Isolation, Antibacterial Activity and Molecular Identification of Avocado Rhizosphere Actinobacteria as Potential Biocontrol Agents of Xanthomonas sp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling of Rhizospheric Soil
2.2. Physical Treatments of Composite Soil Sample
2.3. Isolation of Actinobacteria
2.4. Selection of Isolates with Antibacterial Activity (AA)
2.5. Effect of Culture Media on Antibacterial Activity of Selected Isolates
2.6. Molecular Identification and Phylogenetic Analysis of Selected Actinobacteria
2.7. Statistical Analyses
3. Results
3.1. Soil Treatments to Improve Isolation of Actinobacteria
3.2. Antibacterial Activity of the BVEZ and BVEZMW Isolates
3.3. Effect of Culture Media for Secondary Metabolite Production
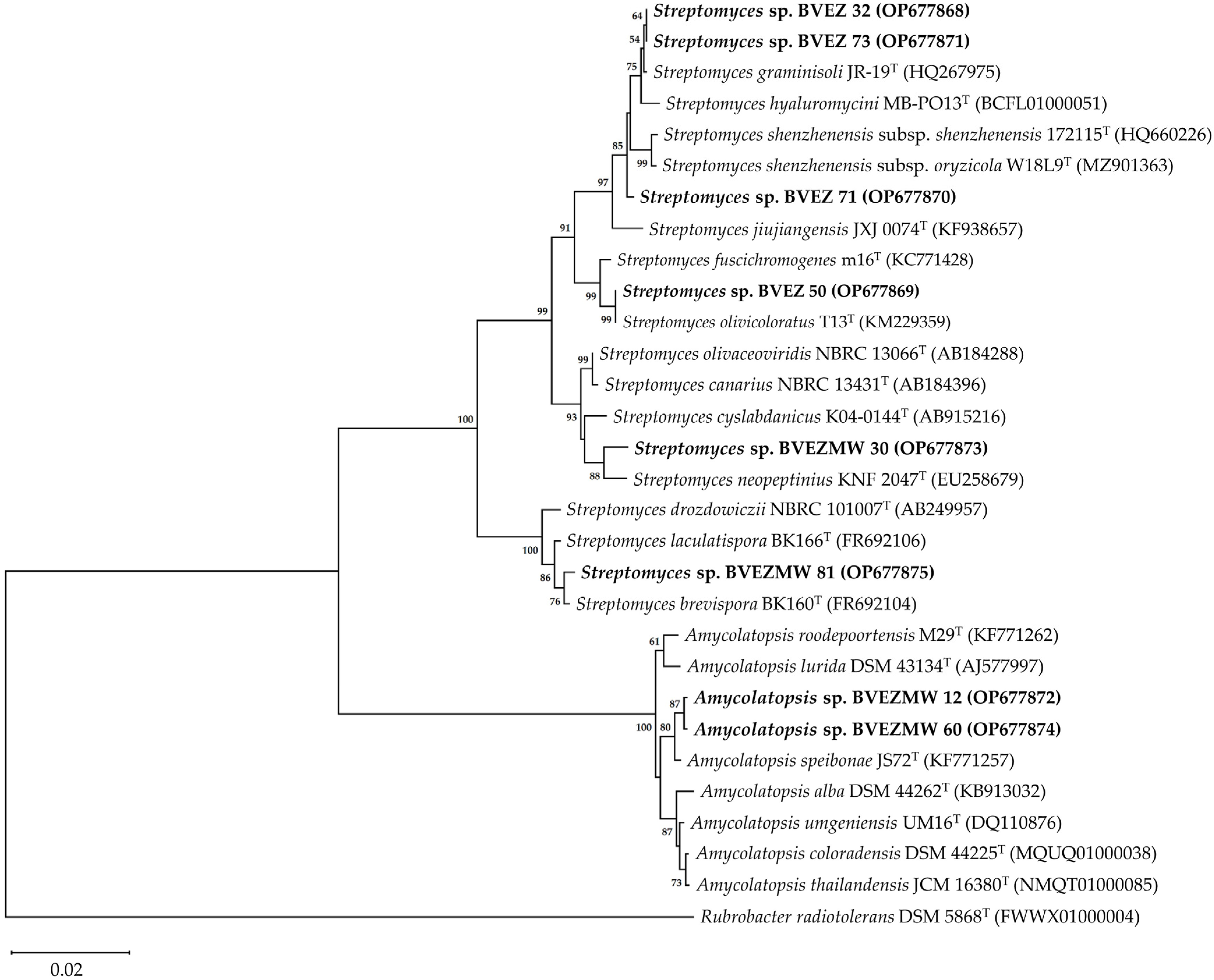
3.4. Identification of the Selected Actinobacteria
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryan, R.P.; Vorhölter, F.-J.; Potnis, N.; Jones, J.B.; Sluys, M.A.V.; Bogdanove, A.J.; Dow, J.M. Pathogenomics of Xanthomonas: Understanding bacterium-plant interactions. Nat. Rev. Microbiol. 2011, 9, 344–355. [Google Scholar] [CrossRef] [PubMed]
- EFSA PLH Panel (EFSA Panel on Plant Health). Scientific Opinion on the pest categorisation of Xanthomonas campestris pv. vesicatoria (Doidge) Dye. EFSA J. 2014, 12, 3720. [Google Scholar] [CrossRef]
- Potnis, N.; Timilsina, S.; Strayer, A.; Shantharaj, D.; Barak, J.D.; Paret, M.L.; Vallad, G.E.; Jones, J.B. Bacterial spot of tomato and pepper: Diverse Xanthomonas species with a wide variety of virulence factors posing a worldwide challenge. Mol. Plant Pathol. 2015, 16, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Areas, M.S.; Gonçalves, R.M.; Soman, J.M.; Souza-Filho, R.C.; Gioria, R.; Silva Junior, T.A.F.; Maringoni, A.C. Resistance of Xanthomonas euvesicatoria strains from Brazilian pepper to copper and zinc sulfates. An. Acad. Bras. Cienc. 2018, 90, 2375–2380. [Google Scholar] [CrossRef]
- Roach, R.; Mann, R.; Gambley, C.G.; Shivas, R.G.; Chapman, T.; Rodoni, B. Pathogenicity and copper tolerance in Australian Xanthomonas species associated with bacterial leaf spot. Crop Prot. 2020, 127, 104923. [Google Scholar] [CrossRef]
- Marin, V.R.; Ferrarezi, J.H.; Vieira, G.; Sass, D.C. Recent advances in the biocontrol of Xanthomonas spp. World J. Microbiol. Biotechnol. 2019, 35, 72. [Google Scholar] [CrossRef]
