Abstract
In this study, bacterial isolates C1-4-7, D2-4-6, and M1-4-11 from Antarctic soil were phenotypically and genotypically characterized, and their antibacterial spectrum and that of cell-free culture supernatant were investigated. Finally, the effect of temperature and culture medium on the production of antimicrobial compounds was investigated. The three bacteria were identified as different strains of the genus Pseudomonas. The three bacteria were multi-drug resistant to antibiotics. They exhibited different patterns of growth inhibition of pathogenic bacteria. M1-4-11 was remarkable for inhibiting the entire set of pathogenic bacteria tested. All three bacteria demonstrated optimal production of antimicrobial compounds at 15 °C and 18 °C. Among the culture media studied, Nutrient broth would be the most suitable to promote the production of antimicrobial compounds. The thermostability exhibited by the antimicrobial molecules secreted, their size of less than 10 kDa, and their protein nature would indicate that these molecules are bacteriocin-like compounds.
1. Introduction
Since the discovery of penicillin in 1928, antibiotics, natural products produced by microorganisms capable of preventing the growth of bacteria and thus curing infectious diseases, have saved millions of lives. In the 1940s, for the first time, life-threatening diseases such as pneumonia or tuberculosis could be controlled and cured with antibiotics such as penicillin and streptomycin, respectively [1]. However, the inappropriate and excessive use of these drugs, both in the health sector and in the food industry, has caused a rapid spread of the phenomenon of antimicrobial resistance and the loss of efficacy of antibiotics in the treatment of some infections [2,3,4]. It has been projected that by 2050, infectious diseases could become the leading cause of death globally, with 10 million deaths each year, if antimicrobial resistance is not adequately addressed [2,5]. This complex situation has generated an urgent need to establish mechanisms to develop new and/or improved strategies to solve the increasing number of infections caused by bacteria resistant to antibiotics. Currently, important efforts are being made to achieve an appropriate use of existing antibiotics and promote the development of new drugs that can be used in clinical settings [5].
Collectively, microorganisms are capable of producing a wide variety of secondary metabolites, and the pharmaceutical industry has developed a few of these for therapeutic use [5,6,7]. These are important metabolites such as acids, alcohols, diacetals, and various antimicrobial compounds [3,8,9,10,11,12].
Bioprospecting of natural environments has led to the identification and commercialization of current antibiotics and continues to reveal new biomolecules with therapeutic potential. Currently, natural products, predominantly from bacteria and fungi, remain the main sources studied to discover new antibiotics [2,13]. In this context, the exploration of unusual new habitats and environments has become important to discover new microorganisms that generate antimicrobial metabolites. Microorganisms living in unique or extreme environments such as mine lakes, hyper-arid deserts, and the Antarctic continent may be promising starting points for finding new antimicrobial compounds [2,10,14,15]. Among the extreme environments, Antarctica has proven to be an interesting source of microbial species with great potential for various applications, including biomedicine through the discovery of antimicrobial compounds [13,16,17].
The antimicrobials produced by bacteria include bacteriocins, small ribosomally synthesized polypeptides, that kill or inhibit the growth of other bacteria [8,18].
Bacteriocins from many bacteria have been reported to be active against human and animal microbial pathogens without showing toxicity, which represents an advantage in the use of these types of compounds as antimicrobials [6]. Additionally, numerous bacteriocins have been identified and studied that show remarkable potential as food preservatives and therapeutic or biocontrol agents [4,19,20]. Therefore, some bacteriocins may be able to replace and/or complement current antibiotics.
In this work, three Antarctic soil bacteria (isolates C1-4-7, D2-4-6, and M1-4-11) with antimicrobial potential [21] were characterized phenotypically and genotypically, and both bacteria and cell-free culture supernatants were tested to determine their phylogenetic spectrum of antimicrobial activity. The effect of growth temperature and culture medium composition on antimicrobial production was also investigated.
2. Materials and Methods
2.1. Origin of the Antarctic Bacteria
The 3 Antarctic bacteria used in the present study were isolated from samples collected on two Antarctic islands belonging to the South Shetland Islands. Bacterial isolate designated C1-4-7 was isolated from a soil sample collected in Fildes Bay on King George Island (62°10′09″ S; 58°55′47″ W). Bacterial isolate D2-4-6 was isolated from a soil sample taken in Chile Bay on Greenwich Island (62°28′53″ S; 59°37′06″ W). Finally, the bacterial isolate designated M1-4-11 was isolated from the rhizosphere of Deschampsia antarctica Desv. collected near the Collins glacier on King George Island (62°10′07″ S; 58°51′06″ W). The Antarctic bacteria were obtained as described in Calisto et al., 2021 [21].
2.2. Morphological and Biochemical Characterization of C1-4-7, D2-4-6, and M1-4-11 Antarctic Bacteria
Morphological and biochemical tests were performed to identify Antarctic bacteria [22]. Bacterial isolates were studied for Gram stain, cell morphology, and sugar fermentation (glucose, fructose, sucrose, and lactose). Catalase activity was determined by the production of bubbles after the addition of a drop of 3% (v/v) hydrogen peroxide solution on the bacteria on a slide. The presence of oxidase activity was tested according to the manufacturer’s instructions for Mastdiscs® ID oxidase (Mast Group Ltd., Bootle, Merseyside, UK). The presence of urease was tested using Urea agar (Becton, Dickinson, and Company, Sparks, MD, USA) according to the manufacturer’s instructions. Simmons citrate agar (AccumixTM Tulip Diagnostics Ltd., Goa, India) was used to test the ability of bacterial isolates to use citrate as the sole carbon source. Additionally, cetrimide agar was used as a medium to favor the growth of Pseudomonas aeruginosa, stimulate the production of its pigments and, in turn, inhibit the growth of other microorganisms.
Microscopy analysis was carried out using an optical light microscope (Motic BA 310 E) with 100X magnification. To perform the scanning electron microscopy (SEM) analyses, the bacterial samples were fixed in 2.5% glutaraldehyde in PBS. A 20 µL drop of the suspension was spread onto a carbon tape-covered coverslip attached to a 12 mm stub. The smear was dried at 37 °C and washed twice with distilled water to remove any remaining electrolyte crystals and glutaraldehyde. The salt-free dry smear was coated with a gold film using a Quorum Q150R ES metalizer. SEM images were obtained using a Carl Zeiss EVO 15 microscope with a tungsten filament under high vacuum and a secondary electron detector at 20 kV.
The enzyme profiles of Antarctic bacteria were determined using API ZYM strips (bioMerieux, Marcy l’Étoile, Lyon, France) according to the manufacturer’s instructions. The incubation time of the API strips was 24 h at 18 °C.
2.3. Antibiotic Susceptibility Profile of Antarctic Bacteria
The susceptibility of Antarctic bacteria to different antibiotics was determined using the disk diffusion method described previously [21]. For this, Mueller–Hinton agar plates and antibiotic disks belonging to different groups such as penicillins (ampicillin, amoxicillin-clavulanic acid, piperacillin-tazobactam), cephalosporins (cefuroxime, cefoxitin, cefotaxime, ceftazidime, cefepime, ceftriaxone), carbapenems (ertapenem, meropenem, imipenem), aminoglycosides (streptomycin, kanamycin, gentamicin, amikacin), quinolones (nalidixic acid, ciprofloxacin, levofloxacin), sulfonamides (sulfafurazole, cotrimoxazole), tetracycline, chloramphenicol, trimethoprim, and bacitracin were used. Bacteria were cultured at 18 °C for 48 h. From this, an inoculum was diluted in sterile distilled water until reaching a turbidity equivalent to 0.5 MacFarland. From this culture, an inoculum was seeded with a sterile swab on plates with Muller–Hinton agar, and on this inoculum, sensidiscs with the selected antibiotics were deposited. The plates were incubated at 18 °C for 24 h. Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923 were used as the control for the susceptibility tests. The diameter of the growth inhibition zone generated by each antibiotic was measured, and it was considered that an isolate was resistant to a given antibiotic when the diameter of the growth inhibition zone was less than or equal to 15 mm, intermediate susceptibility when the diameter was between 16 and 20 mm, and sensitive when the diameter of the growth inhibition zone was equal or greater than 21 mm [17].
