Modulation of C. albicans-Induced Immune Response in Vaginal Epithelial Cells by Garcinoic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. C. albicans Strain and Growth Conditions
2.2. Vaginal Epithelial Cell Line
2.3. α-Tocopherol and Garcinoic Acid
2.4. Analysis of Cell Viability (MTT Assay)
2.5. C. albicans Morphological Analysis
2.6. Protein Extraction and Western Blotting
2.7. Enzyme-Linked Immunosorbent Assay (ELISA) for IL-6, Il-1α, and IL-1β
2.8. Statistical Analysis
3. Results
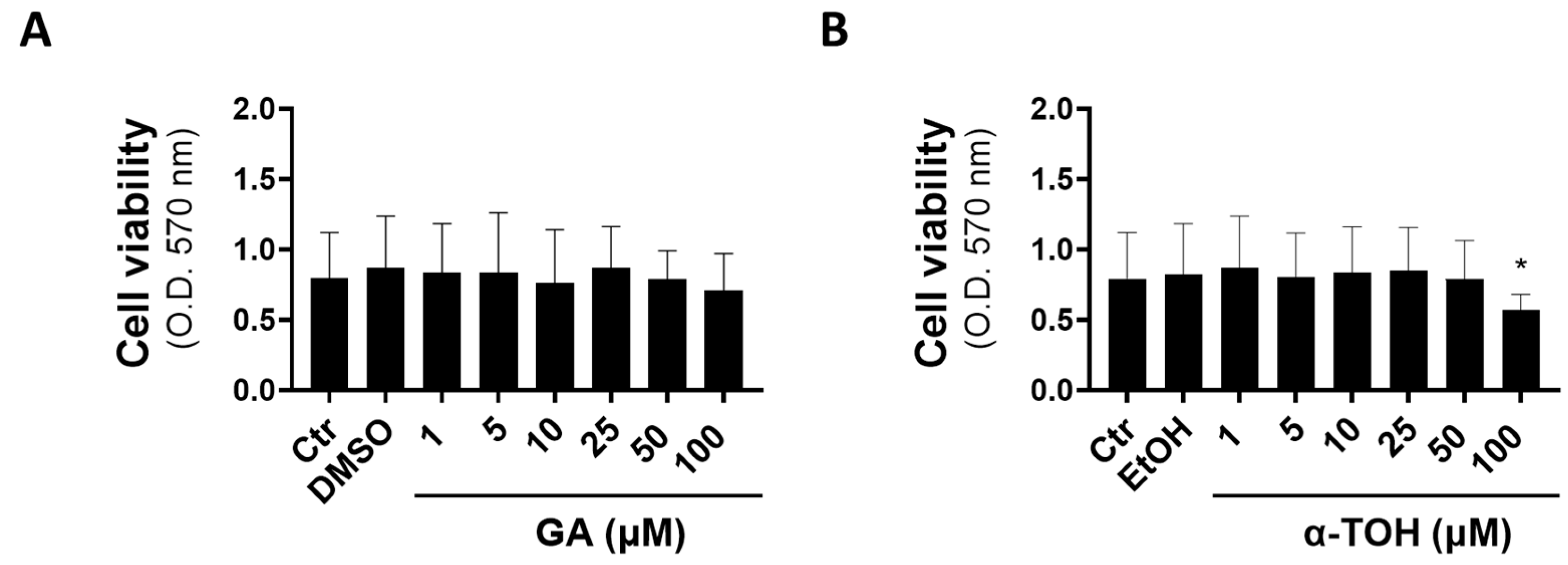
3.1. Effects of GA and α-TOH on Viability of Vaginal Epithelial Cells
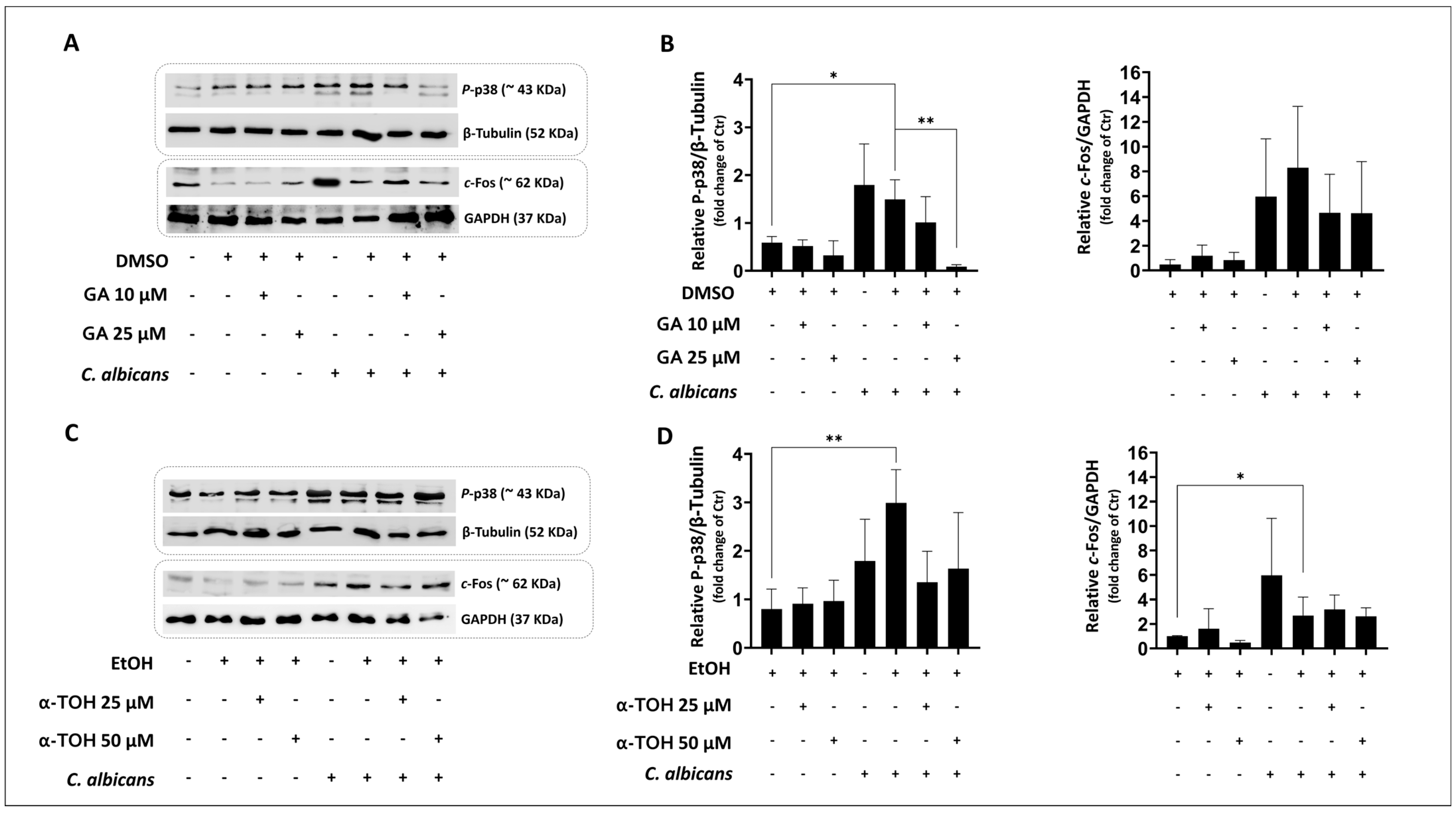
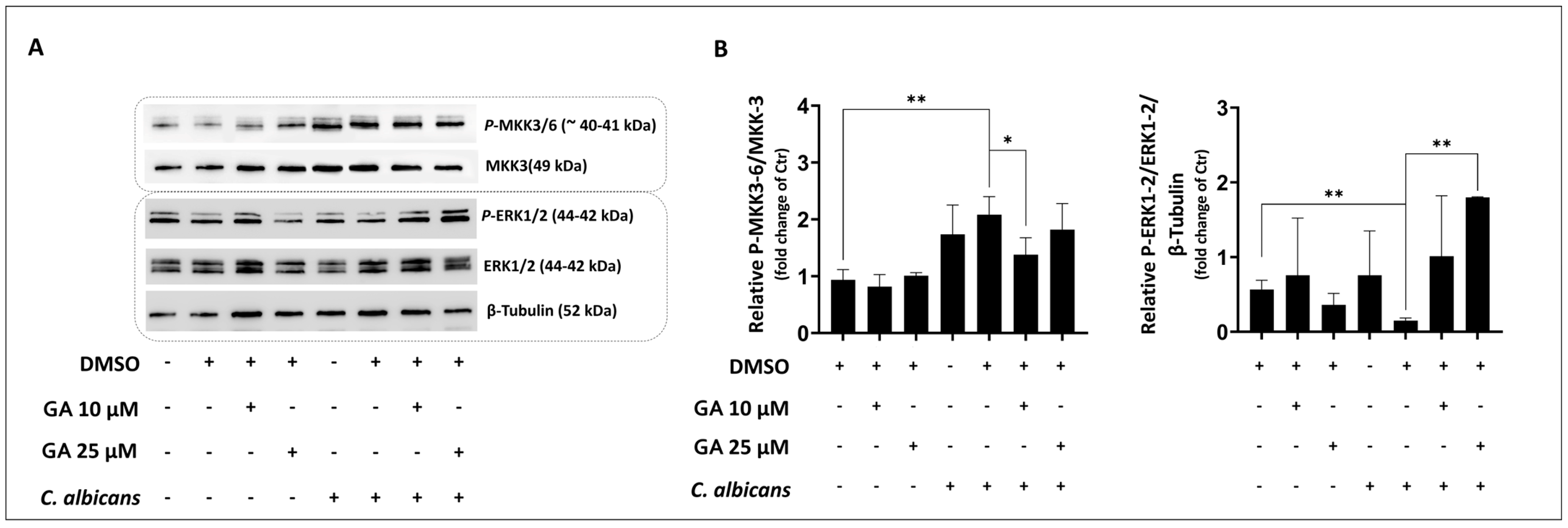
3.2. Evaluation of Effects of GA and α-TOH on Candida albicans-Induced p-38 Activity in Vaginal Epithelial Cells
3.3. Garcinoic Acid Pre-Treatment Reduced IL-6, IL-1α, and IL-1β Secretion in Vaginal Epithelial Cells Exposed to C. albicans Infection
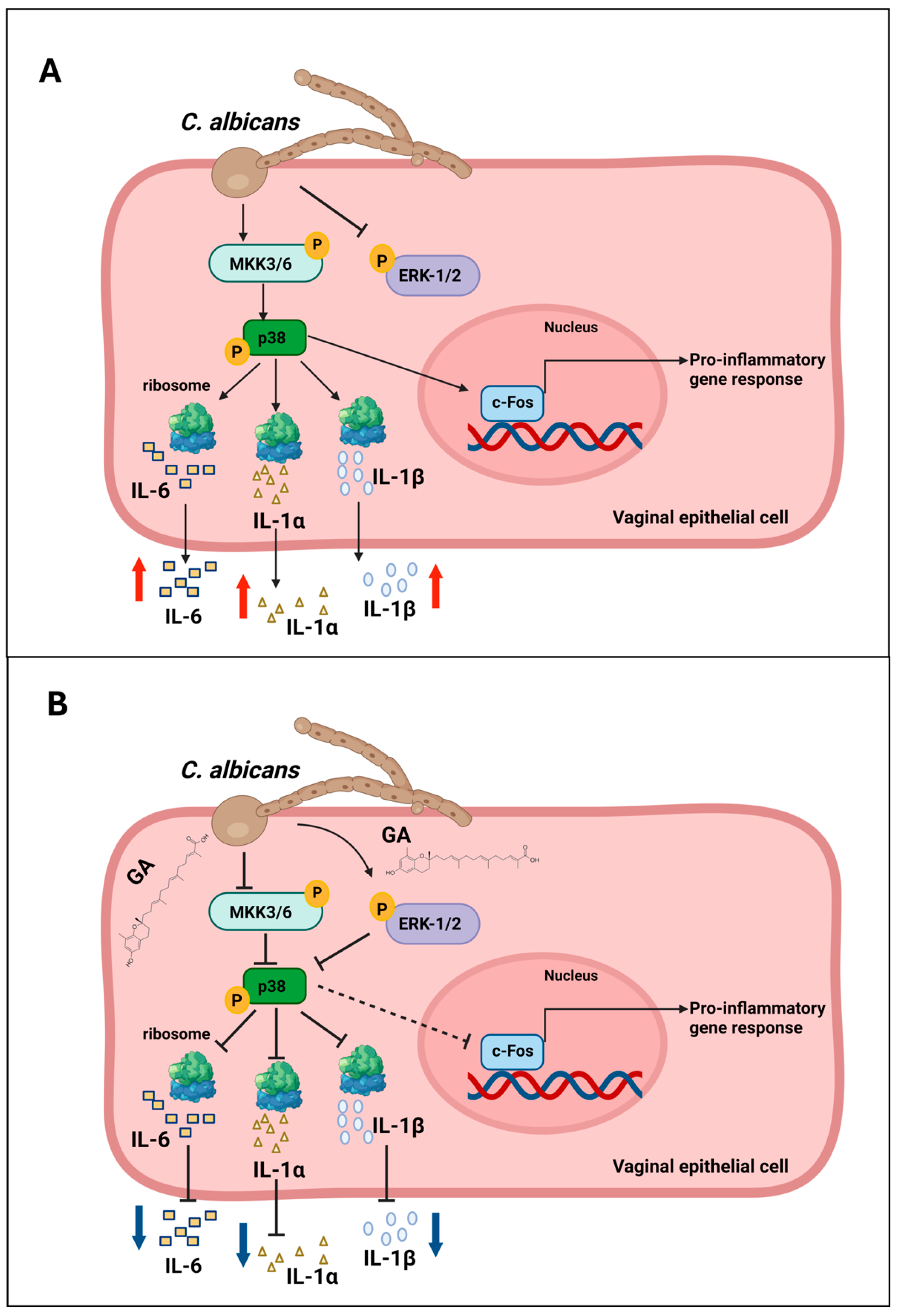
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaziano, R.; Sabbatini, S.; Monari, C. The Interplay between Candida albicans, Vaginal Mucosa, Host Immunity and Resident Microbiota in Health and Disease: An Overview and Future Perspectives. Microorganisms 2023, 11, 1211. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Vaginitis. N. Engl. J. Med. 1997, 337, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Kneale, M.; Sobel, J.D.; Rautemaa-Richardson, R. Global burden of recurrent vulvovaginal candidiasis: A systematic review. Lancet Infect. Dis. 2018, 18, e339–e347. [Google Scholar] [CrossRef] [PubMed]
- Moyes, D.L.; Murciano, C.; Runglall, M.; Islam, A.; Thavaraj, S.; Naglik, J.R. Candida albicans yeast and hyphae are discriminated by MAPK signaling in vaginal epithelial cells. PLoS ONE 2011, 6, e26580. [Google Scholar] [CrossRef]
