Endophytic Fungi Co-Culture: An Alternative Source of Antimicrobial Substances
Abstract
1. Introduction
2. Endophytes and Their Host Plant Interactions
3. Endophytes and Their Metabolites
4. The Influence of the Culture Medium in Co-Cultivations
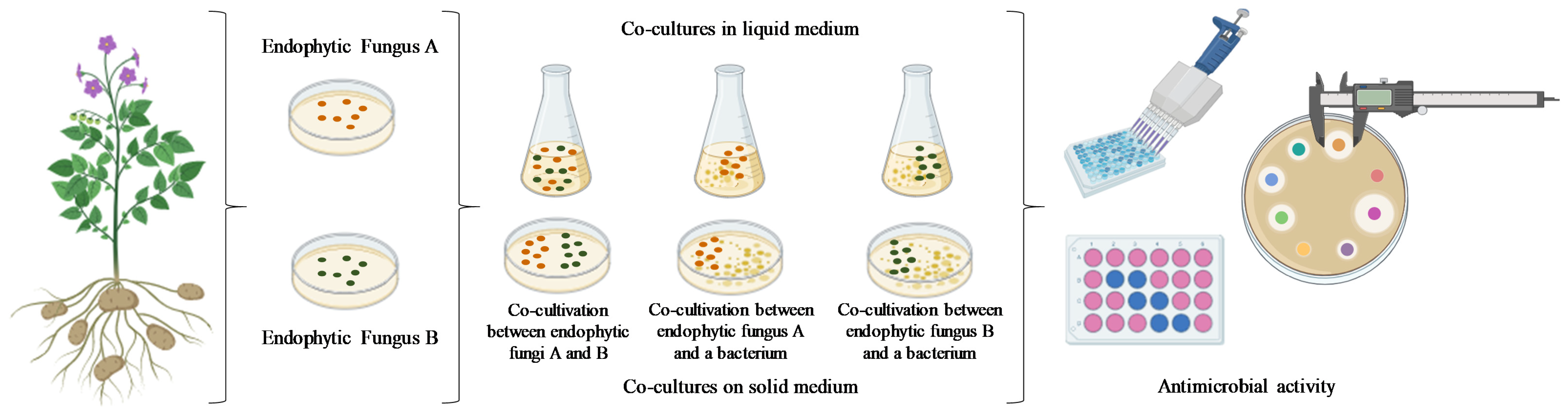
5. Microbial Co-Cultures: Advantages and Challenges
- (1)
- Growth in liquid medium: microorganisms come into direct physical contact;
- (2)
- Solid–liquid interface systems: encapsulation of microorganisms co-cultured in a liquid medium;
- (3)
- Separation by membranes: microorganisms are separated by permeable membranes;
- (4)
- Spatial separations: monocultures are inoculated separately without direct physical contact, but can interact within a shared space;
- (5)
- Microfluidic systems: used primarily in mammalian research, offering better control over fluids and microenvironments [37].
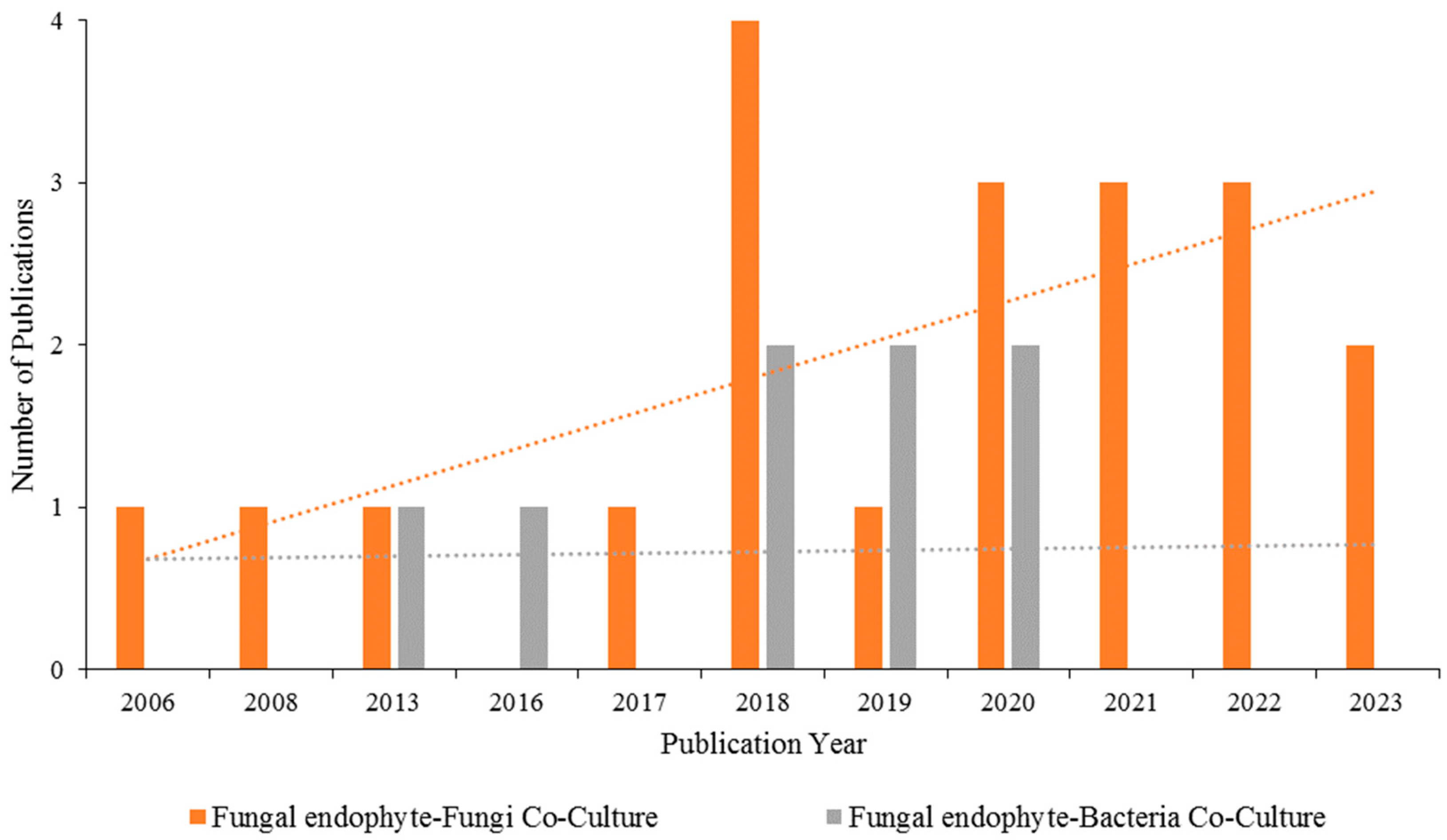
6. Antimicrobial Substances from Co-Cultivation of Endophytic Fungi
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hashem, A.H.; Attia, M.S.; Kandil, E.K.; Fawzi, M.M.; Abdelrahman, A.S.; Khader, M.S.; Khodaira, M.A.; Emam, A.E.; Goma, M.A.; Abdelaziz, A.M. Bioactive compounds and biomedical applications of endophytic fungi: A recent review. Microb. Cell Fact. 2023, 22, 122. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Updates List of Drug-Resistant Bacteria Most Threatening to Human Health. Available online: https://www.who.int/news/item/17-05-2024-who-updates-list-of-drug-resistant-bacteria-most-threatening-to-human-health (accessed on 1 September 2024).