- Guevara-Avendaño, E.; Carrillo, J.D.; Ndinga-Muniania, C.; Moreno, K.; Méndez-Bravo, A.; Guerrero-Analco, J.A.; Eskalen, A.; Reverchon, F. Antifungal activity of avocado rhizobacteria against Fusarium euwallaceae and Graphium spp., associated with Euwallacea spp. nr. fornicatus, and Phytophthora cinnamomi. Antonie Van Leeuwenhoek 2018, 111, 563–572. [Google Scholar] [CrossRef]
- Guevara-Avendaño, E.; Bejarano-Bolívar, A.A.; Kiel-Martínez, A.L.; Ramírez-Vázquez, M.; Méndez-Bravo, A.; von Wobeser, E.A.; Sánchez-Rangel, D.; Guerrero-Analco, J.A.; Eskalen, A.; Reverchon, F. Avocado rhizobacteria emit volatile organic compounds with antifungal activity against Fusarium solani, Fusarium sp. associated with Kuroshio shot hole borer, and Colletotrichum gloeosporioides. Microbiol. Res. 2019, 219, 74–83. [Google Scholar] [CrossRef]
- Vega-Torres, M.G.; Ruiz-Cisneros, M.F.; Pérez-Corral, D.A.; Berlanga-Reyes, D.I.; Ornelas-Paz, J.J.; Rios-Velasco, C.; Cambero-Campos, O.J.; Estrada-Virgen, M.O.; Luna-Esquivel, G.; Denise-Revérchon, F.L. In vitro antifungal activity of antagonistic microorganisms against Fusarium oxysporum from avocado trees rhizosphere of Xalisco, Nayarit, Mexico. Mex. J. Phytopathol. 2019, 37, 57–64. [Google Scholar] [CrossRef]
- Bérdy, J. Thoughts and facts about antibiotics: Where we are now and where we are heading. J. Antibiot. 2012, 65, 385–395. [Google Scholar] [CrossRef]
- Goodfellow, M. Actinobacteria phyl. nov. In Bergey’s Manual of Systematics of Archaea and Bacteria; Trujillo, M.E., Dedysh, S., DeVos, P., Hedlund, B., Kämpfer, P., Rainey, F.A., Whitman, W.B., Eds.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- van der Meij, A.; Worsley, S.F.; Hutchings, M.I.; van Wezel, G.P. Chemical ecology of antibiotic production by actinomycetes. FEMS Microbiol. Rev. 2017, 41, 392–416. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Salwan, R. Biocontrol potential and applications of actinobacteria in agriculture. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, B.P., Gupta, V.K., Passari, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 93–108. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Giovanardi, D.; Stefani, E. Plant growth promoting and biocontrol activity of Streptomyces spp. as endophytes. Int. J. Mol. Sci. 2018, 19, 952. [Google Scholar] [CrossRef] [PubMed]
