Delayed Sowing Reduced Verticillium Wilt by Altering Soil Temperature and Humidity to Enhance Beneficial Rhizosphere Bacteria of Sunflower
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Delayed Sowing Date Reduced the Severity of SVW
3.2. Effect of Sowing Date on Sunflower Yield
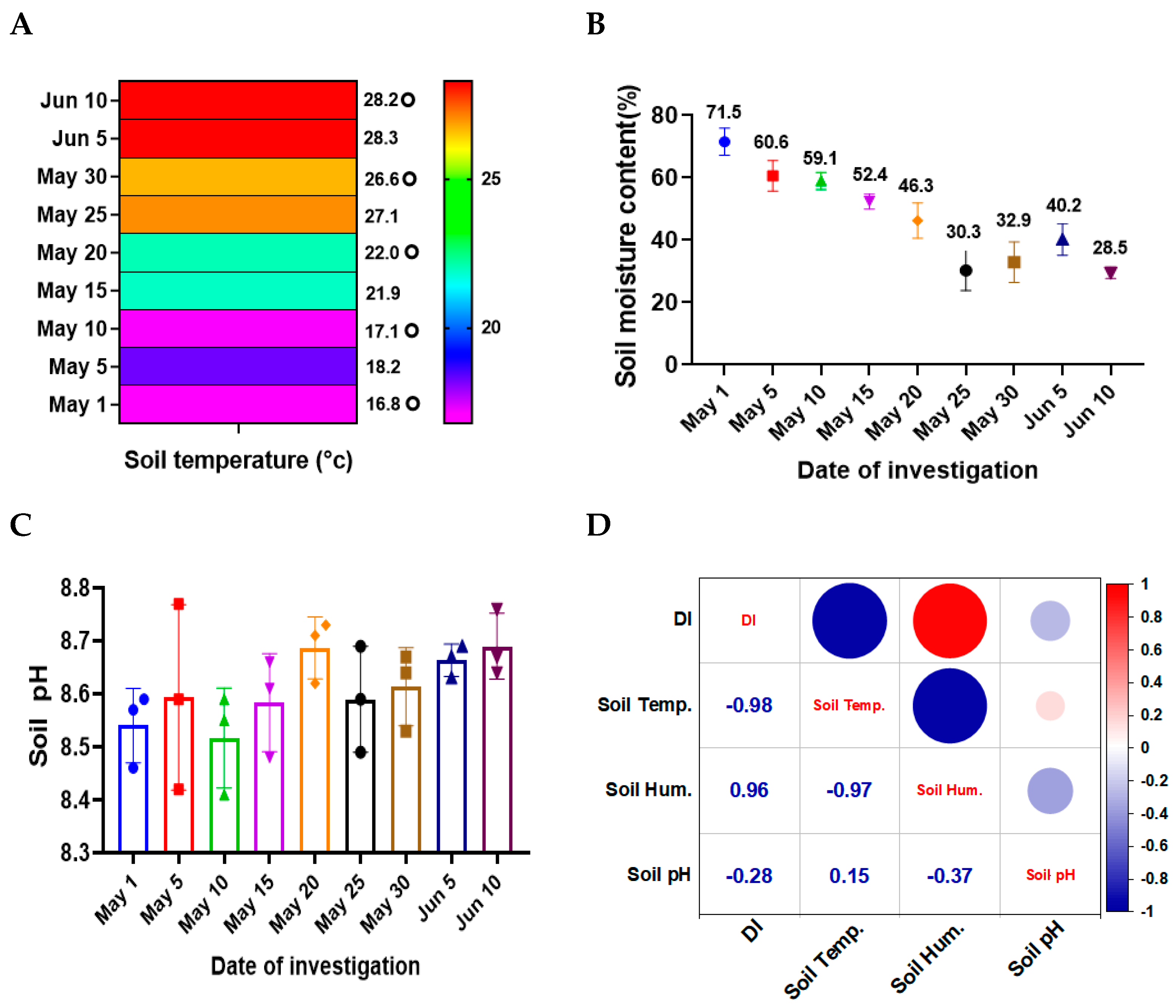
3.3. Correlation Between Soil Temperature, Moisture, and Disease Severity of SVW
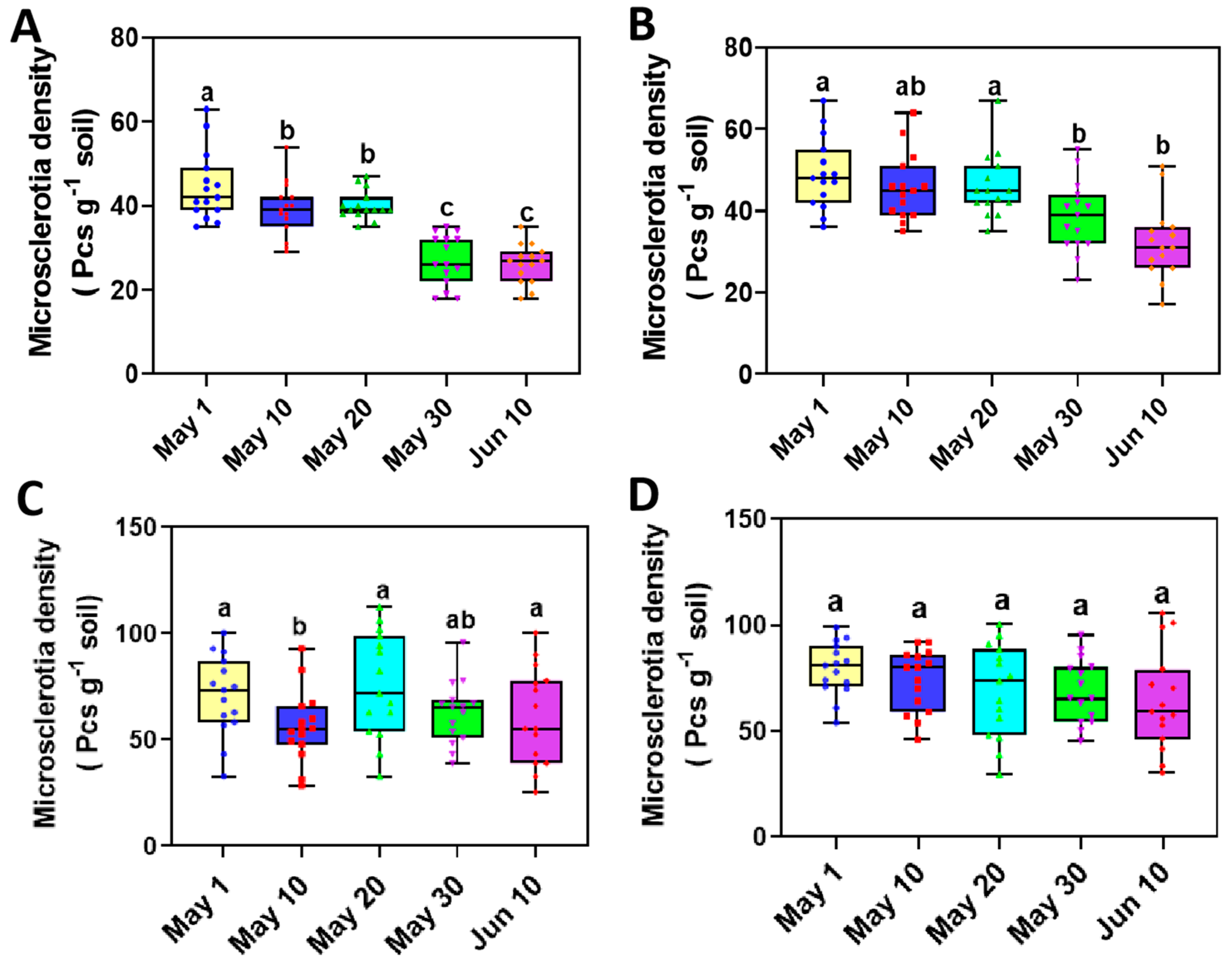
3.4. The Variation of Microsclerotia in Rhizosphere Soil of Sunflower with Delayed Sowing
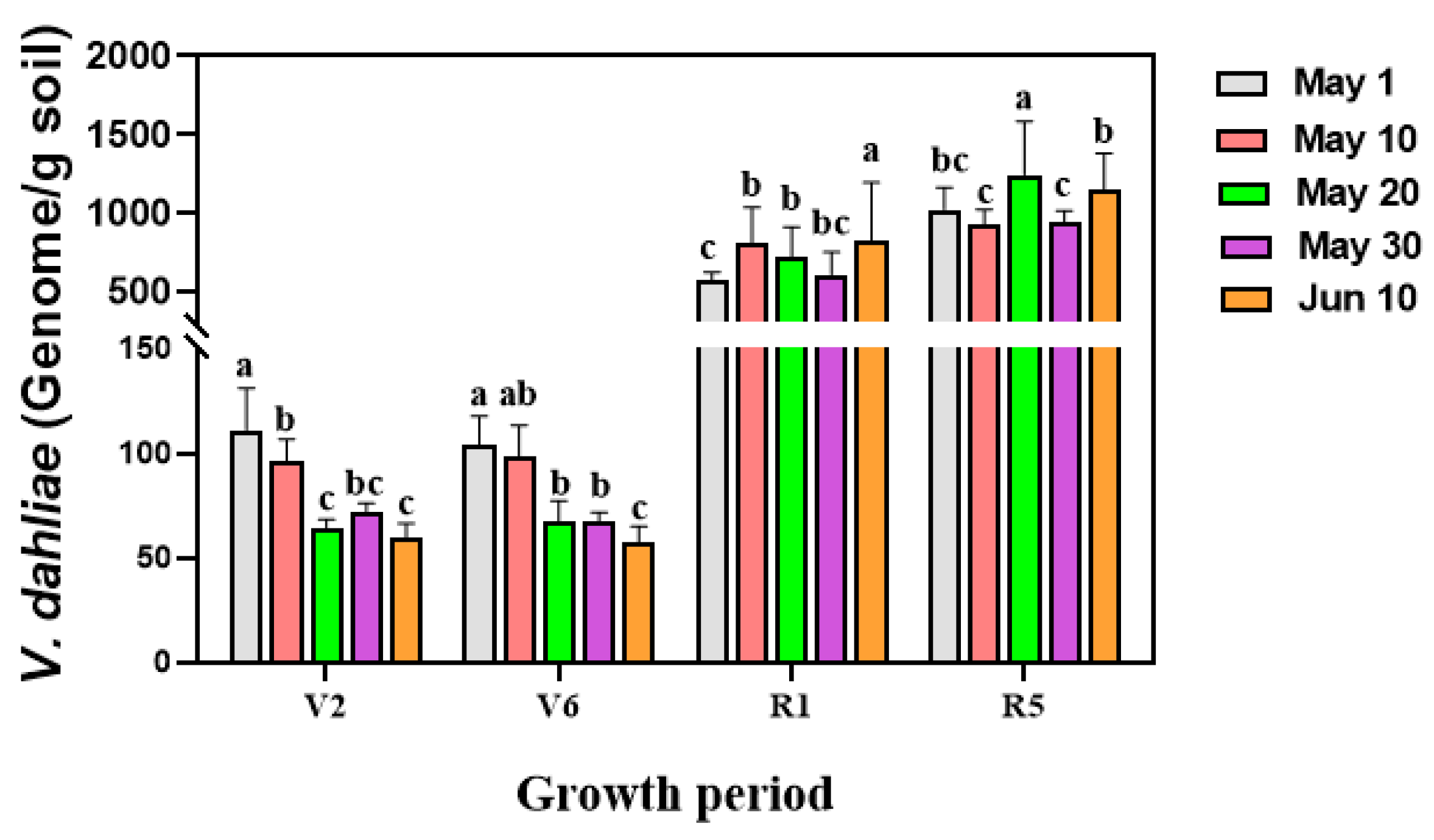
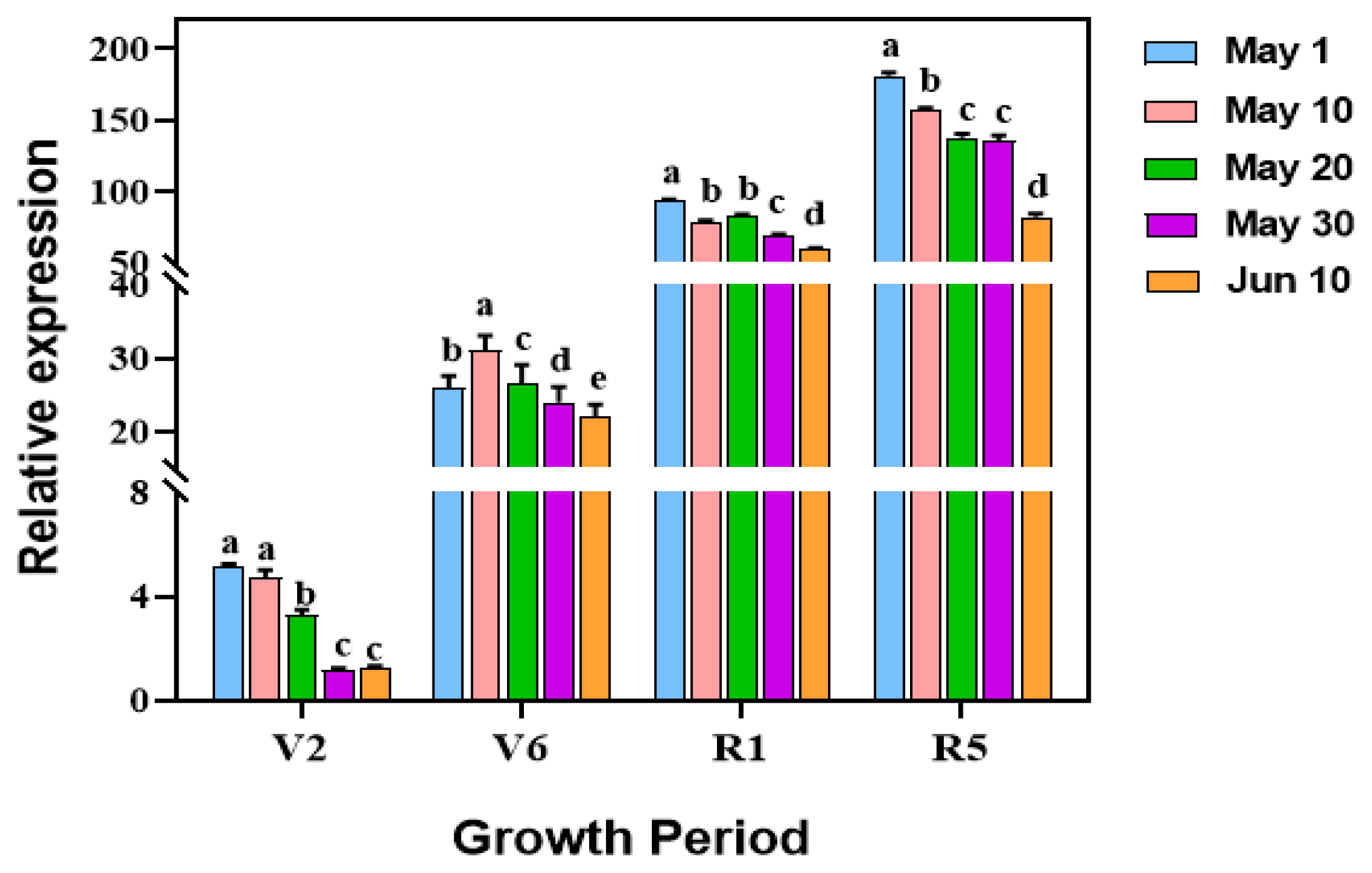
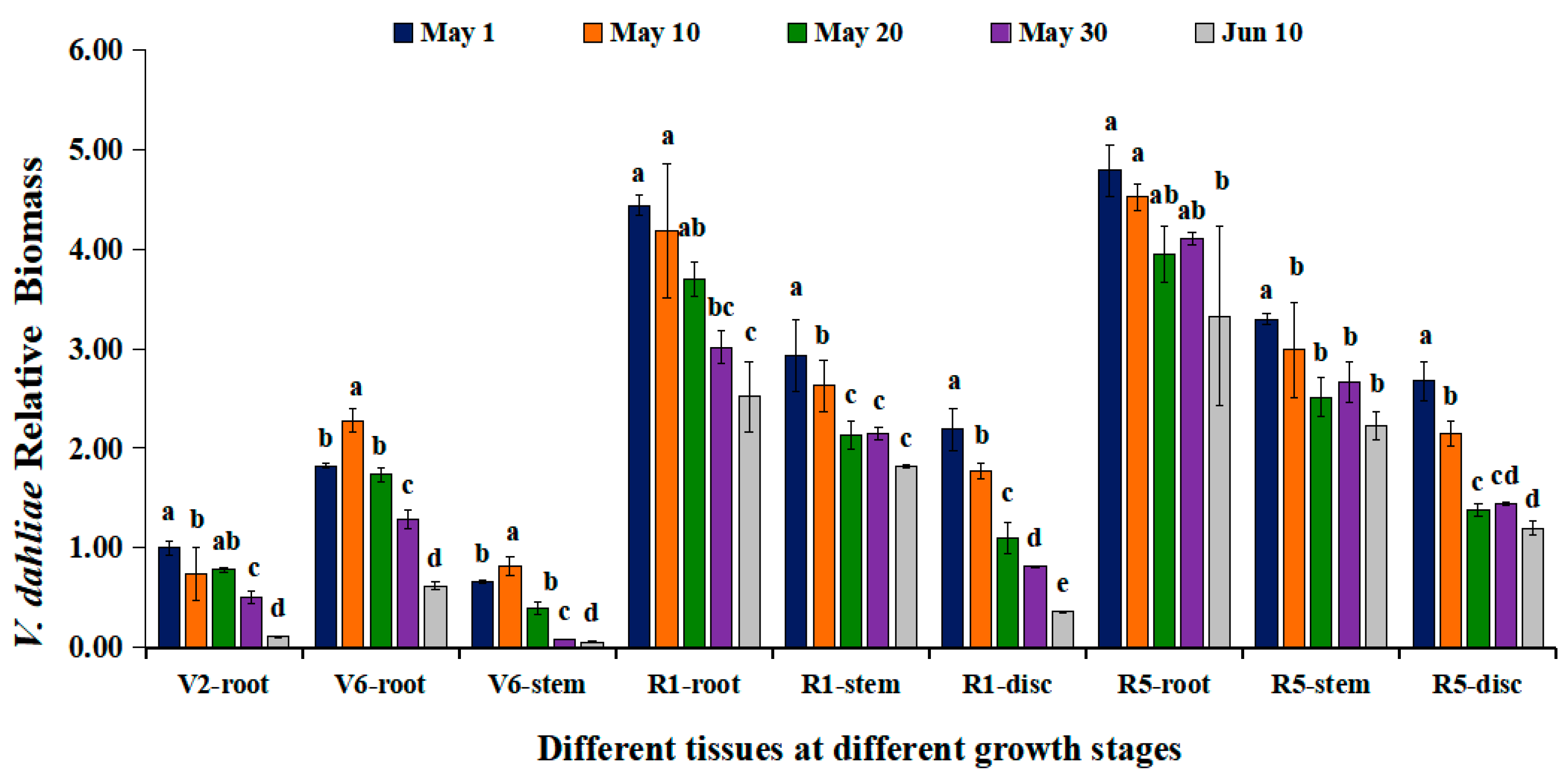
3.5. Decrease in V. dahliae Colonization in Sunflower Roots with Delayed Sowing Dates
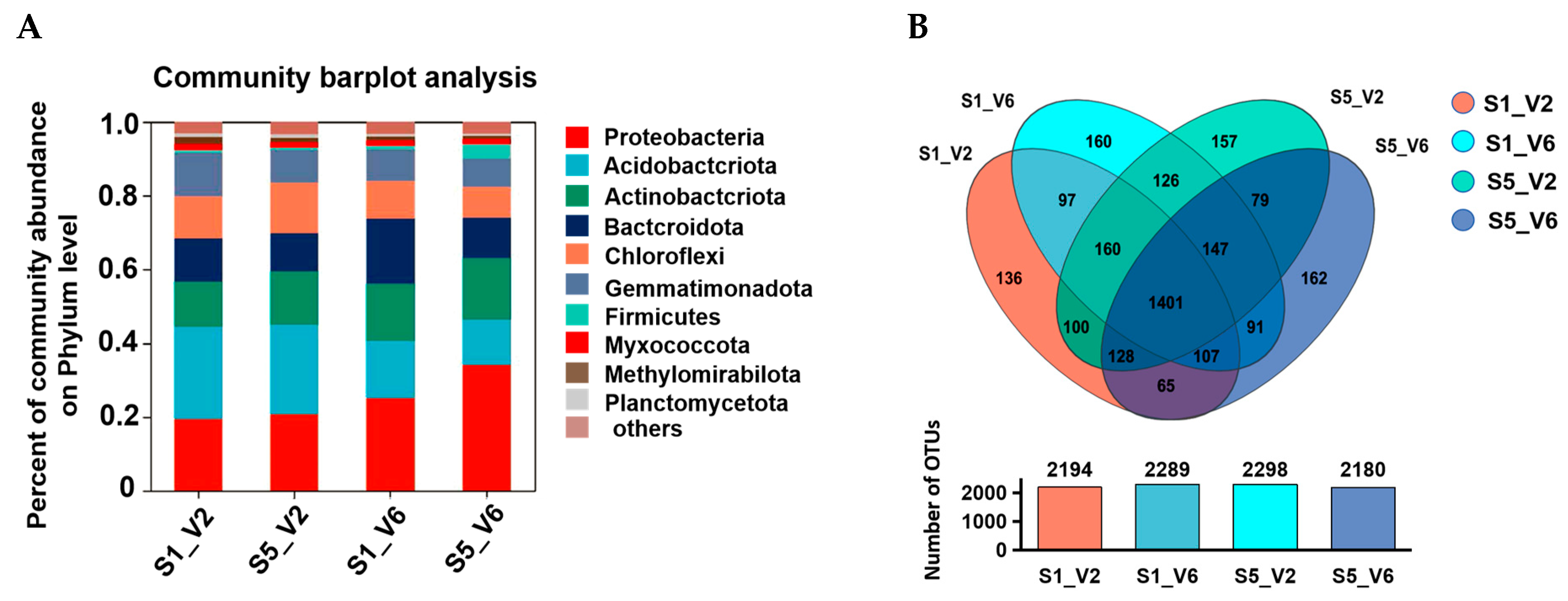
3.6. Effects of Delayed Sowing on Microbial Compositions in Rhizosphere Soil of Sunflower
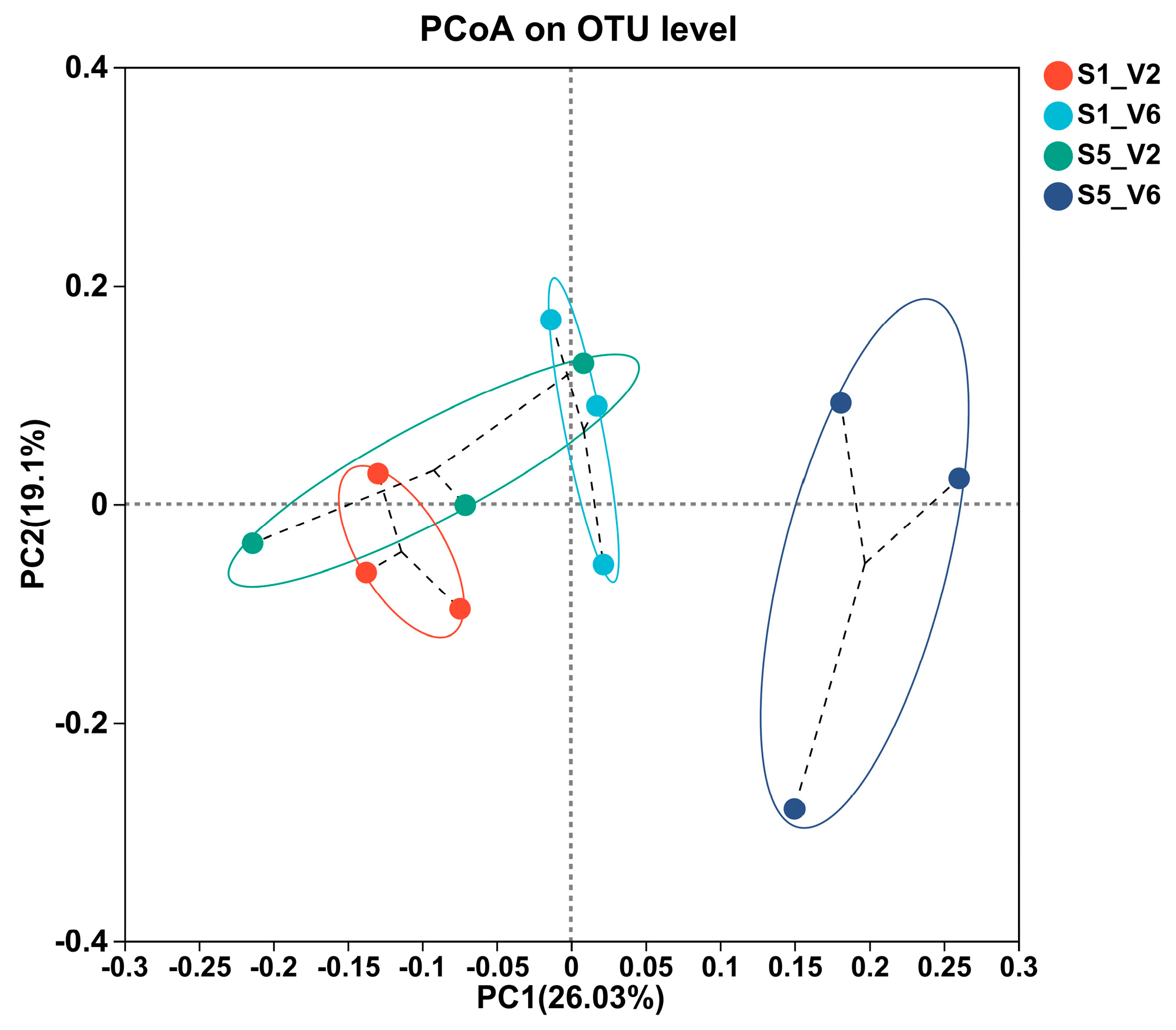
3.7. Beta Diversity
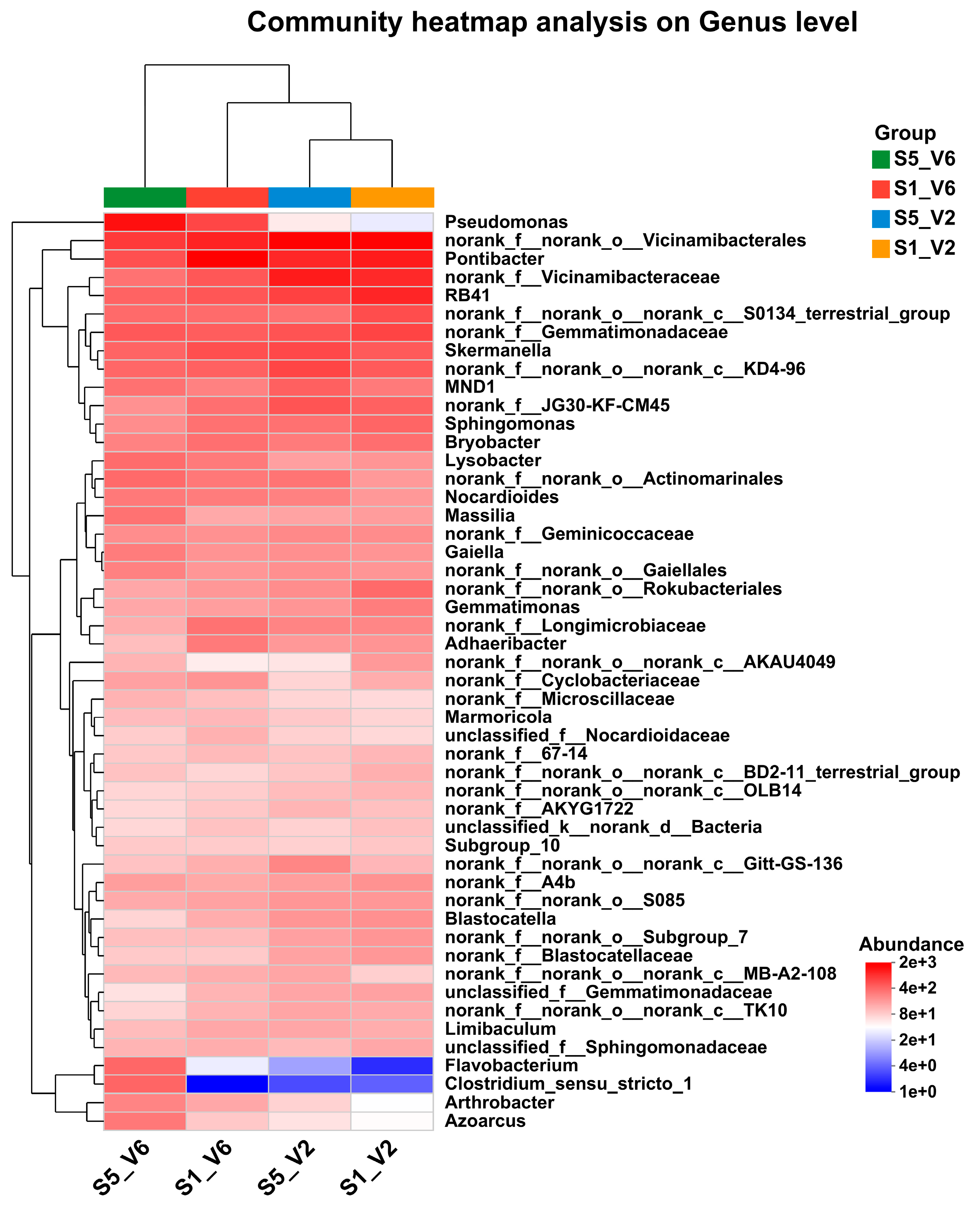
3.8. Analysis of Species Variability
3.9. Isolation and Identification of Bacterial Antagonist V. dahliae
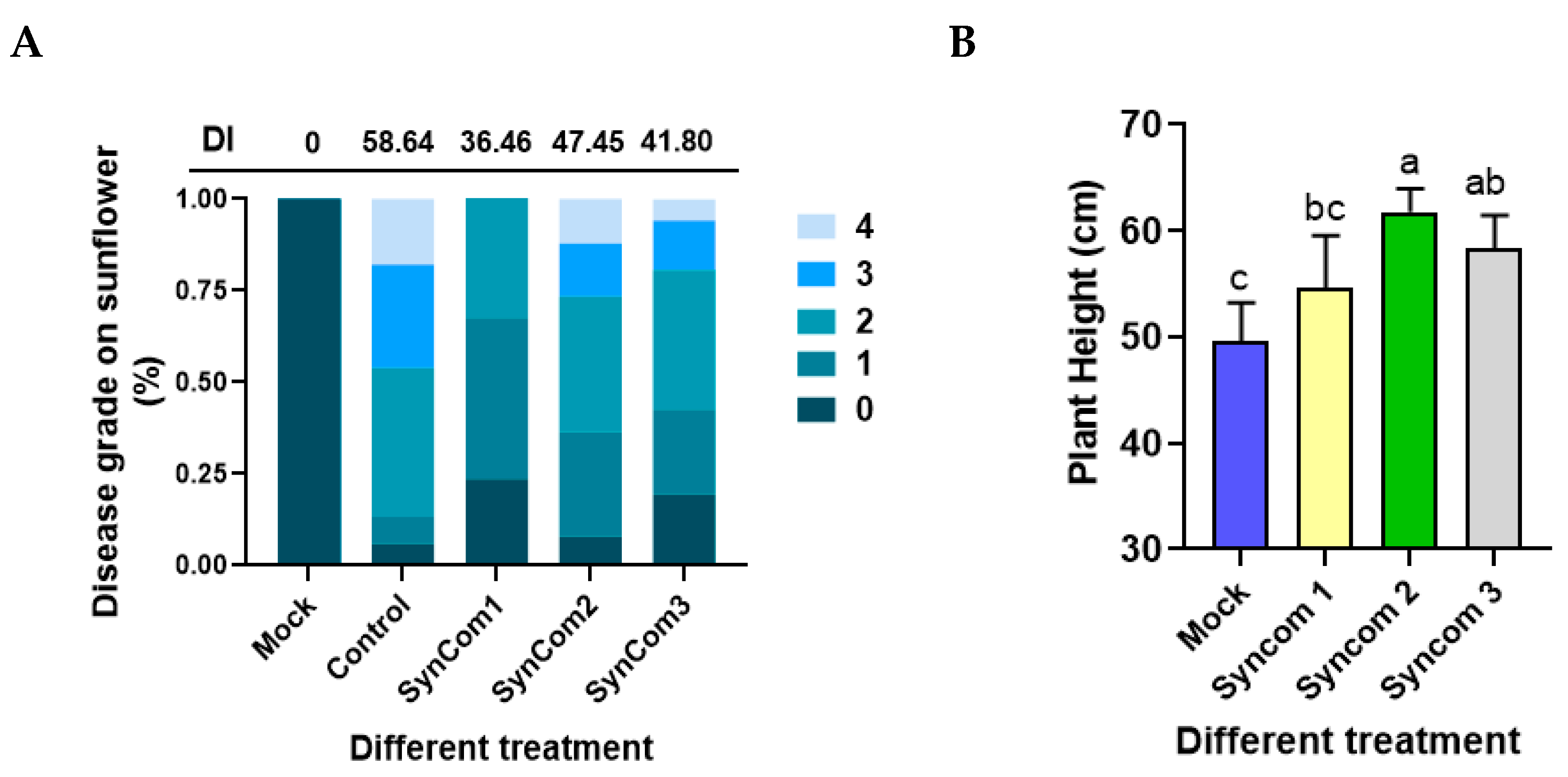
3.10. Antagonist Function of Different SynComs on SVW
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Green, R.J. Soil factors affecting the survival of microsclerotia of Verticillium dahliae. Phytopathology 1980, 70, 353–355. [Google Scholar] [CrossRef]
- Bell, A.A. Verticillium wilt. In Cotton Diseases; Hillocks, R.J., Ed.; CAB International: Wallingford, UK, 1992; pp. 87–126. [Google Scholar]
- Mol, L. Effect of plant roots on the germination of microsclerotia of Verticillium dahliae. II. Quantitative analysis of the luring effects of crops. Eur. J. Plant Pathol. 1955, 101, 673–678. [Google Scholar] [CrossRef]
- Gerik, J.S.; Huisman, O.C. Study of field-grown cotton roots infected with Verticillium dahliae using an immuno enzymatic staining technique. Phytopathology 1988, 78, 1174–1178. [Google Scholar] [CrossRef]
- Zhang, W.W.; Jiang, T.F.; Cui, X.; Qi, F.J.; Jian, G.L. Colonization in cotton plants by a green fluorescent protein labeled strain of Verticillium dahliae. Eur. J. Plant Pathol. 2013, 135, 867–876. [Google Scholar] [CrossRef]
- Rowe, R.C.; Powelson, M.L. Potato early dying: Management challenges in a changing production environment. Plant Dis. 2002, 86, 1184–1193. [Google Scholar] [CrossRef]
- Bubici, G.; Cirulli, M. Integrated Management of Verticillium Wilt of Tomato; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- El-Zik, K.M. Integrated control of Verticillium wilt of cotton. Plant Dis. 1985, 69, 1025–1032. [Google Scholar] [CrossRef]
- Larkin, R.P.; Honeycutt, C.W.; Olanya, O.M. Management of Verticillium wilt of potato with disease-suppressive green manures and as affected by previous cropping history. Plant Dis. 2011, 95, 568–576. [Google Scholar] [CrossRef]
- Navascortés, J.A.; Hau, B.; Jiménezdíaz, R.M. Effect of sowing date, host cultivar, and race of Fusarium oxysporum f. sp. ciceris on development of fusarium wilt of chickpea. Phytopathology 1998, 88, 1338–1346. [Google Scholar] [CrossRef]
- Sharma, P.K.; Handa, A.; Thakur, P.D.; Gupta, D. Management of a potyviral disease of tomato through cultural practices and insecticidal sprays. Environ. Ecol. 2006, 370, 320–323. [Google Scholar]