- Moyes, D.L.; Wilson, D.; Richardson, J.P.; Mogavero, S.; Tang, S.X.; Wernecke, J.; Hofs, S.; Gratacap, R.L.; Robbins, J.; Runglall, M.; et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 2016, 532, 64–68. [Google Scholar] [CrossRef]
- Roselletti, E.; Perito, S.; Sabbatini, S.; Monari, C.; Vecchiarelli, A. Vaginal Epithelial Cells Discriminate Between Yeast and Hyphae of Candida albicans in Women Who Are Colonized or Have Vaginal Candidiasis. J. Infect. Dis. 2019, 220, 1645–1654. [Google Scholar] [CrossRef]
- Nikou, S.A.; Kichik, N.; Brown, R.; Ponde, N.O.; Ho, J.; Naglik, J.R.; Richardson, J.P. Candida albicans Interactions with Mucosal Surfaces during Health and Disease. Pathogens 2019, 8, 53. [Google Scholar] [CrossRef]
- Roselletti, E.; Monari, C.; Sabbatini, S.; Perito, S.; Vecchiarelli, A.; Sobel, J.D.; Cassone, A. A Role for Yeast/Pseudohyphal Cells of Candida albicans in the Correlated Expression of NLRP3 Inflammasome Inducers in Women With Acute Vulvovaginal Candidiasis. Front. Microbiol. 2019, 10, 2669. [Google Scholar] [CrossRef]
- Naglik, J.R.; Konig, A.; Hube, B.; Gaffen, S.L. Candida albicans-epithelial interactions and induction of mucosal innate immunity. Curr. Opin. Microbiol. 2017, 40, 104–112. [Google Scholar] [CrossRef]
- Pellon, A.; Sadeghi Nasab, S.D.; Moyes, D.L. New Insights in Candida albicans Innate Immunity at the Mucosa: Toxins, Epithelium, Metabolism, and Beyond. Front. Cell. Infect. Microbiol. 2020, 10, 81. [Google Scholar] [CrossRef]