- Tanvir, R.; Javeed, A.; Bajwa, A.G. Endophyte bioprospecting in South Asian medicinal plants: An attractive resource for biopharmaceuticals. Appl. Microbiol. Biotechnol. 2017, 101, 1831–1844. [Google Scholar] [CrossRef] [PubMed]
- Pokhriyal, A.; Kapoor, N.; Negi, S.; Sharma, G.; Chandra, S.; Gambhir, L.; Coutinho, H.D.M. Endophytic fungi: Cellular factories of novel medicinal chemistries. Bioorg. Chem. 2024, 150, 107576. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.; Gupta, P.; Jaglan, S.; Roullier, C.; Grovel, O.; Bertrand, S. Expanding the chemical diversity through microorganisms co-culture: Current status and outlook. Biotechnol. Adv. 2020, 40, 107521. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Li, Y.; Liao, S.; Liu, Y. Exploring diverse bioactive secondary metabolites from marine microorganisms using co-culture strategy. Molecules 2023, 28, 6371. [Google Scholar] [CrossRef]
- Zhuang, L.; Zhang, H. Utilizing cross-species co-cultures for discovery of novel natural products. Curr. Opin. Biotechnol. 2021, 69, 252–262. [Google Scholar] [CrossRef]
- Qian, X.; Chen, L.; Sui, Y.; Chen, C.; Zhang, W.; Zhou, J.; Dong, W.; Jiang, M.; Xin, F.; Ochsenreither, K. Biotechnological potential and applications of microbial consortia. Biotechnol. Adv. 2020, 40, 107500. [Google Scholar] [CrossRef]
- Yadav, G.; Meena, M. Bioprospecting of endophytes in medicinal plants of Thar Desert: An attractive resource for biopharmaceuticals. Biotechnol. Rep. 2021, 30, e00629. [Google Scholar] [CrossRef]
- Rosero-Chasoy, G.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Buitrón, G.; Chairez, I.; Ruiz, H.A. Microbial co-culturing strategies for the production of high-value compounds: A reliable framework towards sustainable biorefinery implementation—An overview. Bioresour. Technol. 2021, 321, 124458. [Google Scholar] [CrossRef]
- Compant, S.; Saikkonen, K.; Mitter, B.; Campisano, A.; Mercado-Blanco, J. Editorial special issue: Soil, plants and endophytes. Plant Soil. 2016, 405, 1–11. [Google Scholar] [CrossRef]
- Kusari, S.; Hertweck, C.; Spiteller, M. Chemical ecology of endophytic fungi: Origins of secondary metabolites. Chem. Biol. 2012, 19, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S. Endophytic fungi: Novel sources of anticancer lead molecules. Appl. Microbiol. Biotechnol. 2012, 95, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.A.S.; Polli, A.D.; Polonio, J.C.; Orlandelli, R.C.; Conte, H.; Azevedo, J.L.; Pamphile, J.A. Bioprospection and molecular phylogeny of culturable endophytic fungi associated with yellow passion fruit. Acta Sci. Biol. Sci. 2020, 42, e48321. [Google Scholar] [CrossRef]
- Bulla, L.M.C.; Polonio, J.C.; Portela-Castro, A.L.D.B.; Kava, V.; Azevedo, J.L.; Pamphile, J.A. Activity of the endophytic fungi Phlebia sp. and Paecilomyces formosus in decolourisation and the reduction of reactive dyes’ cytotoxicity in fish erythrocytes. Environ. Monit. Assess. 2017, 189, 88. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.A.S.; Ferreira, A.P.; Polli, A.D.; Silva, A.A.; Ribeiro, A.S.; Azevedo, J.L.; Pamphile, J.A. Plant growth-promoting activity of wild-type and bromate-resistant mutant of the endophytic fungus Colletotrichum karstii. Acta Sci. Technol. 2021, 43, e55457. [Google Scholar] [CrossRef]
- Gómez, O.C.; Moreira, D.M.B.; Hernández, I.L.C.; Lemes, R.M.L.; Luiz, J.H.H. Antimicrobial activity improvement after fractionating organic extracts from Lasiodiplodia sp. fermentation. Brazil. J. Dev. 2021, 7, 3795–3816. [Google Scholar] [CrossRef]