- Subasinghe, H.; Bains, G.S.; Mohan, C. Effect of sowing date and meteorological parameters on the development of white rust disease in Indian mustard (Brassica juncea L.). Curr. Adv. Agric. Sci. 2009, 24, 166–168. [Google Scholar]
- Nehl, D.; Anderson, C. Delayed Sowing as a Best-Bet Approach to Minimise the Impacts of Fusarium Wilt. 2006. Available online: https://www.insidecotton.com/sites/default/files/article-files/307_-_Chris_Anderson_Fusarium_wilt_FINAL_-_background_paper_2006.pdf (accessed on 14 September 2022).
- Guo, C.Q.; Liao, P.A.; Chang-Bin, G.E.; De-Xian, H.E.; Zang, H.C.; Guo, S.J. Effects of main agronomic measures on reducing disease index of sharp eyespot in wheat (Triticum aestivum). J. Triticeae Crops 2008, 3, 537–540. (In Chinese) [Google Scholar]
- Yang, P.; Yi-Lin, G.U.; Luo, Y.; Zhan-Hong, M.A.; Huang, C.Y.; Shi, L. Study on the relationship between the quantity of Puccinia striiformis f.sp. tritici in latent during overwinter and the disease index in Xiangyang area. Acta Phytopathol. Sin. 2016, 5, 13. [Google Scholar]
- Mol, L.; Halteren, J.M.; Scholte, K.; Struik, P.C. Effects of crop species, crop cultivars and isolates of Verticillium dahliae on the population of microsclerotia in the soil, and consequences for crop yield. Plant Pathol. 1996, 45, 205–214. [Google Scholar] [CrossRef]
- Wei, F.; Fan, R.; Dong, H.; Shang, W.; Xu, X.; Zhu, H.; Yang, J.; Hu, X. Threshold microsclerotia inoculum for cotton Verticillium wilt determined through wet sieving and real-time quantitative PCR. Phytopathology 2015, 105, 220–229. [Google Scholar] [CrossRef]
- Leyendecker, P.J. Effects of Certain Cultural Practices on Verticillium Wilt of Cotton in New Mexico. Bull. N. M. Agric. Exp. Stn. 1950, 356, 1–29. [Google Scholar]
- Solís-García, I.A.; Ceballos-Luna, O.; Cortazar-Murillo, E.M.; Desgarennes, D.; Garay-Serrano, E.; Patiño-Conde, V.; Guevara-Avendaño, E.; Méndez-Bravo, A.; Reverchon, F. Phytophthora root rot modifies the composition of the avocado rhizosphere microbiome and increases the abundance of opportunistic fungal pathogens. Front. Microbiol. 2021, 11, 574110. [Google Scholar] [CrossRef] [PubMed]
- Chapelle, E.; Mendes, R.; Bakker, P.A.; Raaijmakers, J.M. Fungal invasion of the rhizosphere microbiome. ISME J. 2016, 10, 265–268. [Google Scholar] [CrossRef]
- Snelders, N.C.; Petti, G.C.; Berg, G.C.M.v.D.; Seidl, M.F.; Thomma, B.P.H.J. An ancient antimicrobial protein co-opted by a fungal plant pathogen for in planta mycobiome manipulation. Proc. Natl. Acad. Sci. USA 2021, 118, e2110968118. [Google Scholar] [CrossRef]
- Tuang, Z.K.; Wu, Z.; Jin, Y.; Wang, Y.; Oo, P.P.Z.; Zuo, G.; Shi, H.; Yang, W. Pst DC3000 infection alleviates subsequent freezing and heat injury to host plants via a salicylic acid-dependent pathway in Arabidopsis. Plant Cell Environ. 2020, 43, 801–817. [Google Scholar] [CrossRef]
- Wu, Z.; Han, S.; Zhou, H.; Tuang, Z.K.; Wang, Y.; Jin, Y.; Shi, H.; Yang, W. Cold stress activates disease resistance in Arabidopsis thaliana through a salicylic acid-dependent pathway. Plant Cell Environ. 2019, 42, 2645–2663. [Google Scholar] [CrossRef]