2.4. Molecular Identification of Antarctic Bacteria
The genomic DNA of bacterial isolates was extracted with a Qiagen Genomic-tip 100/G kit (Qiagen, Courtaboeuf, Les Ulis, France) according to the manufacturer’s protocol. The integrity of the DNA obtained was evaluated by electrophoresis in 1% agarose gels. The DNA concentration and its purity were determined by its absorbance at 260 nm and with the 260/280 nm ratio, respectively, in the Tecan Infinite M200 PRO multiplate reader spectrophotometer.
Antarctic bacteria were identified through 16S rRNA sequence identity analysis. The polymerase chain reaction (PCR) was carried out with bacterial-specific 16S rRNA primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′-CGGTTACCTTGTTACGACTT-3′) using genomic DNA as templates. The PCR conditions were initial denaturation at 95 °C for 3 min, followed by 35 cycles each of denaturation at 95 °C for 1 min, annealing at 55 °C for 1 min, and extension at 72 °C for 1.5 min, and a final extension at 72 °C for 5 min. The PCR products were separated on a 1% agarose gel in 1× TAE buffer, stained with GelRedTM (Biotium Inc., Fremont, CA, USA), and visualized with UV light using a transilluminator. The PCR products were then purified with a GEL/PCR Purification mini-Kit (Favorgen Biotech Corp., Ping-Tung, Taiwan) and sequenced at Austral-Omics of the Universidad Austral de Chile, Valdivia, Chile.
BLAST analysis of 16S rRNA gene sequences was performed using the NCBI nucleotide database to identify similar sequences, and a maximum likelihood phylogenetic tree was used to identify the closest genus/species using IQTREE v.1.6.1 [23] and visualized with iTol v.4 [24]. A phylogenetic tree was created to determine the taxonomic status of Antarctic bacteria from samples collected from Antarctic soil.
2.5. Genotyping of Antarctic Bacteria by Repetitive Elements PCR Amplification
Using genomic DNA obtained from Antarctic bacteria, the sequences enterobacterial repetitive intergenic consensus (ERIC) and interspersed repetitive DNA sequence (BOX) were amplified using PCR to obtain genomic “fingerprint” patterns [25]. ERIC 1R (5′-ATGTAAGCTCCTGGGGATTCAC-3′) and ERIC 2 (5′-AAGTAAGTGACTGGGGTGAGCG-3′) primers were used to perform the ERIC-PCR. The PCR reaction conditions were initial denaturation at 94 °C for 5 min, followed by 35 cycles each of denaturation at 94 °C for 1 min, annealing at 48 °C for 1 min, and extension at 72 °C for 2 min, and a final extension at 72 °C for 5 min. PCR amplifications with BOX primers included only a single primer BOXA1R (5′-CTACGGCAAGGCGACGCTGACG-3′). PCR reaction conditions were an initial denaturation step at 95 °C for 7 min, followed by 30 cycles of denaturation at 90 °C for 30 s, annealing at 52 °C for 1 min, and extension at 65 °C for 8 min with a single final extension at 65 °C for 16 min.
The PCR products were separated on a 1.5% agarose gel in 1× TAE buffer at 50 V for 90 min, stained with GelRedTM (Biotium Inc., Fremont, CA, USA), and visualized with UV light using a transilluminator.
2.6. Screening for Antimicrobial Activity of the Antarctic Bacteria and Their Cell-Free Culture Supernatants
Antimicrobial compound production by C1-4-7, D2-4-6, and M1-4-11 Antarctic bacteria was evaluated by plate activity assays as described previously [21], where it was detected whether the bacterial isolates secrete compounds that have the capacity to inhibit the growth of 18 pathogenic bacteria. The set of reference pathogenic bacteria studied includes Gram-positive bacteria of Bacillus, Enterococcus, Micrococcus, Staphylococcus, and Streptococcus genera, and Gram-negative bacteria of Citrobacter, Enterobacter, Escherichia, Klebsiella, Proteus, Pseudomonas, Salmonella, and Vibrio genera. Briefly, suspensions of overnight-grown pathogenic bacteria cultures were prepared, and an inoculum was seeded with a sterile swab on Muller–Hinton agar plates. Then, an inoculum of 5 µL of Antarctic bacteria, grown at 18 °C for 48 h, was deposited into the indicator bacteria lawn. Plates were incubated at 18 °C for 24 h for adequate growth of Antarctic bacterial isolates and then incubated at 37 °C for growth of indicator pathogenic bacteria. The result of the antagonistic activity was determined by the presence or absence of a growth inhibition zone in the lawn of each pathogenic bacteria analyzed.
The antibacterial activity of the cell-free culture supernatant was tested against the same set of pathogenic bacteria. The Antarctic bacteria were incubated at 18 °C for 60 h. The culture was centrifuged at 10,000 rpm for 20 min. The culture supernatant was concentrated 20-fold by evaporation at 80 °C. The antimicrobial activity of this concentrate was analyzed by the antimicrobial activity plate assay [26]. The culture plates were incubated at 37 °C for 24 h, and the growth inhibition zone in the lawn of each pathogenic bacteria was analyzed. Aliquots of 5 µL of Nut 1/3 broth concentrated 20-fold at 80 °C and 5 µL of E. coli growth supernatant concentrated 20-fold at 80 °C were used as negative controls. The antibiotic ampicillin (10 µg) was used as a positive control.
2.7. Exclusion Membrane Assay
To determine the approximate size of the molecules secreted by Antarctic bacteria that have antimicrobial activity, an antimicrobial activity plate assay [26] was used. On this occasion, the Antarctic bacteria and the cell-free concentrated culture supernatant were inoculated on a cellulose membrane with a 10 kDa exclusion pore (Spectra/Por® 7 MWCO 10,000), previously deposited on a lawn of indicator pathogenic bacteria. The experiments were carried out following the same indications as these for the screening for antimicrobial activity of Antarctic bacteria.
2.8. Sensitivity of the Antimicrobial Agents to Proteinase K Enzyme
The proteinase K enzyme (Promega Corporation, Madison, WI, USA) was used to determine whether the inhibitory compounds produced by the Antarctic bacteria were proteinaceous [27]. The enzyme was prepared at a concentration of 25 mg/mL, according to the manufacturer’s instructions. The 20-fold concentrated supernatants were incubated for 2 h at 37 °C with proteinase K at a final concentration of 10 mg/mL. The antimicrobial activity of proteinase K-treated concentrated supernatant was analyzed by the antimicrobial activity plate assay [26]. Briefly, 5 µL of the concentrated supernatants treated with proteinase K were inoculated onto a lawn of reference pathogenic bacteria (E. coli and S. aureus). Culture plates were incubated at 37 °C for 24 h. The growth inhibition zone in the lawn of the reference bacteria was measured and compared with the growth inhibition zone produced by the concentrated supernatant without enzyme treatment. A 20-fold concentrated Nut 1/3 broth inoculated with nisin (a proteinase K-sensitive bacteriocin) was treated in the same way as the Antarctic bacterial concentrates and served as a positive control to assess proteinase K proteolytic activity.
