Effects of Increasing the Nitrogen–Phosphorus Ratio on the Structure and Function of the Soil Microbial Community in the Yellow River Delta
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of Study Area
2.2. Experiment Design and Soil Sample
2.3. Soil Environmental Factor Determination and Sequencing Analysis
2.4. Statistical Analysis
3. Results
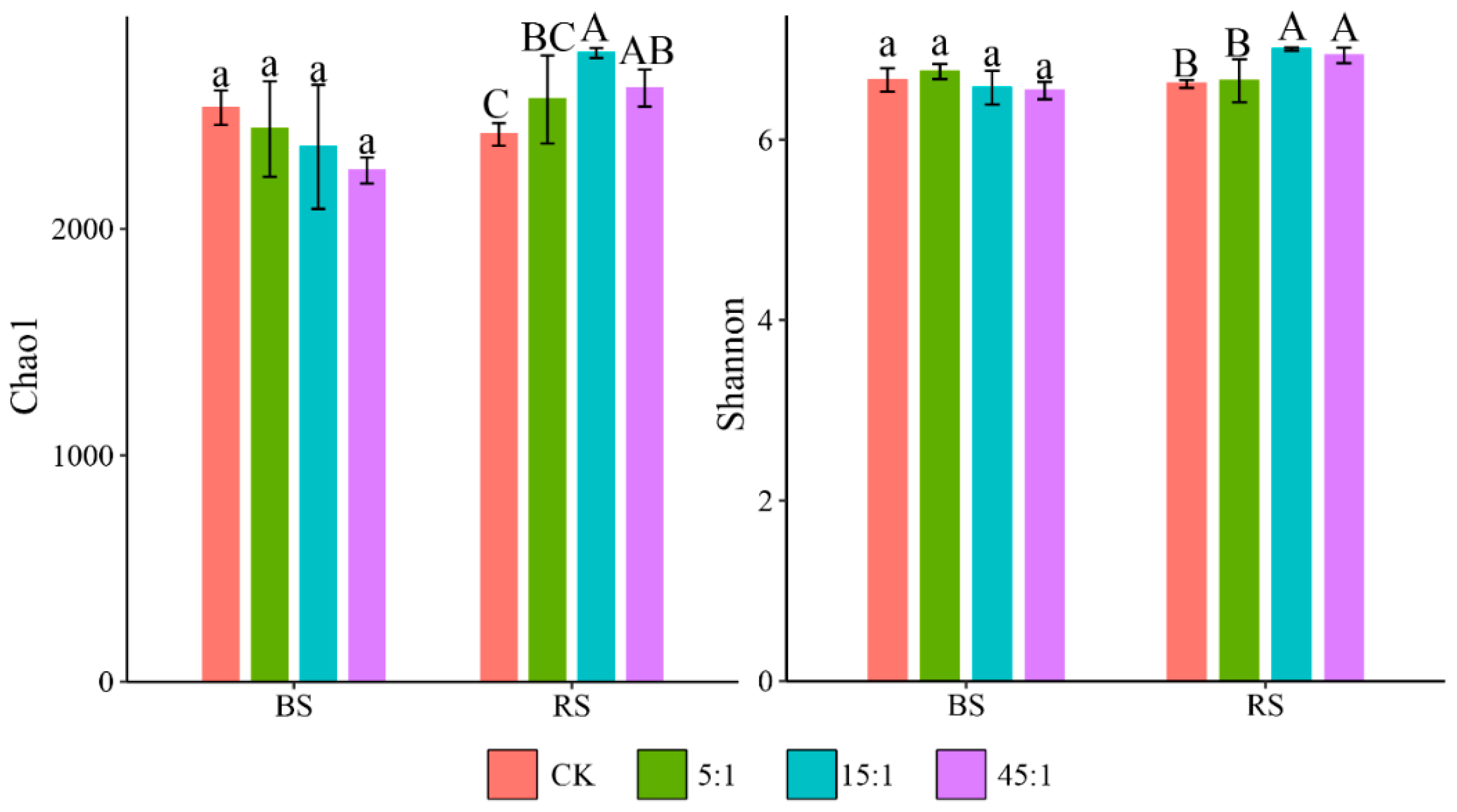
3.1. α-Diversity Analysis
3.2. β-Diversity Analysis
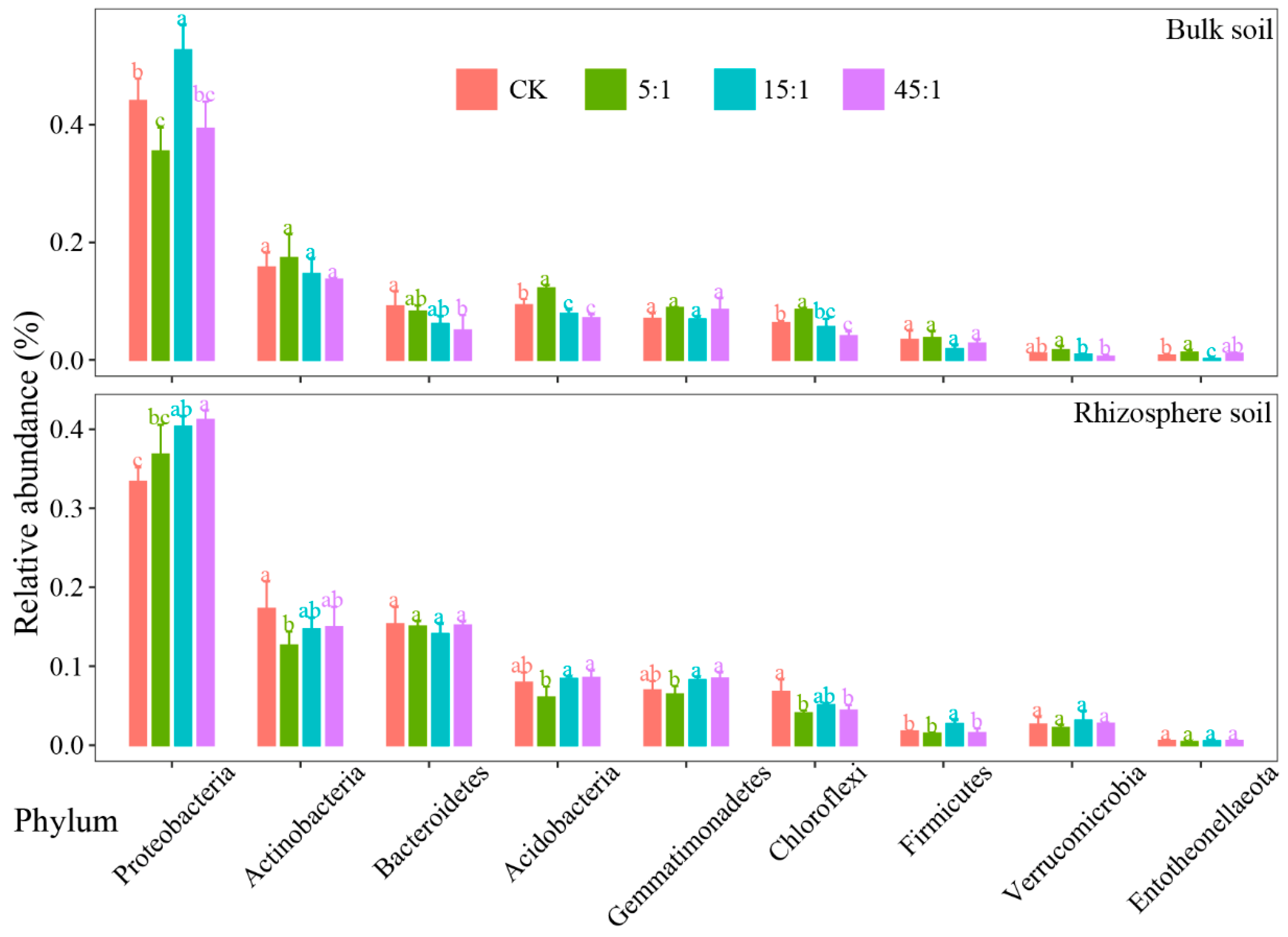
3.3. The Dominant Phylum of the Bacterial Community
3.4. The Genus of the Bacterial Community
3.5. Bacterial Co-Occurrence Network
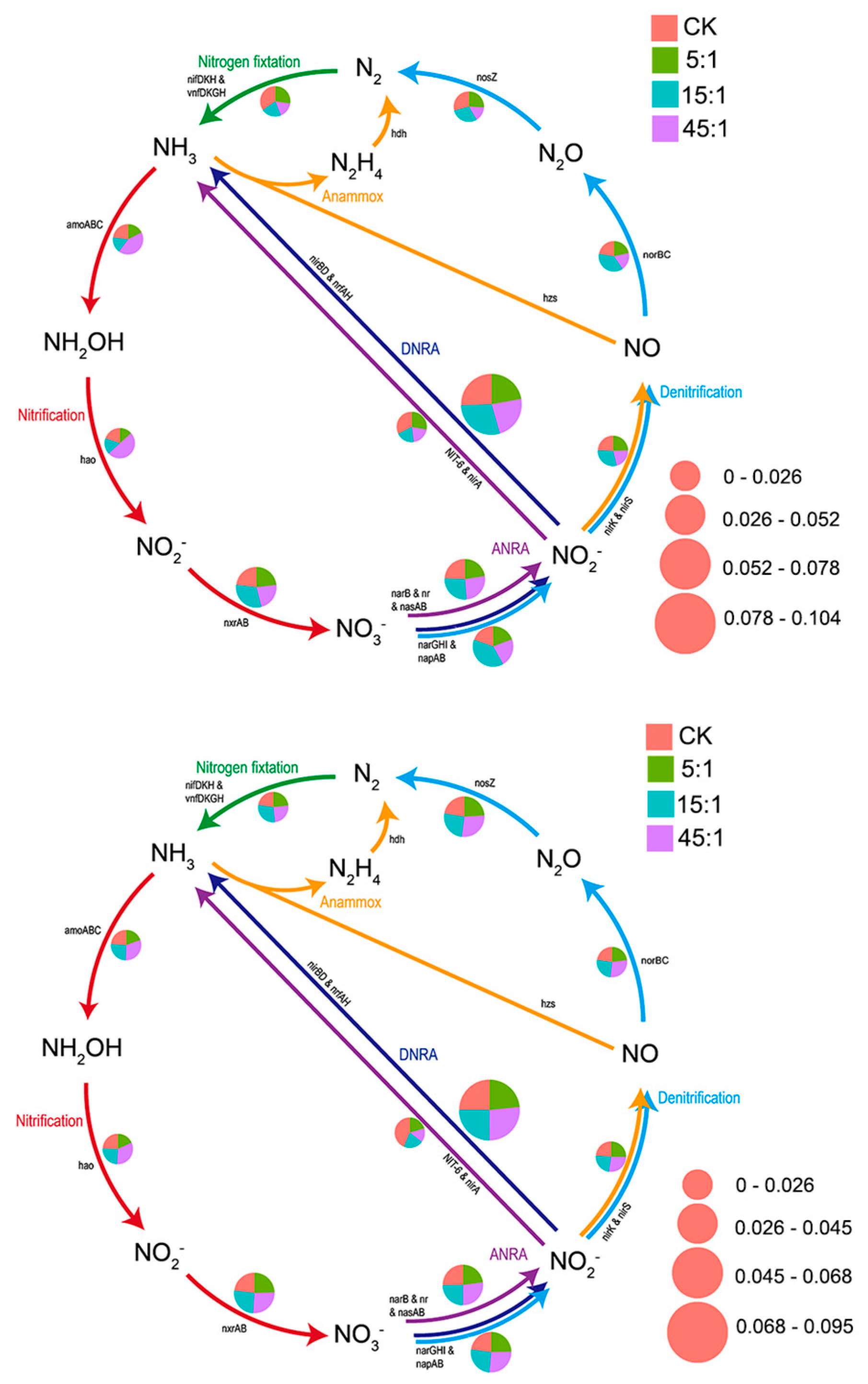
3.6. Function Analysis
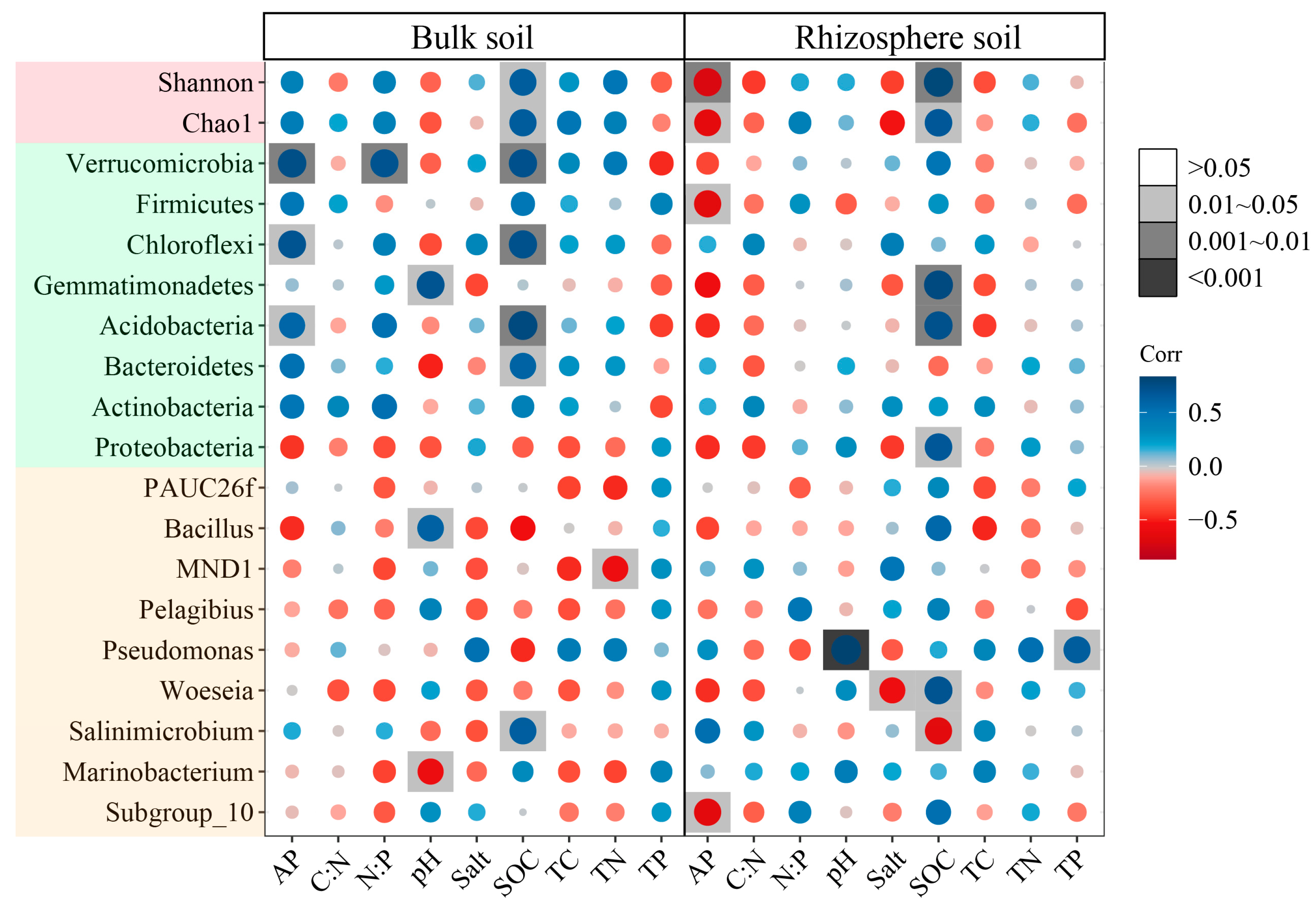
3.7. The Effect of Environmental Factors on Bacterial Community
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zong, N.; Shi, P.L. Differential responses of community structure and production. and the sensitivities of different alpine grasslands tonitrogen addition. Acta Ecol. Sin. 2020, 40, 4000–4010. [Google Scholar] [CrossRef]
- Hao, J.H.; Han, H.K.; Liu, Y.; Li, J.H.; Yang, J.Y.; Ren, B.H.; Bai, L. Phosphorus addition alleviates the inhibition of nitrogen deposition on photosynthesis of Potentilla tanacetifolia. Front. Environ. Sci. 2023, 11, 3389. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Zhu, B.; Homyak, P.M.; Chen, G.; Yao, X.; Wu, D.; Yang, Z.; Lyu, M.; Yang, Y. Long-term nitrogen deposition inhibits soil priming effects by enhancing phosphorus limitation in a subtropical forest. Glob. Chang. Biol. 2023, 29, 4081–4093. [Google Scholar] [CrossRef] [PubMed]
- Penuelas, J.; Sardans, J. The global nitrogen-phosphorus imbalance. Science 2022, 375, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Jia, Y.; He, N.; Zhu, J.; Chen, Z.; Wang, Q.; Piao, S.; Liu, X.; He, H.; Guo, X.; et al. Stabilization of atmospheric nitrogen deposition in China over the past decade. Nat. Geosci. 2019, 12, 424–429. [Google Scholar] [CrossRef]
- Dong, C.; Wang, W.; Liu, H.; Xu, X.; Zeng, H. Temperate grassland shifted from nitrogen to phosphorus limitation induced by degradation and nitrogen deposition: Evidence from soil extracellular enzyme stoichiometry. Ecol. Indic. 2019, 101, 453–464. [Google Scholar] [CrossRef]