- Lv, T.; Zhan, C.; Pan, Q.; Xu, H.; Fang, H.; Wang, M.; Matsumoto, H. Plant pathogenesis: Toward a multidimensional understanding of the microbiome. iMeta 2023, 2, e129. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dai, J.; Rutishauser, T.; Gonsamo, A.; Wu, C.; Ge, Q. Trends and variability in temperature sensitivity of lilac flowering phenology. J. Geophys. Res. Biogeosci. 2018, 123, 807–817. [Google Scholar] [CrossRef]
- Warren, R.J., II; Mordecai, E. Soil moisture mediated interaction between Polygonatum biflorum and leaf spot disease. Plant Ecol. 2010, 209, 1–9. [Google Scholar] [CrossRef]
- Wang, L.; Mazzola, M. Interaction of Brassicaceae seed meal soil amendment and apple rootstock genotype on microbiome structure and replant disease suppression. Phytopathology 2019, 109, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Leach, J.E. High temperature-induced plant disease susceptibility: More than the sum of its parts. Curr. Opin. Plant Biol. 2020, 56, 235–241. [Google Scholar] [CrossRef]
- Gray, S.B.; Brady, S.M. Plant developmental responses to climate change. Dev. Biol. 2016, 419, 64–77. [Google Scholar] [CrossRef]
- Bever, J.D.; Westover, K.M.; Antonovics, J. Incorporating the soil community into plant population dynamics: The utility of the feedback approach. J. Ecol. 1997, 85, 561–573. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Liang, W.; Liu, S. Rhizosphere Microbiome: The Emerging Barrier in Plant-Pathogen Interactions. Front. Microbiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Theis, K.R.; Dheilly, N.M.; Klassen, J.L.; Brucker, R.M.; Baines, J.F.; Bosch, T.C.G.; Cryan, J.F.; Gilbert, S.F.; Goodnight, C.J.; Lloyd, E.A.; et al. Getting the hologenome concept right: An eco-evolutionary framework for hosts and their microbiomes. mSystems 2016, 1, e00028-16. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Tahir, H.A.S.; Gu, Q.; Wu, H.; Raza, W.; Safdar, A.; Huang, Z.; Rajer, F.U.; Gao, X.W. Effect of volatile compounds produced by Ralstonia solanacearum on plant growth promoting and systemic resistance inducing potential of Bacillus volatiles. BMC Plant Biol. 2017, 17, 133. [Google Scholar] [CrossRef] [PubMed]
- Carrión, V.J.; Perez-Jaramillo, J.; Cordovez, V.; Tracanna, V.; de Hollander, M.; Ruiz-Buck, D.; Mendes, L.W.; van Ijcken, W.F.J.; Gomez-Exposito, R.; Elsayed, S.S.; et al. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science 2019, 366, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar]
- Ellouze, W.; Esmaeili Taheri, A.; Bainard, L.D. Soil fungal resources in annual cropping systems and their potential for management. Bio. Med. Res. Int. 2014, 2014, 531824. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.; Finckh, M.R. Plant diseases, and management approaches in organic farming systems. Annu. Rev. Phytopathol. 2016, 54, 25–54. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.; Bardgett, R.D.; Straalen, N.M.V. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecology 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Whitman, W.B.; Coleman, D.C.; Wiebe, W.J. Prokaryotes: The unseen majority. Proc. Natl. Acad. Sci. USA 1998, 95, 6578–6583. [Google Scholar] [CrossRef]
