Metagenomic Analysis Reveals the Effects of Different Land Use Types on Functional Soil Phosphorus Cycling: A Case Study of the Yellow River Alluvial Plain
Abstract
1. Introduction
2. Materials and Methods
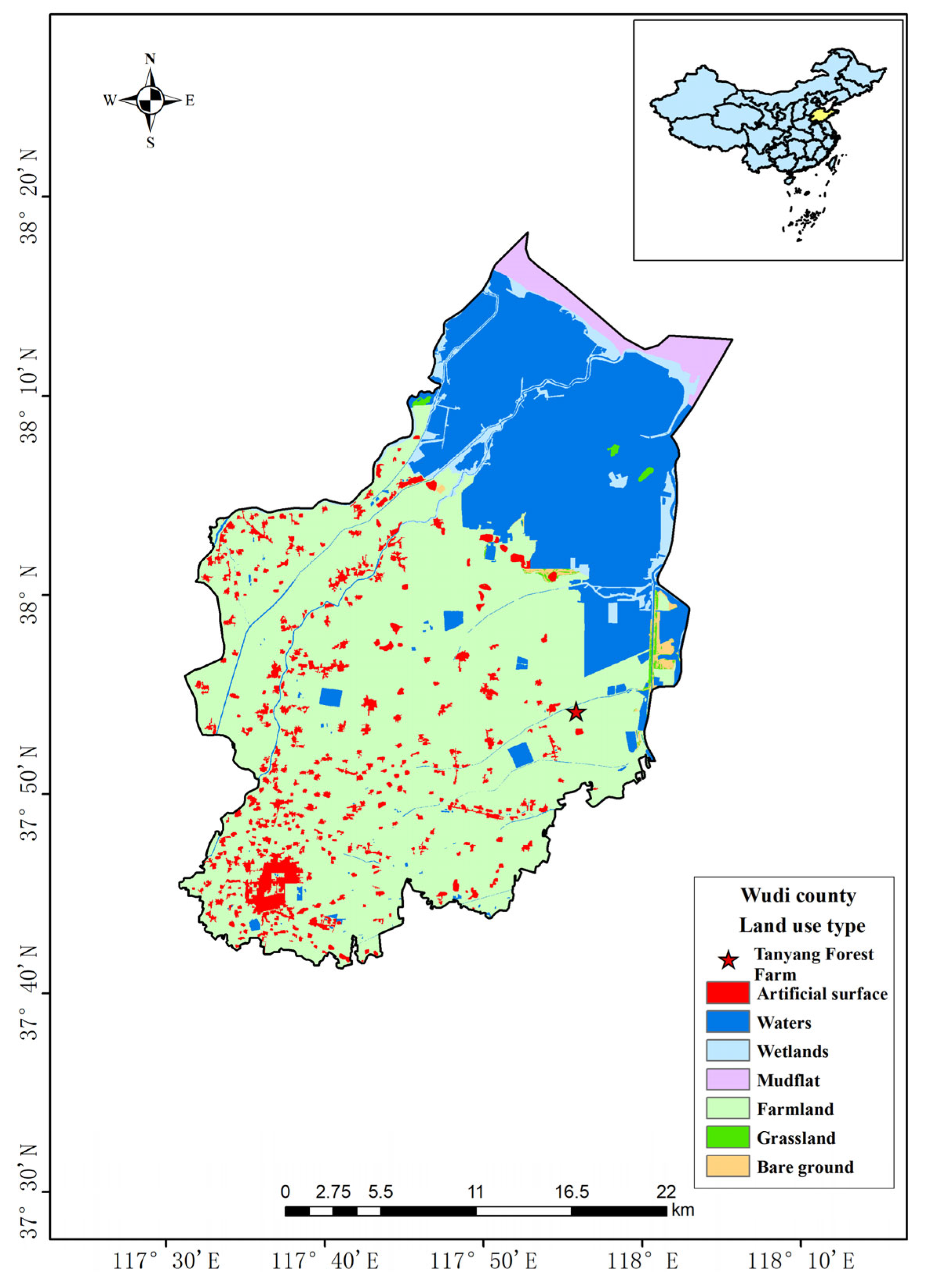
2.1. Study Area
2.2. Soil Sample Collections
2.3. Determination of Physical and Chemical Properties
2.4. Determination of Functional Genes Involved in Soil Phosphorus Cycling
2.5. Statistical Analysis
3. Results
3.1. Differences in Soil Physicochemical Properties Across Land Use Types
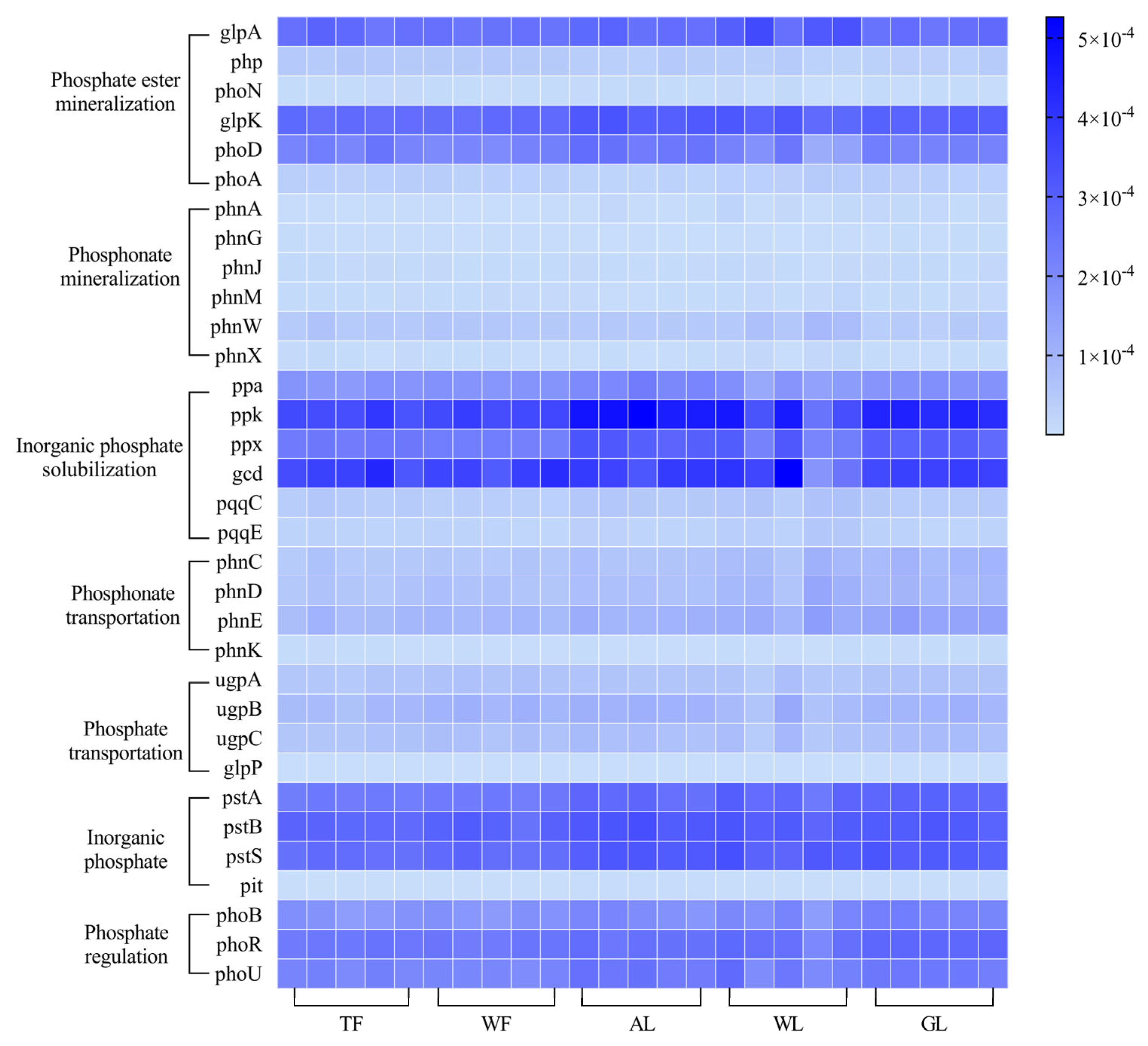
3.2. Functional Gene Composition of Soil Phosphorus Cycling in Different Land Use Types
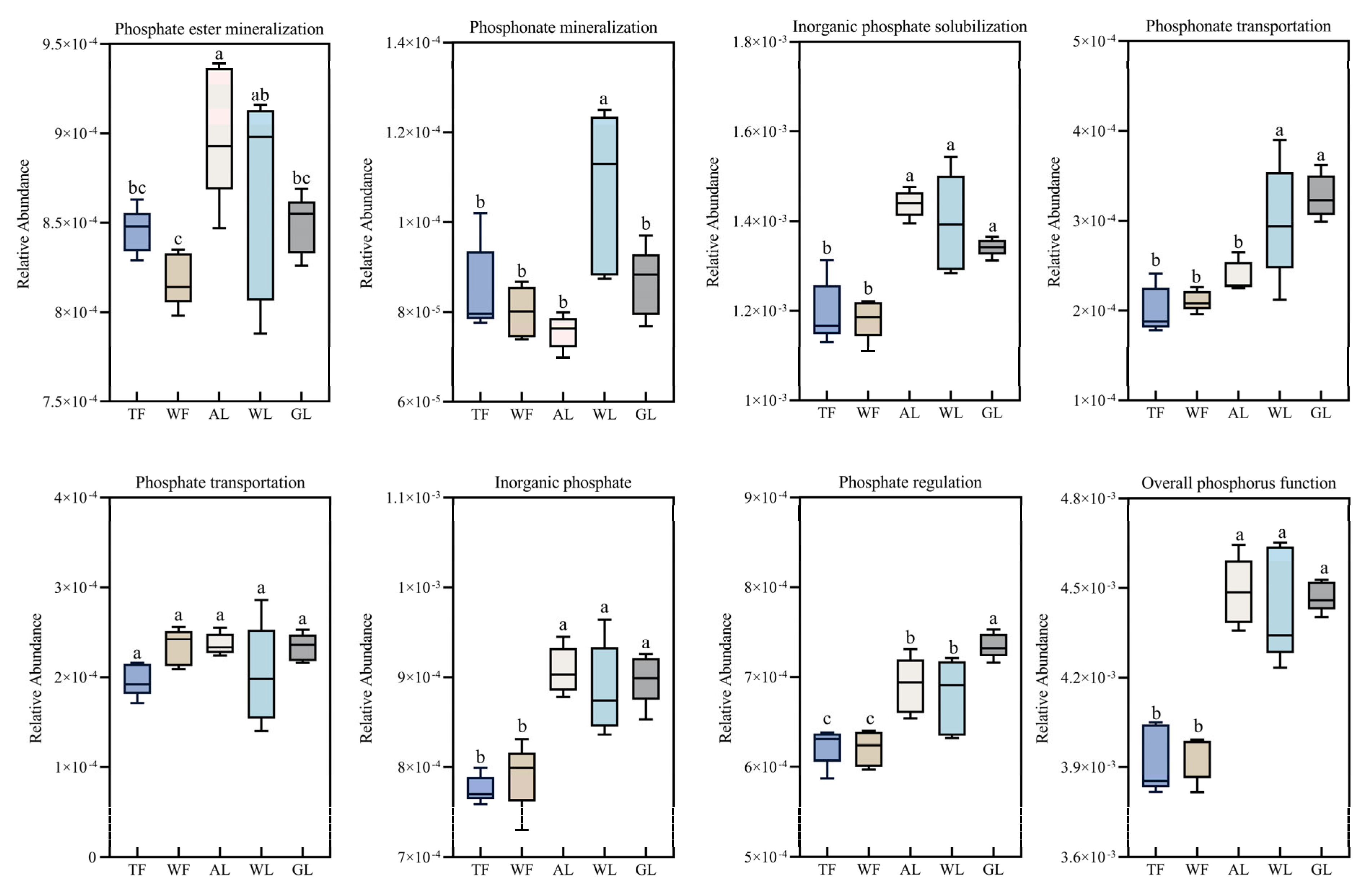
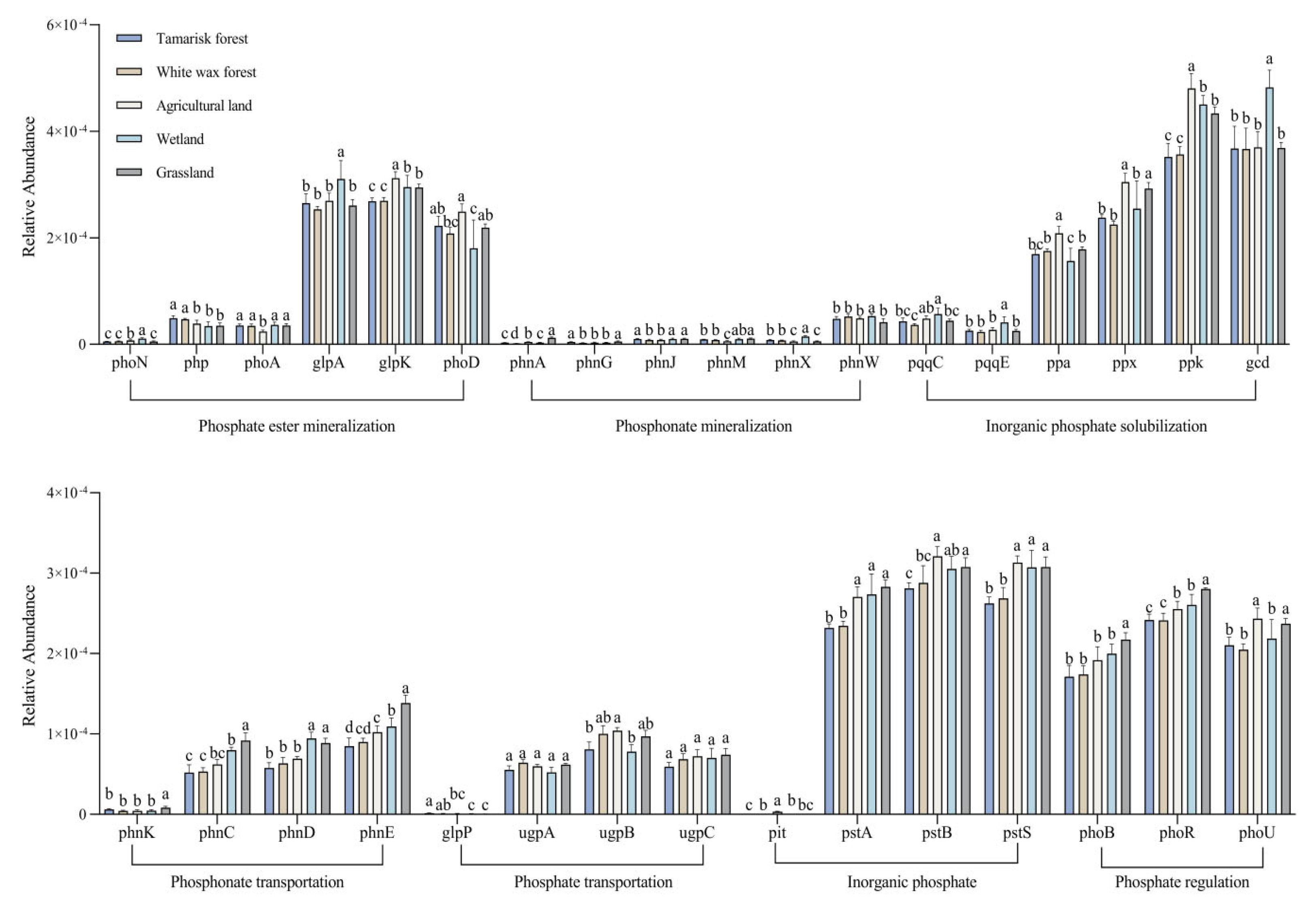
3.3. Differences in the Relative Abundance of Functional Soil Phosphorus-Cycling Genes Across Land Use Types
3.4. Relative Abundance of Soil Phosphorus-Cycling Functional Genes in Relation to Soil Physicochemical Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liang, J.L.; Liu, J.; Jia, P.; Yang, T.T.; Zeng, Q.W.; Zhang, S.C.; Liao, B.; Shu, W.S.; Li, J.T. Novel phosphate-solubilizing bacteria enhance soil phosphorus cycling following ecological restoration of land degraded by mining. ISME J. 2020, 14, 1600–1613. [Google Scholar] [CrossRef] [PubMed]
- Filippelli, G.M. The global phosphorus cycle. Rev. Mineral. Geochem. 2002, 48, 391–425. [Google Scholar] [CrossRef]
- Hong, S.; Lou, Y.; Chen, X.; Huang, Q.; Yang, Q.; Zhang, X.; Li, H.; Huang, G. Identification and Analysis of Long-Term Land Use and Planting Structure Dynamics in the Lower Yellow River Basin. Remote Sens. 2024, 16, 2274. [Google Scholar] [CrossRef]
- Azene, B.; Qiu, P.; Zhu, R.; Pan, K.; Sun, X.; Nigussie, Y.; Yigez, B.; Gruba, P.; Wu, X.; Zhang, L. Response of soil phosphorus fractions to land use change in the subalpine ecosystems of Southeast margin of Qinghai-Tibet Plateau, Southwest China. Ecol. Indic. 2022, 144, 109432. [Google Scholar] [CrossRef]
- Fu, D.; Xu, Z.; Wu, X.; Zhao, L.; Zhu, A.; Duan, C.; Chadwick, D.R.; Jones, D.L. Land use effects on soil phosphorus behavior characteristics in the eutrophic aquatic-terrestrial ecotone of Dianchi Lake, China. Soil Tillage Res. 2021, 205, 104793. [Google Scholar] [CrossRef]
- Yan, X.; Wei, Z.; Hong, Q.; Lu, Z.; Wu, J. Phosphorus fractions and sorption characteristics in a subtropical paddy soil as influenced by fertilizer sources. Geoderma 2017, 295, 80–85. [Google Scholar] [CrossRef]