2.9. Effect of Temperature and Culture Medium on the Growth and Bacteriocin-like Production of the Antarctic Bacteria
The ability of C1-4-7, D2-4-6, and M1-4-11 Antarctic bacteria to grow at different temperatures (4 °C, 10 °C, 15 °C, 18 °C, and 25 °C) was verified using nutrient broth diluted to one-third (Nut 1/3: peptone 1.67 g/L; meat extract 1 g/L; pH: 6.9). Bacterial growths were carried out simultaneously at all temperatures and without shaking.
The growth of bacterial isolates at 18 °C in different culture broths was analyzed. The following culture media were used: Luria Bertani (LB: tryptone 10 g/L; yeast extract 5 g/L; NaCl 10 g/L; pH: 7.1); LB diluted to a third (LB 1/3); nutrient (Nut: peptone 5 g/L; meat extract 3 g/L; pH: 6.9); nut diluted to a third (Nut 1/3); soy trypticase (TSB: casein digest 15 g/L; peptic digest of soy flour 5 g/L; glucose 2.5 g/L; NaCl 5 g/L; pH: 7.1) and minimal medium M63·supplemented with 0.2% glucose (M63 + 0.2%G: KH2PO4 13.6 g/L; (NH4)2SO4 2 g/L; FeSO4·7 H2O 0.5 mg/L; MgSO4·7 H2O 0.24 g/L; glucose 2 g/L; pH: 6.8). No shaking was used. Bacterial growth was monitored at 600 nm using a SPECTRO UV-11 spectrophotometer with 1 cm optical path plastic cuvettes.
After studying the effect of temperature and culture medium on the growth of Antarctic bacteria C1-4-7, D2-4-6, and M1-4-11, it was determined whether these variables affected the production of secreted antimicrobial compounds. For this purpose, the Antarctic bacteria were grown at different temperatures (4 °C, 10 °C, 15 °C, 18 °C, and 25 °C) and in different culture media (LB, LB 1/3, Nut, Nut 1/3, and TSB); then, the antimicrobial activity plate assay was performed, according to the methodology indicated above, and the zone of inhibition produced by the concentrated supernatant of each bacterium on E. coli and S. aureus was measured.
To relate bacterial growth to the production of antimicrobial compounds, the antimicrobial activity was expressed as arbitrary units per milliliter of 20-fold concentrated supernatant (AU/mL). AU was defined as the reciprocal of the highest two-fold dilution exhibiting a clear zone of inhibition on the indicator lawn.
2.10. Statistical Analysis
One-way analysis of variance (ANOVA) and Tukey’s non-parametric test for multiple comparisons were carried out using GraphPad Prism version 9.3.1 software. Differences between samples were considered statistically significant if the p-value was less than 0.05.
3. Results
3.1. Morphological and Biochemical Characterization of Antarctic Bacteria
The C1-4-7, D2-4-6, and M1-4-11 Antarctic bacteria were morphologically characterized as round, shiny colonies with regular borders and later by Gram staining under optical microscopy. It was observed that C1-4-7, D2-4-6, and M1-4-11 correspond to short Gram-negative bacilli. Additionally, the SEM images showed that isolates C1-4-7 ranged in size from 0.47 to 0.64 µm wide by 1.76–2.50 µm long, isolates D2-4-6 ranged from 0.53 to 0.68 µm wide by 1.03–2.57 µm, and finally isolates M1-4-11 ranged from 0.39 to 0.67 µm wide by 1.22–2.54 µm (Figure 1).
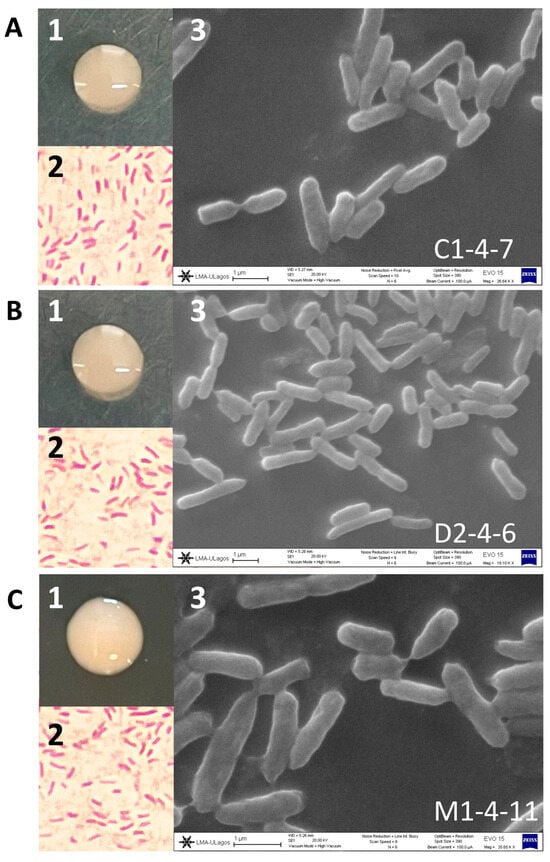
Figure 1.
Morphology and microscopy of Antarctic soil isolates (A) C1-4-7, (B) D2-4-6, and (C) M1-4-11. (1) Bacterial colony. (2) Gram stain microscopy. (3) Electron microscopy.
For the metabolic characterization of the Antarctic bacteria, different biochemical tests were carried out, and the results of these tests are presented in Table 1. The three Antarctic bacteria were positive for the enzyme catalase, oxidase, and urease tests. Additionally, the Antarctic bacteria presented a non-fermentative metabolism and the ability to grow in Simmons citrate, which is a medium that contains sodium citrate and inorganic ammonium salts as the only sources of carbon and nitrogen, respectively. Finally, the three bacteria were able to grow in cetrimide, which is a medium for selective growth of Pseudomonas aeruginosa and other species of the genus Pseudomonas. However, when the isolates were grown on cetrimide agar, no production of characteristic Pseudomonas aeruginosa pigments was observed (Figure S1).

Table 1.
Results of biochemical tests performed on the Antarctic bacteria C1-4-7, D2-4-6, and M1-4-11.
The enzyme activity profile of Antarctic bacteria was determined using the commercial enzyme detection system API-ZYM (bioMerieux, Marcy l’Étoile, France). This colorimetric system allows, through color changes and comparison with controls, to detect the presence or absence of enzymatic activity. The enzymatic activity profiles of isolates C1-4-7, D2-4-6, and M1-4-11 are presented in Figure 2. Additionally, the enzymatic profile of Pseudomonas aeruginosa O400 used as a reference is included. The results show that the three Antarctic bacteria studied have detectable levels of the enzymes acid phosphatase, esterase (C4), and esterase lipase (C8) that participate in lipid metabolism. Additionally, in the three Antarctic bacteria, the enzyme leucine arylamidase (amino-peptidase) related to protein metabolism and the hydrolase naphthol-AS-BI-phosphohydrolase were detected. On the other hand, only C1-4-7 and M1-4-11 bacteria had detectable levels of alkaline phosphatase, and only isolate C1-4-7 had positive levels of valine arylamidase.
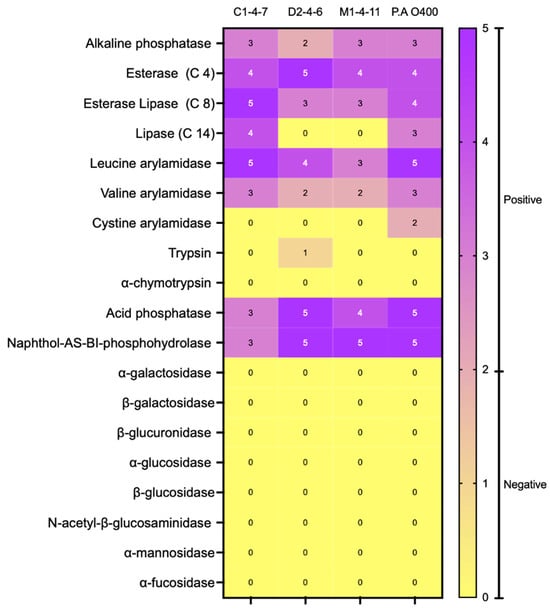
Figure 2.