- Bever, J.D. Soil community feedback and the coexistence of competitors: Conceptual frameworks and empirical tests. New Phytol. 2003, 157, 465–473. [Google Scholar] [CrossRef]
- Thies, J.E. Soil microbial community analysis using terminal restriction fragment length polymorphisms. Soil Sci. Soc. Am. J. 2007, 71, 579–591. [Google Scholar] [CrossRef]
- Moynihan, J.A.; Morrissey, J.P.; Coppoolse, E.R.; Stiekema, W.J.; O’Gara, F.; Boyd, E.F. Evolutionary history of the phl gene cluster in the plant-associated bacterium pseudomonas fluorescens. Appl. Environ. Microbiol. 2009, 75, 2122–2131. [Google Scholar] [CrossRef]
- Benitez, M.S.; McSpadden, G.B.B. Linking sequence to function in soil bacteria: Sequence-directed isolation of novel bacteria contributing to soilborne plant disease suppression. Appl. Environ. Microbiol. 2009, 75, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and soil structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Gans, J.; Wolinsky, M.; Dunbar, J. Computational improvements reveal great bacterial diversity and high metal toxicity in soil. Science 2005, 309, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; Van Der Voort, M.; Schneider, J.H.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.; et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Mallon, C.A.; Elsas, J.D.V.; Salles, J.F. Microbial invasions: The process, patterns, and mechanisms. Trends Microbiol. 2015, 23, 719–729. [Google Scholar] [CrossRef]
- Chater, K.F.; Biro, S.; Lee, K.J. The complex extracellular biology of Streptomyces. FEMS Microbiol. Rev. 2010, 34, 171–198. [Google Scholar] [CrossRef]
- Mendes, L.W.; Raaijmakers, J.M.; de Hollander, M. Influence of resistance breeding in common bean on rhizosphere microbiome composition and function. ISME J. 2018, 12, 212–224. [Google Scholar] [CrossRef]
- Shi, W.; Li, M.; Wei, G.; Tian, R.; Li, C.; Wang, B.; Lin, R.; Shi, C.; Chi, X.; Zhou, B.; et al. The occurrence of potato common scab correlates with the community composition and function of the geocaulosphere soil microbiome. Microbiome 2019, 7, 14. [Google Scholar] [CrossRef]
- Kimotho, R.N.; Maina, S. Unraveling plant-microbe interactions: An integrated omics approaches offer concrete answers? J. Exp. Bot. 2024, 75, 1289–1313. [Google Scholar] [CrossRef]
- Jia, S.; Yang, J.F.; Zhang, J. Field resistance of different sunflower varieties to Verticillium wilt. Chin. J. Plant Prot. 2022, 42, 56–60. (In Chinese) [Google Scholar]
- Zhang, G.; Cao, X.; Dong, B.Z. The effect of delayed sowing date on the incidence of sunflower wilt disease. Chin. J. Plant Prot. 2018, 45, 651–652. (In Chinese) [Google Scholar]
- Cao, X.; Meng, Q.L.; Liu, J.X. Field identification of resistance to Verticillium wilt in different sunflower varieties. Crop J. 2014, 1, 67–72. (In Chinese) [Google Scholar]
- Hu, S.Z.; Qiao, D.M.; Shi, H.B. Analysis of the ecological and physiological characteristics of the root system of oilseed sunflower. Resour. Environ. Arid. Reg. 2006, 20, 6. [Google Scholar]
- Edwards, J.; Johnson, C.; Santos-Medellín, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. USA 2015, 112, E911–E920. [Google Scholar] [CrossRef]