- Betancur, L.A.; Naranjo-Gaybor, S.J.; Vinchira-Villarraga, D.M.; Moreno-Sarmiento, N.C.; Maldonado, L.A.; Suarez-Moreno, Z.R.; Acosta-González, A.; Padilla-Gonzalez, G.F.; Puyana, M.; Castellanos, L.; et al. Marine Actinobacteria as a source of compounds for phytopathogen control: An integrative metabolic-profiling / bioactivity and taxonomical approach. PLoS ONE 2017, 12, e0170148. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Moreno, Z.R.; Vinchira-Villarraga, D.M.; Vergara-Morales, D.I.; Castellanos, L.; Ramos, F.A.; Guarnaccia, C.; Degrassi, G.; Venturi, V.; Moreno-Sarmiento, N. Plant-growth promotion and biocontrol properties of three Streptomyces spp. isolates to control bacterial rice pathogens. Front. Microbiol. 2019, 10, 290. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Han, X.; Li, X.; Zhang, X.; Wang, H.; Zhang, L.; Cao, P.; Wu, Y.; Wang, X.; Zhao, J.; et al. A Streptomyces sp. NEAU-HV9: Isolation, identification, and potential as a biocontrol agent against Ralstonia solanacearum of tomato plants. Microorganisms 2020, 8, 351. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, J.; Chen, Z.; Zhang, H.; Wang, Z.; Zhu, Y.; Liu, B. A Streptomyces sp. strain: Isolation, identification, and potential as a biocontrol agent against soilborne diseases of tomato plants. Biol. Control 2019, 136, 104004. [Google Scholar] [CrossRef]
- Sarwar, A.; Latif, Z.; Zhang, S.; Zhu, J.; Zechel, D.L.; Bechthold, A. Biological control of potato common scab with rare isatropolone C compound produced by plant growth promoting Streptomyces A1RT. Front. Microbiol. 2018, 9, 1126. [Google Scholar] [CrossRef]
- Aeny, T.K.; Prasetyo, J.; Suharjo, R.; Dirmawati, S.R.; Efri, E.; Niswati, A. Isolation and identification of actinomycetes potential as the antagonist of Dickeya zeae pineapple soft rot in Lampung, Indonesia. Biodiversitas 2018, 19, 2052–2058. [Google Scholar] [CrossRef]
- Dias, M.P.; Bastos, M.S.; Xavier, V.B.; Cassel, E.; Astarita, L.V.; Santarém, E.R. Plant growth and resistance promoted by Streptomyces spp. in tomato. Plant Physiol. Biochem. 2017, 118, 479–493. [Google Scholar] [CrossRef]
- Sundin, G.W.; Wang, N. Antibiotic resistance in plan-pathogenic bacteria. Annu. Rev. Phytopathol. 2018, 56, 161–180. [Google Scholar] [CrossRef]
- Kulkarni, M.; Gorthi, S.; Banerjee, G.; Chattopadhyay, P. Production, characterization and optimization of actinomycin D from Streptomyces hydrogenans IB310, a(n antagonistic bacterium against phytopathogens. Biocatal. Agric. Biotechnol. 2017, 10, 69–74. [Google Scholar] [CrossRef]
