Heterologous Expression of the Antiviral Lectin Griffithsin in Probiotic Saccharomyces boulardii and In Vitro Characterization of Its Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmids, Strains, and Media
2.2. The Virus and Cell Model
2.3. The Griffithsin Gene and the Production Strategy
2.4. Construction of the Recombinant Yeast Strains
2.5. Secretion of Griffithsin and Western Blot Analysis
2.6. Confirmation of the High-Production Strains
2.7. Growth Profiles
2.8. Morphology
2.9. Tolerance to Gastrointestinal Pressure
2.10. Hydrophobicity and Auto-Aggregation
2.11. The Antiviral Activity Assay
2.12. Data Analyses
3. Results
3.1. Cloning and Verification of the Gene Expression Cassettes
3.2. Identification of the Secreted Griffithsin
3.3. Selection of the Yeast Strains with High Griffithsin Production
3.4. Characterization of the Properties of Selected Yeast Strains
3.4.1. Growth Characteristics of the S. boulardii Strains
3.4.2. Morphological Characteristics of the S. boulardii Strains
3.4.3. Simulation of Gastrointestinal Stress Tolerance In Vitro
3.4.4. Evaluation of Their Hydrophobicity and Auto-Aggregation In Vitro
3.4.5. Anti-PEDV Activity In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Food and Agriculture Organization of the United Nations. Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria. In Proceedings of the Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria, Amerian Córdoba Park Hotel, Córdoba, Argentina, 1–4 October 2001. [Google Scholar]
- Ivey, K.L.; Hodgson, J.M.; Kerr, D.A.; Thompson, P.L.; Stojceski, B.; Prince, R.L. The effect of yoghurt and its probiotics on blood pressure and serum lipid profile; a randomised controlled trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Buys, N. Effects of probiotics consumption on lowering lipids and CVD risk factors: A systematic review and meta-analysis of randomized controlled trials. Ann. Med. 2015, 47, 430–540. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Christodoulides, S.; Fragkos, K.C.; Scott, S.M.; Whelan, K. The effect of probiotics on functional constipation in adults: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Rizzatti, G.; Plomer, M.; Lopetuso, L.; Scaldaferri, F.; Franceschi, F.; Cammarota, G.; Gasbarrini, A. Bacillus clausii for the Treatment of acute diarrhea in children: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2018, 10, 1074. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017, 2, 6. [Google Scholar] [CrossRef]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastro. Hepat. 2022, 19, 625–637. [Google Scholar] [CrossRef]
- Ulsemer, P.; Toutounian, K.; Kressel, G.; Goletz, C.; Schmidt, J.; Karsten, U.; Hahn, A.; Goletz, S. Impact of oral consumption of heat-treated Bacteroides xylanisolvens DSM 23964 on the level of natural TFα-specific antibodies in human adults. Benef. Microbes 2016, 7, 485–500. [Google Scholar] [CrossRef]
- Ulsemer, P.; Henderson, G.; Toutounian, K.; Löffler, A.; Schmidt, J.; Karsten, U.; Blaut, M.; Goletz, S. Specific humoral immune response to the Thomsen-Friedenreich tumor antigen (CD176) in mice after vaccination with the commensal bacterium Bacteroides ovatus D-6. Cancer Immunol. Immunother. 2013, 62, 875–887. [Google Scholar] [CrossRef]
- Shimbo, I.; Yamaguchi, T.; Odaka, T.; Nakajima, K.; Koide, A.; Koyama, H.; Saisho, H. Effect of Clostridium butyricum on fecal flora in Helicobacter pylori eradication therapy. World J. Gastroenterol. 2005, 11, 7520–7524. [Google Scholar] [CrossRef]
- Woo, T.D.H.; Oka, K.; Takahashi, M.; Hojo, F.; Osaki, T.; Hanawa, T.; Kurata, S.; Yonezawa, H.; Kamiya, S. Inhibition of the cytotoxic effect of Clostridium difficile in vitro by Clostridium butyricum MIYAIRI 588 strain. J. Med. Microbiol. 2011, 60, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Rossi, O.; van Berkel, L.A.; Chain, F.; Tanweer Khan, M.; Taverne, N.; Sokol, H.; Duncan, S.H.; Flint, H.J.; Harmsen, H.J.; Langella, P.; et al. Faecalibacterium prausnitzii A2-165 has a high capacity to induce IL-10 in human and murine dendritic cells and modulates T cell responses. Sci. Rep. 2016, 6, 18507. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yoo, Y.; Hwang, J.; Na, Y.C.; Kim, H.S. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J. Allergy Clin. Immunol. 2016, 137, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Frossard, C.P.; Steidler, L.; Eigenmann, P.A. Oral administration of an IL-10-secreting Lactococcus lactis strain prevents food-induced IgE sensitization. J. Allergy Clin. Immunol. 2007, 119, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Robert, S.; Steidler, L. Recombinant Lactococcus lactis can make the difference in antigen-specific immune tolerance induction, the Type 1 Diabetes case. Microb. Cell. Fact. 2014, 13, S11. [Google Scholar] [CrossRef]
- Li, R.; Wan, X.; Takala, T.M.; Saris, P.E.J. Heterologous expression of the Leuconostoc bacteriocin leucocin c in probiotic yeast Saccharomyces boulardii. Probiotics Antimicrob. 2021, 13, 229–237. [Google Scholar] [CrossRef]
- Gurbatri, C.R.; Radford, G.A.; Vrbanac, L.; Im, J.; Thomas, E.M.; Coker, C.; Taylor, S.R.; Jang, Y.; Sivan, A.; Rhee, K.; et al. Engineering tumor-colonizing E. coli Nissle 1917 for detection and treatment of colorectal neoplasia. Nat. Commun. 2024, 15, 646. [Google Scholar] [CrossRef]
- Food and Drug Administration. Early Clinical Trials with Live Biotherapeutic Products: Chemistry, Manufacturing, and Control Information. Guidance for Industry. 2016. Available online: https://www.fda.gov/files/vaccines,%20blood%20%26%20biologics/published/Early-Clinical-Trials-With-Live-Biotherapeutic-Products--Chemistry--Manufacturing--and-Control-Information--Guidance-for-Industry.pdf (accessed on 11 September 2024).