- d’Enfert, C.; Kaune, A.K.; Alaban, L.R.; Chakraborty, S.; Cole, N.; Delavy, M.; Kosmala, D.; Marsaux, B.; Frois-Martins, R.; Morelli, M.; et al. The impact of the Fungus-Host-Microbiota interplay upon Candida albicans infections: Current knowledge and new perspectives. FEMS Microbiol. Rev. 2021, 45, fuaa060. [Google Scholar] [CrossRef]
- Ardizzoni, A.; Wheeler, R.T.; Pericolini, E. It Takes Two to Tango: How a Dysregulation of the Innate Immunity, Coupled With Candida Virulence, Triggers VVC Onset. Front. Microbiol. 2021, 12, 692491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Peng, J.; Li, D.; Mei, H.; Yu, Y.; Li, X.; She, X.; Liu, W. Divergent EGFR/MAPK-Mediated Immune Responses to Clinical Candida Pathogens in Vulvovaginal Candidiasis. Front. Immunol. 2022, 13, 894069. [Google Scholar] [CrossRef]
- Peters, B.M.; Yano, J.; Noverr, M.C.; Fidel, P.L., Jr. Candida vaginitis: When opportunism knocks, the host responds. PLoS Pathog. 2014, 10, e1003965. [Google Scholar] [CrossRef]
- Tauchen, J.; Frankova, A.; Manourova, A.; Valterova, I.; Lojka, B.; Leuner, O. Garcinia kola: A critical review on chemistry and pharmacology of an important West African medicinal plant. Phytochem. Rev. 2023, 22, 1305–1351. [Google Scholar] [CrossRef]
- Hussain, R.A.; Owegby, A.G.; Parimoo, P.; Waterman, P.G. Kolanone, a novel polyisoprenylated benzophenone with antimicrobial properties from the fruit of Garcinia kola. Planta Med. 1982, 44, 78–81. [Google Scholar] [CrossRef]
- Okoko, T. In vitro antioxidant and free radical scavenging activities of Garcinia kola seeds. Food Chem. Toxicol. 2009, 47, 2620–2623. [Google Scholar] [CrossRef]
- Terashima, K.; Takaya, Y.; Niwa, M. Powerful antioxidative agents based on garcinoic acid from Garcinia kola. Bioorganic Med. Chem. 2002, 10, 1619–1625. [Google Scholar] [CrossRef]