- Tefera, T.; Vidal, S. Effect of inoculation method and plant growth medium on endophytic colonization of sorghum by the entomopathogenic fungus beauveria bassiana. Biocontrol 2009, 54, 663–669. [Google Scholar] [CrossRef]
- Doyle, J. DNA protocols for plants can total DNA isolation. In Molecular Techniques in Taxonomy; Springer: New York, NY, USA, 1991. [Google Scholar]
- Hummon, A.B.; Lim, S.R.; Difilippantonio, M.J.; Ried, T. Isolation and solubilization of proteins after triazole extraction of RNA and DNA from patient material following prolonged storage. Biotechniques 2007, 42, 467–472. [Google Scholar] [CrossRef]
- Yang, J.F.; Zhang, J.; Jia, S.; Zhang, Y.Y.; Zhang, W.B.; Duan, X.J.; Zhao, J. A New Rapid Detection Method for Microsclerotia of Verticillium Wilt Pathogens in Soil of Sunflower Fields. Chin. J. Oil Crop Sci. 2023, 45, 209–214. (In Chinese) [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastq: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatic 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Bacteriol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pommier, T.; Yin, Y.; Wang, J.; Gu, S.; Jousset, A.; Keuskamp, J.; Wang, H.; Wei, Z.; Xu, Y.; et al. Indirect reduction of Ralstonia solanacearum via pathogen helper inhibition. ISME J. 2022, 16, 868–875. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, S.; Wang, Y.; Yang, F.; Tian, Z.; Long, C.-A. Postharvest biocontrol ability and antagonistic mechanism of Bacillus altitudins h217 against Penicillium digitatum in citrus. Postharvest Biol. Technol. 2024, 212, 112911. [Google Scholar] [CrossRef]
- Larkin, R.P.; Fravel, D.R. Efficacy of various fungal and bacterial biocontrol organisms for control of Fusarium wilt of tomato. Plant Dis. 1998, 82, 1022–1028. [Google Scholar] [CrossRef]
- Khaloud, M.A.; Mohamed, S.E. Plant growth-promoting and biocontrol traits of endophytic Bacillus licheniformis against soft rot causing Pythium myristyl in a ginger plant. J. Basic Microbiol. 2024, 64, e202300643. [Google Scholar]
- Kumar, M.; Charishma, K.; Sahu, K.; Sheoran, N.; Patel, A.; Kundu, A.; Kumar, A. Rice leaf associated Chryseobacterium species: An untapped antagonistic flavobacterium displays volatile mediated suppression of rice blast disease. Biol. Control 2021, 161, 104703. [Google Scholar] [CrossRef]
- Lazarovits, G. Management of soil-borne plant pathogens with organic soil amendments: A disease control strategy salvaged from the past. Can. J. Plant Pathol. 2001, 23, 1–7. [Google Scholar] [CrossRef]
- Kayacetin, F. Influence of Sowing Dates and Genotypes on Phenology, Morphology, Yield and Fatty Acid Compounds of Sinapis alba L. for the Energy Industry. Gesunde Pflanz. 2023, 75, 613–623. [Google Scholar] [CrossRef]
- Kakhki, S.H.N.; Taghaddosi, M.V.; Moini, M.R. The impact of seasonal variation on the occurrence of Fusarium root rot diseases, fly infestations, weeds, herbicide application, and yield. Arch. Phytopathol. Plant Prot. 2022, 55, 1410–1429. [Google Scholar] [CrossRef]
- Pathania, M.; Sharma, K.K.; Kumar, V. Management of major insect pests and diseases of maize. Progress. Farming 2022, 9, 25–33. [Google Scholar]