- European Pharmacopoeia Commission. 3053E General monograph on Live Biotherapeutic Products. In European Pharmacopoeia (Supplement 9.7); European Pharmacopoeia Commission: Strasbourg, France, 2019. [Google Scholar]
- Meng, J.; Liu, S.; Wu, X. Engineered probiotics as live biotherapeutics for diagnosis and treatment of human diseases. Crit. Rev. Microbiol. 2024, 50, 300–314. [Google Scholar] [CrossRef]
- Huang, L.; Tang, W.; He, L.; Li, M.; Lin, X.; Hu, A.; Huang, X.; Wu, Z.; Wu, Z.; Chen, S.; et al. Engineered probiotic Escherichia coli elicits immediate and long-term protection against influenza A virus in mice. Nat. Commun. 2024, 15, 6802. [Google Scholar] [CrossRef]
- McFarland, L.V.; Bernasconi, P. Saccharomyces boulardii’: A review of an innovative biotherapeutic agent. Microb. Ecol. Health Dis. 1993, 6, 157–171. [Google Scholar] [CrossRef]
- Czerucka, D.; Piche, T.; Rampal, P. Review article: Yeast as probiotics—Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2007, 26, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Girones, R.; Koutsoumanis, K.; Lindqvist, R.; Nørrung, B.; Robertson, L.; et al. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 7: Suitability of taxonomic units notified to EFSA until september 2017. EFSA J. 2018, 16, e05131. [Google Scholar]
- Fietto, J.L.; Araújo, R.S.; Valadão, F.N.; Fietto, L.G.; Brandão, R.L.; Neves, M.J.; Gomes, F.C.; Nicoli, J.R.; Castro, I.M. Molecular and physiological comparisons between Saccharomyces cerevisiae and Saccharomyces boulardii. Can. J. Microbiol. 2004, 50, 615–621. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.L.; Rajkumar, C.; Cooke, J.; Bulpitt, C.J. Probiotics in prevention of antibiotic associated diarrhoea: Meta-analysis. BMJ 2002, 324, 1361. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, C.M.; Elmer, G.W.; Speelman, P.; McFarland, L.V.; Chinn, J.; van Belle, G. Prevention of antibiotic-associated diarrhea by Saccharomyces boulardii: A prospective study. Gastroenterology 1989, 96, 981–988. [Google Scholar] [CrossRef]
- Szajewska, H.; Mrukowicz, J. Meta-analysis: Non-pathogenic yeast Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment. Pharmacol. Ther. 2005, 22, 365–372. [Google Scholar] [CrossRef]
- Kollaritsch, H.; Holst, H.; Grobara, P.; Wiedermann, G. Prevention of traveler’s diarrhea with Saccharomyces boulardii. Results of a placebo controlled double-blind study. Fortschr. Med. 1993, 111, 152–156. [Google Scholar]
- McFarland, L.V. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel. Med. Infect. Dis. 2007, 5, 97–105. [Google Scholar] [CrossRef]
- Marc, T.S.; Bléhaut, H.; Musiał, C.; Touraine, J.L.J.S.D.H. AIDS-related diarrhea a double-blind trial of Saccharomyces boulardii. Semaine. Des. Hopitaux. 1995, 71, 735–741. [Google Scholar]