- Penuelas, J.; Poulter, B.; Sardans, J.; Ciais, P.; van der Velde, M.; Bopp, L.; Boucher, O.; Godderis, Y.; Hinsinger, P.; Llusia, J.; et al. Human-induced nitrogen-phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 2013, 4, 2934. [Google Scholar] [CrossRef]
- Liu, X.; Wang, G.; Ran, Y.; Qi, D.; Han, G.; Guan, B.; Hao, C. Overall supply level, not the relative supply of nitrogen and phosphorus, affects the plant community composition of a supratidal wetland in the Yellow River Delta. Sci. Total Environ. 2019, 695, 133866. [Google Scholar] [CrossRef]
- Yang, W.; Cai, X.W.; Wang, Y.Q.; Diao, L.F.; Xia, L.; An, S.Q.; Luo, Y.Q.; Cheng, X.L. Increased Soil Bacterial Abundance but Decreased Bacterial Diversity and Shifted Bacterial Community Composition Following Secondary Succession of Old-Field. Forests 2022, 13, 1628. [Google Scholar] [CrossRef]
- Liu, J.; Ma, Q.; Hui, X.; Ran, J.; Ma, Q.; Wang, X.; Wang, Z. Long-term high-P fertilizer input decreased the total bacterial diversity but not phoD-harboring bacteria in wheat rhizosphere soil with available-P deficiency. Soil Biol. Biochem. 2020, 149, 107918. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.; Ao, G.; Qin, W.; Han, M.; Shen, Y.; Liu, M.; Chen, Y.; Zhu, B. Globally nitrogen addition alters soil microbial community structure, but has minor effects on soil microbial diversity and richness. Soil Biol. Biochem. 2023, 179, 108982. [Google Scholar] [CrossRef]
- Hu, J.X.; Huang, C.D.; Zhou, S.X.; Kuzyakov, Y. Nitrogen addition to soil affects microbial carbon use efficiency: Meta-analysis of similarities and differences in C-13 and O-18 approaches. Glob. Chang. Biol. 2022, 28, 4977–4988. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.S.; Hu, B.; Qi, K.B.; Chen, W.J.; Pang, X.Y.; Bao, W.K.; Tian, G.L. Effects of phosphorus addition on soil microbial biomass and community composition in a subalpine spruce plantation. Eur. J. Soil Biol. 2016, 72, 35–41. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Zhang, X.; Mao, Q.; Li, X.; You, Y.; Wang, J.; Zheng, M.; Zhang, W.; Lu, X.; et al. Nitrogen addition reduces soil bacterial richness, while phosphorus addition alters community composition in an old-growth N-rich tropical forest in southern China. Soil Biol. Biochem. 