- Maranguit, D.; Guillaume, T.; Kuzyakov, Y. Land-use change affects phosphorus fractions in highly weathered tropical soils. Catena 2017, 149, 385–393. [Google Scholar] [CrossRef]
- Edwards, K.R.; Picek, T.; Čížková, H.; Máchalová Zemanová, K.; Stará, A. Nutrient addition effects on carbon fluxes in wet grasslands with either organic or mineral soil. Wetlands 2015, 35, 55–68. [Google Scholar] [CrossRef]
- Li, J.; Pu, L.; Zhu, M.; Zhang, J.; Li, P.; Dai, X.; Xu, Y.; Liu, L. Evolution of soil properties following reclamation in coastal areas: A review. Geoderma 2014, 226, 130–139. [Google Scholar] [CrossRef]
- Gao, Y.; Mao, L.; Miao, C.-Y.; Zhou, P.; Cao, J.-J.; Zhi, Y.-E.; Shi, W.-J. Spatial characteristics of soil enzyme activities and microbial community structure under different land uses in Chongming Island, China: Geostatistical modelling and PCR-RAPD method. Sci. Total Environ. 2010, 408, 3251–3260. [Google Scholar] [CrossRef]
- Tian, Q.; Taniguchi, T.; Shi, W.Y.; Li, G.; Yamanaka, N.; Du, S. Land-use types and soil chemical properties influence soil microbial communities in the semiarid Loess Plateau region in China. Sci. Rep. 2017, 7, 45289. [Google Scholar] [CrossRef] [PubMed]
- Santos-Beneit, F. The Pho regulon: A huge regulatory network in bacteria. Front. Microbiol. 2015, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Siles, J.A.; Starke, R.; Martinovic, T.; Fernandes, M.L.; Orgiazzi, A.; Bastida, F. Distribution of phosphorus cycling genes across land uses and microbial taxonomic groups based on metagenome and genome mining. Soil Biol. Biochem. 2022, 174, 108826. [Google Scholar] [CrossRef]
- Lu, J.L.; Jia, P.; Feng, S.W.; Wang, Y.T.; Zheng, J.; Ou, S.N.; Wu, Z.H.; Liao, B.; Shu, W.S.; Liang, J.L.; et al. Remarkable effects of microbial factors on soil phosphorus bioavailability: A country-scale study. Glob. Change Biol. 2022, 28, 4459–4471. [Google Scholar] [CrossRef]
- Neal, A.L.; Rossmann, M.; Brearley, C.; Akkari, E.; Guyomar, C.; Clark, I.M.; Allen, E.; Hirsch, P.R. Land-use influences phosphatase gene microdiversity in soils. Environ. Microbiol. 2017, 19, 2740–2753. [Google Scholar] [CrossRef]
- Lüneberg, K.; Schneider, D.; Siebe, C.; Daniel, R. Drylands soil bacterial community is affected by land use change and different irrigation practices in the Mezquital Valley, Mexico. Sci. Rep. 2018, 8, 1413. [Google Scholar]
- Wu, Y.; Xu, N.; Wang, H.; Li, J.; Zhong, H.; Dong, H.; Zeng, Z.; Zong, C. Variations in the diversity of the soil microbial community and structure under various categories of degraded wetland in Sanjiang Plain, northeastern China. Land Degrad. Dev. 2021, 32, 2143–2156. [Google Scholar] [CrossRef]