- Gómez, O.C.; Luiz, J.H.H. Endophytic fungi isolated from medicinal plants: Future prospects of bioactive natural products from Tabebuia/Handroanthus endophytes. Appl. Microbiol. Biotechnol. 2018, 102, 9105–9119. [Google Scholar] [CrossRef]
- Lata, R.; Chowdhury, S.; Gond, S.K.; White, J.F., Jr. Induction of abiotic stress tolerance in plants by endophytic microbes. Lett. Appl. Microbiol. 2018, 66, 268–276. [Google Scholar] [CrossRef]
- Farhat, H.; Urooj, F.; Sohail, N.; Ansari, M.; Ehteshamul-Haque, S. Evaluation of nematicidal potential of endophytic fungi associated with healthy plants and GC-MS profiling of metabolites of endophytic Fusarium solani. S. Afr. J. Bot. 2022, 146, 146–161. [Google Scholar] [CrossRef]
- Cruz, J.S.; da Silva, C.A.; Hamerski, L. Natural products from endophytic fungi associated with Rubiaceae species. J. Fungi 2020, 6, 128. [Google Scholar] [CrossRef]
- Mohamad, O.A.A.; Li, L.; Ma, J.B.; Hatab, S.; Xu, L.; Guo, J.W.; Rasulov, B.A.; Liu, Y.H.; Hedlund, B.P.; Li, W.J. Evaluation of the antimicrobial activity of endophytic bacterial populations from Chinese traditional medicinal plant licorice and characterization of the bioactive secondary metabolites produced by Bacillus atrophaeus against Verticillium dahliae. Front. Microbiol. 2018, 9, 924. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, P.J.; Challis, G.L. Discovery of microbial natural products by activation of silent biosynthetic gene clusters. Nat. Rev. Microbiol. 2015, 13, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shi, X.; Xing, Y.; Ren, X.X.; Zhang, D.Y.; Li, X.; Xiu, Z.L.; Dong, Y.S. Co-culture of Aspergillus sydowii and Bacillus subtilis induces the production of antibacterial metabolites. Fungal Biol. 2022, 126, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Nai, C.; Meyer, V. From axenic to mixed cultures: Technological advances accelerating a paradigm shift in microbiology. Trends Microbiol. 2018, 26, 538–554. [Google Scholar] [CrossRef]
- Kumar, V.; Ahluwalia, V.; Saran, S.; Kumar, J.; Patel, A.K.; Singhania, R.R. Recent developments on solid-state fermentation for production of microbial secondary metabolites: Challenges and solutions. Bioresour. Technol. 2021, 323, 124566. [Google Scholar] [CrossRef]
- Hu, H.; Catchmark, J.M.; Demirci, A. Co-culture fermentation on the production of bacterial cellulose nanocomposite produced by Komagataeibacter hansenii. Carbohydr. Polym. Technol. Appl. 2021, 2, 100028. [Google Scholar] [CrossRef]
- Diender, M.; Olm, I.P.; Sousa, D.Z. Synthetic co-cultures: Novel avenues for bio-based processes. Curr. Opin. Biotechnol. 2021, 67, 72–79. [Google Scholar] [CrossRef]
- Dos Reis, J.B.A.; Lorenzi, A.S.; do Vale, H.M.M. Methods used for the study of endophytic fungi: A review on methodologies and challenges, and associated tips. Arch. Microbiol. 2022, 204, 675. [Google Scholar] [CrossRef]
- Xu, Z.; Theodoropoulos, C.; Pittman, J.K. Optimization of a Chlorella–Saccharomyces co-culture system for enhanced metabolite productivity. Algal Res. 2024, 79, 103455. [Google Scholar] [CrossRef]
- Perera, I.A.; Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Naidu, R.; Megharaj, M. Combined inorganic nitrogen sources influence the release of extracellular compounds that drive mutualistic interactions in microalgal–bacterial co-cultures. J. Appl. Phycol. 2022, 34, 1311–1322. [Google Scholar] [CrossRef]