- Ravichandran, V.; Ragupathi, N.; Balasubramanian, T.N.; Geethalakshmi, V.; Thambidurai, G.; Selvaraj, R. Influence of weather parameters on the incidence and development of rust (Puccinia arachidis speg.) disease in groundnut. Madras Agric. J. 2004, 10, 39–49. [Google Scholar]
- Ben-Yephet, Y.; Szmulewich, Y. Inoculum levels of Verticillium dahliae in the soils of the hot semi-arid Negev region of Israel. Phytoparasit 1985, 13, 193–200. [Google Scholar] [CrossRef]
- Devay, J.E.; Gutierrez, A.P.; Pullman, G.S.; Wakeman, R.J.; Garber, R.H.; Jeffers, D.P.; Smith, S.N.; Goodell, P.B.; Roberts, P.A. Inoculum Densities of Fusarium oxysporum f. sp. vasinfectum and Meloidogyne incognita in Relation to the Development of Fusarium Wilt and the Phenology of Cotton Plants (Gossypium hirsutum). Phytopathology 1997, 87, 341–346. [Google Scholar]
- Evans, N.; Baierl, A.; Semenov, M.A.; Gladders, P.; Fitt, B.D. The range and severity of a plant disease increased by global warming. J. R. Soc. Interface 2008, 5, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, K.; Fouts, D.E.; Rehm, A.H.; Hill, A.R.; Collmer, A.; Alfano, J.R. The Avr (effector) proteins HrmA (HopPsyA) and AvrPto are secreted in culture from Pseudomonas syringae pathovars via the Hrp (type III) protein secretion system in a temperature- and pH-sensitive manner. J. Bacteriol. 1999, 181, 4790–4797. [Google Scholar] [CrossRef]
- Weingart, H.; Stubner, S.; Schenk, A.; Ullrich, M.S. Impact of temperature on in planta expression of genes involved in the synthesis of the Pseudomonas syringae phytotoxin coronatine. Mol. Plant-Microbe Interact. 2004, 17, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- McCullen, C.A.; Binns, A.N. Agrobacterium tumefaciens and plant cell interactions and activities required for interkingdom macromolecular transfer. Annu. Rev. Cell Dev. Biol. 2006, 22, 101–127. [Google Scholar] [CrossRef]
- Taru Sharma, L.S.R.; Parimal Sinha, P.M. Effect of temperature on rice blast infection process with emphasis on appressoria formation by Magnaporthe oryzae. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1931–1939. [Google Scholar] [CrossRef]
- McCarthy-Neumann, S.; Kobe, R.K. Tolerance of soil pathogens co-varies with shade tolerance across species of tropical tree seedlings. Ecology 2008, 89, 1883–1892. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.A.; Gu€sewell, S.; Harte, J. Response of plant pathogens and herbivores to a warming experiment. Ecology 2004, 85, 2570–2581. [Google Scholar] [CrossRef]
- Qiu, J.; Xie, J.; Chen, Y.; Shen, Z.; Shi, H.; Naqvi, N.I.; Qian, Q.; Liang, Y.; Kou, Y. Warm temperature compromises JA-regulated basal resistance to enhance Magnaporthe oryzae infection in rice. Mol. Plant 2022, 15, 723–739. [Google Scholar] [CrossRef]
- Yao, L.; Jiang, Z.; Wang, Y.; Hu, Y.; Hao, G.; Zhong, W.; Wan, S.; Xin, X.F. High air humidity dampens salicylic acid pathway and NPR1 function to promote plant disease. Embo J. 2023, 42, e113499. [Google Scholar] [CrossRef]
- Mao, X.H.; Liu, K.D.; Jia, X.P.; Wang, X.Z.; Liang, G.S.; Hu, J.T. Effect of adjusting sowing period on the prevention and control of sunflower botrytis. Gansu Agric. Sci. Technol. 2019, 3, 1–4. [Google Scholar]