2018, 127, 22–30. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Q.; He, N.; Smith, M.D.; Elser, J.J.; Du, J.; Yuan, G.; Yu, G.; Yu, Q. Imbalanced atmospheric nitrogen and phosphorus depositions in China: Implications for nutrient limitation. J. Geophys. Res. Biogeosci. 2016, 121, 1605–1616. [Google Scholar] [CrossRef]
- Luo, M.; Moorhead, D.L.; Ochoa-Hueso, R.; Mueller, C.W.; Ying, S.C.; Chen, J. Nitrogen loading enhances phosphorus limitation in terrestrial ecosystems with implications for soil carbon cycling. Funct. Ecol. 2022, 36, 2845–2858. [Google Scholar] [CrossRef]
- Peng, Y.; Peng, Z.; Zeng, X.; Houx, J.H. Effects of nitrogen-phosphorus imbalance on plant biomass production: A global perspective. Plant Soil 2019, 436, 245–252. [Google Scholar] [CrossRef]
- Peng, Y.F.; Guo, D.L.; Yang, Y.H. Global patterns of root dynamics under nitrogen enrichment. Glob. Ecol. Biogeogr. 2017, 26, 102–114. [Google Scholar] [CrossRef]
- Liu, W.; Liu, L.; Yang, X.; Deng, M.; Wang, Z.; Wang, P.; Yang, S.; Li, P.; Peng, Z.; Yang, L.; et al. Long-term nitrogen input alters plant and soil bacterial, but not fungal beta diversity in a semiarid grassland. Glob. Chang. Biol. 2021, 27, 3939–3950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, G.; Xue, B.; Zhang, M.; Tan, Z. Dynamic landscapes and the driving forces in the Yellow River Delta wetland region in the past four decades. Sci. Total Environ. 2021, 787, 147644. [Google Scholar] [CrossRef]
- Li, H.G.; Li, X.R.; Xu, Z.H.; Liang, S.K.; Ding, Y.; Song, D.H.; Guo, H. Nutrient budgets for the Bohai Sea: Implication for ratio imbalance of nitrogen to phosphorus input under intense human activities. Mar. Pollut. Bull. 2022, 179, 113665. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Wang, G.M.; Yu, J.B.; Ran, Y.N.; Guan, B.; Han, G.X. Effects of nitrogen and phosphorus supply on plant community structure of coastal wetland in the Yellow River Delta. Chin. J. Ecol. 2018, 37, 801–809. [Google Scholar] [CrossRef]
- Finks, S.S.; Weihe, C.; Kimball, S.; Allison, S.D.; Martiny, A.C.; Treseder, K.K.; Martiny, J.B.H. Microbial community response to a decade of simulated global changes depends on the plant community. Elem. Sci. Anthr. 2021, 9, 00124. [Google Scholar] [CrossRef]