- Villar-García, J.; Hernández, J.J.; Güerri-Fernández, R.; González, A.; Lerma, E.; Guelar, A.; Saenz, D.; Sorlí, L.; Montero, M.; Horcajada, J.P.; et al. Effect of probiotics (Saccharomyces boulardii) on microbial translocation and inflammation in HIV-treated patients: A double-blind, randomized, placebo-controlled trial. J. Acquir. Immune. Defic. Syndr. 2015, 68, 256–263. [Google Scholar] [CrossRef]
- Dinleyici, E.C.; Kara, A.; Dalgic, N.; Kurugol, Z.; Arica, V.; Metin, O.; Temur, E.; Turel, O.; Guven, S.; Yasa, O.; et al. Saccharomyces boulardii CNCM I-745 reduces the duration of diarrhoea, length of emergency care and hospital stay in children with acute diarrhoea. Benef. Microbes 2015, 6, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Feizizadeh, S.; Salehi-Abargouei, A.; Akbari, V. Efficacy and safety of Saccharomyces boulardii for acute diarrhea. Pediatrics 2014, 134, e176–e191. [Google Scholar] [CrossRef] [PubMed]
- Guslandi, M.; Giollo, P.; Testoni, P.A. A pilot trial of Saccharomyces boulardii in ulcerative colitis. Eur. J. Gastroenterol. Hepatol. 2003, 15, 697–698. [Google Scholar] [CrossRef] [PubMed]
- Guslandi, M.; Mezzi, G.; Sorghi, M.; Testoni, P.A. Saccharomyces boulardii in maintenance treatment of Crohn’s disease. Dig. Dis. Sci. 2000, 45, 1462–1464. [Google Scholar] [CrossRef] [PubMed]
- Maupas, J.; Champemont, P.; Delforge, M. Treatment of irritable bowel syndrome with Saccharomyces boulardii: A double blind, placebo controlled study. Médecine Chir. Dig. 1983, 12, 77–79. [Google Scholar]
- Cremonini, F.; Di Caro, S.; Covino, M.; Armuzzi, A.; Gabrielli, M.; Santarelli, L.; Nista, E.C.; Cammarota, G.; Gasbarrini, G.; Gasbarrini, A. Effect of different probiotic preparations on anti-helicobacter pylori therapy-related side effects: A parallel group, triple blind, placebo-controlled study. Am. J. Gastroenterol. 2002, 97, 2744–2749. [Google Scholar] [CrossRef]
- Duman, D.G.; Bor, S.; Ozütemiz, O.; Sahin, T.; Oğuz, D.; Iştan, F.; Vural, T.; Sandkci, M.; Işksal, F.; Simşek, I.; et al. Efficacy and safety of Saccharomyces boulardii in prevention of antibiotic-associated diarrhoea due to Helicobacter pylori eradication. Eur. J. Gastroenterol. Hepatol. 2005, 17, 1357–1361. [Google Scholar] [CrossRef]
- Lusvarghi, S.; Bewley, C.A. Griffithsin: An antiviral lectin with outstanding therapeutic potential. Viruses. 2016, 8, 296. [Google Scholar] [CrossRef]
- Mori, T.; O’Keefe, B.R.; Sowder, R.C., 2nd; Bringans, S.; Gardella, R.; Berg, S.; Cochran, P.; Turpin, J.A.; Buckheit, R.W., Jr.; McMahon, J.B.; et al. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J. Biol. Chem. 2005, 280, 9345–9353. [Google Scholar] [CrossRef]