- Zheng, X.Q.; Wang, J.; Di, N.; Wang, H.W. Effect of sowing time, phosphorous dosage and herbicide on the occurrence of sunflower broomrape. Plant Quar. 2021, 35, 59–62. [Google Scholar] [CrossRef]
- Luan, F.G.; Zhang, L.L.; Lou, Y.Y. Analysis of microbial diversity and niche in rhizosphere soil of healthy and diseased cotton at the flowering stage in southern Xinjiang. Genet. Mol. Res. 2015, 14, 1602–1611. [Google Scholar] [CrossRef]
- Poudel, R.; Jumpponen, A.; Schlatter, D.; Paulitz, T.C.; Garrett, K.A. Microbiome Networks: A Systems Framework for Identifying Candidate Microbial Assemblages for Disease Management. Phytopathology 2006, 106, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, H.; Wang, J.; Gao, W.; Sun, X.; Xiong, Q.; Shu, X.; Miao, Y.; Shen, Q.; Xun, W.; et al. Nonpathogenic Pseudomonas syringae derivatives and its metabolites trigger the plant “cry for help” response to assemble disease suppressing and growth promoting rhizomicrobiome. Nat. Commun. 2024, 15, 1907. [Google Scholar] [CrossRef]
- Haas, D.; Défago, G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 2005, 3, 307–319. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Z.; Chen, L.; Xun, W.; Shu, X.; Chen, Y.; Sun, X.; Wang, Z.; Ren, Y.; Shen, Q.; et al. Root colonization by beneficial rhizobacteria. FEMS Microbiol. Rev. 2024, 48, 66. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Tabassum, B.; Fathi Abd Allah, E. Bacillus subtilis: A plant-growth-promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y. The Function of Root Exudates in the Root Colonization by Beneficial Soil Rhizobacteria. Biology 2014, 13, 95. [Google Scholar] [CrossRef] [PubMed]

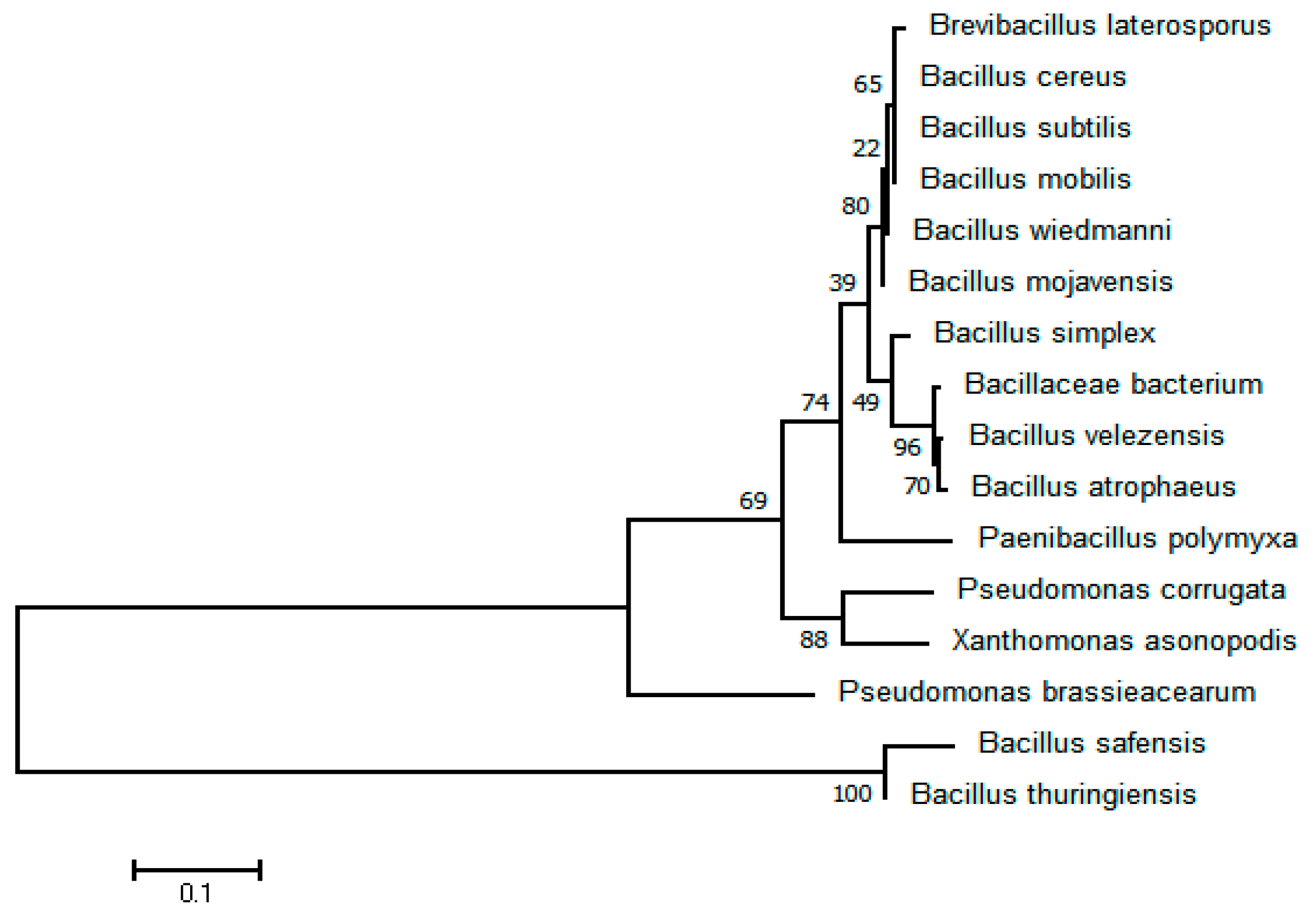
| Location | Year | Sowing Dates | Sites | Coordinates | Sunflower Varieties and Verticillium Susceptibility z | Previous Crop |
|---|---|---|---|---|---|---|
| 1 | 2017 | 20 April, 1 May, 10 May, 20 May, 30 May | Minqin, WuWei, Gansu | N: 38°43′45″, E: 103°32′10″ | LD5009 (S) T562 (R) | Sunflower |
| 2 | 2018 | 1 May, 10 May, 20 May, 30 May, 10 June | Wuyuan, Inner Mongolia | N: 41°12′30″, E: 107°48′20″ | LD5009 (S) KY-7 (R) | Sunflower |
| 3 | 2019 | 1 May, 10 May, 20 May, 30 May, 10 June | Wulateqianqi, Inner Mongolia | N: 40°32′21″, E: 109°21′35″ | LD5009 (S) JK601 (R) | Sunflower |
| Number | Explanation |
|---|---|
| S1-V2 | Sunflower V2 growth period, the first sowing date (S1) |
| S1-V6 | Sunflower V6 growth period, the first sowing date (S1) |
| S5-V2 | Sunflower V2 growth period, the fifth sowing date (S5) |
| S5-V6 | Sunflower V6 growth period, the fifth sowing date (S5) |
| Synthetic Composition | Strain Species | Strain Number |
|---|---|---|
| SynComs 1 | Bacillus thuringiensis | A12-23 |
| Bacillus atrophaeus | T4-42 | |
| Pseudomonas brassieacearum | G9-2 | |
| Bacillaceae bucterium | A1-67 | |
| Bacillus cereus | N3-16 | |
| SynComs 2 | Flavobacterium flevense | S5-L-3 |
| Arthrobacter gandavensis | S5-B-1 | |
| Azoarcus sp. | S5-A-1 | |
| SynComs 3 | Bacillus thuringiensis | A12-23 |
| Bacillus atrophaeus | T4-42 | |
| Pseudomonas brassieacearum | G9-2 | |
| Bacillaceae bucterium | A1-67 | |
| Bacillus cereus | N3-16 | |
| Flavobacterium flevense | S5-L-3 | |
| Arthrobacter gandavensis | S5-B-1 | |
| Azoarcus sp. | S5-A-1 |