- Li, W.; Sun, H.; Cao, M.; Wang, L.; Fang, X.; Jiang, J. Diversity and Structure of Soil Microbial Communities in Chinese Fir Plantations and Cunninghamia lanceolata–Phoebe bournei Mixed Forests at Different Successional Stages. Forests 2023, 14, 1977. [Google Scholar] [CrossRef]
- Olatunji, O.A.; Pan, K.; Tariq, A.; Zhang, L.; Wu, X.; Sun, X.; Luo, H.; Song, D.; Li, N. The responses of soil microbial community and enzyme activities of Phoebe zhennan cultivated under different soil moisture conditions to phosphorus addition. Iforest-Biogeosci. For. 2018, 11, 751. [Google Scholar] [CrossRef]
- Mirzakhaninafchi, H.; Mani, I.; Hasan, M.; Nafchi, A.M.; Parray, R.A.; Kumar, D. Development of prediction models for soil nitrogen management based on electrical conductivity and moisture content. Sensors 2022, 22, 6728. [Google Scholar] [CrossRef]
- Page, A.L. Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy, Inc.: Madison, WI, USA, 1982. [Google Scholar]
- Bremner, J.M.; Shaw, K. Denitrification in soil. I. Methods of investigation. J. Agric. Sci. 1958, 51, 22–39. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, J.; Kong, Q. Potassium-solubilizing activity of bacillus aryabhattai SK1-7 and its growth-promoting effect on Populus alba L. Forests 2020, 11, 1348. [Google Scholar] [CrossRef]
- Wen, Y.; You, J.; Zhu, J.; Hu, H.; Gao, J.; Huang, J. Long-term green manure application improves soil K availability in red paddy soil of subtropical China. J. Soils Sediments 2021, 21, 63–72. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Ren, C.; Zhao, F.; Kang, D.; Yang, G.; Han, X.; Tong, X.; Feng, Y.; Ren, G. Linkages of C: N: P stoichiometry and bacterial community in soil following afforestation of former farmland. For. Ecol. Manag. 2016, 376, 59–66. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Orellana, L.H.; Johnston, E.R.; Hatt, J.K.; Löffler, F.E.; Ayala-del-Río, H.L.; González, G.; Konstantinidis, K.T. Metagenomic characterization of soil microbial communities in the Luquillo experimental forest (Puerto Rico) and implications for nitrogen cycling. Appl. Environ. Microbiol. 2021, 87, e00546-21. [Google Scholar] [CrossRef]
- Noguchi, H.; Park, J.; Takagi, T. MetaGene: Prokaryotic gene finding from environmental genome shotgun sequences. Nucleic Acids Res. 2006, 34, 5623–5630. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Bergkemper, F.; Schöler, A.; Engel, M.; Lang, F.; Krüger, J.; Schloter, M.; Schulz, S. Phosphorus depletion in forest soils shapes bacterial communities towards phosphorus recycling systems. Environ. Microbiol. 2016, 18, 1988–2000. [Google Scholar] [CrossRef]
