One-Step Multiplex Real-Time Fluorescent Quantitative Reverse Transcription PCR for Simultaneous Detection of Four Waterfowl Viruses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viruses and Clinical Samples
2.2. Methods
2.2.1. Primers and TaqMan Probes
2.2.2. Extraction of Nucleic Acid
2.2.3. Construction of Standard Plasmid
2.2.4. Optimization of Single qRT-PCR Reaction System
2.2.5. Optimization of Multiplex qRT-PCR Detection
2.2.6. Specificity Analysis of Multiplex qRT-PCR
2.2.7. Sensitivity Analysis of Multiplex qRT-PCR
2.2.8. Repeatability Analysis of Multiplex qRT-PCR
2.2.9. Detection of Clinical Samples by Multiplex qRT-PCR
3. Results
3.1. Construction of Standard Recombinant Plasmid
3.2. The Optimal Parameters of Multiplex qRT-PCR
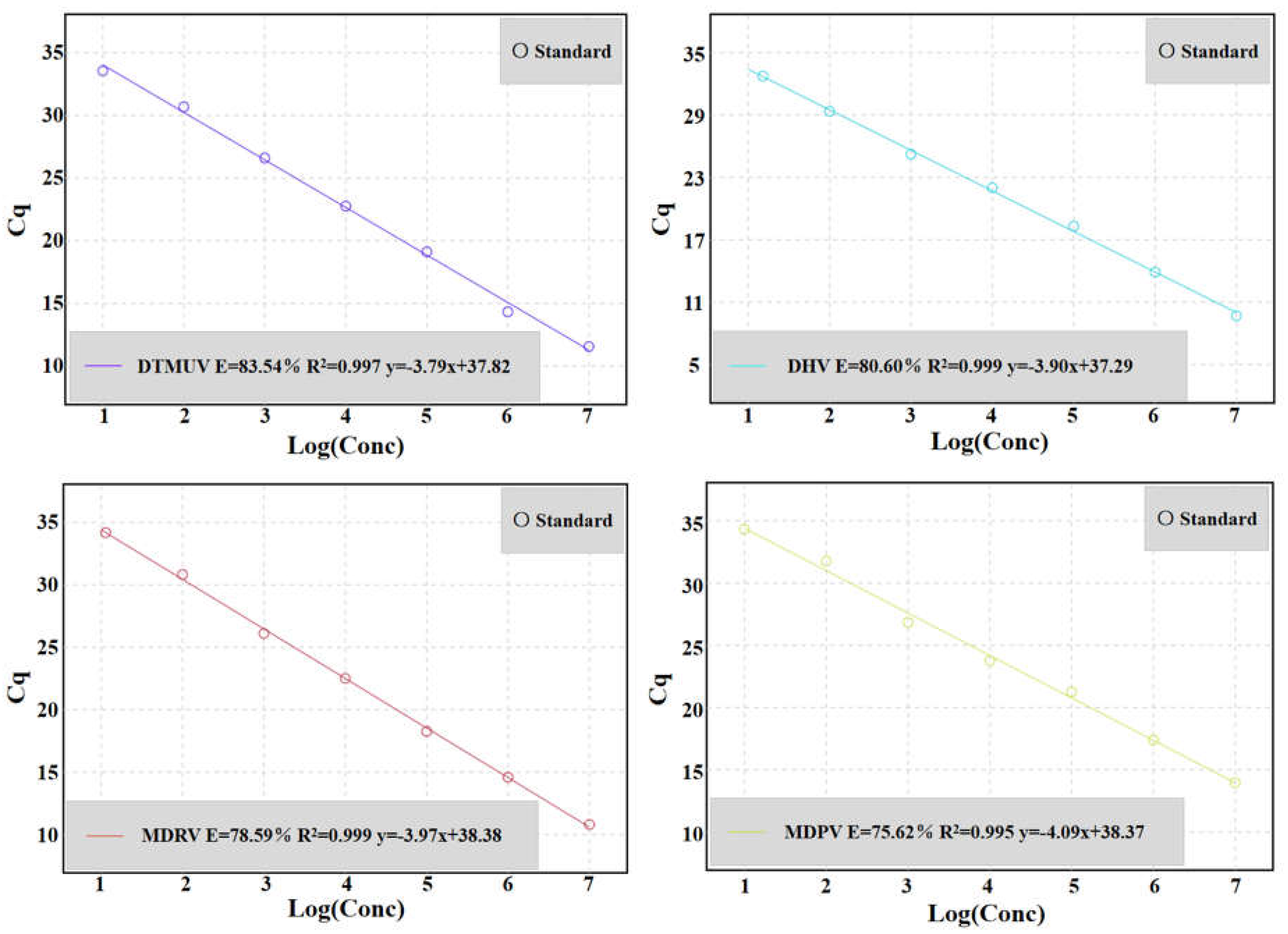
3.3. Standard Curve of Multiplex qRT-PCR
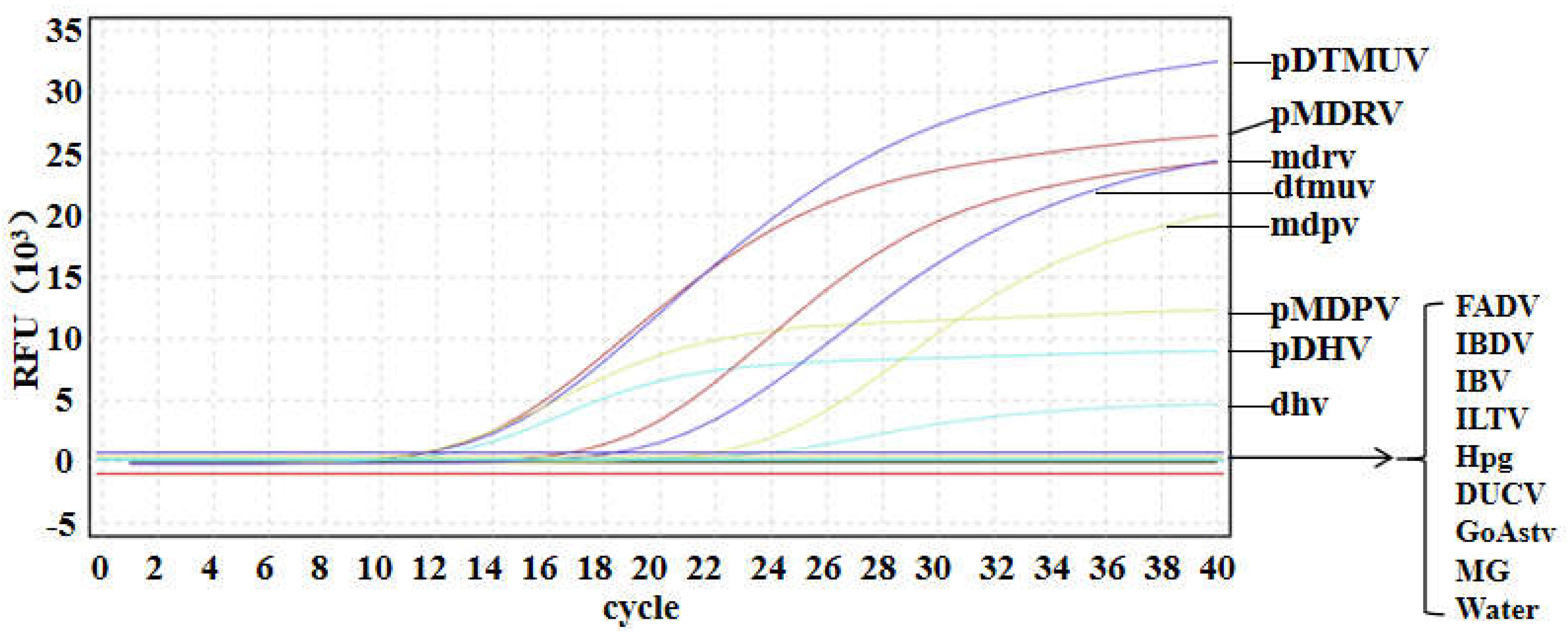
3.4. Specificity of Multiplex qRT-PCR
3.5. Sensitivity of Multiplex qRT-PCR
3.6. Repeatability of Multiplex qRT-PCR
3.7. Multiplex qRT-PCR Detection of Clinical Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, Z.; Zhang, C.; Liu, Y.; Liu, Y.; Ye, W.; Han, J.; Ma, G.; Zhang, D.; Xu, F.; Gao, X.; et al. Tembusu virus in ducks, china. Emerg. Infect. Dis. 2011, 17, 1873–1875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, S.; Mahalingam, S.; Wang, M.; Cheng, A. An updated review of avian-origin Tembusu virus: A newly emerging avian Flavivirus. J. Gen. Virol. 2017, 98, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Yu, S.; Duan, Y.; Hu, Y.; Qiu, X.; Tan, L.; Sun, Y.; Wang, M.; Cheng, A.; Ding, C. Effect of age on the pathogenesis of DHV-1 in Pekin ducks and on the innate immune responses of ducks to infection. Arch. Virol. 2014, 159, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Yun, T.; Yu, B.; Ni, Z.; Ye, W.; Chen, L.; Hua, J.; Zhang, C. Isolation and genomic characterization of a classical Muscovy duck reovirus isolated in Zhejiang, China. Infect. Genet. Evol. 2013, 20, 444–453. [Google Scholar] [CrossRef]
- Zhang, X.L.; Shao, J.W.; Li, X.W.; Mei, M.M.; Guo, J.Y.; Li, W.F.; Huang, W.J.; Chi, S.H.; Yuan, S.; Li, Z.L.; et al. Molecular characterization of two novel reoviruses isolated from Muscovy ducklings in Guangdong, China. BMC Vet. Res. 2019, 15, 143. [Google Scholar] [CrossRef]
- Li, K.P.; Hsu, Y.C.; Lin, C.A.; Chang, P.C.; Shien, J.H.; Liu, H.Y.; Yen, H.; Ou, S.C. Molecular Characterization and Pathogenicity of the Novel Recombinant Muscovy Duck Parvovirus Isolated from Geese. Animals 2021, 11, 3211. [Google Scholar] [CrossRef]
- Dong, J.; Bingga, G.; Sun, M.; Li, L.; Liu, Z.; Zhang, C.; Guo, P.; Huang, Y.; Zhang, J. Application of high-resolution melting curve analysis for identification of Muscovy duck parvovirus and goose parvovirus. J. Virol. Methods 2019, 266, 121–125. [Google Scholar] [CrossRef]
- Fan, W.; Sun, Z.; Shen, T.; Xu, D.; Huang, K.; Zhou, J.; Song, S.; Yan, L. Analysis of Evolutionary Processes of Species Jump in Waterfowl Parvovirus. Front. Microbiol. 2017, 8, 421. [Google Scholar] [CrossRef]
- Sanisuriwong, J.; Yurayart, N.; Thontiravong, A.; Tiawsirisup, S. Duck Tembusu virus detection and characterization from mosquitoes in duck farms, Thailand. Transbound. Emerg. Dis. 2020, 67, 1082–1088. [Google Scholar] [CrossRef]
- Tunterak, W.; Prakairungnamthip, D.; Ninvilai, P.; Tiawsirisup, S.; Oraveerakul, K.; Sasipreeyajan, J.; Amonsin, A.; Thontiravong, A. Patterns of duck Tembusu virus infection in ducks, Thailand: A serological study. Poult. Sci. 2021, 100, 537–542. [Google Scholar] [CrossRef]