- Betancur, L.A.; Forero, A.M.; Romero-Otero, A.; Sepúlveda, L.Y.; Moreno-Sarmiento, N.C.; Castellanos, L.; Ramos, F.A. Cyclic tetrapeptides from the marine strain Streptomyces sp. PNM-161a with activity against rice and yam phytopathogens. J. Antibiot. 2019, 72, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Wattana-Amorn, P.; Charoenwongsa, W.; Williams, C.; Crump, M.P.; Apichaisataienchote, B. Antibacterial activity of cyclo(L-Pro-L-Tyr) and cyclo(D-Pro-L-Tyr) from Streptomyces sp. strain 22-4 against phytopathogenic bacteria. Nat. Prod. Res. 2016, 30, 1980–1983. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.T.; Park, A.R.; Van Le, V.; Hwang, I.; Kim, J.-C. Exploration of a multifunctional biocontrol agent Streptomyces sp. JCK-8055 for the management of apple fire blight. Appl. Microbiol. Biotechnol. 2024, 108, 49. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Gálvez, N.; Luengo-Uribe, P.; Mancilla, S.; Maurin, A.; Torres, C.; Ruiz, P.; France, A.; Acuña, I.; Urrutia, H. Antagonistic activity of endophytic actinobacteria from native potatoes (Solanum tuberosum subsp. tuberosum L.) against Pectobacterium carotovorum subsp. carotovorum and Pectobacterium atrosepticum. BMC Microbiol. 2021, 21, 335. [Google Scholar] [CrossRef]
- Li, Y.V.; Terekhova, L.P.; Gapochka, M.G. Isolation of actinomycetes from soil using extremely high frequency radiation. Microbiology 2002, 71, 105–108. [Google Scholar] [CrossRef]
- Hayakawa, M. Studies on the isolation and distribution of rare actinomycetes in soil. Actinomycetologica 2008, 22, 12–19. [Google Scholar] [CrossRef]
- Wang, D.S.; Xue, Q.H.; Zhu, W.J.; Zhao, J.; Duan, J.L.; Shen, G.H. Microwave irradiation is a useful tool for improving isolation of actinomycetes from soil. Microbiology 2013, 82, 102–110. [Google Scholar] [CrossRef]
- Trinidad-Cruz, J.R.; Rincón-Enríquez, G.; Evangelista-Martínez, Z.; Guízar-González, C.; Enríquez-Vara, J.N.; López-Pérez, L.; Quiñones-Aguilar, E.E. Actinobacteria from avocado rhizosphere: Antagonistic activity against Colletotrichum gloeosporioides and Xanthomonas sp. Rev. Terra Latinoam. 2021, 39, e802. [Google Scholar] [CrossRef]
- Evangelista-Martínez, Z. Preliminary study on some actinomycetes and evaluation of their potential antagonism against fungal pathogens. Br. Microbiol. Res. J. 2014, 4, 272–281. [Google Scholar] [CrossRef]
- Shirling, E.B.; Gottlieb, D. Methods for characterization of Streptomyces species1. Int. J. Syst. Evol. Microbiol. 1966, 16, 313–340. [Google Scholar] [CrossRef]
