; McLaggan, D.; Booth, I.R. Inhibition of Escherichia coli growth by acetic acid: A problem with methionine biosynthesis and homocysteine toxicity. Microbiology 2002, 148, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Blount, P.; Iscla, I. Life with Bacterial Mechanosensitive Channels, from Discovery to Physiology to Pharmacological Target. Microbiol. Mol. Biol. Rev. 2020, 84, e00055-19. [Google Scholar] [CrossRef]
- Ramsey, K.; Britt, M.; Maramba, J.; Ushijima, B.; Moller, E.; Anishkin, A.; Häse, C.; Sukharev, S. The dynamic hypoosmotic response of Vibrio cholerae relies on the mechanosensitive channel MscS. iScience 2024, 27, 110001. [Google Scholar] [CrossRef] [PubMed]
- Schumann, U.; Edwards, M.D.; Rasmussen, T.; Bartlett, W.; van West, P.; Booth, I.R. YbdG in Escherichia coli is a threshold-setting mechanosensitive channel with MscM activity. Proc. Natl. Acad. Sci. USA 2010, 107, 12664–12669. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.D.; Black, S.; Rasmussen, T.; Rasmussen, A.; Stokes, N.R.; Stephen, T.-L.; Miller, S.; Booth, I.R. Characterization of three novel mechanosensitive channel activities in Escherichia coli. Channels 2012, 6, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Lu, P.; Xue, J.; Zhao, N.; Zhang, Y.; Dong, L.; Zhang, X.; Li, P.; Hu, Y.; Wang, J.; et al. The lipoprotein NlpD in Cronobacter sakazakii responds to acid stress and regulates macrophage resistance and virulence by maintaining membrane integrity. Virulence 2021, 12, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Pivetti, C.D.; Yen, M.-R.; Miller, S.; Busch, W.; Tseng, Y.-H.; Booth, I.R.; Saier, M.H. Two families of mechanosensitive channel proteins. Microbiol. Mol. Biol. R. 2003, 67, 66–85. [Google Scholar] [CrossRef]
- Wang, X.Y.; Li, P.; Du, X.J.; Wang, S. Effect of glutathione-transport-related gene gsiD on desiccation tolerance of Cronobacter sakazakii and its related regulatory mechanism. Appl. Environ. Microbiol. 2024, 90, e0156223. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.G.; Yousef, A.E. Carvacrol and thymol combat desiccation resistance mechanisms in Salmonella enterica serovar tennessee. Microorganisms 2021, 10, 44. [Google Scholar] [CrossRef]
- Sarah Finn, S.; Condell, O.; McClure, P.; Amézquita, A.; Fanning, S. Mechanisms of survival, responses and sources of Salmonella in low-moisture environments. Front. Microbiol. 2013, 4, 331. [Google Scholar] [CrossRef]
- Jovanovich, S.B.; Martinell, M.; Record, M.T.J.; Burgess, R.R. Rapid response to osmotic upshift by osmoregulated genes in Escherichia coli and Salmonella typhimurium. J. Bacteriol. 1988, 170, 534–539. [Google Scholar] [CrossRef]
- Kempf, B.; Bremer, E. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 1998, 170, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Scherber, C.M.; Schottel, J.L.; Aksan, A. Membrane phase behavior of Escherichia coli during desiccation, rehydration, and growth recovery. Biochim. Biophys. Acta. 2009, 1788, 2427–2435. [Google Scholar] [CrossRef] [PubMed]
- Lamark, T.; Røkenes, T.P.; McDougall, J.; Strøm, A.R. The complex bet promoters of Escherichia coli: Regulation by oxygen (ArcA), choline (BetI), and osmotic stress. J. Bacteriol. 1996, 178, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Fouladkhah, A. Outbreak history, biofilm formation, and preventive measures for control of Cronobacter sakazakii in infant formula and infant care settings. Microorganisms 2019, 7, 77. [Google Scholar] [CrossRef]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth. Adv. Colloid. Interface. Sci. 2018, 261, 1–14. [Google Scholar] [CrossRef]
- Irene, I.; Paul, B. Sensing and responding to membrane tension: The bacterial MscL channel as a model system. Biophys. J. 2012, 103, 169–174. [Google Scholar] [CrossRef]
- Gu, S.; Zhang, Y.; Wu, Y. Effects of sound exposure on the growth and intracellular macromolecular synthesis of E. coli k-12. PeerJ 2016, 4, e1920. [Google Scholar] [CrossRef]
- Sukharev, S.I.; Blount, P.; Martinac, B.; Blattner, F.R.; Kung, C. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature 1994, 368, 265–268. [Google Scholar] [CrossRef]
- Barron, J.C.; Forsythe, S.J. Dry stress and survival time of Enterobacter sakazakii and other Enterobacteriaceae in dehydrated powdered infant formula. J. Food. Prot. 2007, 70, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, R.R.; Morgan, E.; Peters, S.E.; Pleasance, S.J.; Hudson, D.L.; Davies, H.M.; Wang, J.; Van Diemen, P.M.; Buckley, A.M.; Bowen, A.J.; et al. Comprehensive assignment of roles for Salmonella typhimurium genes in intestinal colonization of food-producing animals. PLoS. Genet. 2013, 9, e1003456. [Google Scholar] [CrossRef]
- Glogauer, M.; Arora, P.; Yao, G.; Sokholov, I.; Ferrier, J.; McCulloch, C.A. Calcium ions and tyrosine phosphorylation interact coordinately with actin to regulate cytoprotective responses to stretching. J. Cell. Sci. 1997, 110 Pt 1, 11–21. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, D.; Zhang, Z.; Li, P.; Du, X. Effects of mscM Gene on Desiccation Resistance in Cronobacter sakazakii. Microorganisms 2024, 12, 2464. https://doi.org/10.3390/microorganisms12122464
Zhu D, Zhang Z, Li P, Du X. Effects of mscM Gene on Desiccation Resistance in Cronobacter sakazakii. Microorganisms. 2024; 12(12):2464. https://doi.org/10.3390/microorganisms12122464
Chicago/Turabian StyleZhu, Dongdong, Zhengyang Zhang, Ping Li, and Xinjun Du. 2024. "Effects of mscM Gene on Desiccation Resistance in Cronobacter sakazakii" Microorganisms 12, no. 12: 2464. https://doi.org/10.3390/microorganisms12122464
APA StyleZhu, D., Zhang, Z., Li, P., & Du, X. (2024). Effects of mscM Gene on Desiccation Resistance in Cronobacter sakazakii. Microorganisms, 12(12), 2464. https://doi.org/10.3390/microorganisms12122464