- Platt, G.S.; Way, H.J.; Bowen, E.T.; Simpson, D.I.; Hill, M.N.; Kamath, S.; Bendell, P.J.; Heathcote, O.H. Arbovirus infections in Sarawak, October 1968–February 1970 Tembusu and Sindbis virus isolations from mosquitoes. Ann. Trop. Med. Parasitol. 1975, 69, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Ninvilai, P.; Nonthabenjawan, N.; Limcharoen, B.; Tunterak, W.; Oraveerakul, K.; Banlunara, W.; Amonsin, A.; Thontiravong, A. The presence of duck Tembusu virus in Thailand since 2007: A retrospective study. Transbound. Emerg. Dis. 2018, 65, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, J.; Yang, Y.; Zeng, Y.; Liu, S. Innate immune responses to duck Tembusu virus infection. Vet. Res. 2020, 51, 87. [Google Scholar] [CrossRef]
- He, Y.; Wang, A.; Chen, S.; Wu, Z.; Zhang, J.; Wang, M.; Jia, R.; Zhu, D.; Liu, M.; Yang, Q.; et al. Differential immune-related gene expression in the spleens of duck Tembusu virus-infected goslings. Vet. Microbiol. 2017, 212, 39–47. [Google Scholar] [CrossRef]
- Yang, Q.; Ding, Y.; Yao, W.; Chen, S.; Jiang, Y.; Yang, L.; Bao, G.; Yang, K.; Fan, S.; Du, Q.; et al. Pathogenicity and Interspecies Transmission of Cluster 3 Tembusu Virus Strain TMUV HQ-22 Isolated from Geese. Viruses 2023, 15, 2449. [Google Scholar] [CrossRef]
- Tang, Y.; Diao, Y.; Yu, C.; Gao, X.; Ju, X.; Xue, C.; Liu, X.; Ge, P.; Qu, J.; Zhang, D. Characterization of a Tembusu virus isolated from naturally infected house sparrows (Passer domesticus) in Northern China. Transbound. Emerg. Dis. 2013, 60, 152–158. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Chen, H.; Ti, J.; Yang, G.; Zhang, L.; Lu, Y.; Diao, Y. Evidence of possible vertical transmission of Tembusu virus in ducks. Vet. Microbiol. 2015, 179, 149–154. [Google Scholar] [CrossRef]
- Pulmanausahakul, R.; Ketsuwan, K.; Jaimipuk, T.; Smith, D.R.; Auewarakul, P.; Songserm, T. Detection of antibodies to duck tembusu virus in human population with or without the history of contact with ducks. Transbound. Emerg. Dis. 2022, 69, 870–873. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, R.; Wu, Q.; Chen, J.; Wang, A.; Wu, Z.; Sun, F.; Zhu, S. Advancements in Research on Duck Tembusu Virus Infections. Viruses 2024, 16, 811. [Google Scholar] [CrossRef]
- Yang, S.; Huang, Y.; Shi, Y.; Bai, X.; Yang, P.; Chen, Q. Tembusu Virus entering the central nervous system caused nonsuppurative encephalitis without disrupting the blood-brain barrier. J. Virol. 2021, 95, e02191–e02220. [Google Scholar] [CrossRef]
- Huang, Y.; Chu, X.; Zhang, Y.; Yang, S.; Shi, Y.; Wu, J.; Chen, Q. Duck Tembusu virus infection causes testicular atrophy. Theriogenology 2022, 188, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Xu, Q.; Zhang, R.H.; Yang, L.; Li, J.X.; Xie, Z.J.; Zhu, Y.L.; Jiang, S.J.; Si, X.K. Improved duplex RT-PCR assay for differential diagnosis of mixed infection of duck hepatitis A virus type 1 and type 3 in ducklings. J. Virol. Methods 2013, 192, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Fehér, E.; Jakab, S.; Bali, K.; Kaszab, E.; Nagy, B.; Ihász, K.; Bálint, Á.; Palya, V.; Bányai, K. Genomic Epidemiology and Evolution of Duck Hepatitis A Virus. Viruses 2021, 13, 1592. [Google Scholar] [CrossRef] [PubMed]
- Doan, H.T.; Le, X.T.; Do, R.T.; Hoang, C.T.; Nguyen, K.T.; Le, T.H. Molecular genotyping of duck hepatitis A viruses (DHAV) in Vietnam. J. Infect. Dev. Ctries. 2016, 10, 988–995. [Google Scholar] [CrossRef]