- O’Keefe, B.R.; Giomarelli, B.; Barnard, D.L.; Shenoy, S.R.; Chan, P.K.; McMahon, J.B.; Palmer, K.E.; Barnett, B.W.; Meyerholz, D.K.; Wohlford-Lenane, C.L.; et al. Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. J. Virol. 2010, 84, 2511–2521. [Google Scholar] [CrossRef]
- Millet, J.K.; Séron, K.; Labitt, R.N.; Danneels, A.; Palmer, K.E.; Whittaker, G.R.; Dubuisson, J.; Belouzard, S. Middle East respiratory syndrome coronavirus infection is inhibited by griffithsin. Antiviral. Res. 2016, 133, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Takebe, Y.; Saucedo, C.J.; Lund, G.; Uenishi, R.; Hase, S.; Tsuchiura, T.; Kneteman, N.; Ramessar, K.; Tyrrell, D.L.; Shirakura, M.; et al. Antiviral lectins from red and blue-green algae show potent in vitro and in vivo activity against hepatitis C virus. PLoS ONE 2013, 8, e64449. [Google Scholar] [CrossRef] [PubMed]
- Meuleman, P.; Albecka, A.; Belouzard, S.; Vercauteren, K.; Verhoye, L.; Wychowski, C.; Leroux-Roels, G.; Palmer, K.E.; Dubuisson, J. Griffithsin has antiviral activity against hepatitis C virus. Antimicrob. Agents Chemother. 2011, 55, 5159–5167. [Google Scholar] [CrossRef] [PubMed]
- Ishag, H.Z.; Li, C.; Wang, F.; Mao, X. Griffithsin binds to the glycosylated proteins (E and prM) of Japanese encephalitis virus and inhibit its infection. Virus Res. 2016, 215, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, X.; Zhang, H.; Cheng, H.; Hou, L.; Zheng, Q.; Hou, J. In vitro antiviral activity of Griffithsin against porcine epidemic diarrhea virus. Virus Genes 2019, 55, 174–181. [Google Scholar] [CrossRef]
- Jin, Y.; Yu, S.; Liu, J.J.; Yun, E.J.; Lee, J.W.; Jin, Y.S.; Kim, K.H. Production of neoagarooligosaccharides by probiotic yeast Saccharomyces cerevisiae var. boulardii engineered as a microbial cell factory. Microb. Cell. Fact. 2021, 20, 160. [Google Scholar] [CrossRef]
- Li, R.; Yassami, S.; Kiviniemi, E.A.; Qiao, W.; Takala, T.M.; Saris, P.E.J. Listeria decontamination of chicken meat with beer brewed with bacteriocin producing Saccharomyces boulardii. LWT-Food. Sci. Technol. 2021, 152, 112323. [Google Scholar] [CrossRef]
- Li, R.; Tan, X.; Li, S.; Jin, Y.; Li, S.; Li, S.; Takala, T.M.; Saris, P.E.J. Cloning, expression, characterization, and tissue distribution of Cystatin C from silver carp (Hypophthalmichthys molitrix). J. Agric. Food. Chem. 2021, 69, 5144–5154. [Google Scholar] [CrossRef]
- Walker, J.M. The bicinchoninic acid (BCA) assay for protein quantitation. Methods. Mol. Biol. 1994, 32, 5–8. [Google Scholar]
- Regmi, P.R.; Sauer, W.C.; Zijlstra, R.T. Prediction of in vivo apparent total tract energy digestibility of barley in grower pigs using an in vitro digestibility technique. J. Anim. Sci. 2008, 86, 2619–2626. [Google Scholar] [CrossRef]