- Intra, B.; Mungsuntisuk, I.; Nihira, T.; Igarashi, Y.; Panbangred, W. Identification of actinomycetes from plant rhizospheric soils with antagonist activity against Colletotrichum spp., the causative agent of anthracnose disease. BMC Res. Notes 2011, 4, 98. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, M.D.; Kharel, M.K.; Bosserman, M.A.; Rohr, J. Laboratory maintenance of Streptomyces species. Curr. Protoc. Microbiol. 2010, 18, 10E.1.1–10E.1.8. [Google Scholar] [CrossRef] [PubMed]
- López-Vielma, C.; Solís-Sánchez, A.; Quiñones-Aguilar, E.; Qui-Zapata, J.; Rincón-Enríquez, G. Aislamiento del agente causal de la mancha bacteriana de chile en las regiones productoras de Jalisco, Zacatecas y Michoacán. Rev. Biotecnol. Sustentab. 2016, 1, 141–144. [Google Scholar]
- Daniels, M.J.; Barber, C.E.; Turner, P.C.; Cleary, W.G.; Sawczyc, M.K. Isolations of mutations of Xanthomonas campestris pv. campestris showing altered pathogenicity. J. Gen. Microbiol. 1984, 130, 2447–2455. [Google Scholar] [CrossRef]
- Salamoni, S.P.; Mann, M.B.; Campos, F.S.; Franco, A.C.; Germani, J.C.; Van Der Sand, S.T. Preliminary characterization of some Streptomyces species isolated from a composting process and their antimicrobial potential. World J. Microbiol. Biotechnol. 2010, 26, 1847–1856. [Google Scholar] [CrossRef]
- Shomura, T.; Yoshida, J.; Amano, S.; Kojima, M.; Inouye, S.; Niida, T. Studies on Actinomycetales producing antibiotics only on agar culture. I. Screening, taxonomy and morphology-productivity relationship of Streptomyces halstedii, strain SF-1993. J. Antibiot. 1979, 32, 427–435. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA and whole genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, J.; Kafshnouchi, M.; Ranjbaran, M. A Study on actinobacterial diversity of Hampoeil cave and screening of their biological activities. Saudi J. Biol. Sci. 2019, 26, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Komarova, A.S.; Likhacheva, A.A.; Lapygina, E.V.; Maksimova, I.A.; Pozdnyakov, A.I. Impact of electromagnetic microwaves on the germination of spores of Streptomyces xanthochromogenes in a peat soil and in a liquid nutrient medium. Eurasian Soil Sci. 2010, 43, 72–75. [Google Scholar] [CrossRef]
- Likhacheva, A.A.; Komarova, A.S.; Luk’yanov, A.A.; Gorlenko, M.V.; Terekhov, A.S. The influence of SHF radiation on soil streptomycetes. Eurasian Soil Sci. 2006, 39, 854–858. [Google Scholar] [CrossRef]