- Sheng, X.D.; Zhang, W.P.; Zhang, Q.R.; Gu, C.Q.; Hu, X.Y.; Cheng, G.F. Apoptosis induction in duck tissues during duck hepatitis A virus type 1 infection. Poult. Sci. 2014, 93, 527–534. [Google Scholar] [CrossRef]
- Hisham, I.; Ellakany, H.F.; Selim, A.A.; Abdalla, M.A.M.; Zain El-Abideen, M.A.; Kilany, W.H.; Ali, A.; Elbestawy, A.R. Comparative Pathogenicity of Duck Hepatitis A Virus-1 Isolates in Experimentally Infected Pekin and Muscovy Ducklings. Front. Vet. Sci. 2020, 7, 234. [Google Scholar] [CrossRef]
- Yugo, D.M.; Hauck, R.; Shivaprasad, H.L.; Meng, X.J. Hepatitis Virus Infections in Poultry. Avian Dis. 2016, 60, 576–588. [Google Scholar] [CrossRef]
- Cova, L.; Lambert, V.; Chevallier, A.; Hantz, O.; Fourel, I.; Jacquet, C.; Pichoud, C.; Boulay, J.; Chomel, B.; Vitvitski, L.; et al. Evidence for the presence of duck hepatitis B virus in wild migrating ducks. J. Gen. Virol. 1986, 67 Pt 3, 537–547. [Google Scholar] [CrossRef]
- Freiman, J.S.; Cossart, Y.E. Natural duck hepatitis B virus infection in Australia. Aust. J. Exp. Biol. Med. Sci. 1986, 64 Pt 5, 477–484. [Google Scholar] [CrossRef]
- Sridhar, G.; Valliammai, T.; Varalakshmi, C.S.; Udayasankar, K.; Panchanadam, M.; Ramakrishna, J.; Gopal, K.V.; Jayaraman, K.; Thyagarajan, S.P. Duck hepatitis B virus (DHBV) infection in Indian domestic ducks: A pilot study. Trop. Anim. Health Prod. 1993, 25, 229–233. [Google Scholar] [CrossRef]
- Kim, M.C.; Kwon, Y.K.; Joh, S.J.; Kwon, J.H.; Lindberg, A.M. Differential diagnosis between type-specific duck hepatitis virus type 1 (DHV-1) and recent Korean DHV-1-like isolates using a multiplex polymerase chain reaction. Avian Pathol. 2008, 37, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zhu, D.; Cheng, A.; Wang, M.; Chen, S.; Jia, R.; Liu, M.; Sun, K.; Zhao, X.; Yang, Q.; et al. Molecular epidemiology of duck hepatitis a virus types 1 and 3 in China, 2010–2015. Transbound. Emerg. Dis. 2018, 65, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Yehia, N.; Erfan, A.M.; Omar, S.E.; Soliman, M.A. Dual Circulation of Duck Hepatitis A Virus Genotypes 1 and 3 in Egypt. Avian Dis. 2021, 65, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Fanyi, M.; Li, X.J.; Zhang, Z.; Liu, S.; Zhang, Y. Goose haemorrhagic hepatitis caused by a new subtype duck hepatitis type 1 virus. Vet. Microbiol. 2011, 152, 280–283. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, Y.; Lan, J.; Xie, Z.; Zhang, X.; Jiang, S. Evidence of possible vertical transmission of duck hepatitis A virus type 1 in ducks. Transbound. Emerg. Dis. 2021, 68, 267–275. [Google Scholar] [CrossRef]
- Yang, C.; Shah, P.T.; Bahoussi, A.N.; Wu, C.; Wang, L.; Xing, L. Duck hepatitis a virus: Full-length genome-based phylogenetic and phylogeographic view during 1986–2020. Virus Res. 2023, 336, 199216. [Google Scholar] [CrossRef]
- Niu, Y.; Ma, H.; Ding, Y.; Li, Z.; Sun, Y.; Li, M.; Shi, Y. The pathogenicity of duck hepatitis A virus types 1 and 3 on ducklings. Poult. Sci. 2019, 98, 6333–6339. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, M.; Huang, M.; Xiao, S.; Lin, F.; Chen, S.; Chen, S. Muscovy Duck Reovirus Infection Disrupts the Composition of Intestinal Microbiota in Muscovy Ducklings. Curr. Microbiol. 2020, 77, 769–778. [Google Scholar] [CrossRef]