- Vargha, A.; Delaney, H.D. The Kruskal-Wallis test and stochastic homogeneity. J. Educ. Behav. Stat. 1998, 23, 170–192. [Google Scholar] [CrossRef]
- Bewick, V.; Cheek, L.; Ball, J. Statistics review 9: One-way analysis of variance. Crit. Care 2004, 8, 1–7. [Google Scholar]
- Sedgwick, P. Spearman’s rank correlation coefficient. bmj 2014, 349, g7327. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bai, J.; Zhao, Q.; Lu, Q.; Xia, Z. Five-year changes in soil organic carbon and total nitrogen in coastal wetlands affected by flow-sediment regulation in a Chinese delta. Sci. Rep. 2016, 6, 21137. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Dong, Y.; Peng, Q.; Xiao, S.; He, Y.; Liu, X.; Sun, L.; Jia, J.; Yang, Z. Effects of a conversion from grassland to cropland on the different soil organic carbon fractions in Inner Mongolia, China. J. Geogr. Sci. 2012, 22, 315–328. [Google Scholar] [CrossRef]
- Gogoi, N.; Choudhury, M.; Ul Hasan, M.S.; Changmai, B.; Baruah, D.; Samanta, P. Soil organic carbon pool in diverse land utilization patterns in North-East India: An implication for carbon sequestration. Environ. Dev. Sustain. 2024, 1–27. [Google Scholar] [CrossRef]
- Babauta, J.T.; Nguyen, H.D.; Harrington, T.D.; Renslow, R.; Beyenal, H. pH, redox potential and local biofilm potential microenvironments within Geobacter sulfurreducens biofilms and their roles in electron transfer. Biotechnol. Bioeng. 2012, 109, 2651–2662. [Google Scholar] [CrossRef]
- Ramesh, T.; Bolan, N.S.; Kirkham, M.B.; Wijesekara, H.; Kanchikerimath, M.; Rao, C.S.; Sandeep, S.; Rinklebe, J.; Ok, Y.S.; Choudhury, B.U.; et al. Soil organic carbon dynamics: Impact of land use changes and management practices: A review. Adv. Agron. 2019, 156, 1–107. [Google Scholar]
- Liu, Y.; Li, S.; Sun, X.; Yu, X. Variations of forest soil organic carbon and its influencing factors in east China. Ann. For. Sci. 2016, 73, 501–511. [Google Scholar] [CrossRef]
- Gelaw, A.M.; Singh, B.R.; Lal, R. Soil organic carbon and total nitrogen stocks under different land uses in a semi-arid watershed in Tigray, Northern Ethiopia. Agric. Ecosyst. Environ. 2014, 188, 256–263. [Google Scholar] [CrossRef]