Enzyme activity assay (API-ZYM) of Antarctic bacteria C1-4-7, D2-4-6, and M1-4-11. According to the instructions indicated by the manufacturer, a negative result is obtained when the intensity of the observed color is between 0 and 2, while positive results correspond to values between 3 and 5.
The Antarctic soil isolates all possessed some of the biochemical characteristics typical of Pseudomonas species.
3.2. Molecular Identification of Antarctic Bacteria
For molecular identification of the C1-4-7, D2-4-6, and M1-4-11 Antarctic bacteria, 16S rRNA sequence analysis of Antarctic bacteria C1-4-7, D2-4-6, and M1-4-11 was performed by phylogeny (Figure 3). The rooted tree shows the 16S rRNA sequence of the Antarctic bacteria clusters with the homologous sequences of microorganisms belonging to the genus Pseudomonas. As shown in Table 2, the Antarctic bacteria studied showed between 99.93% and 100% identify with bacteria of the genus Pseudomonas when BLAST analysis of 16S rRNA gene sequences was performed. This result, together with the results obtained in the morphological characterization of the Antarctic bacteria, allows us to classify the isolates as members of the genus Pseudomonas. Further characterization and comparison using the complete sequence of the bacterial genome could allow in the future to identify the species to which the Antarctic bacteria studied in this work belong.
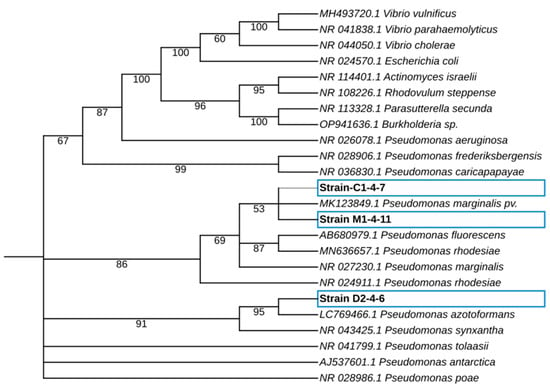
Figure 3.
Maximum-likelihood phylogenetic tree-based 16S rRNA gene sequences of Antarctic bacteria C1-4-7, D2-4-6, and M1-4-11. The phylogenetic tree was obtained using the IQTREE v2.2.0 software, and the iTol program was used to visualize the phylogenetic tree. The percentage of bootstrap values is shown next to the branches based on 10,000 bootstrap replications.

Table 2.
Identification of Antarctic bacteria based on 16S ribosomal DNA sequencing and sequence analysis by BLAST alignment.
3.3. Genotyping of Antarctic Bacteria by Detection of Repetitive Elements Amplified by PCR
When performing genomic identification using ERIC-PCR (Figure 4A) and BOX-PCR (Figure 4B) of Pseudomonas sp. C1-4-7, D2-4-6, and M1-4-11, the generation of different amplicon patterns was observed for each bacterium studied. PCR with ERIC and BOX primers allowed the visualization of fragments of different lengths that produced different fingerprints for Pseudomonas sp. C1-4-7, D2-4-6, and M1-4-11, indicating that they are different bacteria and confirming their phylogeny and enzyme profile.
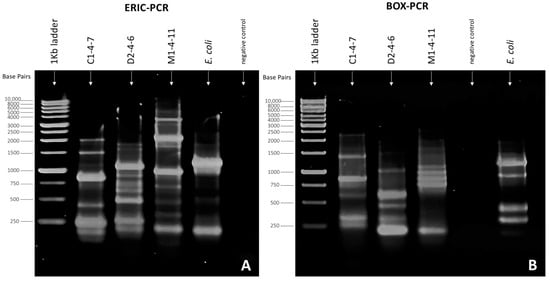
Figure 4.
Fingerprinting patterns obtained for Pseudomonas sp. C1-4-7, D2-4-6, and M1-4-11 using (A) ERIC-PCR and (B) BOX-PCR. Electrophoresis conditions: 10 μL sample, 1.5% agarose gel, 50 Volt, and 90 min.
3.4. Antibiotic Susceptibility Profile of Antarctic Pseudomonas sp. C1-4-7, D2-4-6, and M1-4-11
Table 3 shows the susceptibility of the isolates to antimicrobial agents. The three bacteria studied are multi-resistant since they present resistance to three or more groups of antibiotics.

Table 3.
Antibiotic susceptibility profile of Antarctic bacteria C1-4-7, D2-4-6, and M1-4-11.
The Antarctic bacteria (soil isolates) showed resistance or intermediate susceptibility to 16 of the 24 antibiotics studied. Collectively, they showed resistance to at least one antibiotic from each group tested.
3.5. Effect of Temperature and Culture Medium on the Growth of the Antarctic Bacteria C1-4-7, D2-4-6, and M1-4-11
Growth curves of the three isolates cultured in Nut 1/3 broth are shown in Figure 5. When analyzing the growth curves, it can be observed that as the temperature increases, the growth rate of the Antarctic bacteria increases; however, similar optical density (OD) values were reached at 150 h of growth, except for the curves observed at 4 °C.
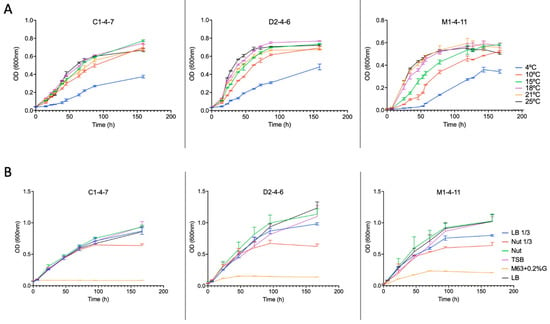
Figure 5.
Growth curves of Antarctic bacteria C1-4-7, D2-4-6, and M1-4-11 (A) at different temperatures and (B) in different culture media at 18 °C.
The growth of Pseudomonas sp. C1-4-7, D2-4-6, and M1-4-11 in different culture broths (Nut, Nut 1/3, LB, LB 1/3, TBS and M63 + 0.2%G) was analyzed. The growth curves obtained are shown in Figure 5. Bacterial growth is very slight for Pseudomonas sp. M1-4-11 and D2-4-6 and practically undetectable for Pseudomonas sp. C1-4-7 when the M63 + 0.2%G broth is used for bacterial growth.
3.6. Screening for Antimicrobial Activity of the Antarctic Bacteria C1-4-7, D2-4-6, and M1-4-11 and Their Cell-Free Culture Supernatants Concentrated at 80 °C
The ability of the Pseudomonas sp. C1-4-7, D2-4-6, and M1-4-11 to inhibit the growth of a set of 18 human pathogenic bacteria was analyzed (Table 4; Figure S2). The three strains of Antarctic Pseudomonas presented different patterns of growth inhibition of the human pathogenic bacteria studied. The Pseudomonas sp. C1-4-7 secreted antimicrobial compounds with the ability to inhibit the growth of mainly Gram-negative pathogenic bacteria (Salmonella enterica serotype Enteritidis, Klebsiella oxytocic, Vibrio parahaemolyticus VpKX, Klebsiella pneumoniae, Escherichia coli XL-1 Blue, Enterobacter aerogenes). Pseudomonas sp. D2-4-6 secreted compounds that inhibit the growth of only Gram-positive pathogenic bacteria (Staphylococcus saprophyticus, Micrococcus sp., Staphylococcus aureus, Streptococcus agalactiae). Finally, the Pseudomonas sp. M1-4-11 secreted antimicrobial compounds capable of inhibiting all pathogenic bacteria studied.

Table 4.