| Location | Sites | Sunflower Varieties | Average Disease Index of SVW at Different Sowing Dates | |||||
|---|---|---|---|---|---|---|---|---|
| 20 April | 1 May | 10 May | 20 May | 30 May | 10 June | |||
| Field 1 | Minqin, GS | LD5009(S) | 55.01 a | 46.04 b | 34.38 c | 25.59 d | 23.02 d | —— |
| T562 | 27.86 a | 19.12 b | 22.57 b | 14.79 bc | 9.43 c | —— | ||
| Field 2 | Wuyuan, IM | LD5009(S) | —— | 66.56 a | 57.75 ab | 30.63 b | 13.99 c | 13.30 c |
| KY-7(R) | —— | 39.87 a | 29.36 b | 19.94 c | 5.94 d | 6.96 d | ||
| Field 3 | Wulateqianqi, IM | LD5009(S) | —— | 64.94 a | 56.62 b | 43.18 c | 39.33 c | 28.77 d |
| JK601(R) | —— | 32.93 a | 18.57 b | 12.90 bc | 15.09 bc | 5.45 c | ||
| Pot experiment | Greenhouse, IMAU | LD5009(S) | —— | 54.15 a | 56.94 a | 45.69 b | 28.16 c | 15.43 d |
| Location | Sunflower Varieties | Subplot Yield and TKW of Sunflower at Different Sowing Dates | ||||||
|---|---|---|---|---|---|---|---|---|
| 20 April | 1 May | 10 May | 20 May | 30 May | 10 June | |||
| Field 1 | LD5009(S) | Yield (Kg/P) z | 3.34 b | 3.48 b | 3.92 b | 4.39 ab | 4.67 a | —— |
| TKW (g) | 80.44 b | 88.96 ab | 85.50 ab | 97.06 a | 96.78 a | —— | ||
| T562 | Yield (Kg/P) z | 2.46 b | 2.72 ab | 3.63 a | 3.68 a | 3.87 a | —— | |
| TKW (g) | 51.80 b | 56.24 ab | 59.82 ab | 68.98 a | 69.76 a | —— | ||
| Field 2 | LD5009(S) | Yield (Kg/P) z | —— | 4.41 c | 4.87 bc | 5.49 abc | 6.31 ab | 6.43 a |
| TKW (g) | —— | 100.42 b | 120.60 ab | 123.24 ab | 126.81 ab | 140.88 a | ||
| KY-7(R) | Yield (Kg/P) z | —— | 7.58 b | 8.73 a | 8.31 ab | 8.1 ab | 7.74 ab | |
| TKW (g) | —— | 155.63 b | 165.27 ab | 169.01 ab | 170.13 ab | 189.45 a | ||
| Field 3 | LD5009(S) | Yield (Kg/P) z | —— | 3.76 b | 3.84 b | 4.38 b | 4.93 ab | 5.67 a |
| TKW (g) | —— | 93.42 b | 111.62 ab | 115.05 a | 117.67 a | 120.43 a | ||
| JK601(R) | Yield (Kg/P) z | —— | 4.46 b | 5.72 ab | 6.63 a | 6.68 a | 6.87 a | |
| TKW (g) | —— | 103.04 b | 126.42 ab | 136.20 ab | 140.88 a | 146.21 a | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Jia, S.; Li, T.; Zhang, J.; Zhang, Y.; Hao, J.; Zhao, J. Delayed Sowing Reduced Verticillium Wilt by Altering Soil Temperature and Humidity to Enhance Beneficial Rhizosphere Bacteria of Sunflower. Microorganisms 2024, 12, 2416. https://doi.org/10.3390/microorganisms12122416
Yang J, Jia S, Li T, Zhang J, Zhang Y, Hao J, Zhao J. Delayed Sowing Reduced Verticillium Wilt by Altering Soil Temperature and Humidity to Enhance Beneficial Rhizosphere Bacteria of Sunflower. Microorganisms. 2024; 12(12):2416. https://doi.org/10.3390/microorganisms12122416
Chicago/Turabian StyleYang, Jianfeng, Shuo Jia, Tie Li, Jian Zhang, Yuanyuan Zhang, Jianjun Hao, and Jun Zhao. 2024. "Delayed Sowing Reduced Verticillium Wilt by Altering Soil Temperature and Humidity to Enhance Beneficial Rhizosphere Bacteria of Sunflower" Microorganisms 12, no. 12: 2416. https://doi.org/10.3390/microorganisms12122416
APA StyleYang, J., Jia, S., Li, T., Zhang, J., Zhang, Y., Hao, J., & Zhao, J. (2024). Delayed Sowing Reduced Verticillium Wilt by Altering Soil Temperature and Humidity to Enhance Beneficial Rhizosphere Bacteria of Sunflower. Microorganisms, 12(12), 2416. https://doi.org/10.3390/microorganisms12122416