- Zhong, L.; Qiguo, Z. Organic carbon content and distribution in soils under different land uses in tropical and subtropical China. Plant Soil 2001, 231, 175–185. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Huang, C. Spatial variability of soil total nitrogen and soil total phosphorus under different land uses in a small watershed on the Loess Plateau, China. Geoderma 2009, 150, 141–149. [Google Scholar] [CrossRef]
- Liu, J.; Cade-Menun, B.J.; Yang, J.; Hu, Y.; Liu, C.W.; Tremblay, J.; LaForge, K.; Schellenberg, M.; Hamel, C.; Bainard, L.D. Long-term land use affects phosphorus speciation and the composition of phosphorus cycling genes in agricultural soils. Front. Microbiol. 2018, 9, 1643. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Jiang, M.T.; Li, S.; Ni, H.W.; Sun, B.; Liang, Y.T. Functional Genomics Analysis of Nitrogen and Phosphorus Transformation in Maize Rhizosphere Microorganisms. Environ. Sci. 2023, 44, 7014–7023. [Google Scholar]
- Wang, H.; Chen, J.; Ruan, Y.; Sun, W.; Wang, S.; Wang, H.; Zhang, Y.; Guo, J.; Wang, Y.; Guo, H.; et al. Metagenomes reveal the effect of crop rotation systems on phosphorus cycling functional genes and soil phosphorus avail–ability. Agric. Ecosyst. Environ. 2024, 364, 108886. [Google Scholar] [CrossRef]
- Wu, X.H.; Wang, R.; Gao, C.Q.; Gao, S.; Du, L.L.; Asif, K.; Million, B.; Shengli, G. Variations of soil properties effect on microbial community structure and functional structure under land uses. Acta Ecol. Sin. 2021, 41, 7989–8002. [Google Scholar]
- Guan, X.; Wang, C.; Li, C.; Li, J.; Xu, L.; Zhang, J.; Zhang, J.; He, N. Soil Phosphorus Distribution and Its Influencing Factors in Different Grassland Types on the Qinghai-Tibet Plateau. J. Soil Water Conserv. 2022, 36, 351–359. [Google Scholar]
- Zhi, R.; Deng, J.; Xu, Y.; Xu, M.; Zhang, S.; Han, X.; Yang, G.; Ren, C. Altered microbial P cycling genes drive P availability in soil after afforestation. J. Environ. Manag. 2023, 328, 116998. [Google Scholar] [CrossRef]
- Li, J.; Wu, B.; Zhang, D.; Cheng, X. Elevational variation in soil phosphorus pools and controlling factors in alpine areas of Southwest China. Geoderma 2023, 431, 116361. [Google Scholar] [CrossRef]
- Li, W.; Shao, X.; Wu, M. Soil alkaline phosphatase activity and its relationship with phosphorus forms of Hangzhou Bay Intertidal Wetland. Acta Entiae Circumstantiae 2013, 33, 3341–3349. [Google Scholar]
- Li, C.; Ma, X.; Wang, Y.; Sun, Q.; Chen, M.; Zhang, C.; Ding, S.; Dai, Z. Root-mediated acidification, phosphatase activity and the phosphorus-cycling microbial community enhance phosphorus mobilization in the rhizosphere of wetland plants. Water Res. 2024, 255, 121548. [Google Scholar] [CrossRef]