- Wallert, M.; Bauer, J.; Kluge, S.; Schmolz, L.; Chen, Y.C.; Ziegler, M.; Searle, A.K.; Maxones, A.; Schubert, M.; Thurmer, M.; et al. The vitamin E derivative Garcinoic acid from Garcinia kola nut seeds attenuates the inflammatory response. Redox Biol. 2019, 24, 101166. [Google Scholar] [CrossRef]
- Mazzini, F.; Betti, M.; Netscher, T.; Galli, F.; Salvadori, P. Configuration of the vitamin E analogue Garcinoic acid extracted from Garcinia Kola seeds. Chirality 2009, 21, 519–524. [Google Scholar] [CrossRef]
- Tian, H.; Li, Y.F.; Jiao, G.L.; Sun, W.Y.; He, R.R. Unveiling the antioxidant superiority of α-tocopherol: Implications for vitamin E nomenclature and classification. Free Radic. Biol. Med. 2024, 216, 46–49. [Google Scholar] [CrossRef]
- Galli, F.; Azzi, A.; Birringer, M.; Cook-Mills, J.M.; Eggersdorfer, M.; Frank, J.; Cruciani, G.; Lorkowski, S.; Ozer, N.K. Vitamin E: Emerging aspects and new directions. Free Radic. Biol. Med. 2017, 102, 16–36. [Google Scholar] [CrossRef]
- Upaganlawar, A.B.; Wankhede, N.L.; Kale, M.B.; Umare, M.D.; Sehgal, A.; Singh, S.; Bhatia, S.; Al-Harrasi, A.; Najda, A.; Nurzyńska-Wierdak, R.; et al. Interweaving epilepsy and neurodegeneration: Vitamin E as a treatment approach. Biomed. Pharmacother. 2021, 143, 112146. [Google Scholar] [CrossRef]
- Barros, S.; Ribeiro, A.P.D.; Offenbacher, S.; Loewy, Z.G. Anti-Inflammatory Effects of Vitamin E in Response to Candida albicans. Microorganisms 2020, 8, 804. [Google Scholar] [CrossRef]
- Zingg, J.M. Vitamin E: Regulatory Role on Signal Transduction. IUBMB Life 2019, 71, 456–478. [Google Scholar] [CrossRef]
- Yu, Z.Q.; Wang, L.M.; Yang, W.X. How vitamin E and its derivatives regulate tumour cells via the MAPK signalling pathway? Gene 2022, 808, 145998. [Google Scholar] [CrossRef]