- Wang, D.; Shi, J.; Yuan, Y.; Zheng, L.; Zhang, D. Complete sequence of a reovirus associated with necrotic focus formation in the liver and spleen of Muscovy ducklings. Vet. Microbiol. 2013, 166, 109–122. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, G.; Wang, Q.; Wang, S.; Chi, X.; Huang, Y.; Wei, H.; Wu, B.; Huang, S.; Chen, J.L. Muscovy duck reovirus infection rapidly activates host innate immune signaling and induces an effective antiviral immune response involving critical interferons. Vet. Microbiol. 2015, 175, 232–243. [Google Scholar] [CrossRef]
- Gaudry, D.; Charles, J.M.; Tektoff, J. [A new disease expressing itself by a viral pericarditis in Barbary ducks]. C. R. Acad. Hebd. Seances Acad. Sci. D 1972, 274, 2916–2919. [Google Scholar] [PubMed]
- Malkinson, M.; Perk, K.; Weisman, Y. Reovirus infection of young Muscovy ducks (Cairina moschata). Avian Pathol. 1981, 10, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Yun, T.; Yu, B.; Ni, Z.; Ye, W.; Chen, L.; Hua, J.; Zhang, C. Genomic characteristics of a novel reovirus from Muscovy duckling in China. Vet. Microbiol. 2014, 168, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Benavente, J.; Martínez-Costas, J. Avian reovirus: Structure and biology. Virus Res. 2007, 123, 105–119. [Google Scholar] [CrossRef]
- Heffels-Redmann, U.; Muller, H.; Kaleta, E.F. Structural and biological characteristics of reoviruses isolated from Muscovy ducks (Cairina moschata). Avian Pathol. 1992, 21, 481–491. [Google Scholar] [CrossRef]
- Liu, Z.; Liao, L.; Chen, Q.; Lin, S.; Luo, Y.; Qin, T.; Li, J.; Wang, Q.; Wu, B.; Huang, Y.; et al. Effects of Hericium erinaceus polysaccharide on immunity and apoptosis of the main immune organs in Muscovy duck reovirus-infected ducklings. Int. J. Biol. Macromol. 2021, 171, 448–456. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, H.; Zhu, E.; Li, J.; Wang, Q.; Zhou, W.; Qin, T.; Wu, X.; Wu, B.; Huang, Y. Hericium erinaceus polysaccharide facilitates restoration of injured intestinal mucosal immunity in Muscovy duck reovirus-infected Muscovy ducklings. Int. J. Biol. Macromol. 2018, 107, 1151–1161. [Google Scholar] [CrossRef]
- Wan, C.; Chen, C.; Cheng, L.; Chen, H.; Fu, Q.; Shi, S.; Fu, G.; Liu, R.; Huang, Y. Specific detection of Muscovy duck parvovirus infection by TaqMan-based real-time PCR assay. BMC Vet. Res. 2018, 14, 267. [Google Scholar] [CrossRef]
- Dong, H.V.; Tran, G.T.H.; Nguyen, H.T.T.; Nguyen, T.M.; Trinh, D.Q.; Le, V.P.; Choowongkomon, K.; Rattanasrisomporn, J. Epidemiological Analysis and Genetic Characterization of Parvovirus in Ducks in Northern Vietnam Reveal Evidence of Recombination. Animals 2022, 12, 2846. [Google Scholar] [CrossRef]
- Chen, H.; Dou, Y.; Tang, Y.; Zheng, X.; Niu, X.; Yang, J.; Yu, X.; Diao, Y. Experimental reproduction of beak atrophy and dwarfism syndrome by infection in cherry valley ducklings with a novel goose parvovirus-related parvovirus. Vet. Microbiol. 2016, 183, 16–20. [Google Scholar] [CrossRef]
- Woolcock, P.R.; Jestin, V.; Shivaprasad, H.L.; Zwingelstein, F.; Arnauld, C.; McFarland, M.D.; Pedersen, J.C.; Senne, D.A. Evidence of Muscovy duck parvovirus in Muscovy ducklings in California. Vet. Rec. 2000, 146, 68–72. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, Y.; Hu, Z.; Zhang, L.; Shao, G.; Xie, Z.; Nie, Y.; Li, W.; Li, Y.; Chen, L.; et al. Recombinant Muscovy Duck Parvovirus Led to Ileac Damage in Muscovy Ducklings. Viruses 2022, 14, 1471. [Google Scholar] [CrossRef] [PubMed]