- Sharma, P.; Thakur, D. Antimicrobial biosynthetic potential and diversity of culturable soil actinobacteria from forest ecosystems of Northeast India. Sci. Rep. 2020, 10, 4104. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Kim, J. Streptomyces olivicoloratus sp. nov., an antibiotic-producing bacterium isolated from soil. Int. J. Syst. Evol. Microbiol. 2015, 65, 3262–3270. [Google Scholar] [CrossRef]
- Das, R.; Romi, W.; Das, R.; Sharma, H.K.; Thakur, D. Antimicrobial potentiality of actinobacteria isolated from two microbiologically unexplored forest ecosystems of Northeast India. BMC Microbiol. 2018, 18, 71. [Google Scholar] [CrossRef]
- Maiti, P.K.; Das, S.; Sahoo, P.; Mandal, S. Streptomyces sp. SM01 isolated from Indian soil produces a novel antibiotic picolinamycin effective against multi drug resistant bacterial strains. Sci. Rep. 2020, 10, 10092. [Google Scholar] [CrossRef]
- Han, S.I.; Lee, H.; Whang, K. Isolation and taxonomical characterization of Streptomyces sp. JR-24 with antibacterial activity of bacterial leaf spot of pepper (Xanthomonas axonopodis pv. vesicatoria). Korean J. Microbiol. 2010, 46, 359–365. [Google Scholar]
- Lee, H.J.; Whang, K.S. Streptomyces graminisoli sp. nov. and Streptomyces rhizophilus sp. nov., isolated from bamboo (Sasa borealis) rhizosphere soil. Int. J. Syst. Evol. Microbiol. 2014, 64, 1546–1551. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.V.L.; Martins, S.C.S.; de Siqueira, K.A.; Soares, M.A.; Martins, C.M. Characterization of actinobacteria from the semiarid region, and their antagonistic effect on strains of rhizobia. Afr. J. Biotechnol. 2017, 16, 499–507. [Google Scholar] [CrossRef]
- Hong, K.; Gao, A.-H.; Xie, Q.-Y.; Gao, H.; Zhuang, L.; Lin, H.-P.; Yu, H.-P.; Yao, X.-S.; Goodfellow, M.; Ruan, J.-S. Actinomycetes for marine drug discovery isolated from mangrove soils and plants in China. Mar. Drugs 2009, 7, 24–44. [Google Scholar] [CrossRef]
- Hu, H.; Lin, H.-P.; Xie, Q.; Li, L.; Xie, X.-Q.; Sun, M.; Hong, K. Streptomyces shenzhenensis sp. nov., a novel actinomycete isolated from mangrove sediment. Antonie Van Leeuwenhoek 2011, 100, 631–637. [Google Scholar] [CrossRef]
- Kaewkla, O.; Sukpanoa, S.; Suriyachadkun, C.; Chamroensaki, N.; Chumroenphat, T.; Franco, C.M.M. Streptomyces spinosus sp. nov. and Streptomyces shenzhenensis subsp. oryzicola subsp. nov. endophytic actinobacteria isolated from Jasmine rice and their genome mining for potential as antibiotic producers and plant growth promoters. Antonie Van Leeuwenhoek 2022, 115, 871–888. [Google Scholar] [CrossRef]