- Betti, M.; Minelli, A.; Canonico, B.; Castaldo, P.; Magi, S.; Aisa, M.C.; Piroddi, M.; Di Tomaso, V.; Galli, F. Antiproliferative effects of tocopherols (vitamin E) on murine glioma C6 cells: Homologue-specific control of PKC/ERK and cyclin signaling. Free Radic. Biol. Med. 2006, 41, 464–472. [Google Scholar] [CrossRef]
- Schmolz, L.; Wallert, M.; Rozzino, N.; Cignarella, A.; Galli, F.; Glei, M.; Werz, O.; Koeberle, A.; Birringer, M.; Lorkowski, S. Structure-Function Relationship Studies In Vitro Reveal Distinct and Specific Effects of Long-Chain Metabolites of Vitamin E. Mol. Nutr. Food Res. 2017, 61, 1700562. [Google Scholar] [CrossRef]
- Wallert, M.; Schmolz, L.; Koeberle, A.; Krauth, V.; Glei, M.; Galli, F.; Werz, O.; Birringer, M.; Lorkowski, S. alpha-Tocopherol long-chain metabolite alpha-13′-COOH affects the inflammatory response of lipopolysaccharide-activated murine RAW264.7 macrophages. Mol. Nutr. Food Res. 2015, 59, 1524–1534. [Google Scholar] [CrossRef]
- Olajide, O.A.; Iwuanyanwu, V.U.; Lepiarz-Raba, I.; Al-Hindawi, A.A.; Aderogba, M.A.; Sharp, H.L.; Nash, R.J. Garcinia kola and Garcinoic acid suppress SARS-CoV-2 spike glycoprotein S1-induced hyper-inflammation in human PBMCs through inhibition of NF-kappaB activation. Phytother. Res. 2021, 35, 6963–6973. [Google Scholar] [CrossRef]
- Austermeier, S.; Pekmezović, M.; Porschitz, P.; Lee, S.; Kichik, N.; Moyes, D.L.; Ho, J.; Kotowicz, N.K.; Naglik, J.R.; Hube, B.; et al. Albumin Neutralizes Hydrophobic Toxins and Modulates Candida albicans Pathogenicity. mBio 2021, 17, e1010037. [Google Scholar] [CrossRef]