- Kasemiire, A.; Avohou, H.T.; De Bleye, C.; Sacre, P.Y.; Dumont, E.; Hubert, P.; Ziemons, E. Design of experiments and design space approaches in the pharmaceutical bioprocess optimization. Eur. J. Pharm. Biopharm. 2021, 166, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.; Islam, M.A.; Mishra, P.; Muzahid, A.J.M.; Yousuf, A.; Khan, M.M.R.; Faizal, C.K.M. Yeast and bacteria co-culture-based lipid production through bioremediation of palm oil mill effluent: A statistical optimization. Biomass Conv. Bioref. 2023, 13, 2947–2958. [Google Scholar] [CrossRef]
- Padmaperuma, G.; Kapoore, R.V.; Gilmour, D.J.; Vaidyanathan, S. Microbial consortia: A critical look at microalgae co-cultures for enhanced biomanufacturing. Crit. Rev. Biotechnol. 2018, 38, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Marmann, A.; Aly, A.H.; Lin, W.; Wang, B.; Proksch, P. Co-cultivation—A powerful emerging tool for enhancing the chemical diversity of microorganisms. Mar. Drugs 2014, 12, 1043–1065. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, S.; Schumpp, O.; Bohni, N.; Monod, M.; Gindro, K.; Wolfender, J.L. De novo production of metabolites by fungal co-culture of Trichophyton rubrum and Bionectria ochroleuca. J. Nat. Prod. 2013, 76, 1157–1165. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Padmaperuma, G.; Maneein, S.; Vaidyanathan, S. Co-culturing microbial consortia: Approaches for applications in biomanufacturing and bioprocessing. Crit. Rev. Biotechnol. 2022, 42, 46–72. [Google Scholar] [CrossRef]
- Brenner, K.; You, L.; Arnold, F.H. Engineering microbial consortia: A new frontier in synthetic biology. Trends Biotechnol. 2008, 26, 483–489. [Google Scholar] [CrossRef]
- Müller, C.; Caspers, B.A.; Gadau, J.; Kaiser, S. The power of infochemicals in mediating individualized niches. Trends Ecol. Evol. 2020, 35, 981–989. [Google Scholar] [CrossRef]
- Masset, J.; Calusinska, M.; Hamilton, C.; Hiligsmann, S.; Joris, B.; Wilmotte, A.; Thonart, P. Fermentative hydrogen production from glucose and starch using pure strains and artificial co-cultures of Clostridium spp. Biotechnol. Biofuels 2012, 5, 35. [Google Scholar] [CrossRef]
- Angelis, S.; Novak, A.C.; Sydney, E.B.; Soccol, V.T.; Carvalho, J.C.; Pandey, A.; Noseda, M.D.; Tholozan, J.L.; Lorquin, J.; Soccol, C.R. Co-culture of microalgae, cyanobacteria, and macromycetes for exopolysaccharides production: Process preliminary optimization and partial characterization. Appl. Biochem. Biotechnol. 2012, 167, 1092–1106. [Google Scholar] [CrossRef]
- Guo, T.; Song, X.; Chen, L.; Zhang, W. Using OMICS technologies to analyze the mechanisms of synthetic microbial co-culture systems: A review. Chin. J. Biotechnol. 2022, 38, 460–477. [Google Scholar] [CrossRef]
- Mittermeier, F.; Bäumler, M.; Arulrajah, P.; García Lima, J.D.J.; Hauke, S.; Stock, A.; Weuster-Botz, D. Artificial microbial consortia for bioproduction processes. Eng. Life Sci. 2023, 23, e2100152. [Google Scholar] [CrossRef] [PubMed]
- Boruta, T. Computation-aided studies related to the induction of specialized metabolite biosynthesis in microbial co-cultures: An introductory overview. Comput. Struct. Biotechnol. J. 2023, 21, 4021–4029. [Google Scholar] [CrossRef]
- Jones, J.A.; Vernacchio, V.R.; Sinkoe, A.L.; Collins, S.M.; Ibrahim, M.H.A.; Lachance, D.M.; Hahn, J.; Koffas, M.A.G. Experimental and computational optimization of an Escherichia coli co-culture for the efficient production of flavonoids. Metab. Eng. 2016, 35, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xi, B.; Lu, L. Strategies to enhance production of metabolites in microbial co-culture systems. Bioresour. Technol. 2024, 406, 131049. [Google Scholar] [CrossRef] [PubMed]