- Venterink, H.O.; Güsewell, S. Competitive interactions between two meadow grasses under nitrogen and phosphorus limitation. Funct. Ecol. 2010, 24, 877–886. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, G.; Jiang, S.; Liu, Y.-X. Wekemo Bioincloud: A user-friendly platform for meta-omics data analyses. iMeta 2024, 3, e175. [Google Scholar] [CrossRef]
- Zhao, X.C.; Du, Z.W.; Liu, S.E.; Wang, Q.K. Effects of nitrogen addition on soil microbial respiration and its temperature sensitivity in a Duolun grassland ecosystem. Acta Ecol. Sin. 2020, 40, 1551–1561. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, M.; Jiang, X.; Guan, D.; Wei, D.; Zhao, B.; Chen, S.; Cao, F.; Li, L.; Yang, X.; et al. Impact of 36 years of nitrogen fertilization on microbial community composition and soil carbon cycling-related enzyme activities in rhizospheres and bulk soils in northeast China. Appl. Soil Ecol. 2019, 136, 148–157. [Google Scholar] [CrossRef]
- Feng, J.; Zhu, B. Global patterns and associated drivers of priming effect in response to nutrient addition. Soil Biol. Biochem. 2021, 153, 108118. [Google Scholar] [CrossRef]
- Liu, W.X.; Jiang, L.; Yang, S.; Wang, Z.; Tian, R.; Peng, Z.Y.; Chen, Y.L.; Zhang, X.X.; Kuang, J.L.; Ling, N.; et al. Critical transition of soil bacterial diversity and composition triggered by nitrogen enrichment. Ecology 2020, 101, e03053. [Google Scholar] [CrossRef]
- Wang, Y.J.; Huang, Q.Q.; Gao, H.; Zhang, R.Q.; Yang, L.; Guo, Y.R.; Li, H.K.; Awasthi, M.K.; Li, G.C. Long-term cover crops improved soil phosphorus availability in a rain-fed apple orchard. Chemosphere 2021, 275, 130093. [Google Scholar] [CrossRef]
- Xu, C.H.; Xu, X.; Ju, C.H.; Chen, H.Y.H.; Wilsey, B.J.; Luo, Y.Q.; Fan, W. Long-term, amplified responses of soil organic carbon to nitrogen addition worldwide. Glob. Chang. Biol. 2021, 27, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Fornara, D.A.; Yang, W.Q.; Peng, Y.; Peng, C.H.; Liu, Z.L.; Wu, F.Z. Influence of multiple global change drivers on terrestrial carbon storage: Additive effects are common. Ecol. Lett. 2017, 20, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, S.; Taylor, P.; Richter, A.; Porporato, A.; Agren, G.I. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 2012, 196, 79–91. [Google Scholar] [CrossRef]