- Hu, X.; Gu, H.; Liu, J.; Wei, D.; Zhu, P.; Cui, X.A.; Zhou, B.; Chen, X.; Jin, J.; Liu, X.; et al. Metagenomic strategies uncover the soil bioavailable phosphorus improved by organic fertilization in Mollisols. Agric. Ecosyst. Environ. 2023, 349, 108462. [Google Scholar] [CrossRef]
- Liang, J.L.; Feng, S.W.; Lu, J.L.; Wang, X.N.; Li, F.L.; Guo, Y.Q.; Liu, S.Y.; Zhuang, Y.Y.; Zhong, S.J.; Zheng, J.; et al. Hidden diversity and potential ecological function of phosphorus acquisition genes in widespread terrestrial bacteriophages. Nat. Commun. 2024, 15, 2827. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhao, Z.; Lv, F.; Wang, R.; Gong, Q.; Zhai, B.; Wang, Z.; Zhao, Z.; Li, Z. Metagenomic exploration of the interactions between N and P cycling and SOM turnover in an apple orchard with a cover crop fertilized for 9 years. Biol. Fertil. Soils 2019, 55, 365–381. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, G.; Chen, H.; Chen, C.; Wang, J.; Ai, S.; Wei, D.; Li, D.; Ma, B.; Tang, C.; et al. Long-term nutrient inputs shift soil microbial functional profiles of phosphorus cycling in diverse agroecosystems. ISME J. 2020, 14, 757–770. [Google Scholar] [CrossRef] [PubMed]
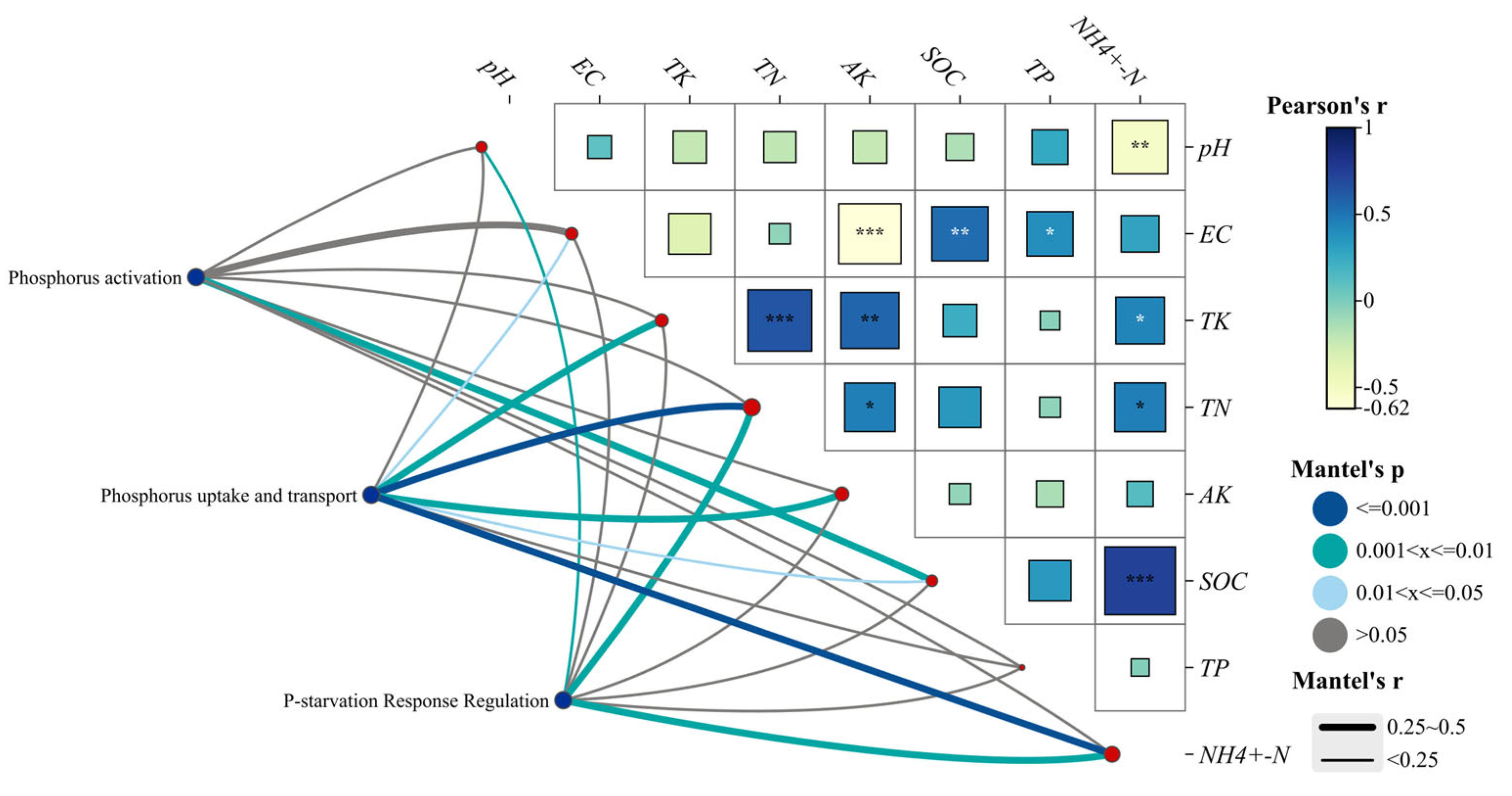
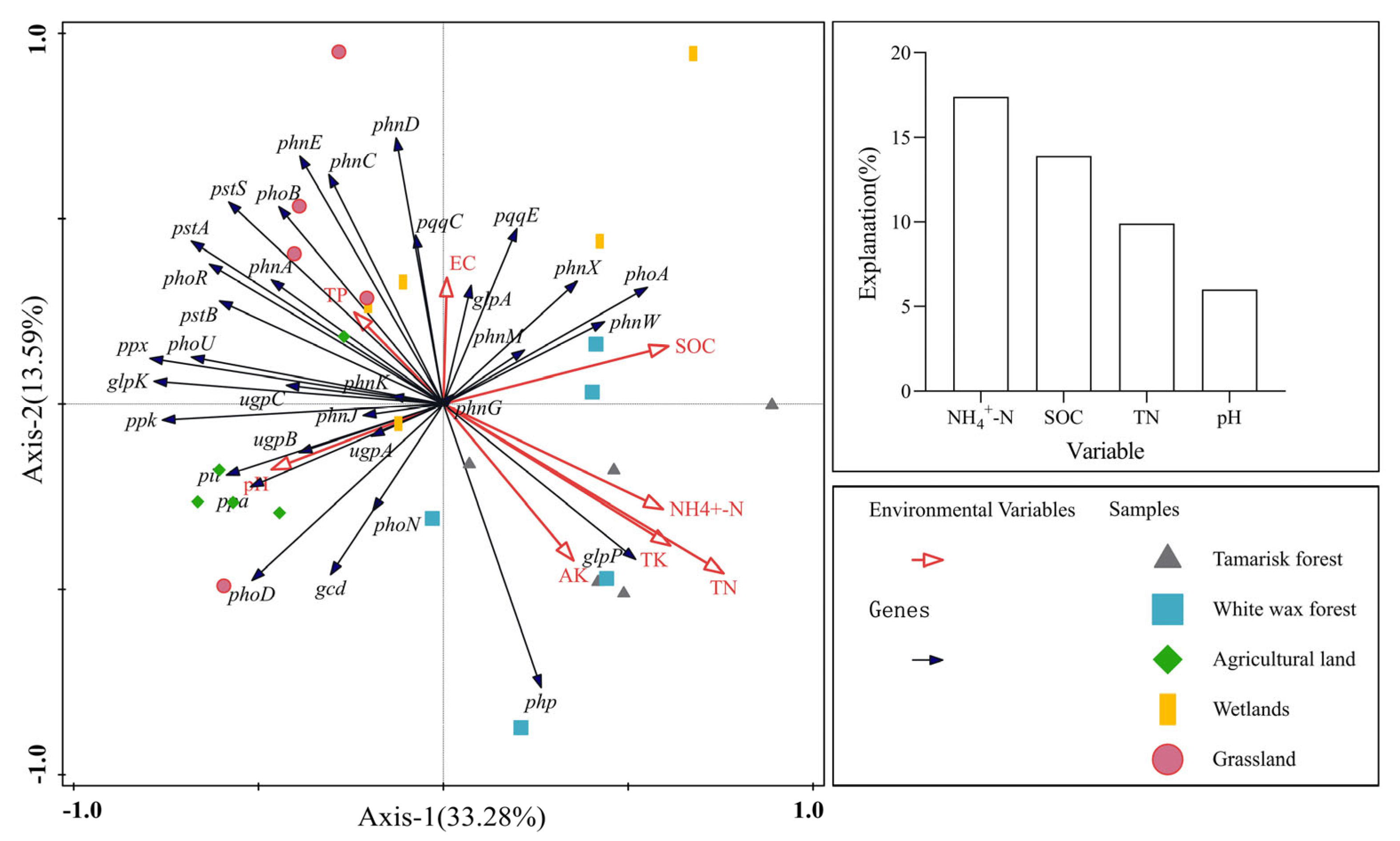
| KO Number | Gene | Product Name | Functional Gene Grouping | Function in the Nitrogen Cycle |
|---|---|---|---|---|
| K00111 | glpA | Glycerol-3-phosphate dehydrogenase | Phosphate ester mineralization | Phosphorus activation |
| K07048 | php | Phosphotriesterase-related protein | Phosphate ester mineralization | Phosphorus activation |
| K09474 | phoN | Acid phosphatase (class A) | Phosphate ester mineralization | Phosphorus activation |
| K01077 | phoA | Alkaline phosphatase | Phosphate ester mineralization | Phosphorus activation |
| K00864 | glpK | JNMGlycerol kinase | Phosphate ester mineralization | Phosphorus activation |
| K01113 | phoD | Alkaline phosphatase D | Phosphate ester mineralization | Phosphorus activation |
| K06193 | phnA | Phosphonoacetate hydrolase | Phosphonate mineralization | Phosphorus activation |
| K06166 | phnG | Alpha-D-ribose 1-methylphosphonate 5-triphosphate synthase subunit | Phosphonate mineralization | Phosphorus activation |
| K06163 | phnJ | α-D-ribose 1-methylphosphonate 5-phosphate C-P-lyase | Phosphonate mineralization | Phosphorus activation |
| K06162 | phnM | Alpha-D-ribose 1-methylphosphonate 5-triphosphate diphosphatase | Phosphonate mineralization | Phosphorus activation |