Antagonistic effect of Antarctic bacteria C1-4-7, D2-4-6, and M1-4-11 against bacterial pathogens.
Additionally, to determine if the compounds with antimicrobial activity secreted by the Antarctic bacteria are thermostable, the bacteria-free culture supernatants were concentrated at 80 °C. Then, the ability of these concentrates to inhibit the growth of pathogenic bacteria was determined (Table 4; Figure S3). It can be noted that both the Pseudomonas sp. M1-4-11 and its concentrated cell-free supernatant can inhibit the entire set of pathogenic bacteria studied. In the case of Pseudomonas sp. D2-4-6, the concentrated cell-free supernatant was observed to inhibit the growth of a greater number of pathogenic bacteria than the bacterial isolate.
3.7. Exclusion Membrane Assay
Antimicrobial activity plate assays were performed using 10 kDa pore size exclusion membranes located between the reference pathogenic bacteria and the Antarctic bacteria or cell-free culture supernatant concentrate. It was observed that the compounds with antimicrobial activity were able to cross the exclusion membrane and inhibit the growth of the reference pathogenic bacteria (Figure S4). This result indicates that the Antarctic bacteria studied secrete antimicrobial compounds that are less than 10 kDa in size.
3.8. Sensitivity of the Antimicrobial Agents to Proteinase K Enzyme
The antimicrobial compounds produced by Pseudomonas sp. C1-4-7, D2-4-6, and M1-4-11 were sensitive to Proteinase K treatment. After treating the cell-free concentrated supernatants with Proteinase K, none of them showed antimicrobial activity on the reference pathogenic bacteria (E. coli and S. aureus) (Figure S5). This result indicated that the antimicrobial compounds produced by these Pseudomonas sp. from Antarctica are proteinaceous.
3.9. Effect of Temperature and Culture Medium on the Bacteriocin-like Production of the Antarctic Bacteria C1-4-7, D2-4-6, and M1-4-11
To determine at what stage of growth of the Pseudomonas sp. C1-4-7, D2-4-6 and M1-4-11 bacteria the antimicrobial compounds are produced, the growth curves of the bacteria were compared with the antimicrobial activity of concentrated supernatants obtained at various time points during bacterial growth (0, 18, 42, 66, and 90 h). A time of 90 h was chosen since the three Antarctic bacteria reached the stationary phase at that time. Figure 6 shows the results obtained when the experiments were carried out at 18 °C and using LB as a culture medium. S. aureus was used as an indicator of pathogenic bacteria for Pseudomonas sp. D2-4-6 and M1-4-7 and E. coli for Pseudomonas sp. C1-4-7.
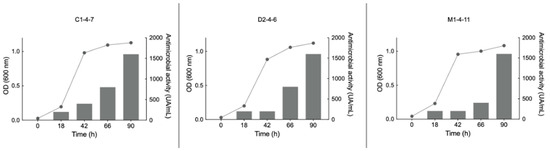
Figure 6.
Relationship between growth curves (left axis line graph) and growth inhibition zone (right axis bar graph) produced by the concentrated supernatant of Antarctic bacteria C1-4-7, D2-4-6, and M1-4-11 on reference pathogenic bacteria. For C1-4-7, E. coli was used as a reference and for D1-4-6 and M1-4-11, S. aureus was used as a reference. UA/mL: arbitrary unit per milliliter.
To determine whether the temperature and the culture broths used for the growth of Antarctic bacteria affect the production of bacteriocins, the growth inhibition zone of reference pathogenic bacteria produced by Pseudomonas sp. C1-4-7, D2-4-6, and M1-4-11 at different temperatures and using different culture broths were analyzed.
Figure 7 shows the results obtained for the different growth temperatures studied using nut 1/3 broth. It can be observed for Pseudomonas sp. C1-4-7 that the inhibition zone is only significantly lower when the bacterium grows at 25 °C. For Pseudomonas sp. D2-4-6, no growth inhibition zone was observed for S. aureus when the bacteria grew at 4 °C. At 18 °C, a significantly larger inhibition zone was obtained than that observed for the other temperatures studied. Finally, for Pseudomonas sp. M1-4-7, it was observed that at 10 °C and 15 °C, significantly larger inhibition zones were obtained for both E. coli and S. aureus.
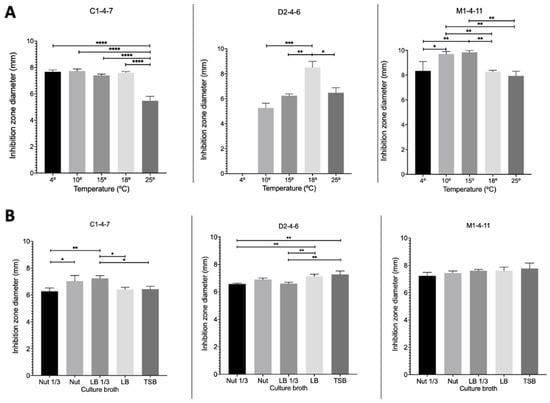
Figure 7.
Inhibition zones at different temperatures (A) and in different culture broths (B) produced by Pseudomonas sp. C1-4-7, D2-4-6, and M1-4-11 on reference pathogenic bacteria. For C1-4-7, E. coli was used as a reference and for D1-4-6 and M1-4-11, S. aureus was used as a reference. **** p < 0.0001; *** p < 0.001; ** p < 0.01; * p < 0.05 (n = 3).
Figure 7 shows the results obtained for the growth inhibition zone of reference pathogenic bacteria produced by Antarctic bacteria when grown in different culture broths at 18 °C. Figure 7 shows that for Pseudomonas sp. C1-4-7, significantly greater growth inhibition zones were obtained for E. coli when nut and LB 1/3 broths were used for bacterial growth. In the case of Pseudomonas sp. D2-4-7, the largest growth inhibition zones for S. aureus were obtained when the bacteria were grown in LB and TSB broths. In contrast to the other strains studied, Pseudomonas sp. D2-4-7 did not exhibit growth inhibition of S. aureus at 4 °C. Finally, for Pseudomonas sp. M1-4-11, the growth inhibition zone of E. coli was not modified depending on the culture broth studied.
4. Discussion
The data on Antarctic microorganisms that produce antimicrobial compounds are scarce compared to other widely studied environments [28,29]. Bacteria isolated from the Antarctic environment currently remain a potential and underexplored source of new antimicrobial compounds, which may be advantageous in food, therapeutic, health, and industrial applications in the future.
In the first part of this study, we worked on the identification and characterization of three bacteria isolated from Antarctic soil samples collected in sectors far from human influence. These bacteria were selected because they belong to different sampling sites and because they secrete antibacterial molecules with different growth inhibition patterns against a set of eight reference human pathogenic bacteria [21]. The results of the analysis of the 16S rDNA gene sequences confirmed that the Antarctic bacteria belong to the genus Pseudomonas. The ERIC-PCR and BOX-PCR genomic fingerprinting of Pseudomonas sp. C1-4-7, D2-4-6, and M1-4-11 indicated that the three bacteria are different from each other. The metabolic and enzymatic characteristics of the Antarctic bacterial isolates are like those reported by other authors for bacteria of the genus Pseudomonas isolated from soil samples [30,31,32].
Pseudomonas strains are found in large numbers in all the major natural environments (terrestrial, freshwater, and marine) and form intimate associations with plants and animals [33]. The population of such environments involves a struggle for living space and organic nutrients with many other microorganisms. Pseudomonas display a highly versatile metabolism, and several secondary metabolites affecting other bacteria and fungi that are attracted to common niches have been identified [34]. Different authors have reported the presence of this bacterial genus in Antarctica, both at the soil and sediment level [10,17,31,35,36] and associated with the growth of vascular plants such as D. antarctica [37].