- Huang, Y.; Adams, M.C. In vitro assessment of the upper gastrointestinal tolerance of potential probiotic dairy propionibacteria. Int. J. Food Microbiol. 2004, 91, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Surono, I.; Meriluoto, J.; Salminen, S. Indigenous dadih lactic acid bacteria: Cell-surface properties and interactions with pathogens. J. Food. Sci. 2007, 72, M89–M93. [Google Scholar] [CrossRef] [PubMed]
- Syazni; Yanagisawa, M.; Kasuu, M.; Ariga, O.; Nakasaki, K. Direct production of ethanol from neoagarobiose using recombinant yeast that secretes α-neoagarooligosaccharide hydrolase. Enzyme. Microb. Technol. 2016, 85, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Kong, I.I.; Zhang, G.C.; Jayakody, L.N.; Kim, H.; Xia, P.F.; Kwak, S.; Sung, B.H.; Sohn, J.H.; Walukiewicz, H.E.; et al. Metabolic engineering of probiotic Saccharomyces boulardii. Appl. Environ. Microbiol. 2016, 82, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Terciolo, C.; Dobric, A.; Ouaissi, M.; Siret, C.; Breuzard, G.; Silvy, F.; Marchiori, B.; Germain, S.; Bonier, R.; Hama, A.; et al. Saccharomyces boulardii CNCM I-745 restores intestinal barrier integrity by regulation of E-cadherin recycling. J. Crohns. Colitis. 2017, 11, 999–1010. [Google Scholar] [CrossRef]
- Douradinha, B.; Reis, V.C.; Rogers, M.B.; Torres, F.A.; Evans, J.D.; Marques, E.T., Jr. Novel insights in genetic transformation of the probiotic yeast Saccharomyces boulardii. Bioengineered 2014, 5, 21–29. [Google Scholar] [CrossRef]
- Wang, T.; Sun, H.; Zhang, J.; Liu, Q.; Wang, L.; Chen, P.; Wang, F.; Li, H.; Xiao, Y.; Zhao, X. The establishment of Saccharomyces boulardii surface display system using a single expression vector. Fungal. Genet. Biol. 2014, 64, 1–10. [Google Scholar] [CrossRef]
- Latorre-García, L.; Adam, A.C.; Polaina, J. Overexpression of the glucoamylase-encoding STA1 gene of Saccharomyces cerevisiae var. diastaticus in laboratory and industrial strains of Saccharomyces. World. J. Microb. Blot. 2008, 24, 2957–2963. [Google Scholar] [CrossRef]
- Michael, S.; Keubler, L.M.; Smoczek, A.; Meier, M.; Gunzer, F.; Pöhlmann, C.; Krause-Buchholz, U.; Hedrich, H.J.; Bleich, A. Quantitative phenotyping of inflammatory bowel disease in the IL-10-deficient mouse by use of noninvasive magnetic resonance imaging. Inflamm. Bowel. Dis. 2013, 19, 185–193. [Google Scholar] [CrossRef]
- Bagherpour, G.; Ghasemi, H.; Zand, B.; Zarei, N.; Roohvand, F.; Ardakani, E.M.; Azizi, M.; Khalaj, V. Oral administration of recombinant Saccharomyces boulardii expressing ovalbumin-CPE fusion protein induces antibody response in mice. Front. Microbiol. 2018, 9, 723. [Google Scholar] [CrossRef]