| K03430 | phnW | 2-aminoethylphosphonate-pyruvate transaminase | Phosphonate mineralization | Phosphorus activation |
| K05306 | phnX’ | Phosphonoacetaldehyde hydrolase | Phosphonate mineralization | Phosphorus activation |
| K01507 | ppa | Inorganic pyrophosphatase | Inorganic phosphate solubilization | Phosphorus activation |
| K00937 | ppk | Polyphosphate kinase | Inorganic phosphate solubilization | Phosphorus activation |
| K01524 | ppx | Exopolyphosphatase | Inorganic phosphate solubilization | Phosphorus activation |
| K00117 | gcd | Quinoprotein glucose dehydrogenase | Inorganic phosphate solubilization | Phosphorus activation |
| K06137 | pqqC | Pyrroloquinoline-quinone synthase | Inorganic phosphate solubilization | Phosphorus activation |
| K06139 | pqqE | PqqA peptide cyclase | Inorganic phosphate solubilization | Phosphorus activation |
| K02041 | phnC | Phosphonate transport system ATP-binding protein | Phosphonate transportation | Phosphorus uptake and transport and transport |
| K02044 | phnD | Phosphonate transport system substrate-binding protein | Phosphonate transportation | Phosphorus uptake and transport and transport |
| K02042 | phnE | Phosphonate transport system permease protein | Phosphonate transportation | Phosphorus uptake and transport and transport |
| K05781 | phnK | Phosphonate transport system ATP-binding protein | Phosphonate transportation | Phosphorus uptake and transport and transport |
| K05814 | ugpA | Sn-glycerol 3-phosphate transport system permease protein | Phosphate transportation | Phosphorus uptake and transport and transport |
| K05813 | ugpB | Sn-glycerol 3-phosphate transport system substrate-binding protein | Phosphate transportation | Phosphorus uptake and transport and transport |
| K05816 | ugpC | Sn-glycerol 3-phosphate transport system ATP-binding protein | Phosphate transportation | Phosphorus uptake and transport and transport |
| K02443 | glpP | Glycerol uptake operon anti-terminator | Phosphate transportation | Phosphorus uptake and transport and transport |
| K02038 | pstA | Phosphate transport system permease protein | Inorganic phosphate | Phosphorus uptake and transport and transport |
| K02036 | pstB | Phosphate transport system ATP-binding protein | Inorganic phosphate | Phosphorus uptake and transport and transport |
| K02040 | pstS | Phosphate transport system substrate-binding protein | Inorganic phosphate | Phosphorus uptake and transport and transport |