- Spohn, M.; Potsch, E.M.; Eichorst, S.A.; Woebken, D.; Wanek, W.; Richter, A. Soil microbial carbon use efficiency and biomass turnover in a long-term fertilization experiment in a temperate grassland. Soil Biol. Biochem. 2016, 97, 168–175. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.; Su, Y.; Gong, X.; Yao, B.; Cheng, L. Phosphorus Coupled with High Nitrogen Addition Exerts a Great Influence on Soil Bacterial Community in a Semiarid Grassland. Microb. Ecol. 2023, 86, 2993–3002. [Google Scholar] [CrossRef]
- Wang, J.; Liao, L.; Ye, Z.; Liu, H.; Zhang, C.; Zhang, L.; Liu, G.; Wang, G. Different bacterial co-occurrence patterns and community assembly between rhizosphere and bulk soils under N addition in the plant–soil system. Plant Soil 2022, 471, 697–713. [Google Scholar] [CrossRef]
- Li, W.; Lv, X.; Ruan, J.; Yu, M.; Song, Y.B.; Yu, J.; Dong, M. Variations in Soil Bacterial Composition and Diversity in Newly Formed Coastal Wetlands. Front Microbiol. 2018, 9, 3256. [Google Scholar] [CrossRef]
- Shao, P.; Liang, C.; Rubert-Nason, K.; Li, X.; Xie, H.; Bao, X. Secondary successional forests undergo tightly-coupled changes in soil microbial community structure and soil organic matter. Soil Biol. Biochem. 2019, 128, 56–65. [Google Scholar] [CrossRef]
- Li, X.M.; Chen, Q.L.; He, C.; Shi, Q.; Chen, S.C.; Reid, B.J.; Zhu, Y.G.; Sun, G.X. Organic Carbon Amendments Affect the Chemodiversity of Soil Dissolved Organic Matter and Its Associations with Soil Microbial Communities. Environ. Sci. Technol. 2019, 53, 50–59. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Wang, J.; Wang, J.D.; Zhang, Y.C. Long-term fertilization with high nitrogen rates decreased diversity and stability of diazotroph communities in soils of sweet potato. Appl. Soil Ecol. 2022, 170, 104266. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Sha, M.H.; Xu, J.; Zheng, Z.C.; Fa, K.Y. Enhanced atmospheric nitrogen deposition triggered little change in soil microbial diversity and structure in a desert ecosystem. Glob. Ecol. Conserv. 2021, 31, e01879. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Zhang, Y.Y.; Dong, W.G.; Zhang, Y.Q.; Zhang, G.X.; Sun, Z.P.; Yang, L.J. Vermicompost can suppress Fusarium oxysporum f. sp. lycopersici via generation of beneficial bacteria in a long-term tomato monoculture soil. Plant Soil 2019, 440, 491–505. [Google Scholar] [CrossRef]