- Bader, J.; Mast-Gerlach, E.; Popović, M.K.; Bajpai, R.; Stahl, U. Relevance of microbial coculture fermentations in biotechnology. J. Appl. Microbiol. 2010, 109, 371–387. [Google Scholar] [CrossRef]
- Li, Q.; Lin, W.; Zhang, X.; Wang, M.; Zheng, Y.; Wang, X.; Gao, G.; Li, Y.; Zhao, D.; Zhang, C. Transcriptomics integrated with metabolomics reveal the competitive relationship between co-cultured Trichoderma asperellum HG1 and Bacillus subtilis Tpb55. Microbiol. Res. 2024, 280, 127598. [Google Scholar] [CrossRef]
- Xu, S.; Li, M.; Hu, Z.; Shao, Y.; Ying, J.; Zhang, H. The potential use of fungal co-culture strategy for discovery of new secondary metabolites. Microorganisms 2023, 11, 464. [Google Scholar] [CrossRef]
- Knowles, S.L.; Raja, H.A.; Roberts, C.D.; Oberlies, N.H. Fungal–fungal co-culture: A primer for generating chemical diversity. Nat. Prod. Rep. 2022, 39, 1557–1573. [Google Scholar] [CrossRef]
- Pawlowska, T.E. Symbioses between fungi and bacteria: From mechanisms to impacts on biodiversity. Curr. Opin. Microbiol. 2024, 80, 102496. [Google Scholar] [CrossRef]
- Thuan, N.H.; Tatipamula, V.B.; Canh, N.X.; Van Giang, N. Recent advances in microbial co-culture for production of value-added compounds. 3 Biotech 2022, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of antibiotics and antibiotic resistance, and their impacts on drug development: A narrative review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Q.; Li, X.M.; Li, X.; Li, H.L.; Meng, L.H.; Wang, B.G. New citrinin analogues produced by coculture of the marine algal-derived endophytic fungal strains Aspergillus sydowii EN-534 and Penicillium citrinum EN-535. Phytochem. Lett. 2018, 25, 191–195. [Google Scholar] [CrossRef]
- Li, C.; Sarotti, A.M.; Yang, B.; Turkson, J.; Cao, S. A new N-methoxypyridone from the co-cultivation of Hawaiian endophytic fungi Camporesia sambuci FT1061 and Epicoccum sorghinum FT1062. Molecules 2017, 22, 1166. [Google Scholar] [CrossRef] [PubMed]
- Chagas, F.O.; Dias, L.G.; Pupo, M.T. A mixed culture of endophytic fungi increases the production of antifungal polyketides. J. Chem. Ecol. 2013, 39, 1335–1342. [Google Scholar] [CrossRef]
- Halecker, S.; Wennrich, J.-P.; Rodrigo, S.; Andrée, N.; Rabsch, L.; Baschien, C.; Steinert, M.; Stadler, M.; Surup, F.; Schulz, B. Fungal endophytes for biocontrol of ash dieback: The antagonistic potential of Hypoxylon rubiginosum. Fungal Ecol. 2020, 45, 100918. [Google Scholar] [CrossRef]
- Yin, H.Y.; Yang, X.Q.; Wang, D.L.; Zhao, T.D.; Wang, C.F.; Yang, Y.B.; Ding, Z.T. Antifeedant and antiphytopathogenic metabolites from co-culture of endophyte Irpex lacteus, phytopathogen Nigrospora oryzae, and entomopathogen Beauveria bassiana. Fitoterapia 2021, 148, 104781. [Google Scholar] [CrossRef]
- Yang, S.; Su, S.; Cen, R.H.; Sun, L.J.; Yang, X.Q.; Yang, Y.B.; Ding, Z.T. A new butenolide from the co-culture of the endophyte Irpex lacteus, the phytopathogenic Nigrospora oryzae, and the host Dendrobium officinale. Chem. Nat. Compd. 2022, 58, 404–406. [Google Scholar] [CrossRef]
- Chen, J.X.; Xia, D.D.; Yang, X.Q.; Yang, Y.B.; Ding, Z.T. The antifeedant and antifungal cryptic metabolites isolated from tobacco endophytes induced by host medium and coculture. Fitoterapia 2022, 163, 105335. [Google Scholar] [CrossRef]
- Zhou, Q.Y.; Yang, X.Q.; Zhang, Z.X.; Wang, B.Y.; Hu, M.; Yang, Y.B.; Zhou, H.; Ding, Z.T. New azaphilones and tremulane sesquiterpene from endophytic Nigrospora oryzae cocultured with Irpex lacteus. Fitoterapia 2018, 130, 26–30. [Google Scholar] [CrossRef]