- Wozniakowski, G.; Kozdrun, W.; Samorek-Salamonowicz, E. Genetic variance of Derzsy’s disease strains isolated in Poland. J. Mol. Genet. Med. 2009, 3, 210–216. [Google Scholar] [CrossRef]
- Maurin-Bernaud, L.; Goutebroze, S.; Merdy, O.; Chanay, A.; Cozette, V.; Le Gros, F.X. Efficacy of a new attenuated duck parvovirosis vaccine in Muscovy ducks. Vet. Rec. 2014, 175, 281. [Google Scholar] [CrossRef]
- Zhao, H.; Xie, Z.; Xie, L.; Deng, X.; Xie, Z.; Luo, S.; Huang, L.; Huang, J.; Zeng, T. Molecular characterization of the full muscovy duck parvovirus, isolated in Guangxi, China. Genome Announc. 2014, 2, e01249–e01314. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, Z.; Huang, Y.; Yu, R.; Dong, S.; Li, Z.; Zhang, Y. Identification of a recombinant Muscovy Duck parvovirus (MDPV) in Shanghai, China. Vet. Microbiol. 2014, 174, 560–564. [Google Scholar] [CrossRef]
- Glávits, R.; Zolnai, A.; Szabó, E.; Ivanics, E.; Zarka, P.; Mató, T.; Palya, V. Comparative pathological studies on domestic geese (Anser anser domestica) and Muscovy ducks (Cairina moschata) experimentally infected with parvovirus strains of goose and Muscovy duck origin. Acta Vet. Hung. 2005, 53, 73–89. [Google Scholar] [CrossRef]
- Fu, Q.; Huang, Y.; Wan, C.; Fu, G.; Qi, B.; Cheng, L.; Shi, S.; Chen, H.; Liu, R.; Chen, Z. Genomic and pathogenic analysis of a Muscovy duck parvovirus strain causing short beak and dwarfism syndrome without tongue protrusion. Res. Vet. Sci. 2017, 115, 393–400. [Google Scholar] [CrossRef]
- Chen, H.; Dou, Y.; Tang, Y.; Zhang, Z.; Zheng, X.; Niu, X.; Yang, J.; Yu, X.; Diao, Y. Isolation and Genomic Characterization of a Duck-Origin GPV-Related Parvovirus from Cherry Valley Ducklings in China. PLoS ONE 2015, 10, e0140284. [Google Scholar] [CrossRef]
- Niu, X.; Wang, H.; Wei, L.; Zhang, M.; Yang, J.; Chen, H.; Tang, Y.; Diao, Y. Epidemiological investigation of H9 avian influenza virus, Newcastle disease virus, Tembusu virus, goose parvovirus and goose circovirus infection of geese in China. Transbound. Emerg. Dis. 2018, 65, e304–e316. [Google Scholar] [CrossRef]
- Petz, L.N.; Turell, M.J.; Padilla, S.; Long, L.S.; Reinbold-Wasson, D.D.; Smith, D.R.; O’Guinn, M.L.; Melanson, V.R.; Lee, J.S. Development of conventional and real-time reverse transcription polymerase chain reaction assays to detect Tembusu virus in Culex tarsalis mosquitoes. Am. J. Trop. Med. Hyg. 2014, 91, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Zhang, X.; Gao, Y.; Song, S.; Xu, D.; Yan, L. Development and application of multiplex PCR method for simultaneous detection of seven viruses in ducks. BMC Vet. Res. 2019, 15, 103. [Google Scholar] [CrossRef]
- Gong, H.; Fan, Y.; Zhou, P.; Li, Y.; Hu, X.; Jin, H.; Luo, R. Identification of a linear epitope within domain I of Duck Tembusu virus envelope protein using a novel neutralizing monoclonal antibody. Dev. Comp. Immunol. 2021, 115, 103906. [Google Scholar] [CrossRef]
- Kim, M.C.; Kwon, Y.K.; Joh, S.J.; Kwon, J.H.; Kim, J.H.; Kim, S.J. Development of one-step reverse transcriptase-polymerase chain reaction to detect duck hepatitis virus type 1. Avian Dis. 2007, 51, 540–545. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, X.; Wang, S.; Wang, J.; Huang, M.; Xiao, S.; Cheng, X.; Chen, S.; Chen, X.; Lin, F.; et al. A TaqMan-MGB real-time RT-PCR assay with an internal amplification control for rapid detection of Muscovy duck reovirus. Mol. Cell. Probes 2020, 52, 101575. [Google Scholar] [CrossRef]