- Bartolini, D.; De Franco, F.; Torquato, P.; Marinelli, R.; Cerra, B.; Ronchetti, R.; Schon, A.; Fallarino, F.; De Luca, A.; Bellezza, G.; et al. Garcinoic Acid Is a Natural and Selective Agonist of Pregnane X Receptor. J. Med. Chem. 2020, 63, 3701–3712. [Google Scholar] [CrossRef]
- Marinelli, R.; Torquato, P.; Bartolini, D.; Mas-Bargues, C.; Bellezza, G.; Gioiello, A.; Borras, C.; De Luca, A.; Fallarino, F.; Sebastiani, B.; et al. Garcinoic acid prevents beta-amyloid (Abeta) deposition in the mouse brain. J. Biol. Chem. 2020, 295, 11866–11876. [Google Scholar] [CrossRef]
- Varga, Z.; Kosaras, E.; Komodi, E.; Katko, M.; Karpati, I.; Balla, J.; Paragh, G.; Aisa, M.C.; Galli, F. Effects of tocopherols and 2,2′-carboxyethyl hydroxychromans on phorbol-ester-stimulated neutrophils. J. Nutr. Biochem. 2008, 19, 320–327. [Google Scholar] [CrossRef]
- Bartolini, D.; Torquato, P.; Barola, C.; Russo, A.; Rychlicki, C.; Giusepponi, D.; Bellezza, G.; Sidoni, A.; Galarini, R.; Svegliati-Baroni, G.; et al. Nonalcoholic fatty liver disease impairs the cytochrome P-450-dependent metabolism of alpha-tocopherol (vitamin E). J. Nutr. Biochem. 2017, 47, 120–131. [Google Scholar] [CrossRef]
- Torquato, P.; Bartolini, D.; Giusepponi, D.; Saluti, G.; Galarini, R.; Russo, A.; Birringer, M.; Lorkowski, S.; Borras, C.; Viña, J.; et al. Alpha-(13′-hydroxy)-6-hydroxychroman, the main product of alpha-tocopherol metabolism in human hepatocytes, regulates CYP4F2 and PPAR-γ expression. Free Radic. Biol. Med. 2017, 108 (Suppl. S1), S16. [Google Scholar] [CrossRef]
- Wächtler, B.; Wilson, D.; Hube, B. Candida albicans adhesion to and invasion and damage of vaginal epithelial cells: Stage-specific inhibition by clotrimazole and bifonazole. Antimicrob. Agents Chemother. 2011, 55, 4436–4439. [Google Scholar] [CrossRef]