- Gou, Y.C.; Wang, Z.K.; Zhang, Z.P.; Wei, H.; Meng, P.P.; Zeng, Y.H. Advance in role mechanisms of plant growth promoting rhizobacteria. Chin. J. Appl. Environ. Biol. 2022, 1, 1–19. [Google Scholar] [CrossRef]
- Chen, W.; Gao, Y.; Yang, J.; Fan, F.J.; Zhang, W.G.; Li, J.Y.; Zhou, C.; Shi, G.L.; Tong, F.; Fan, G.P. Taxonomical and functional bacterial community selection in the rhizosphere of the rice genotypes with different nitrogen use efficiencies. Plant Soil 2022, 470, 111–125. [Google Scholar] [CrossRef]
- Duan, X.; Yu, X.F.; Li, Z.; Wang, Q.G.; Liu, Z.P.; Zou, Y.C. Iron-bound organic carbon is conserved in the rhizosphere soil of freshwater wetlands. Soil Biol. Biochem. 2020, 149, 107949. [Google Scholar] [CrossRef]
- Chai, Y.N.; Schachtman, D.P. Root exudates impact plant performance under abiotic stress. Trends Plant Sci. 2022, 27, 80–91. [Google Scholar] [CrossRef]
- Fu, Y.; Luo, Y.; Tang, C.; Li, Y.; Guggenberger, G.; Xu, J. Succession of the soil bacterial community as resource utilization shifts from plant residues to rhizodeposits. Soil Biol. Biochem. 2022, 173, 108785. [Google Scholar] [CrossRef]
- Loganathachetti, D.S.; Alhashmi, F.; Chandran, S.; Mundra, S. Irrigation water salinity structures the bacterial communities of date palm (Phoenix dactylifera)-associated bulk soil. Front. Plant Sci. 2022, 13, 944637. [Google Scholar] [CrossRef]
- Xie, Y.M.; Tian, X.L.; Liu, Y.; Zhao, K.; Li, Y.M.; Luo, K.; Wang, B.; Dong, S.L. Nitrogen removal capability and mechanism of a novel heterotrophic nitrifying-aerobic denitrifying strain H1 as a potential candidate in mariculture wastewater treatment. Environ. Sci. Pollut. Res. 2023, 48, 106366–106377. [Google Scholar] [CrossRef]
- Wang, H.Y.; Wang, R.F.; Zhong, W.; Kong, Q.; Ma, Q.L.; Zong, K.J. Advance in Structural Characteristics and lnfluence Factors of Functional Microbial Communities in the Soils of the Wetlands in the Yellow River Delta. Wetl. Sci. 2022, 20, 111–118. [Google Scholar] [CrossRef]