- Bazioli, J.M.; Fill, T.P.; Rocha, M.C.; Malavazi, I.; Rodrigues Filho, E.; de Medeiros, L.S. Perylenequinones production induced by co-culturing Setophoma sp. and Penicillium brasilianum. Phytochem. Lett. 2020, 40, 76–83. [Google Scholar] [CrossRef]
- Wang, D.L.; Yang, X.Q.; Shi, W.Z.; Cen, R.H.; Yang, Y.B.; Ding, Z.T. The selective anti-fungal metabolites from Irpex lacteus and applications in the chemical interaction of Gastrodia elata, Armillaria sp., and endophytes. Fitoterapia 2021, 155, 105035. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.M.; Tan, M.H.; Lu, L.W.; Zhang, R.H.; Guo, Z.Y.; Liu, C.X.; Yang, J.; Zou, K.; Proksch, P. Chinoketides A and B, two new antimicrobial polyketides from the endophytes of Distylium chinense with the “Black-Box” co-culture method. Nat. Prod. Sci. 2018, 24, 159–163. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Yang, X.Q.; Zhou, Q.Y.; Wang, B.Y.; Hu, M.; Yang, Y.B.; Zhou, H.; Ding, Z.T. New azaphilones from Nigrospora oryzae co-cultured with Beauveria bassiana. Molecules 2018, 23, 1816. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.M.; Yang, X.Q.; Chen, J.X.; Wang, T.; Li, T.R.; Liao, F.R.; Liu, R.T.; Yang, Y.B.; Ding, Z.T. A new butenolide with antifungal activity from solid co-cultivation of Irpex lacteus and Nigrospora oryzae. Nat. Prod. Res. 2023, 37, 2243–2247. [Google Scholar] [CrossRef]
- Costa, M.F.; Borges, M.S.; Chapla, V.M.; Biasetto, C.R.; Nascimento, I.R.; Bolzani, V.S.; Araujo, A.R. Evaluation of the metabolic production from the co-culture of Saccharicola sp. and Botryosphaeria parva, an endophytic fungi associated with Eugenia jambolana Lam. J. Braz. Chem. Soc. 2023, 34, 1347–1352. [Google Scholar] [CrossRef]
- Cen, R.H.; Li, S.Y.; Yang, Y.B.; Yang, X.Q.; Ding, Z.T. Novel antifungal and antifeedant metabolites from Penicillium chrysogenum co-cultured with Nemania primolutea and Aspergillus fumigatus. Chem. Biodivers. 2023, 20, e202300004. [Google Scholar] [CrossRef]
- Yang, W.; Yuan, J.; Tan, Q.; Chen, Y.; Zhu, Y.; Jiang, H.; Zou, G.; Zang, Z.; Wang, B.; She, Z. Peniazaphilones A–I, produced by co-culturing of mangrove endophytic fungi, Penicillium sclerotiorum THSH-4 and Penicillium sclerotiorum ZJHJJ-18. Chin. J. Chem. 2021, 39, 3404–3412. [Google Scholar] [CrossRef]
- Li, H.L.; Li, X.M.; Yang, S.Q.; Cao, J.; Li, Y.H.; Wang, B.G. Induced terreins production from marine red algal-derived endophytic fungus Aspergillus terreus EN-539 co-cultured with symbiotic fungus Paecilomyces lilacinus EN-531. J. Antibiot. 2020, 73, 108–111. [Google Scholar] [CrossRef]
- Zhu, F.; Lin, Y. Marinamide, a novel alkaloid and its methyl ester produced by the application of mixed fermentation technique to two mangrove endophytic fungi from the South China Sea. Chin. Sci. Bull. 2006, 51, 1426–1430. [Google Scholar] [CrossRef]
- Zhang, C.L.; Zheng, B.Q.; Lao, J.P.; Mao, L.J.; Chen, S.Y.; Kubicek, C.P.; Lin, F.C. Clavatol and patulin formation as the antagonistic principle of Aspergillus clavatonanicus, an endophytic fungus of Taxus mairei. Appl. Microbiol. Biotechnol. 2008, 78, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Li, M.; Wang, Y.; Bai, X.; Chen, J.; Li, X.; Wang, H.; Zhang, H. Chemical investigation of a co-culture of Aspergillus fumigatus D and Fusarium oxysporum R1. Rec. Nat. Prod. 2021, 15, 130–135. [Google Scholar] [CrossRef]
- Akone, S.H.; Mándi, A.; Kurtán, T.; Hartmann, R.; Lin, W.; Daletos, G.; Proksch, P. Inducing secondary metabolite production by the endophytic fungus Chaetomium sp. through fungal–bacterial co-culture and epigenetic modification. Tetrahedron 2016, 72, 6340–6347. [Google Scholar] [CrossRef]