| K16322 | pit | Low-affinity inorganic phosphate transporter | Inorganic phosphate | Phosphorus uptake and transport and transport |
| K07657 | phoB | Phosphate regulon response regulator | Phosphate regulation | P-starvation response regulation |
| K07636 | phoR | Phosphate regulon sensor histidine kinase | Phosphate regulation | P-starvation response regulation |
| K02039 | phoU | Negative regulator of PhoR/PhoB two-component regulator | Phosphate regulation | P-starvation response regulation |
| Soil Type | pH | EC (ms/cm) | TK (g/kg) | TN (g/kg) | AK (mg/kg) | TP (g/kg) | SOC (g/kg) | NH4+-N (mg/kg) |
|---|---|---|---|---|---|---|---|---|
| Tamarisk forest | 7.6 ± 0.13 c | 1.46 ± 0.35 b | 8.64 ± 3.13 a | 1.34 ± 0.31 a | 353.56 ± 48.22 a | 1.54 ± 0.23 a | 2.6 ± 0.13 c | 16.73 ± 0.29 a |
| White wax forest | 8.3 ± 0.13 b | 1.22 ± 0.85 b | 6.89 ± 1.45 a | 1.35 ± 0.18 a | 311.30 ± 36.04 ab | 1.25 ± 0.19 b | 8.56 ± 0.31 b | 12.06 ± 0.44 c |
| Farmland | 7.59 ± 0.3 c | 1.28 ± 0.53 b | 3.83 ± 1.79 b | 0.61 ± 0.14 b | 265.72 ± 37.43 b | 1.36 ± 0.05 ab | 1.0 ± 0.18 d | 11.90 ± 0.25 c |
| Wetlands | 9.22 ± 0.4 a | 3.35 ± 1.6 a | 3.54 ± 2.32 b | 0.87 ± 0.16 b | 205.00 ± 51.33 c | 1.58 ± 0.26 a | 9.42 ± 0.24 a | 15.09 ± 0.25 b |
| Grassland | 9.13 ± 0.32 a | 1.41 ± 0.22 b | 3.41 ± 0.21 b | 0.68 ± 0.29 b | 276.34 ± 21.27 b | 1.60 ± 0.15 a | 2.86 ± 0.17 c | 6.30 ± 0.23 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, M.; Liu, Y.; Feng, C.; Li, Z. Metagenomic Analysis Reveals the Effects of Different Land Use Types on Functional Soil Phosphorus Cycling: A Case Study of the Yellow River Alluvial Plain. Microorganisms 2024, 12, 2194. https://doi.org/10.3390/microorganisms12112194
Wen M, Liu Y, Feng C, Li Z. Metagenomic Analysis Reveals the Effects of Different Land Use Types on Functional Soil Phosphorus Cycling: A Case Study of the Yellow River Alluvial Plain. Microorganisms. 2024; 12(11):2194. https://doi.org/10.3390/microorganisms12112194
Chicago/Turabian StyleWen, Ming, Yu Liu, Chaoyang Feng, and Zhuoqing Li. 2024. "Metagenomic Analysis Reveals the Effects of Different Land Use Types on Functional Soil Phosphorus Cycling: A Case Study of the Yellow River Alluvial Plain" Microorganisms 12, no. 11: 2194. https://doi.org/10.3390/microorganisms12112194
APA StyleWen, M., Liu, Y., Feng, C., & Li, Z. (2024). Metagenomic Analysis Reveals the Effects of Different Land Use Types on Functional Soil Phosphorus Cycling: A Case Study of the Yellow River Alluvial Plain. Microorganisms, 12(11), 2194. https://doi.org/10.3390/microorganisms12112194