- Yin, Y.; Xiong, C.; Shi, K.; Long, F.; Feng, S.; Qu, S.; Lu, W.; Huang, M.; Lin, C.; Sun, W.; et al. Multiplex digital PCR: A superior technique to qPCR for the simultaneous detection of duck Tembusu virus, duck circovirus, and new duck reovirus. Front. Vet. Sci. 2023, 10, 1222789. [Google Scholar] [CrossRef]
- Yin, Y.W.; Xiong, C.; Shi, K.C.; Xie, S.Y.; Long, F.; Li, J.; Zheng, M.; Wei, X.K.; Feng, S.; Qu, S.; et al. Development and application of a multiplex qPCR assay for the detection of duck circovirus, duck Tembusu virus, Muscovy duck reovirus, and new duck reovirus. Virus Genes. 2023, 59, 91–99. [Google Scholar] [CrossRef]
- Liu, J.T.; Chen, Y.H.; Pei, Y.F.; Yu, Q.; Afumba, R.; Dong, H. Rapid and visual detection of an isolated and identified goose parvovirus (GPV) strain by a loop-mediated isothermal amplification assay. Vet. Res. Forum 2023, 14, 7–12. [Google Scholar] [CrossRef]
- Niu, X.; Chen, H.; Yang, J.; Yu, X.; Ti, J.; Wang, A.; Diao, Y. Development of a TaqMan-based real-time PCR assay for the detection of Novel GPV. J. Virol. Methods 2016, 237, 32–37. [Google Scholar] [CrossRef]
- Agrimonti, C.; Bottari, B.; Sardaro, M.L.S.; Marmiroli, N. Application of real-time PCR (qPCR) for characterization of microbial populations and type of milk in dairy food products. Crit. Rev. Food Sci. Nutr. 2019, 59, 423–442. [Google Scholar] [CrossRef]
- Sonawane, G.G.; Tripathi, B.N. Comparison of a quantitative real-time polymerase chain reaction (qPCR) with conventional PCR, bacterial culture and ELISA for detection of Mycobacterium avium subsp. paratuberculosis infection in sheep showing pathology of Johne’s disease. Springerplus 2013, 2, 45. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, J.; De Preter, K.; Lefever, S.; Nuytens, J.; De Vloed, F.; Derveaux, S.; Hellemans, J.; Speleman, F.; Vandesompele, J. Measurable impact of RNA quality on gene expression results from quantitative PCR. Nucleic Acids Res. 2011, 39, e63. [Google Scholar] [CrossRef]
- Thornton, B.; Basu, C. Rapid and simple method of qPCR primer design. Methods Mol. Biol. 2015, 1275, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Hijjawi, N.; Yang, R.; Hatmal, M.; Yassin, Y.; Mharib, T.; Mukbel, R.; Mahmoud, S.A.; Al-Shudifat, A.E.; Ryan, U. Comparison of ELISA, nested PCR and sequencing and a novel qPCR for detection of Giardia isolates from Jordan. Exp. Parasitol. 2018, 185, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Bashir, I.M.; Otsyula, N.; Awinda, G.; Spring, M.; Schneider, P.; Waitumbi, J.N. Comparison of PfHRP-2/pLDH ELISA, qPCR and microscopy for the detection of plasmodium events and prediction of sick visits during a malaria vaccine study. PLoS ONE 2013, 8, e56828. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.; Bustin, S.A. Reliability of real-time reverse-transcription PCR in clinical diagnostics: Gold standard or substandard? Expert Rev. Mol. Diagn. 2009, 9, 187–197. [Google Scholar] [CrossRef]
- Bustin, S.A. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 2000, 25, 169–193. [Google Scholar] [CrossRef]
- Weis, J.H.; Tan, S.S.; Martin, B.K.; Wittwer, C.T. Detection of rare mRNAs via quantitative RT-PCR. Trends Genet. 1992, 8, 263–264. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, M.; Tang, Z.; Xu, D.; Luo, Y.; Gao, Y.; Yan, L. Development and application of a triplex real-time PCR assay for simultaneous detection of avian influenza virus, Newcastle disease virus, and duck Tembusu virus. BMC Vet. Res. 2020, 16, 203. [Google Scholar] [CrossRef]