- Ola, A.R.; Thomy, D.; Lai, D.; Brötz-Oesterhelt, H.; Proksch, P. Inducing secondary metabolite production by the endophytic fungus Fusarium tricinctum through co-culture with Bacillus subtilis. J. Nat. Prod. 2013, 76, 2094–2099. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, M.F.; Kurtán, T.; Mándi, A.; Müller, W.E.G.; Fouad, M.A.; Kamel, M.S.; Liu, Z.; Ebrahim, W.; Daletos, G.; Proksch, P. Induced secondary metabolites from the endophytic fungus Aspergillus versicolor through bacterial co-culture and OSMAC approaches. Tetrahedron Lett. 2018, 59, 2647–2652. [Google Scholar] [CrossRef]
- Chagas, F.O.; Pupo, M.T. Chemical interaction of endophytic fungi and actinobacteria from Lychnophora ericoides in co-cultures. Microbiol. Res. 2018, 212, 10–16. [Google Scholar] [CrossRef]
- Moussa, M.; Ebrahim, W.; Bonus, M.; Gohlke, H.; Mándi, A.; Kurtán, T.; Hartmann, R.; Kalscheuer, R.; Lin, W.; Liu, Z.; et al. Co-culture of the fungus Fusarium tricinctum with Streptomyces lividans induces production of cryptic naphthoquinone dimers. RSC Adv. 2019, 9, 1491–1500. [Google Scholar] [CrossRef]
- Wang, Z.R.; Li, G.; Ji, L.X.; Wang, H.H.; Gao, H.; Peng, X.P.; Lou, H.X. Induced production of steroids by co-cultivation of two endophytes from Mahonia fortunei. Steroids 2019, 145, 1–4. [Google Scholar] [CrossRef]
- Bathini, T.; Alhadrami, H.A.; Hassan, M.H.A.; Hassan, H.M.; Behery, F.A.; Bawazeer, M.; Yaseen, M.; Belbahri, L.; Rateb, M.E. Induction of cryptic antifungal pulicatin derivatives from Pantoea agglomerans by microbial co-culture. Biomolecules 2020, 10, 268. [Google Scholar] [CrossRef]
- Moussa, M.; Ebrahim, W.; Kalscheuer, R.; Liu, Z.; Proksch, P. Co-culture of the bacterium Pseudomonas aeruginosa with the fungus Fusarium tricinctum induces bacterial antifungal and quorum sensing signaling molecules. Phytochem. Lett. 2020, 36, 37–41. [Google Scholar] [CrossRef]


| Co-Cultivation of Endophytic Fungi and Fungi | Antimicrobials | References |
|---|---|---|
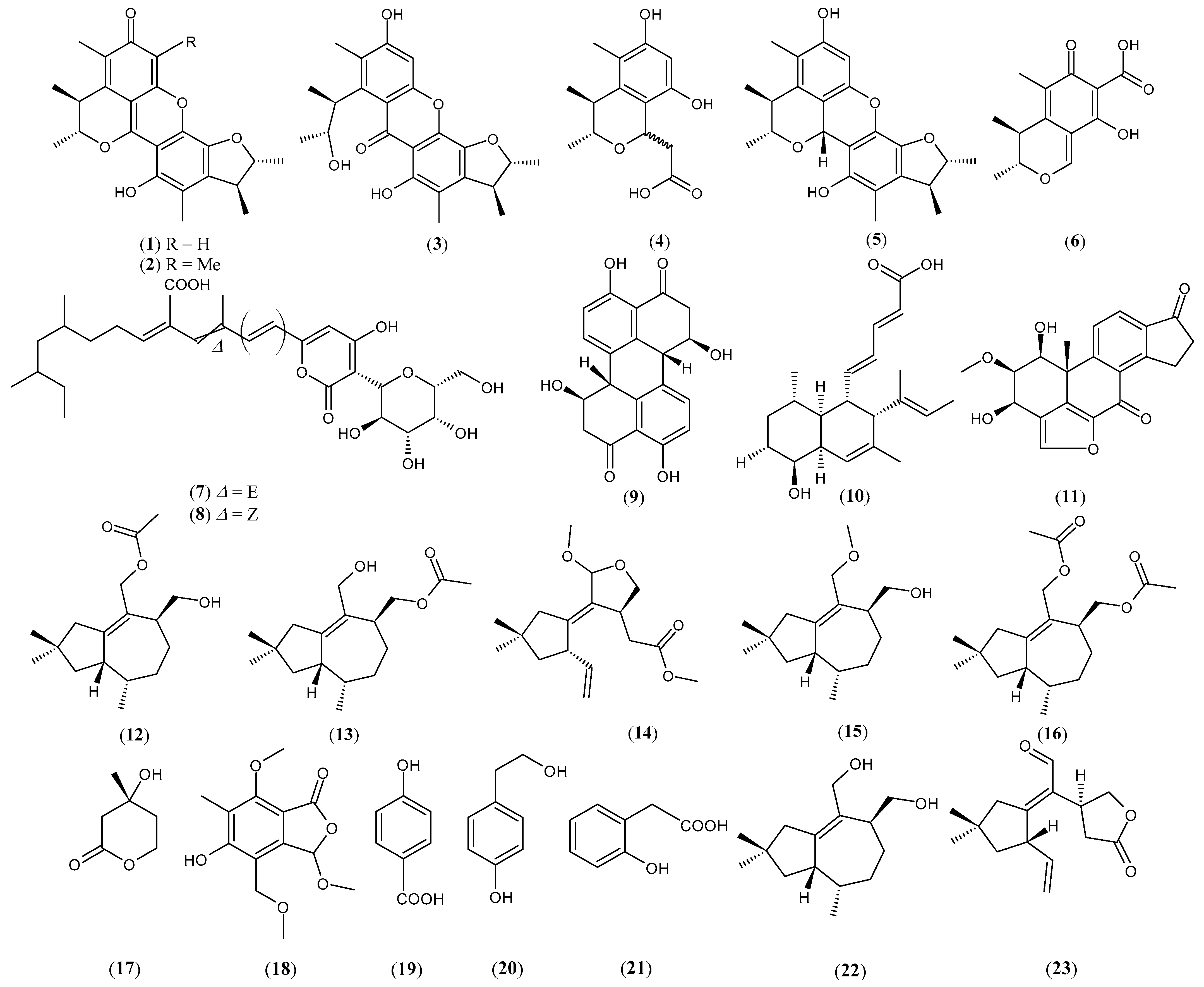
| Aspergillus sydowii and Penicillium citrinum | Penicitrinone A (1) Penicitrinone F (2) Seco-penicitrinol A (3) Penicitrinol L (4) Penicitrinol A (5) Citrinin (6) | [54] |
| Camporesia sambuci and Epicoccum sorghinum | D8646-2-6 (7) Iso-D8646-2-6 (8) | [55] |
| Alternaria tenuissima and Nigrospora sphaerica | Stemphyperylenol (9) | [56] |
| Hypoxylon rubiginosum and Hymenoscyphus fraxineus | Phomopsidin (10) Viridiol (11) | [57] |
| Irpex lacteus, Nigrospora oryzae, and Beauveria bassiana | Nigpexin A (12) Nigpexin B (13) Nigpexin C (14) Nigpexin D (15) Nigpexin E (16) Mevalonolactone (17) Microsphaerophthalide F (18) p-hydroxybenzoic acid (19) Tyrosol (20) 2-hydroxyphenylacetic acid (21) Tremulenediol A (22) 11-aldehyde-5,6-seco-1,6(13)-tremuladien-5,12-olide (23) β-sitosterol (24) Scytalone (25) 4,6,8-trihydroxy-3,4-dihydronaphthalen-1(2H)-one (26) (3S,4R)-3,4-dihydroxypentanoic acid (27) | [58] |
| Irpex lacteus and Nigrospora oryzae | Nigrolactin (28) | [59] |
| Nigrospora sp. and Stagonosporopsis sp. | Nigrolactone (29) Multiplolide B (30) 4β-acetoxyprobotryane-9β,15α-diol (31) | [60] |
| Nigrospora oryzae and Irpex lacteus | Conocenol B (32) Nigrosirpexin A (33) Nigirpexin D (34) | [61] |
| Setophoma sp. and Penicillium brasilianum | Stemphyperylenol (9) | [62] |
| Irpex lacteus and Armillaria sp. | Irpexlactin B (35) Conocenol B (32) 11,12-dihydroxy-1-tremulen-5-one (36) 11,12-epoxy-5,6-secotremula-1,6(13)-dien-5,12-olide (37) Irpexlacte B (38) 2,3-dihydroxydodacane-4,7-dione (39) | [63] |