- Zhou, W.G.; Ding, D.W.; Yang, Q.S.; Ling, J.; Ahmad, M.; Lin, X.C.; Lin, L.Y.; Zhang, Y.; Dong, J.D. Diversity and abundance of diazotrophic communities of seagrass Halophila ovalis based on genomic and transcript level in Daya Bay, South China Sea. Arch. Microbiol. 2021, 203, 5577–5589. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.D.; Wang, K.; Li, M.D.; Duan, C.J. Genome Sequencing and Analysis of Sphingobium sp. LF-16. Genom. Appl. Biol. 2020, 39, 599–605. [Google Scholar] [CrossRef]
- Mo, Y.Y.; Peng, F.; Gao, X.F.; Xiao, P.; Logares, R.; Jeppesen, E.; Ren, K.X.; Xue, Y.Y.; Yang, J. Low shifts in salinity determined assembly processes and network stability of microeukaryotic plankton communities in a subtropical urban reservoir. Microbiome 2021, 9, 128. [Google Scholar] [CrossRef]
- Sun, M.Y.; Li, M.C.; Zhou, Y.Q.; Liu, J.I.; Shi, W.C.; Wu, X.L.; Xie, B.H.; Deng, Y.; Gao, Z. Nitrogen deposition enhances the deterministic process of the prokaryotic community and increases the complexity of the microbial co-network in coastal wetlands. Sci. Total Environ. 2023, 856, 158939. [Google Scholar] [CrossRef]
- Madhaiyan, M.; Poonguzhali, S.; Lee, J.S.; Lee, K.C.; Sundaram, S. Flavobacterium glycines sp. nov., a facultative methylotroph isolated from the rhizosphere of soybean. Int. J. Syst. Evol. Microbiol. 2010, 60, 2187–2192. [Google Scholar] [CrossRef]
- Tian, G.L.; Qiu, H.S.; Li, D.W.; Wang, Y.T.; Zhen, B.; Li, H.Z.; Niu, Q.L.; Qi, D.L.; Zhou, X.G. Little environmental adaptation and high stability of bacterial communities in rhizosphere rather than bulk soils in rice fields. Appl. Soil Ecol. 2022, 169, 104183. [Google Scholar] [CrossRef]
- Yan, K.; Lu, D.S.; Ding, C.J.; Wang, Y.; Tian, Y.R.; Su, X.H.; Dong, Y.F.; Wang, Y.P. Rare and abundant bacterial communities in poplar rhizosphere soils respond differently to genetic effects. Sci. Total Environ. 2024, 908, 168216. [Google Scholar] [CrossRef]
- Jousset, A.; Bienhold, C.; Chatzinotas, A.; Gallien, L.; Gobet, A.; Kurm, V.; Küsel, K.; Rillig, M.C.; Rivett, D.W.; Salles, J.F.; et al. Where less may be more: How the rare biosphere pulls ecosystems strings. ISME J. 2017, 11, 853–862. [Google Scholar] [CrossRef]