- Zucchi, T.D.; Kim, B.Y.; Kshetrimayum, J.D.; Weon, H.Y.; Kwon, S.W.; Goodfellow, M. Streptomyces brevispora sp. nov. and Streptomyces laculatispora sp. nov., actinomycetes isolated from soil. Int. J. Syst. Evol. Microbiol. 2012, 62, 478–483. [Google Scholar] [CrossRef]
- Zhao, K.; Li, J.; Zhang, X.; Chen, Q.; Liu, M.; Ao, X.; Gu, Y.; Liao, D.; Xu, K.; Ma, M.; et al. Actinobacteria associated with Glycyrrhiza inflata Bat. are diverse and have plant growth promoting and antimicrobial activity. Sci. Rep. 2018, 8, 13661. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, H.M.; Chang, C.; Hwang, I.C.; Oh, H.; Ahn, J.S.; Kim, K.D.; Hwang, B.K.; Kim, B.S. Biological evaluation of neopeptins isolated from a Streptomyces strain. Pest Manag. Sci. 2007, 63, 1208–1214. [Google Scholar] [CrossRef]
- Han, J.H.; Hwang, I.C.; Cho, S.H.; Jang, C.; Kim, N.G.; Yu, S.H.; Yu, Y.M.; Kim, S.B. Description of Streptomyces neopeptinius sp. nov., an actinobacterium with broad spectrum antifungal activities. J. Microbiol. 2008, 46, 295–299. [Google Scholar] [CrossRef]
- Tan, G.Y.A.; Goodfellow, M. Amycolatopsis. In Bergey’s Manual of Systematics of Archaea and Bacteria; Trujillo, M.E., Dedysh, S., DeVos, P., Hedlund, B., Kämpfer, P., Rainey, F.A., Whitman, W.B., Eds.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Everest, G.J.; le Roes-Hill, M.; Rohland, J.; Enslin, S.; Meyers, P.R. Amycolatopsis roodepoortensis sp. nov. and Amycolatopsis speibonae sp. nov.: Antibiotic-producing actinobacteria isolated from South African soils. J. Antibiot. 2014, 67, 813–818. [Google Scholar] [CrossRef] [PubMed]
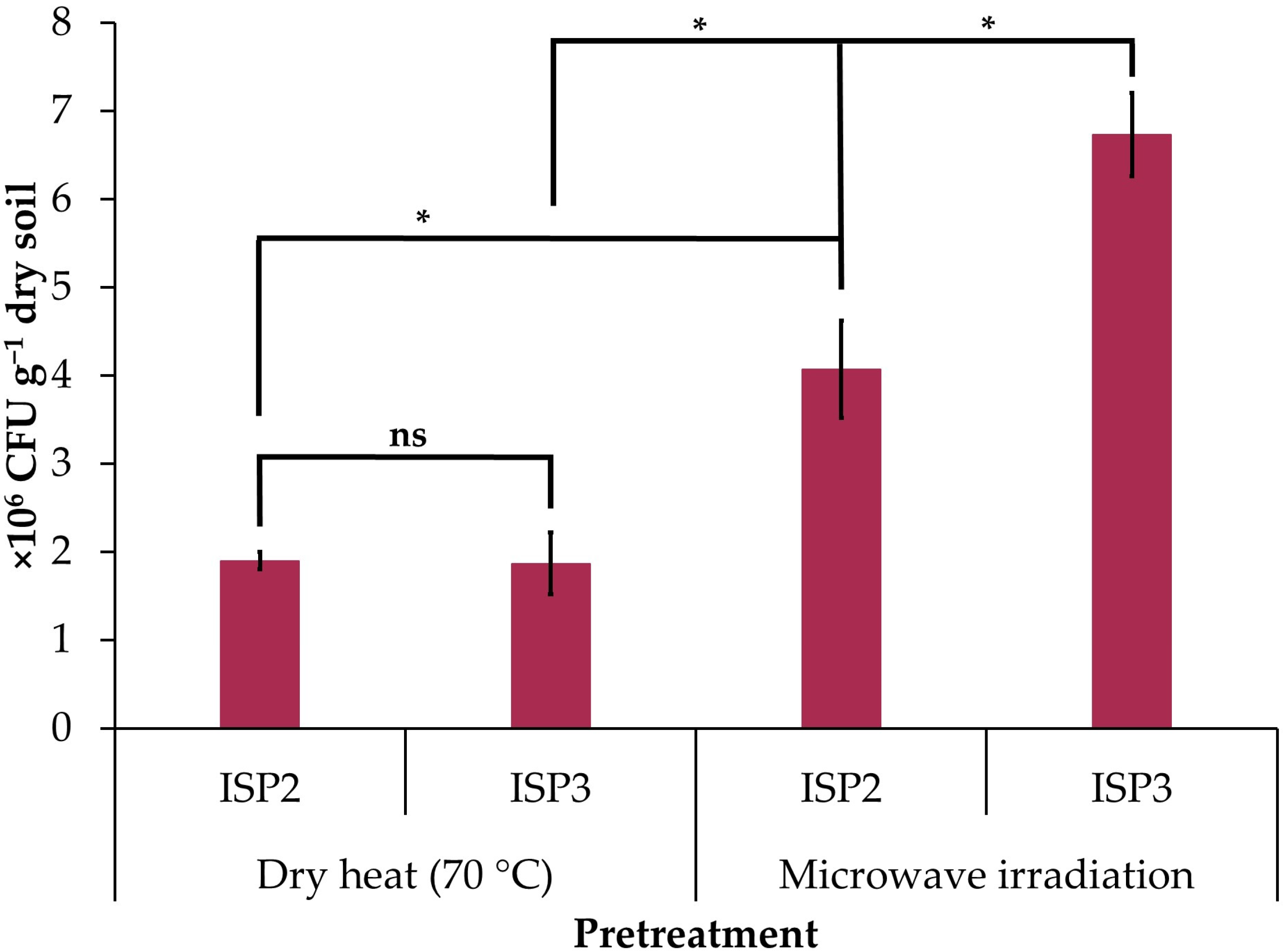
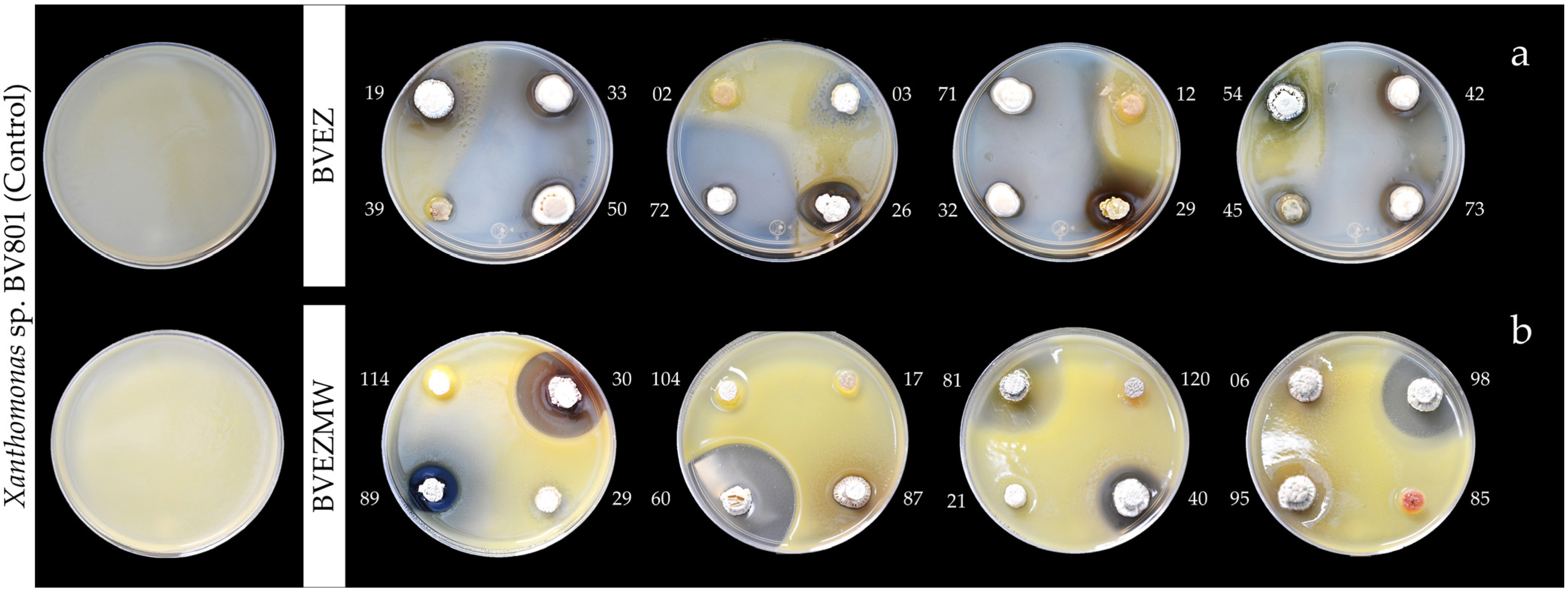
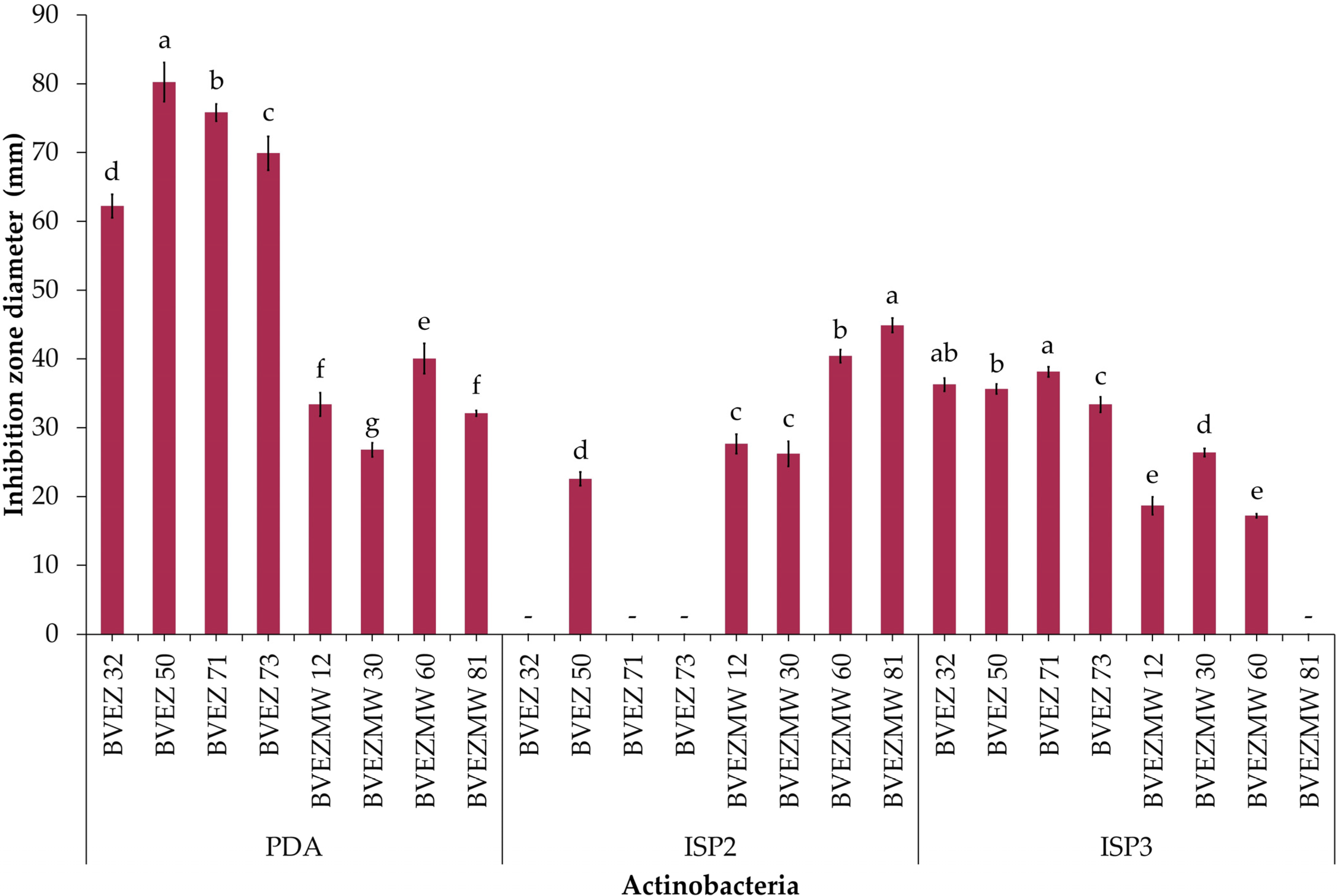
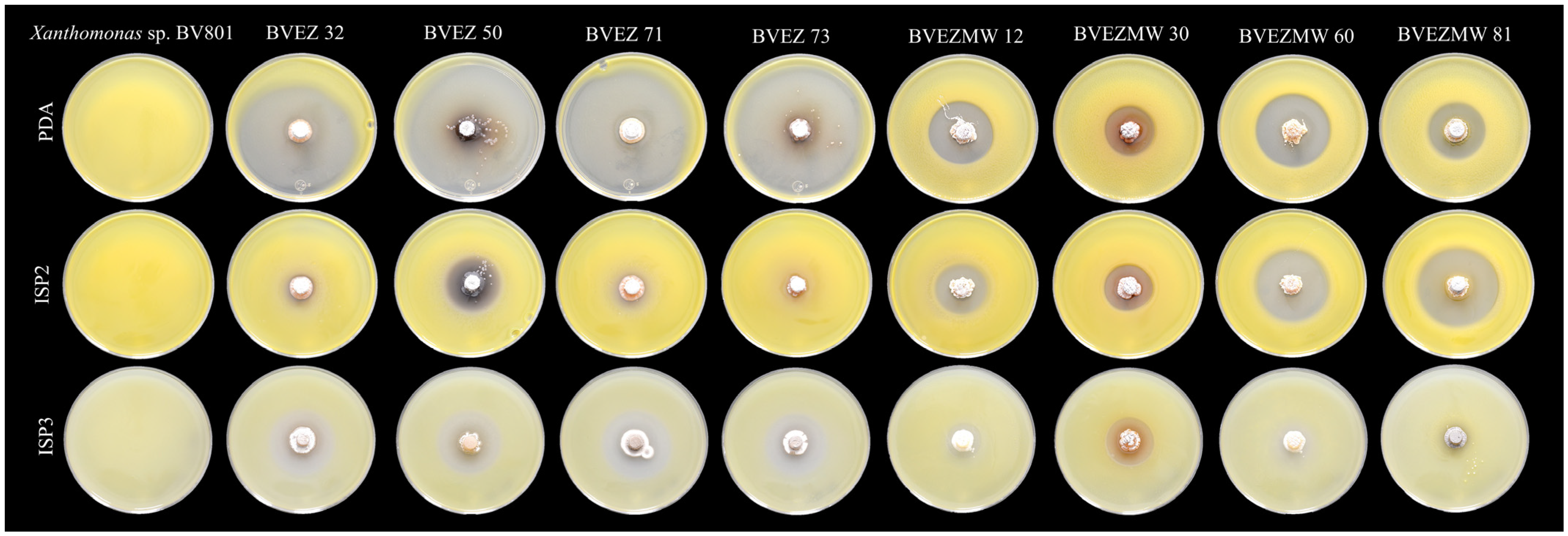
| Soil Pretreatment | Culture Medium | Number of Isolates | Code |
|---|---|---|---|
| Dry heat (70 °C) | ISP2 | 23 | BVEZ 01–23 |
| ISP3 | 53 | BVEZ 24–76 | |
| Microwave irradiation | ISP2 | 61 | BVEZMW 01–61 |
| ISP3 | 61 | BVEZMW 62–122 |
| Dry Heat | Microwave Irradiation | ||
|---|---|---|---|
| Isolate | Inhibition Zone (mm) † | Isolate | Inhibition Zone (mm) † |
| BVEZ 03 | 12.76 ± 1.02 k* | BVEZMW 03 | 27.65 ± 2.16 d* |
| BVEZ 26 | 20.34 ± 0.54 hi | BVEZMW 05 | 20.98 ± 1.33 efg |
| BVEZ 28 | 54.08 ± 2.06 cd | BVEZMW 08 | 28.48 ± 0.49 d |
| BVEZ 29 | 38.82 ± 0.17 f | BVEZMW 12 | 55.47 ± 1.57 a |
| BVEZ 31 | 23.79 ± 0.67 h | BVEZMW 19 | 12.22 ± 0.68 jk |
| BVEZ 32 | 82.37 ± 0.78 a | BVEZMW 30 | 34.63 ± 0.39 c |