| Endophytes from the leaves of the plant Distylium chinense | Chinoketide A (40) Chinoketide B (41) Xylarphthalide A (42) | [64] |
| Nigrospora oryzae and Beauveria bassiana | Nigbeauvin A (43) | [65] |
| Irpex lacteus and Nigrospora oryzae | Butenolide irperide (44) Lactedine (45) Conocenol B (32) Nigirpexin C (46) Tremulenediol A (22) (+)-(3S,6R,7R)-tremulene-6,11,12-triol (47) | [66] |
| Saccharicola sp. and Botryosphaeria parva | cis-4-hydroxymellein (48) 7-hydroxymellein (49) | [67] |
| Penicillium chrysogenum, Nemania primolutea, and Aspergillus fumigatus | Nemmolutin A (50) Penigenumin (51) Penemin (52) Xylabisboein B (53) Xylarenolide (54) 4-(2-hydroxybutynoxy)benzoic acid (55) 5-hydroxymellein (56) Penicilligenin (57) Monasone B (58) Monasone A (59) Monaspurpurone (60) | [68] |
| Penicillium sclerotiorum THSH-4 and Penicillium sclerotiorum ZJHJJ-18 | Peniazaphilone A (61) Scleratioramine (62) WB (63) | [69] |
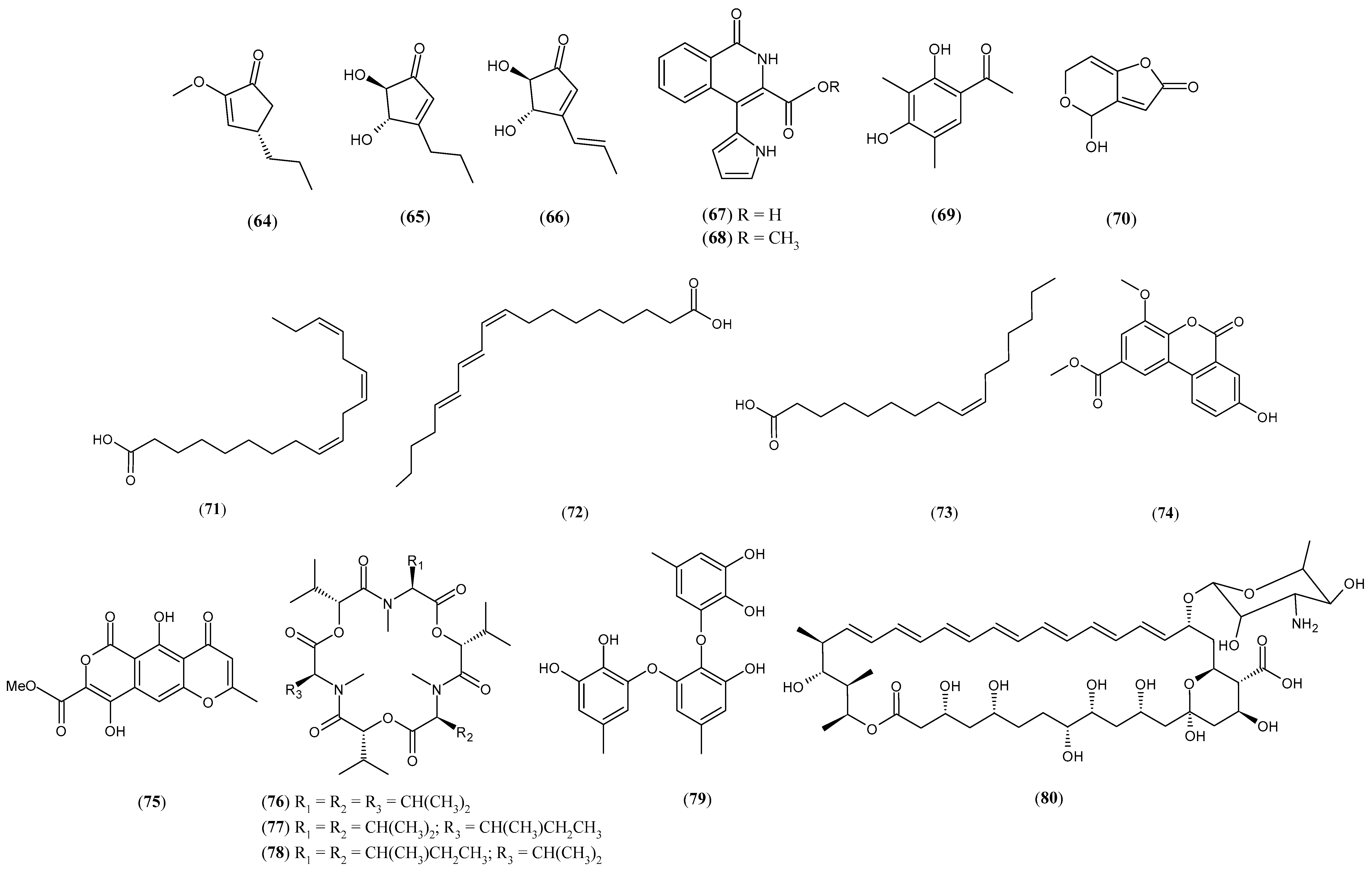
| Aspergillus terreus and Paecilomyces lilacinus | Asperterrein (64) Dihydroterrein (65) Terrein (66) | [70] |
| Marine fungal strains 1924 and 3893 isolated from a plant in a mangrove | Marinamide (67) Methyl ester (68) | [71] |
| Aspergillus clavatonanicus and Pythium ultimum | Clavatol (69) Patulin (70) | [72] |
| Aspergillus fumigatus and Fusarium oxysporum | α-linolenic acid (71) α-elaeostearic acid (72) Palmitoleic acid (73) | [73] |
| Co-Cultivation of Endophytic Fungi and Bacteria | Antimicrobials | References |
| Chaetomium sp. and Bacillus subtilis | Serkydayn (74) | [74] |
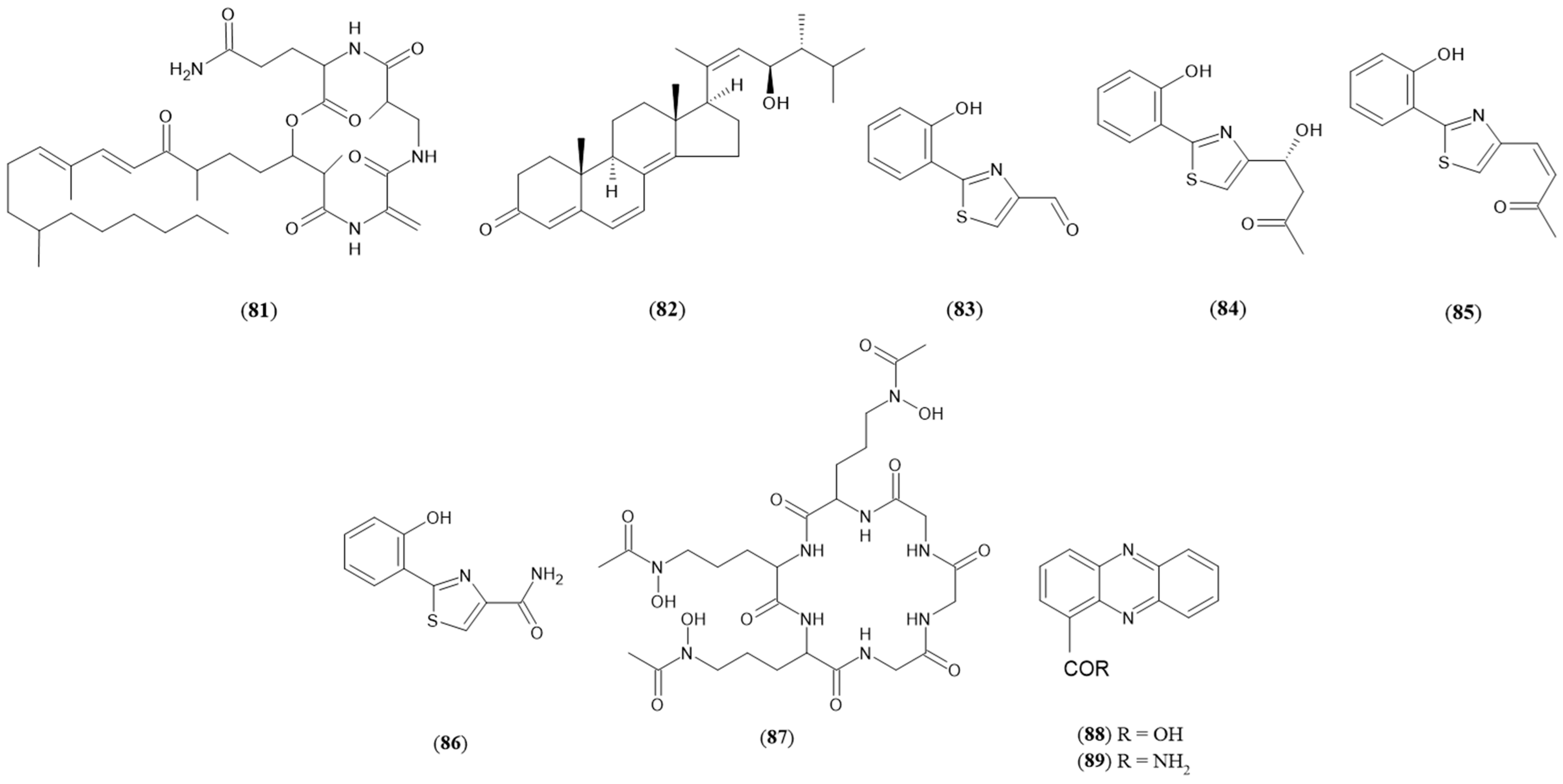
| Fusarium tricinctum and Bacillus subtilis | Lateropyrone (75) Enniatin B (76) Enniatin B1 (77) Enniatin A1 (78) | [75] |
| Aspergillus versicolor and Bacillus subtilis | Sydowiol B (79) | [76] |
| Phomopsis sp. and Streptomyces albospinus | Amphotericin B (80) | [77] |
| Fusarium tricinctum and Streptomyces lividans | Lateropyrone (75) Enniatin B (76) Enniatin B1 (77) Enniatin A1 (78) Fusaristatin A (81) | [78] |
| Pleosporales sp. and Bacillus wiedmannii | 23R-hydroxy-(20Z,24R)-ergosta-4,6,8(14),20(22)-tetraen-3-one (82) | [79] |
| Penicillium citrinum and Pantoea aggolomerans | Aeruginaldehyde (83) Pulicatin H (84) Pulicatin I (85) Pulicatin F (86) Desferrichrome (87) | [80] |
| Fusarium tricinctum and Pseudomonas aeruginosa | Enniatin B (76) Enniatin B1 (77) Enniatin A1 (78) Fusaristatin A (81) Phenazine-1-carboxylic acid (88) Phenazine-1-carboxamide (89) | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tironi, L.S.; Carletto, L.B.; Silva, E.O.; Schripsema, J.; Luiz, J.H.H. Endophytic Fungi Co-Culture: An Alternative Source of Antimicrobial Substances. Microorganisms 2024, 12, 2413. https://doi.org/10.3390/microorganisms12122413
Tironi LS, Carletto LB, Silva EO, Schripsema J, Luiz JHH. Endophytic Fungi Co-Culture: An Alternative Source of Antimicrobial Substances. Microorganisms. 2024; 12(12):2413. https://doi.org/10.3390/microorganisms12122413
Chicago/Turabian StyleTironi, Lucas Silva, Lucilene Bento Carletto, Eliane Oliveira Silva, Jan Schripsema, and Jaine Honorata Hortolan Luiz. 2024. "Endophytic Fungi Co-Culture: An Alternative Source of Antimicrobial Substances" Microorganisms 12, no. 12: 2413. https://doi.org/10.3390/microorganisms12122413
APA StyleTironi, L. S., Carletto, L. B., Silva, E. O., Schripsema, J., & Luiz, J. H. H. (2024). Endophytic Fungi Co-Culture: An Alternative Source of Antimicrobial Substances. Microorganisms, 12(12), 2413. https://doi.org/10.3390/microorganisms12122413