- Durmusoglu, D.; Al’Abri, I.S.; Collins, S.P.; Cheng, J.; Eroglu, A.; Beisel, C.L.; Crook, N. In Situ biomanufacturing of small molecules in the mammalian gut by probiotic Saccharomyces boulardii. ACS Synth. Biol. 2021, 10, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Chang, J.H.; Chang, Y.C.; Mou, K.Y. Treatment of murine colitis by Saccharomyces boulardii secreting atrial natriuretic peptide. J. Mol. Med. 2020, 98, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhu, Y.; Zhang, Y.; Hamza, T.; Yu, H.; Saint Fleur, A.; Galen, J.; Yang, Z.; Feng, H. A probiotic yeast-based immunotherapy against Clostridioides difficile infection. Sci. Transl. Med. 2020, 12, eaax4905. [Google Scholar] [CrossRef] [PubMed]
- Palma, M.L.; Garcia-Bates, T.M.; Martins, F.S.; Douradinha, B. Genetically engineered probiotic Saccharomyces cerevisiae strains mature human dendritic cells and stimulate Gag-specific memory CD8(+) T cells ex vivo. Appl. Microbiol. Biotechnol. 2019, 103, 5183–5192. [Google Scholar] [CrossRef] [PubMed]
- Vamvaka, E.; Arcalis, E.; Ramessar, K.; Evans, A.; O’Keefe, B.R.; Shattock, R.J.; Medina, V.; Stöger, E.; Christou, P.; Capell, T. Rice endosperm is cost-effective for the production of recombinant griffithsin with potent activity against HIV. Plant Biotechnol. J. 2016, 14, 1427–1437. [Google Scholar] [CrossRef]
- Tang, R.; Guo, L.; Fan, Q.; Zhang, L.; Wang, Y.; Zhang, X.; Shi, D.; Wu, Y.; Shi, H.; Liu, J.; et al. Porcine deltacoronavirus infection is inhibited by Griffithsin in cell culture. Vet. Microbiol. 2022, 264, 109299. [Google Scholar] [CrossRef]
- Petrova, M.I.; van den Broek, M.F.L.; Spacova, I.; Verhoeven, T.L.A.; Balzarini, J.; Vanderleyden, J.; Schols, D.; Lebeer, S. Engineering Lactobacillus rhamnosus GG and GR-1 to express HIV-inhibiting griffithsin. Int. J. Antimicrob. Agents 2018, 52, 599–607. [Google Scholar] [CrossRef]

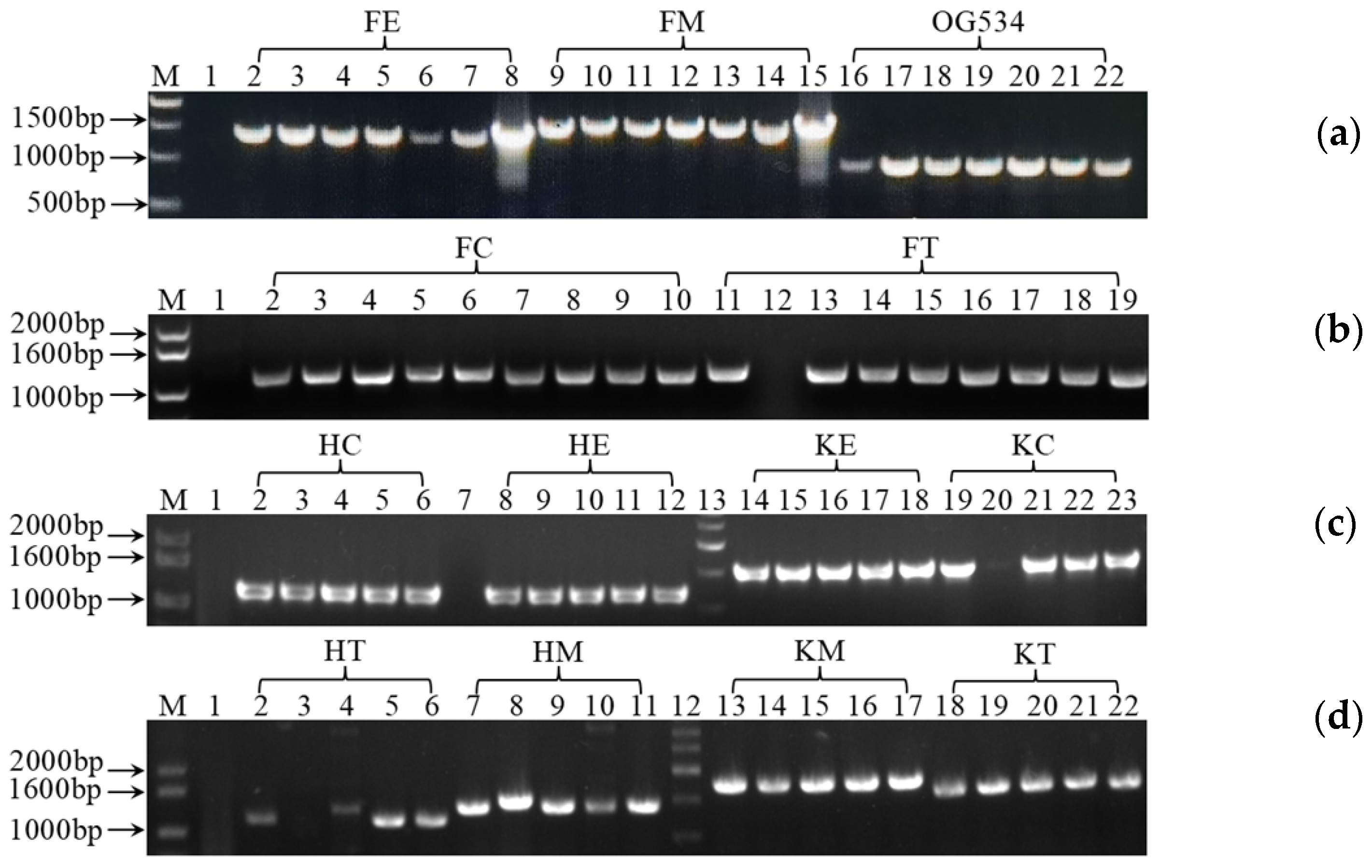
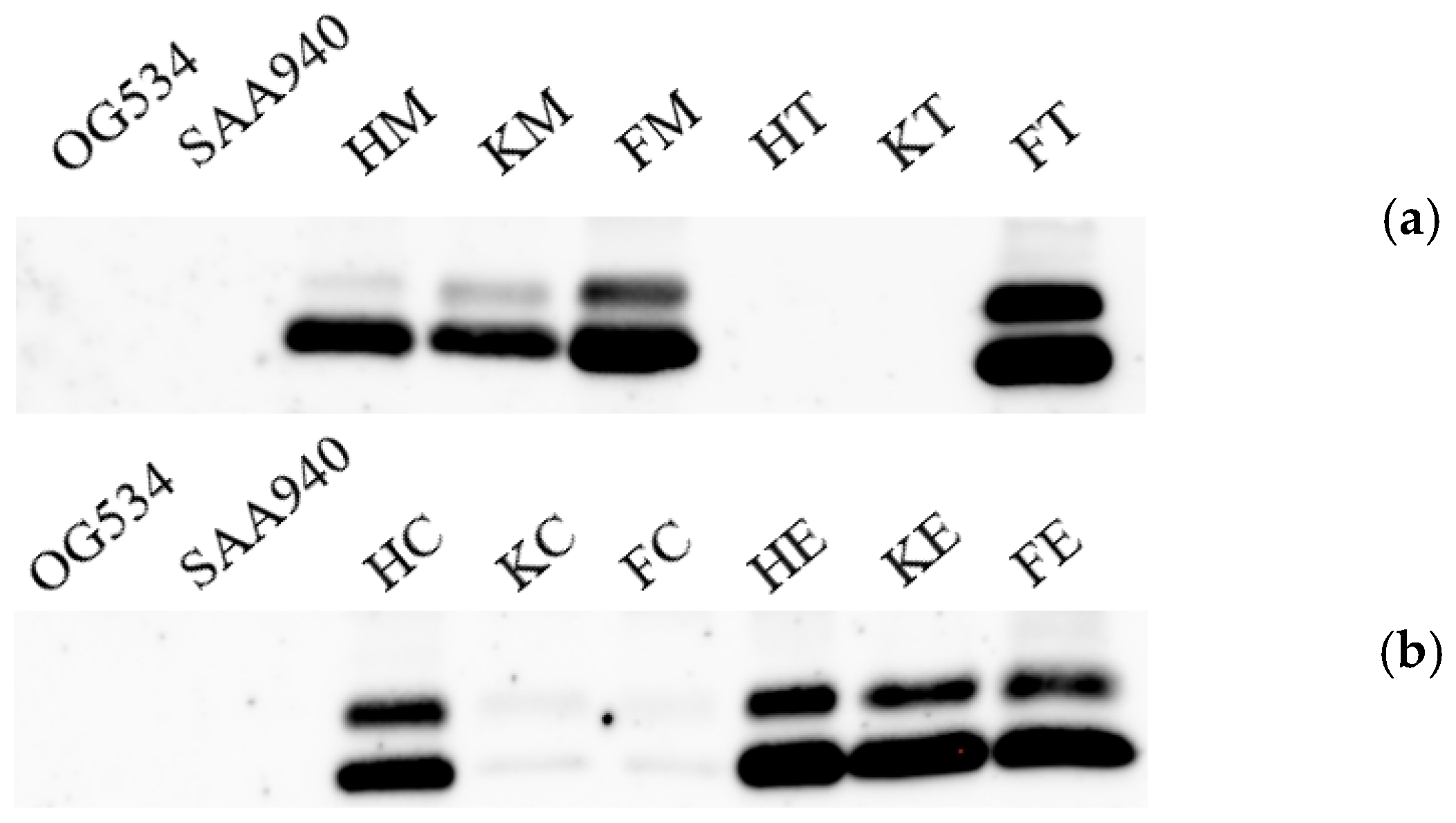






| Plasmids and Strains | Name | Descriptions | Source |
|---|---|---|---|
| Vector plasmid | pSF-TEF1-URA3 | Shuttle vector plasmid of E. coli and yeast containing constitutive TEF1 promoter from Saccharomyces cerevisiae and URA3 gene for auxotrophic selection. | Prof. Per E. J. Saris University of Helsinki, Helsinki, Finland |
| Recombinant plasmids for griffithsin secretion | pSF-TEF1-SED1-GRFT | Griffithsin expression plasmid containing TEF1 promoter and SED1 signal sequence from S. boulardii. | Designed in this study, constructed by GenScript Biotechnology Co., Ltd., Nanjing, China |
| pSF-TEF1-αMF-GRFT | Griffithsin expression plasmid containing TEF1 promoter and αMF signal sequence from S. boulardii. | ||