The Pseudomonas sp. C1-4-7, D2-4-6, and M1-4-11 showed multi-drug resistance, which correlates with several studies that have reported the presence of resistance to different antibiotics in bacteria isolated from Antarctic soils [17,36,38,39,40]. Tomova et al. (2015) [17] suggest that the antibiotic multiresistance detected in their study could be associated with an anthropogenic impact on the Antarctic islands Deception and Galíndez. On the other hand, Marcoleta et al. (2022) [39] found a higher general resistance in bacteria from areas not intervened by human activities. In their study, they detected Pseudomonas resistant to 10 or more different antibiotics, a result similar to that observed in this study where bacteria were isolated from soil samples obtained in sectors without anthropogenic intervention. This supports our initial findings reported in Calisto et al. (2021) [21].
The results of the first part of this study suggest that these Antarctic bacteria are potential sources of genes encoding for both antimicrobial compounds and resistance to antibiotics. From an ecological point of view, these two capabilities probably provided the competitive advantage to these Antarctic bacteria to survive in the harsh environment [36,41], while on the other hand, these same characteristics may be of interest for biomedical applications.
The second part of this work aimed to analyze the antimicrobial activity of Pseudomonas sp. C1-4-7, D2-4-6, and M1-4-11 and their cell-free supernatants. It was observed that each bacterium studied showed different patterns of growth inhibition of the set of pathogenic bacteria tested. The growth inhibition of all pathogenic bacteria tested by Pseudomonas sp. M1-4-11 is remarkable. When the antimicrobial activity of the cell-free supernatant concentrated at 80 °C of Pseudomonas sp. M1-4-11 was analyzed, the same inhibition pattern observed for the bacterium was found. However, for the cell-free concentrated supernatant of Pseudomonas sp. C1-4-7, a different pattern of antimicrobial activity was observed. The concentrated supernatant was able to inhibit the growth of pathogenic bacteria that Pseudomonas sp. C1-4-7 could not inhibit, but this concentration lost the capacity to inhibit other pathogens that the bacteria could directly inhibit. Finally, the concentrated supernatant of Pseudomonas sp. D2-4-6 showed antimicrobial activity in a group of Gram-negative pathogenic bacteria on which bacteria did not present antimicrobial activity. The loss of antimicrobial activity of the concentrated supernatant in relation to the bacteria could be because the bacteria could secrete antimicrobial compounds that are not stable at high temperatures; therefore, they degrade when concentrating the supernatant at 80 °C. On the other hand, the increase in the antimicrobial activity of the concentrated supernatant with respect to the activity presented by the bacteria could be explained if it is considered that the bacteria can secrete small amounts of some thermostable antimicrobial compounds; therefore, the antimicrobial activity of these compounds is only evidenced by concentrating the supernatant 20 times.
Different authors have reported that Antarctic bacteria are a potential source of antimicrobial compounds, and bacteria with antimicrobial activity have been isolated from various Antarctic habitats, predominantly soils, sediments, and seawater [2,10,13,14,15,16,17,28,41,42,43]. Several authors have reported bacteria of the genus Pseudomonas isolated from Antarctic soils that can inhibit the growth of different groups of reference bacteria [10,17,36,43,44]. Wong et al. (2011) [36] identified five bacteria of the genus Pseudomonas that showed different patterns of growth inhibition of pathogenic bacteria. Among the 24 isolates obtained by Tomova et al. (2015) [17], the Pseudomonas sp. A1-1 exhibited the broadest inhibitory spectrum, being active against all reference bacteria and yeast cultures tested. On the other hand, Silva et al. (2018) [10] highlighted Pseudomonas sp. isolate 99 for showing a broad antimicrobial spectrum, in addition to antiproliferative and antiparasitic activity. Recently, Orellana et al. (2022) [43] reported 11 bacteria of the genus Pseudomonas isolated from the rhizosphere of Deschampsia antarctica Desv. with the ability to inhibit the growth of different human pathogenic bacteria. None of the works mentioned above report the antimicrobial capacity of the bacteria and their cell-free supernatant.
Despite the diversity of studies in the academic literature pointing to the commercial and therapeutic potential of Antarctic bacteria in the field of antimicrobials, in the study carried out by Silva et al. (2022) [29], which was based on a bibliographic search by patent documents, only four patent documents were found mentioning Antarctic bacteria, which were associated with five bacteria identified as Janthinobacterium sp., Flavobacterium sp., Streptomyces radiopugnans, Streptomyces sp., and Bacillus sp. This situation could be indicate that many of the studies that have reported Antarctic bacteria with antimicrobial activity have been limited only to identifying these bacteria in different environmental matrices but have not continued to study the characteristics of the compounds produced by these bacteria.
The last part of this study focused on performing an initial characterization of the antimicrobial compounds secreted by Pseudomonas sp. C1-4-7, D2-4-6, and M1-4-11 and analyzing how the temperature and culture media used for the growth of these Antarctic bacteria affect the production of antimicrobial compounds.
The results in Figure 6 and Figure 7 show that the growth of Pseudomonas sp. C1-4-7, D2-4-6, and M1-4-11 and the production of antimicrobial compounds are different for each of the bacteria under the same growth conditions. However, it could be indicated that the three bacteria have an optimal production of antimicrobial compounds between 15 °C and 18 °C. Among the culture media studied, nut would be the most suitable for favoring the production of antimicrobial compounds for Pseudomonas sp. C1-4-7, D2-4-6, and M1-4-11.
The thermostability exhibited by antimicrobial compounds secreted by Pseudomonas sp. C1-4-7, D2-4-6, and M1-4-11 when concentrating the cell-free supernatant at 80 °C, the size less than 10 kDa, and the protein nature of the antimicrobial molecules would indicate that these compounds are bacteriocins [20,45]. Bacteriocins are small peptides or polypeptides produced by Gram-positive and Gram-negative bacteria, ribosomally synthesized, and very heterogeneous regarding their size, structure, mechanisms of action, spectrum of activity, biochemical properties, and target cell receptors [46,47]. It is considered that the majority of bacteria generate at least one antimicrobial peptide for self-preservation and competitive advantages in their ecological niche [45].
In this work, we studied three Pseudomonas obtained from Antarctic soil. It was found that these three Pseudomonas were able to produce antimicrobial bacteriocin-like compounds, which have different patterns of inhibition of human pathogenic bacteria. It was also found that the production of bacteriocin-like compounds can be modified with temperature and with the culture mediums used for bacterial growth. These results indicate that bacteriocin-like compounds secreted by Antarctic bacteria C1-4-7, D2-4-6, and M1-4-11 could be promising new antimicrobial compounds with potential therapeutic and food preservation applications.
Among the future perspectives of this study, we propose to sequence the complete genomes to identify the genes responsible for encoding the bacteriocin-like compounds in each of the Pseudomonas. This could lead to their subsequent expression in a heterologous system to ultimately improve their production.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms12030530/s1, Figure S1: Growth of Antarctic isolates C1-4-7, -D2-4-6, and M1-4-11 on selective medium for Pseudomonas cetrimide agar; Figure S2: Example of the antagonistic effect of Antarctic bacteria C1-4-7, D2-4-6, and M1-4-11 against reference bacterial pathogens; Figure S3: Example of antimicrobial activity of 20-fold concentrated supernatant of isolates (A) C1-4-7, (B) D2-4-6, and (C) M1-4-11 against reference bacterial pathogens (S. aureus, E. coli); Figure S4: Antimicrobial activity of 20-fold concentrated supernatant of isolates C1-4-7, D2-4-6, and M1-4-11 via a 10 kDa MWCO membrane; Figure S5: Effect of proteinase K (PK) on the antimicrobial activity of 20-fold concentrated supernatants of bacterial isolates C1-4-7, D2-4-6, and M1-4-11.