| BVEZ 33 | 48.23 ± 2.11 e | BVEZMW 31 | 21.94 ± 1.54 ef |
| BVEZ 40 | 16.67 ± 1.05 ijk | BVEZMW 37 | 15.71 ± 0.36 ij |
| BVEZ 42 | 55.15 ± 2.38 cd | BVEZMW 40 | 23.12 ± 1.44 e |
| BVEZ 46 | 19.69 ± 1.51 hij | BVEZMW 50 | 16.36 ± 0.89 hi |
| BVEZ 50 | 70.64 ± 2.05 b | BVEZMW 51 | 11.40 ± 0.35 k |
| BVEZ 64 | 53.21 ± 1.36 d | BVEZMW 60 | 44.57 ± 0.58 b |
| BVEZ 65 | 22.35 ± 1.47 h | BVEZMW 68 | 21.80 ± 1.28 ef |
| BVEZ 67 | 48.20 ± 0.95 e | BVEZMW 70 | 28.75 ± 0.61 d |
| BVEZ 68 | 33.53 ± 2.15 g | BVEZMW 74 | 21.71 ± 1.49 ef |
| BVEZ 71 | 66.13 ± 1.78 b | BVEZMW 75 | 19.35 ± 1.24 fgh |
| BVEZ 72 | 52.19 ± 1.88 de | BVEZMW 76 | 17.69 ± 1.35 ghi |
| BVEZ 73 | 58.10 ± 1.57 c | BVEZMW 81 | 37.24 ± 0.85 c |
| BVEZ 76 | 15.60 ± 0.77 jk | BVEZMW 88 | 16.83 ± 1.37 hi |
| BVEZMW 89 | 18.94 ± 1.40 fghi | ||
| BVEZMW 98 | 33.96 ± 0.90 c | ||
| BVEZMW 99 | 37.13 ± 0.47 c | ||
| BVEZMW 107 | 28.41 ± 0.95 d | ||
| BVEZMW 117 | 17.32 ± 0.73 hi | ||
| BVEZMW 121 | 17.63 ± 0.99 ghi | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinidad-Cruz, J.R.; Rincón-Enríquez, G.; Evangelista-Martínez, Z.; López-Pérez, L.; Quiñones-Aguilar, E.E. Isolation, Antibacterial Activity and Molecular Identification of Avocado Rhizosphere Actinobacteria as Potential Biocontrol Agents of Xanthomonas sp. Microorganisms 2024, 12, 2199. https://doi.org/10.3390/microorganisms12112199
Trinidad-Cruz JR, Rincón-Enríquez G, Evangelista-Martínez Z, López-Pérez L, Quiñones-Aguilar EE. Isolation, Antibacterial Activity and Molecular Identification of Avocado Rhizosphere Actinobacteria as Potential Biocontrol Agents of Xanthomonas sp. Microorganisms. 2024; 12(11):2199. https://doi.org/10.3390/microorganisms12112199
Chicago/Turabian StyleTrinidad-Cruz, Jesús Rafael, Gabriel Rincón-Enríquez, Zahaed Evangelista-Martínez, Luis López-Pérez, and Evangelina Esmeralda Quiñones-Aguilar. 2024. "Isolation, Antibacterial Activity and Molecular Identification of Avocado Rhizosphere Actinobacteria as Potential Biocontrol Agents of Xanthomonas sp." Microorganisms 12, no. 11: 2199. https://doi.org/10.3390/microorganisms12112199
APA StyleTrinidad-Cruz, J. R., Rincón-Enríquez, G., Evangelista-Martínez, Z., López-Pérez, L., & Quiñones-Aguilar, E. E. (2024). Isolation, Antibacterial Activity and Molecular Identification of Avocado Rhizosphere Actinobacteria as Potential Biocontrol Agents of Xanthomonas sp. Microorganisms, 12(11), 2199. https://doi.org/10.3390/microorganisms12112199