| pSF-TEF1-STA1-GRFT | Griffithsin expression plasmid containing TEF1 promoter from S. boulardii and STA1 signal sequence from S. diastaticus. | ||
| pSF-TEF1-CL-GRFT | Griffithsin expression plasmid containing TEF1 promoter from S. boulardii and CL signal sequence from chicken lysozyme. | ||
| pSF-TDH3-SED1-GRFT | Griffithsin expression plasmid containing TDH3 promoter and SED1 signal sequence from S. boulardii. | ||
| pSF-TDH3-αMF-GRFT | Griffithsin expression plasmid containing TDH3 promoter and αMF signal sequence from S. boulardii. | ||
| pSF-TDH3-STA1-GRFT | Griffithsin expression plasmid containing TDH3 promoter from S. boulardii and STA1 signal sequence from S. diastaticus. | ||
| pSF-TDH3-CL-GRFT | Griffithsin expression plasmid containing TDH3 promoter from S. boulardii and CL signal sequence from chicken lysozyme. | ||
| pSF-PGK1-SED1-GRFT | Griffithsin expression plasmid containing PGK1 promoter and SED1 signal sequence from S. boulardii. | ||
| pSF-PGK1-αMF-GRFT | Griffithsin expression plasmid containing PGK1 promoter and αMF signal sequence from S. boulardii. | ||
| pSF-PGK1-STA1-GRFT | Griffithsin expression plasmid containing PGK1 promoter from S. boulardii and STA1 signal sequence from S. diastaticus. | ||
| pSF-PGK1-CL-GRFT | Griffithsin expression plasmid containing PGK1 promoter from S. boulardii and CL signal sequence from chicken lysozyme. | ||
| Host strains | S. boulardii SAA940 (SAA940) | URA3-deficient strain of Saccharomyces boulardii CNCM I-745, host strain for griffithsin secretion | Prof. Vahid Khalaj, the Pasteur Institute of Iran, Tehran, Iran |
| E. coli DH5α | Intermediate host strain for recombinant plasmids | Novizan Biotechnology Co., Ltd., Nanjing, China | |
| RERecombinant S. boulardii strains | S. boulardii OG534 (OG534) | S. boulardii SAA940 carrying pSF-TEF1-URA3 (OG534), vector control strain | This study |
| S. boulardii FE (FE) | S. boulardii SAA940 carrying plasmid pSF-TEF1-SED1-GRFT | ||
| S. boulardii FM (FM) | S. boulardii SAA940 carrying plasmid pSF-TEF1-αMF -GRFT | ||
| S. boulardii FT (FT) | S. boulardii SAA940 carrying plasmid pSF-TEF1-STA1-GRFT | ||
| S. boulardii FC (FC) | S. boulardii SAA940 carrying plasmid pSF-TEF1-CL-GRFT | ||
| S. boulardii HE (HE) | S. boulardii SAA940 carrying plasmid pSF-TDH3-SED1-GRFT | ||
| S. boulardii HM (HM) | S. boulardii SAA940 carrying plasmid pSF-TDH3-αMF-GRFT | ||
| S. boulardii HT (HT) | S. boulardii SAA940 carrying plasmid pSF-TDH3-STA1-GRFT | ||
| S. boulardii HC (HC) | S. boulardii SAA940 carrying plasmid pSF-TDH3-CL-GRFT | ||
| S. boulardii KE (KE) | S. boulardii SAA940 carrying plasmid pSF-PGK1-SED1-GRFT | ||
| S. boulardii KM (KM) | S. boulardii SAA940 carrying plasmid pSF-PGK1-αMF-GRFT | ||