- Remy, G.; Risco, A.M.; Inesta-Vaquera, F.A.; Gonzalez-Teran, B.; Sabio, G.; Davis, R.J.; Cuenda, A. Differential activation of p38MAPK isoforms by MKK6 and MKK3. Cell. Signal. 2010, 22, 660–667. [Google Scholar] [CrossRef]
- Luchetti, F.; Betti, M.; Canonico, B.; Arcangeletti, M.; Ferri, P.; Galli, F.; Papa, S. ERK MAPK activation mediates the antiapoptotic signaling of melatonin in UVB-stressed U937 cells. Free Radic. Biol. Med. 2009, 46, 339–351. [Google Scholar] [CrossRef]
- Russell, C.M.; Rybak, J.A.; Miao, J.; Peters, B.M.; Barrera, F.N. Candidalysin: Connecting the pore forming mechanism of this virulence factor to its immunostimulatory properties. J. Biol. Chem. 2023, 299, 102829. [Google Scholar] [CrossRef]
- Nikou, S.A.; Zhou, C.; Griffiths, J.S.; Kotowicz, N.K.; Coleman, B.M.; Green, M.J.; Moyes, D.L.; Gaffen, S.L.; Naglik, J.R.; Parker, P.J. The Candida albicans toxin candidalysin mediates distinct epithelial inflammatory responses through p38 and EGFR-ERK pathways. Sci. Signal. 2022, 15, eabj6915. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.P.; Willems, H.M.E.; Moyes, D.L.; Shoaie, S.; Barker, K.S.; Tan, S.L.; Palmer, G.E.; Hube, B.; Naglik, J.R.; Peters, B.M. Candidalysin Drives Epithelial Signaling, Neutrophil Recruitment, and Immunopathology at the Vaginal Mucosa. Infect. Immun. 2018, 86, e00645-17. [Google Scholar] [CrossRef] [PubMed]
- Dinh, C.P.; Ville, A.; Neukirch, K.; Viault, G.; Temml, V.; Koeberle, A.; Werz, O.; Schuster, D.; Stuppner, H.; Richomme, P.; et al. Structure-based design, semi-synthesis and anti-inflammatory activity of tocotrienolic amides as 5-lipoxygenase inhibitors. Eur. J. Med. Chem. 2020, 202, 112518. [Google Scholar] [CrossRef] [PubMed]