Author Contributions
Conceptualization: G.C. and N.C.; methodology and formal analysis: N.C., C.I., L.N., P.O., C.A. and G.C; data curation C.I.; writing original draft preparation N.C., C.I. and L.N.; writing review and editing: N.C., C.I., L.N., P.O., C.G., L.S., A.G., A.R.G., M.T., P.C.-C., M.G. and G.C.; sampling: N.C., C.G., A.G. and M.G.; funding acquisition, project administration, and supervision: G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the DIUA189-2020, DIUA181-2020, and DIUA260-2023 projects of the Universidad Autónoma de Chile.
Data Availability Statement
The study data are present in the main text, and for further inquiries please contact the corresponding author.
Acknowledgments
The authors are thankful for the CIMAA project of the Department of Chemical Engineering of the Universidad de Magallanes and the project MA-01-12 of the Chilean Antarctic Institute (INACH) for their support in the taking of samples. We also thank the Ph.D. program of biomedical science at Universidad Autónoma de Chile. Acknowledgments to Mauricio Quiroz for his support in electron microscopy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cole, S.T. Who Will Develop New Antibacterial Agents? Phil. Trans. R. Soc. B 2014, 369, 20130430. [Google Scholar] [CrossRef] [PubMed]
- Baricz, A.; Teban, A.; Chiriac, C.M.; Szekeres, E.; Farkas, A.; Nica, M.; Dascălu, A.; Oprișan, C.; Lavin, P.; Coman, C. Investigating the Potential Use of an Antarctic Variant of Janthinobacterium Lividum for Tackling Antimicrobial Resistance in a One Health Approach. Sci. Rep. 2018, 8, 15272. [Google Scholar] [CrossRef] [PubMed]
- Joerger, R. Alternatives to Antibiotics: Bacteriocins, Antimicrobial Peptides and Bacteriophages. Poult. Sci. 2003, 82, 640–647. [Google Scholar] [CrossRef]
- Yang, S.-C.; Lin, C.-H.; Sung, C.T.; Fang, J.-Y. Antibacterial Activities of Bacteriocins: Application in Foods and Pharmaceuticals. Front. Microbiol. 2014, 5, 241. [Google Scholar] [CrossRef]
- Mishra, B.; Reiling, S.; Zarena, D.; Wang, G. Host Defense Antimicrobial Peptides as Antibiotics: Design and Application Strategies. Curr. Opin. Chem. Biol. 2017, 38, 87–96. [Google Scholar] [CrossRef]
- Ahmad, V.; Jamal, Q.M.S.; Siddiqui, M.U.; Shukla, A.K.; Alzohairy, M.A.; Al Karaawi, M.A.; Kamal, M.A. Methods of Screening-Purification and Antimicrobial Potentialities of Bacteriocin in Health Care. Curr. Drug Metab. 2017, 18, 814–830. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Gallo, R.L. Antimicrobial Peptides: Old Molecules with New Ideas. J. Investig. Dermatol. 2012, 132, 887–895. [Google Scholar] [CrossRef]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A Viable Alternative to Antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef]
- Juturu, V.; Wu, J.C. Microbial Production of Bacteriocins: Latest Research Development and Applications. Biotechnol. Adv. 2018, 36, 2187–2200. [Google Scholar] [CrossRef]
- Silva, T.R.; Duarte, A.W.F.; Passarini, M.R.Z.; Ruiz, A.L.T.G.; Franco, C.H.; Moraes, C.B.; de Melo, I.S.; Rodrigues, R.A.; Fantinatti-Garboggini, F.; Oliveira, V.M. Bacteria from Antarctic Environments: Diversity and Detection of Antimicrobial, Antiproliferative, and Antiparasitic Activities. Polar Biol. 2018, 41, 1505–1519. [Google Scholar] [CrossRef]
- Katz, L.; Baltz, R.H. Natural Product Discovery: Past, Present, and Future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rendon, D.; Passari, A.K.; Ruiz-Villafán, B.; Rodríguez-Sanoja, R.; Sánchez, S.; Demain, A.L. Impact of Novel Microbial Secondary Metabolites on the Pharma Industry. Appl. Microbiol. Biotechnol. 2022, 106, 1855–1878. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Montero, K.; Lamilla, C.; Abanto, M.; Maruyama, F.; Jorquera, M.A.; Santos, A.; Martinez-Urtaza, J.; Barrientos, L. Antarctic Streptomyces Fildesensis So13.3 Strain as a Promising Source for Antimicrobials Discovery. Sci. Rep. 2019, 9, 7488. [Google Scholar] [CrossRef] [PubMed]
- Asencio, G.; Lavin, P.; Alegría, K.; Domínguez, M.; Bello, H.; González-Rocha, G.; González-Aravena, M. Antibacterial Activity of the Antarctic Bacterium Janthinobacterium Sp. SMN 33.6 against Multi-Resistant Gram-Negative Bacteria. Electron. J. Biotechnol. 2014, 17, 1–5. [Google Scholar] [CrossRef]
- Maida, I.; Fondi, M.; Papaleo, M.C.; Perrin, E.; Orlandini, V.; Emiliani, G.; De Pascale, D.; Parrilli, E.; Tutino, M.L.; Michaud, L.; et al. Phenotypic and Genomic Characterization of the Antarctic Bacterium Gillisia Sp. CAL575, a Producer of Antimicrobial Compounds. Extremophiles 2014, 18, 35–49. [Google Scholar] [CrossRef]
- Tedesco, P.; Maida, I.; Palma Esposito, F.; Tortorella, E.; Subko, K.; Ezeofor, C.C.; Zhang, Y.; Tabudravu, J.; Jaspars, M.; Fani, R.; et al. Antimicrobial Activity of Monoramnholipids Produced by Bacterial Strains Isolated from the Ross Sea (Antarctica). Mar. Drugs 2016, 14, 83. [Google Scholar] [CrossRef] [PubMed]
- Tomova, I.; Stoilova-Disheva, M.; Lazarkevich, I.; Vasileva-Tonkova, E. Antimicrobial Activity and Resistance to Heavy Metals and Antibiotics of Heterotrophic Bacteria Isolated from Sediment and Soil Samples Collected from Two Antarctic Islands. Front. Life Sci. 2015, 8, 348–357. [Google Scholar] [CrossRef]
- Baquero, F.; Lanza, V.F.; Baquero, M.-R.; del Campo, R.; Bravo-Vázquez, D.A. Microcins in Enterobacteriaceae: Peptide Antimicrobials in the Eco-Active Intestinal Chemosphere. Front. Microbiol. 2019, 10, 2261. [Google Scholar] [CrossRef]
- Indira, M.; Venkateswarulu, T.C.; Vidya Prabhakar, K.; Abraham Peele, K.; Krupanidhi, S. Isolation and Characterization of Bacteriocin Producing Enterococcus Casseliflavus and Its Antagonistic Effect on Pseudomonas Aeruginosa. Karbala Int. J. Mod. Sci. 2018, 4, 361–368. [Google Scholar] [CrossRef]
- Kumariya, R.; Garsa, A.K.; Rajput, Y.S.; Sood, S.K.; Akhtar, N.; Patel, S. Bacteriocins: Classification, Synthesis, Mechanism of Action and Resistance Development in Food Spoilage Causing Bacteria. Microb. Pathog. 2019, 128, 171–177. [Google Scholar] [CrossRef]
- Calisto, N.; Navarro, L.; Orellana, P.; Wiese, G.; Gómez, C.; Cortés-Cortés, P.; Gutiérrez-Moraga, A.; Gidekel, M.; Corsini, G. Resistencia a antibióticos y actividad antimicrobiana de aislados bacterianos de suelo antártico. An. Inst. Patagon. 2021, 49, 1–15. [Google Scholar] [CrossRef]