- Liu, H.; Shi, K.; Zhao, J.; Yin, Y.; Chen, Y.; Si, H.; Qu, S.; Long, F.; Lu, W. Development of a one-step multiplex qRT-PCR assay for the detection of African swine fever virus, classical swine fever virus and atypical porcine pestivirus. BMC Vet. Res. 2022, 18, 43. [Google Scholar] [CrossRef]
- Li, H.; Wan, C.; Wang, Z.; Tan, J.; Tan, M.; Zeng, Y.; Huang, J.; Huang, Y.; Su, Q.; Kang, Z.; et al. Rapid diagnosis of duck Tembusu virus and goose astrovirus with TaqMan-based duplex real-time PCR. Front. Microbiol. 2023, 14, 1146241. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Dong, J.; Li, L.; Lin, Q.; Sun, J.; Liu, Z.; Shen, H.; Zhang, J.; Ren, T.; Zhang, C. Recombinant Newcastle disease virus (NDV) expressing Duck Tembusu virus (DTMUV) pre-membrane and envelope proteins protects ducks against DTMUV and NDV challenge. Vet. Microbiol. 2018, 218, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Y.; Dong, J.; Zhang, J.; Zhang, C.; Sun, M.; Cao, Y. The truncated E protein of DTMUV provide protection in young ducks. Vet. Microbiol. 2020, 240, 108508. [Google Scholar] [CrossRef]
- Dermody, T.S.; Schiff, L.A.; Nibert, M.L.; Coombs, K.M.; Fields, B.N. The S2 gene nucleotide sequences of prototype strains of the three reovirus serotypes: Characterization of reovirus core protein sigma 2. J. Virol. 1991, 65, 5721–5731. [Google Scholar] [CrossRef]
- Yu, T.F.; Li, M. Identification of recombination among VP1 gene of Muscovy duck parvovirus from the Mainland of China. Vet. Microbiol. 2016, 195, 78–80. [Google Scholar] [CrossRef]

| Primers and Probes | Sequence (5′→3′) | Concentration (μM) | Product Size (bp) |
|---|---|---|---|
| DTMUV-F | AAGCTTTCACGTCAACAC | 10 | 176 |
| DTMUV-R | CATGCCTTGAGTAATCCACGA | 10 | |
| DTMUV-Q | ACTGAGCCAAAATCCCATGC | 10 | |
| DHV-F | ACTTTTCTGGTTTTGACGG | 10 | 173 |
| DHV-R | TGAGCACATACCACCTTC | 10 | |
| DHV-Q | TTCACAAGGGCTGGATCGTT | 10 | |
| MDRV-F | CCCAATGTTGTGGCGTTCTA | 10 | 65 |
| MDRV-R | ATGGTGCGGGAAGCAAAC | 10 | |
| MDRV-Q | ATTATGGCGCGCCTCCAACGG | 10 | |
| MDPV-F | TTTACGGATGACGAGCATCAAC | 10 | 70 |
| MDPV-R | GGAACGGCGGCATGGT | 10 | |
| MDPV-Q | CCCGTATGTCCTGGGCTCGGC | 10 |
| Plasmid | Concentration (Copies/μL) | 2.68 × 106 | 2.68 × 105 | 2.68 × 104 | 2.68 × 103 | 2.68 × 102 | 2.68 × 101 | 2.68 × 100 | 2.68 × 10−1 |
|---|---|---|---|---|---|---|---|---|---|
| DTMUV | Singleplex qRT-PCR | 11.38 | 13.89 | 19.02 | 21.93 | 26.72 | 31.06 | 33.17 | (none) |
| Multiplex qRT-PCR | 11.56 | 14.33 | 19.12 | 22.76 | 26.60 | 30.68 | 33.55 | (none) | |
| DHV | Singleplex qRT-PCR | 9.78 | 13.47 | 17.19 | 21.34 | 24.67 | 30.01 | 31.93 | (none) |
| Multiplex qRT-PCR | 9.69 | 13.90 | 18.31 | 22.00 | 25.24 | 29.34 | 32.74 | (none) | |
| MDRV | Singleplex qRT-PCR | 10.49 | 15.19 | 18.43 | 22.61 | 27.07 | 31.23 | 35.02 | (none) |
| Multiplex qRT-PCR | 10.80 | 14.58 | 18.27 | 22.51 | 26.11 | 30.83 | 34.19 | (none) | |
| MDPV | Singleplex qRT-PCR | 9.17 | 13.43 | 17.61 | 21.54 | 25.18 | 30.50 | 33.48 | (none) |
| Multiplex qRT-PCR | 9.75 | 13.86 | 18.50 | 21.50 | 25.21 | 31.11 | 34.18 | (none) |
| Plasmid | Concentration (Copies/μL) | Cq Values of Intra-Assay | Cq Value of Inter-Asssay | ||||
|---|---|---|---|---|---|---|---|
| SD | CV (%) | SD | CV (%) | ||||
| DTMUV | 2.68 × 106 | 11.29 | 0.36 | 1.34 | 11.31 | 0.28 | 1.36 |
| 2.68 × 104 | 19.45 | 0.15 | 0.96 | 19.54 | 0.16 | 1.23 | |
| 2.68 × 102 | 26.69 | 0.18 | 0.83 | 26.73 | 0.20 | 0.78 | |