| S. boulardii KT (KT) | S. boulardii SAA940 carrying plasmid pSF-PGK1-STA1-GRFT | ||
| S. boulardii KC (KC) | S. boulardii SAA940 carrying plasmid pSF-PGK1-CL-GRFT |
| S. boulardii Strains | |||||||
|---|---|---|---|---|---|---|---|
| Digestive Fluids | Incubation Time | SAA940 | OG534 | FM | FT | HE | HC |
| Stomach | 1 h | 96.46 ± 1.62 ab | 97.46 ± 0.00 a | 91.85 ± 0.71 bc | 89.99 ± 2.24 c | 97.29 ± 0.80 a | 93.71 ± 3.52 abc |
| 2 h | 96.56 ± 1.43 a | 95.88 ± 0.00 a | 93.06 ± 2.26 ab | 86.32 ± 1.49 c | 96.51 ± 0.89 a | 91.25 ± 2.40 b | |
| Small intestine | 2 h | 96.72 ± 0.39 a | 97.27 ± 1.45 a | 89.18 ± 0.18 b | 89.21 ± 0.64 b | 97.49 ± 1.47 a | 89.05 ± 1.65 b |
| 4 h | 95.94 ± 0.68 a | 97.81 ± 1.31 a | 89.61 ± 0.43 b | 90.87 ± 1.71 b | 97.18 ± 0.61 a | 91.20 ± 1.65 b | |
| Large intestine | 2 h | 98.43 ± 1.65 a | 98.31 ± 1.00 a | 95.36 ± 1.94 a | 89.57 ± 1.14 b | 96.98 ± 0.66 a | 90.2 ± 2.64 b |
| 4 h | 97.47 ± 1.17 a | 98.74 ± 1.97 a | 93.79 ± 1.86 b | 89.4 ± 0.90 c | 97.41 ± 0.62 a | 90.8 ± 0.83 bc | |
| 6 h | 97.68 ± 1.67 a | 97.03 ± 1.56 a | 91.64 ± 5.1 ab | 92.43 ± 1.37 ab | 97.18 ± 0.95 a | 90.35 ± 1.48 b | |
| 8 h | 96.34 ± 0.79 a | 96.06 ± 0.00 a | 91.91 ± 4.38 ab | 92.32 ± 0.00 ab | 96.75 ± 0.00 a | 90.6 ± 1.62 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Li, R.; Jiang, T.; Lv, J.; Jiang, Y.; Zhou, X.; Chen, H.; Li, M.; Wu, A.; Yu, B.; et al. Heterologous Expression of the Antiviral Lectin Griffithsin in Probiotic Saccharomyces boulardii and In Vitro Characterization of Its Properties. Microorganisms 2024, 12, 2414. https://doi.org/10.3390/microorganisms12122414
Tang J, Li R, Jiang T, Lv J, Jiang Y, Zhou X, Chen H, Li M, Wu A, Yu B, et al. Heterologous Expression of the Antiviral Lectin Griffithsin in Probiotic Saccharomyces boulardii and In Vitro Characterization of Its Properties. Microorganisms. 2024; 12(12):2414. https://doi.org/10.3390/microorganisms12122414
Chicago/Turabian StyleTang, Jie, Ran Li, Tingyu Jiang, Jiachen Lv, Yuwei Jiang, Xingjian Zhou, Hong Chen, Meiliang Li, Aimin Wu, Bing Yu, and et al. 2024. "Heterologous Expression of the Antiviral Lectin Griffithsin in Probiotic Saccharomyces boulardii and In Vitro Characterization of Its Properties" Microorganisms 12, no. 12: 2414. https://doi.org/10.3390/microorganisms12122414
APA StyleTang, J., Li, R., Jiang, T., Lv, J., Jiang, Y., Zhou, X., Chen, H., Li, M., Wu, A., Yu, B., Takala, T. M., Saris, P. E. J., Li, S., & Fang, Z. (2024). Heterologous Expression of the Antiviral Lectin Griffithsin in Probiotic Saccharomyces boulardii and In Vitro Characterization of Its Properties. Microorganisms, 12(12), 2414. https://doi.org/10.3390/microorganisms12122414