- Pan, C.C.; Feng, Q.; Li, Y.L.; Li, Y.Q.; Liu, L.D.; Yu, X.Y.; Ren, S.L. Rare soil bacteria are more responsive in desertification restoration than abundant bacteria. Environ. Sci. Pollut. Res. 2022, 29, 33323–33334. [Google Scholar] [CrossRef]
- Capek, P.T.; Manzoni, S.; Kastovská, E.; Wild, B.; Diáková, K.; Bárta, J.; Schnecker, J.; Blasi, C.; Martikainen, P.J.; Alves, R.J.E.; et al. A plant-microbe interaction framework explaining nutrient effects on primary production. Nat. Ecol. Evol. 2018, 2, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.B.; Wang, F.H.; Hu, C.S.; Liu, B.B. Metagenomics reveals taxon-specific responses of the nitrogen-cycling microbial community to long-term nitrogen fertilization. Soil Biol. Biochem. 2021, 156, 108214. [Google Scholar] [CrossRef]
- Song, L.; Niu, S.L. Increased soil microbial AOB, amoA and narG abundances sustain long-term positive responses of nitrification and denitrification to N deposition. Soil Biol. Biochem. 2022, 166, 108539. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Zhang, Z.; Sun, J.; Li, T.; Fu, Z.; Hu, R.; Zhang, Y. Effects of Increasing the Nitrogen–Phosphorus Ratio on the Structure and Function of the Soil Microbial Community in the Yellow River Delta. Microorganisms 2024, 12, 2419. https://doi.org/10.3390/microorganisms12122419
Ma J, Zhang Z, Sun J, Li T, Fu Z, Hu R, Zhang Y. Effects of Increasing the Nitrogen–Phosphorus Ratio on the Structure and Function of the Soil Microbial Community in the Yellow River Delta. Microorganisms. 2024; 12(12):2419. https://doi.org/10.3390/microorganisms12122419
Chicago/Turabian StyleMa, Jinzhao, Zehao Zhang, Jingkuan Sun, Tian Li, Zhanyong Fu, Rui Hu, and Yao Zhang. 2024. "Effects of Increasing the Nitrogen–Phosphorus Ratio on the Structure and Function of the Soil Microbial Community in the Yellow River Delta" Microorganisms 12, no. 12: 2419. https://doi.org/10.3390/microorganisms12122419
APA StyleMa, J., Zhang, Z., Sun, J., Li, T., Fu, Z., Hu, R., & Zhang, Y. (2024). Effects of Increasing the Nitrogen–Phosphorus Ratio on the Structure and Function of the Soil Microbial Community in the Yellow River Delta. Microorganisms, 12(12), 2419. https://doi.org/10.3390/microorganisms12122419