| DHV | 2.68 × 106 | 9.82 | 0.24 | 1.41 | 9.76 | 0.15 | 1.21 |
| 2.68 × 104 | 17.63 | 0.17 | 0.68 | 17.59 | 0.16 | 0.93 | |
| 2.68 × 102 | 24.84 | 0.15 | 0.72 | 24.91 | 0.15 | 0.98 | |
| MDRV | 2.68 × 106 | 10.58 | 0.16 | 1.37 | 10.62 | 0.23 | 1.27 |
| 2.68 × 104 | 18.52 | 0.14 | 1.04 | 18.47 | 0.18 | 1.32 | |
| 2.68 × 102 | 26.37 | 0.25 | 0.81 | 26.32 | 0.25 | 0.98 | |
| MDPV | 2.68 × 106 | 9.09 | 0.21 | 1.28 | 8.95 | 0.13 | 0.84 |
| 2.68 × 104 | 18.70 | 0.18 | 0.79 | 18.64 | 0.16 | 1.15 | |
| 2.68 × 102 | 25.36 | 0.15 | 0.87 | 25.47 | 0.15 | 0.63 | |
| Date | Numbers | DTMUV (%) | DHV (%) | MDRV (%) | MDPV (%) | DTMUV + DHV (%) | DHV + MDRV (%) | MDRV + MDPV (%) | DTMUV + MDPV (%) |
|---|---|---|---|---|---|---|---|---|---|
| October 2021 | 12 | 1 (8.33) | 1 (8.33) | 0 (0) | 0 (0) | 1 (8.33) | 0 (0) | 0 (0) | 0 (0) |
| November 2021 | 10 | 0 (0) | 0 (0) | 0 (0) | 1 (10.00) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| January 2022 | 9 | 1 (11.11) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| April 2022 | 27 | 2 (7.40) | 1 (3.70) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| May 2022 | 39 | 5 (12.82) | 1 (2.56) | 2 (5.13) | 1 (2.56) | 1 (2.56) | 0 (0) | 0 (0) | 0 (0) |
| October 2022 | 35 | 3 (8.57) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.86) | 0 (0) |
| November 2022 | 20 | 0 (0) | 1 (5.00) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| December 2022 | 34 | 2 (5.89) | 0 (0) | 0 (0) | 3 (8.82) | 0 (0) | 0 (0) | 0 (0) | 1 (2.94) |
| January 2023 | 26 | 2 (7.69) | 0 (0) | 1 (3.85) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| March 2023 | 21 | 1 (4.76) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| April 2023 | 19 | 0 (0) | 2 (10.53) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| September 2023 | 16 | 0 (0) | 1 (6.25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| October 2023 | 28 | 2 (7.14) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3.57) | 0 (0) | 0 (0) |
| December 2023 | 30 | 3 (10.00) | 0 (0) | 2 (6.67) | 1 (3.33) | 0 (0) | 0 (0) | 0 (0) | 2 (6.67) |
| Total | 326 | 22 (6.75) | 7 (2.15) | 5 (1.53) | 6 (1.84) | 2 (0.61) | 1 (0.31) | 1 (0.31) | 3 (0.92) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Liu, H.; Cheng, J.; Pan, S.; Yang, W.; Wei, X.; Cheng, Y.; Xu, T.; Si, H. One-Step Multiplex Real-Time Fluorescent Quantitative Reverse Transcription PCR for Simultaneous Detection of Four Waterfowl Viruses. Microorganisms 2024, 12, 2423. https://doi.org/10.3390/microorganisms12122423
Wang C, Liu H, Cheng J, Pan S, Yang W, Wei X, Cheng Y, Xu T, Si H. One-Step Multiplex Real-Time Fluorescent Quantitative Reverse Transcription PCR for Simultaneous Detection of Four Waterfowl Viruses. Microorganisms. 2024; 12(12):2423. https://doi.org/10.3390/microorganisms12122423
Chicago/Turabian StyleWang, Chenchen, Huixin Liu, Junze Cheng, Sijia Pan, Wenwen Yang, Xiaofang Wei, Yue Cheng, Ting Xu, and Hongbin Si. 2024. "One-Step Multiplex Real-Time Fluorescent Quantitative Reverse Transcription PCR for Simultaneous Detection of Four Waterfowl Viruses" Microorganisms 12, no. 12: 2423. https://doi.org/10.3390/microorganisms12122423
APA StyleWang, C., Liu, H., Cheng, J., Pan, S., Yang, W., Wei, X., Cheng, Y., Xu, T., & Si, H. (2024). One-Step Multiplex Real-Time Fluorescent Quantitative Reverse Transcription PCR for Simultaneous Detection of Four Waterfowl Viruses. Microorganisms, 12(12), 2423. https://doi.org/10.3390/microorganisms12122423