- Holt, J.G.; Krieg, N.R.; Sneath, P.H.A.; Staley, J.T.; Williams, S.T. Bergey’s Manual of Determinative Bacteriology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1994; ISBN 978-0-683-00603-2. [Google Scholar]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent Updates and New Developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [PubMed]
- Koeuth, T.; Versalovic, J.; Lupski, J.R. Differential Subsequence Conservation of Interspersed Repetitive Streptococcus Pneumoniae BOX Elements in Diverse Bacteria. Genome Res. 1995, 5, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Corsini, G.; Karahanian, E.; Tello, M.; Fernandez, K.; Rivero, D.; Saavedra, J.M.; Ferrer, A. Purification and Characterization of the Antimicrobial Peptide Microcin N: Properties of the Antimicrobial Peptide Microcin N. FEMS Microbiol. Lett. 2010, 312, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Daba, G.M.; Mostafa, F.A.; Saleh, S.A.A.; Elkhateeb, W.A.; Awad, G.; Nomiyama, T.; Zendo, T.; El-Dein, A.N. Purification, Amino Acid Sequence, and Characterization of Bacteriocin GA15, a Novel Class IIa Bacteriocin Secreted by Lactiplantibacillus Plantarum GCNRC_GA15. Int. J. Biol. Macromol. 2022, 213, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Montero, K.; Barrientos, L. Advances in Antarctic Research for Antimicrobial Discovery: A Comprehensive Narrative Review of Bacteria from Antarctic Environments as Potential Sources of Novel Antibiotic Compounds Against Human Pathogens and Microorganisms of Industrial Importance. Antibiotics 2018, 7, 90. [Google Scholar] [CrossRef]
- Silva, M.B.; Feitosa, A.O.; Lima, I.G.O.; Bispo, J.R.S.; Santos, A.C.M.; Moreira, M.S.A.; Câmara, P.E.A.S.; Rosa, L.H.; Oliveira, V.M.; Duarte, A.W.F.; et al. Antarctic Organisms as a Source of Antimicrobial Compounds: A Patent Review. Acad. Bras. Cienc. 2022, 94, e20210840. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-Growth-Promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Rodríguez-Rojas, F.; Tapia, P.; Castro-Nallar, E.; Undabarrena, A.; Muñoz-Díaz, P.; Arenas-Salinas, M.; Díaz-Vásquez, W.; Valdés, J.; Vásquez, C. Draft Genome Sequence of a Multi-Metal Resistant Bacterium Pseudomonas Putida ATH-43 Isolated from Greenwich Island, Antarctica. Front. Microbiol. 2016, 7, 1777. [Google Scholar] [CrossRef]
- See-Too, W.S.; Salazar, S.; Ee, R.; Convey, P.; Chan, K.-G.; Peix, Á. Pseudomonas Versuta Sp. Nov., Isolated from Antarctic Soil. Syst. Appl. Microbiol. 2017, 40, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Spiers, A.J.; Buckling, A.; Rainey, P.B. The Causes of Pseudomonas Diversity. Microbiology 2000, 146, 2345–2350. [Google Scholar] [CrossRef] [PubMed]
- Ghequire, M.G.K.; De Mot, R. Ribosomally Encoded Antibacterial Proteins and Peptides from Pseudomonas. FEMS Microbiol. Rev. 2014, 38, 523–568. [Google Scholar] [CrossRef]
- Smith, J.J.; Tow, L.A.; Stafford, W.; Cary, C.; Cowan, D.A. Bacterial Diversity in Three Different Antarctic Cold Desert Mineral Soils. Microb. Ecol. 2006, 51, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Tam, H.; Alias, S.; González, M.; González-Rocha, G.; Domínguez-Yévenes, M. Pseudomonas and Pedobacter Isolates from King George Island Inhibited the Growth of Foodborne Pathogens. Pol. Polar Res. 2011, 32, 3–14. [Google Scholar] [CrossRef]
- García-Echauri, S.A.; Gidekel, M.; Gutiérrez-Moraga, A.; Santos, L.; De León-Rodríguez, A. Isolation and Phylogenetic Classification of Culturable Psychrophilic Prokaryotes from the Collins Glacier in the Antarctica. Folia Microbiol. 2011, 56, 209–214. [Google Scholar] [CrossRef]
- De Souza, M.-J.; Nair, S.; Loka Bharathi, P.A.; Chandramohan, D. Metal and Antibiotic-Resistance in Psychrotrophic Bacteria from Antarctic Marine Waters. Ecotoxicology 2006, 15, 379–384. [Google Scholar] [CrossRef]
- Marcoleta, A.E.; Arros, P.; Varas, M.A.; Costa, J.; Rojas-Salgado, J.; Berríos-Pastén, C.; Tapia-Fuentes, S.; Silva, D.; Fierro, J.; Canales, N.; et al. The Highly Diverse Antarctic Peninsula Soil Microbiota as a Source of Novel Resistance Genes. Sci. Total Environ. 2022, 810, 152003. [Google Scholar] [CrossRef]
- Na, G.; Zhang, W.; Gao, H.; Wang, C.; Li, R.; Zhao, F.; Zhang, K.; Hou, C. Occurrence and Antibacterial Resistance of Culturable Antibiotic-Resistant Bacteria in the Fildes Peninsula, Antarctica. Mar. Pollut. Bull. 2021, 162, 111829. [Google Scholar] [CrossRef]
- Lo Giudice, A.; Brilli, M.; Bruni, V.; De Domenico, M.; Fani, R.; Michaud, L. Bacterium-Bacterium Inhibitory Interactions among Psychrotrophic Bacteria Isolated from Antarctic Seawater (Terra Nova Bay, Ross Sea): Antagonism among Psychrotrophic Antarctic Marine Bacteria. FEMS Microbiol. Ecol. 2007, 60, 383–396. [Google Scholar] [CrossRef]
- Bratchkova, A.; Ivanova, V. Bioactive Metabolites Produced by Microorganisms Collected in Antarctica and the Arctic. Biotechnol. Biotechnol. Equip. 2011, 25, 1–7. [Google Scholar] [CrossRef]
- Orellana, P.; Pavón, A.; Calisto, N.; Wiese, G.; Navarro, L.; Cortés-Cortés, P.; Gidekel, M.; Gutiérrez-Moraga, A.; Corsini, G. Identificación de bacterias antárticas con actividad antimicrobiana aisladas de la rizósfera de Deschampsia antarctica Desv. An. Inst. Patagon. 2022, 50, 11. [Google Scholar] [CrossRef]
- O’Brien, A.; Sharp, R.; Russell, N.J.; Roller, S. Antarctic Bacteria Inhibit Growth of Food-Borne Microorganisms at Low Temperatures. FEMS Microbiol. Ecol. 2004, 48, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Gradisteanu Pircalabioru, G.; Popa, L.I.; Marutescu, L.; Gheorghe, I.; Popa, M.; Czobor Barbu, I.; Cristescu, R.; Chifiriuc, M.-C. Bacteriocins in the Era of Antibiotic Resistance: Rising to the Challenge. Pharmaceutics 2021, 13, 196. [Google Scholar] [CrossRef]
- Nishie, M.; Nagao, J.-I.; Sonomoto, K. Antibacterial Peptides “Bacteriocins”: An Overview of Their Diverse Characteristics and Applications. Biocontrol. Sci. 2012, 17, 1–16. [Google Scholar] [CrossRef]
- Walsh, C.J.; Guinane, C.M.; Hill, C.; Ross, R.P.; O’Toole, P.W.; Cotter, P.D. In Silico Identification of Bacteriocin Gene Clusters in the Gastrointestinal Tract, Based on the Human Microbiome Project’s Reference Genome Database. BMC Microbiol. 2015, 15, 183. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).