| pg/mL Mean ± SD | UCV | GA (25 μM) | C. albicans | C. albicans + GA (25 μM) |
|---|---|---|---|---|
| IL-6 | 18.19 ± 2.46 | 20.10 ± 1.43 | 55.19 ± 16.30 | 28.17 ± 1.61 ** |
| IL-1α | 85.58 ± 2.79 | 81.15 ± 4.35 | 90.23 ± 6.09 | 80.66 ± 4.56 * |
| IL-1β | 4.71 ± 1.80 | 3.81 ± 0.40 | 27.03 ± 2.41 | 13.10 ± 2.96 **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabbatini, S.; Zatini, L.; Narducci, E.; Rosati, L.; Ardizzoni, A.; Mencacci, A.; Rende, M.; Pericolini, E.; Galli, F.; Bartolini, D.; et al. Modulation of C. albicans-Induced Immune Response in Vaginal Epithelial Cells by Garcinoic Acid. Microorganisms 2024, 12, 2455. https://doi.org/10.3390/microorganisms12122455
Sabbatini S, Zatini L, Narducci E, Rosati L, Ardizzoni A, Mencacci A, Rende M, Pericolini E, Galli F, Bartolini D, et al. Modulation of C. albicans-Induced Immune Response in Vaginal Epithelial Cells by Garcinoic Acid. Microorganisms. 2024; 12(12):2455. https://doi.org/10.3390/microorganisms12122455
Chicago/Turabian StyleSabbatini, Samuele, Linda Zatini, Eleonora Narducci, Lucrezia Rosati, Andrea Ardizzoni, Antonella Mencacci, Mario Rende, Eva Pericolini, Francesco Galli, Desirée Bartolini, and et al. 2024. "Modulation of C. albicans-Induced Immune Response in Vaginal Epithelial Cells by Garcinoic Acid" Microorganisms 12, no. 12: 2455. https://doi.org/10.3390/microorganisms12122455
APA StyleSabbatini, S., Zatini, L., Narducci, E., Rosati, L., Ardizzoni, A., Mencacci, A., Rende, M., Pericolini, E., Galli, F., Bartolini, D., & Monari, C. (2024). Modulation of C. albicans-Induced Immune Response in Vaginal Epithelial Cells by Garcinoic Acid. Microorganisms, 12(12), 2455. https://doi.org/10.3390/microorganisms12122455











