Effects of Different Types and Ratios of Dry Tea Residues on Nutrient Content, In Vitro Rumen Fermentation, and the Bacterial Community of Ensiled Sweet Sorghum
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Chemical Composition and Fermentation Quality Analysis
2.3. Total Phenolic Content
2.4. Analysis of Free Amino Acids
2.5. Bacterial Community Analysis
2.5.1. Sequencing DNA Extraction and PCR
2.5.2. Data Analysis
2.6. In Vitro Rumen Fermentation
2.7. Statistical Analysis
3. Results
3.1. Chemical Composition of the Raw Materials
3.2. Fermentation Quality and Nutrient Composition in Sweet Sorghum Silage with the Addition of Tea Residues
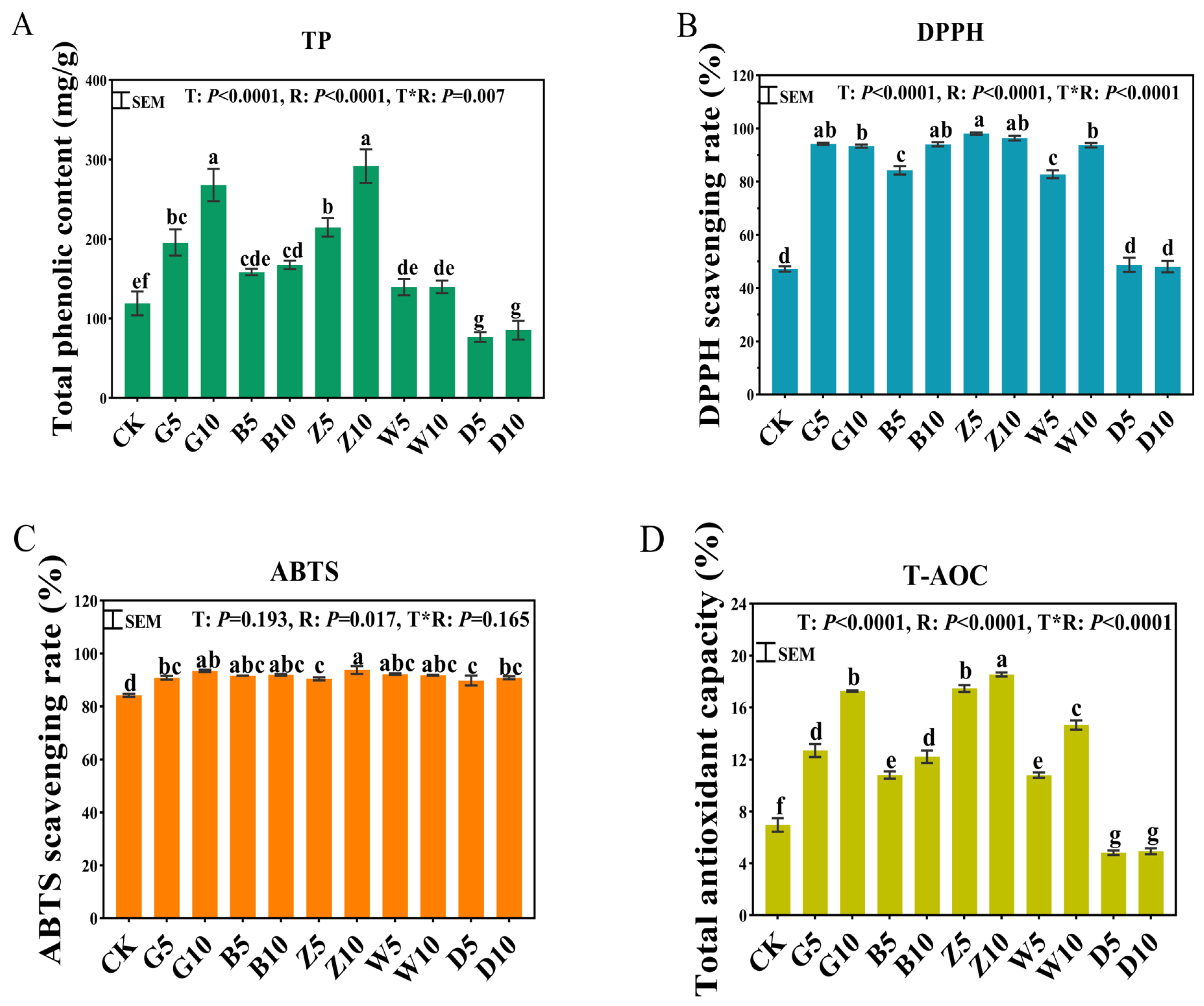
3.3. Total Phenolics and Antioxidant Capacity in Sweet Sorghum Silage with the Addition of Tea Residues
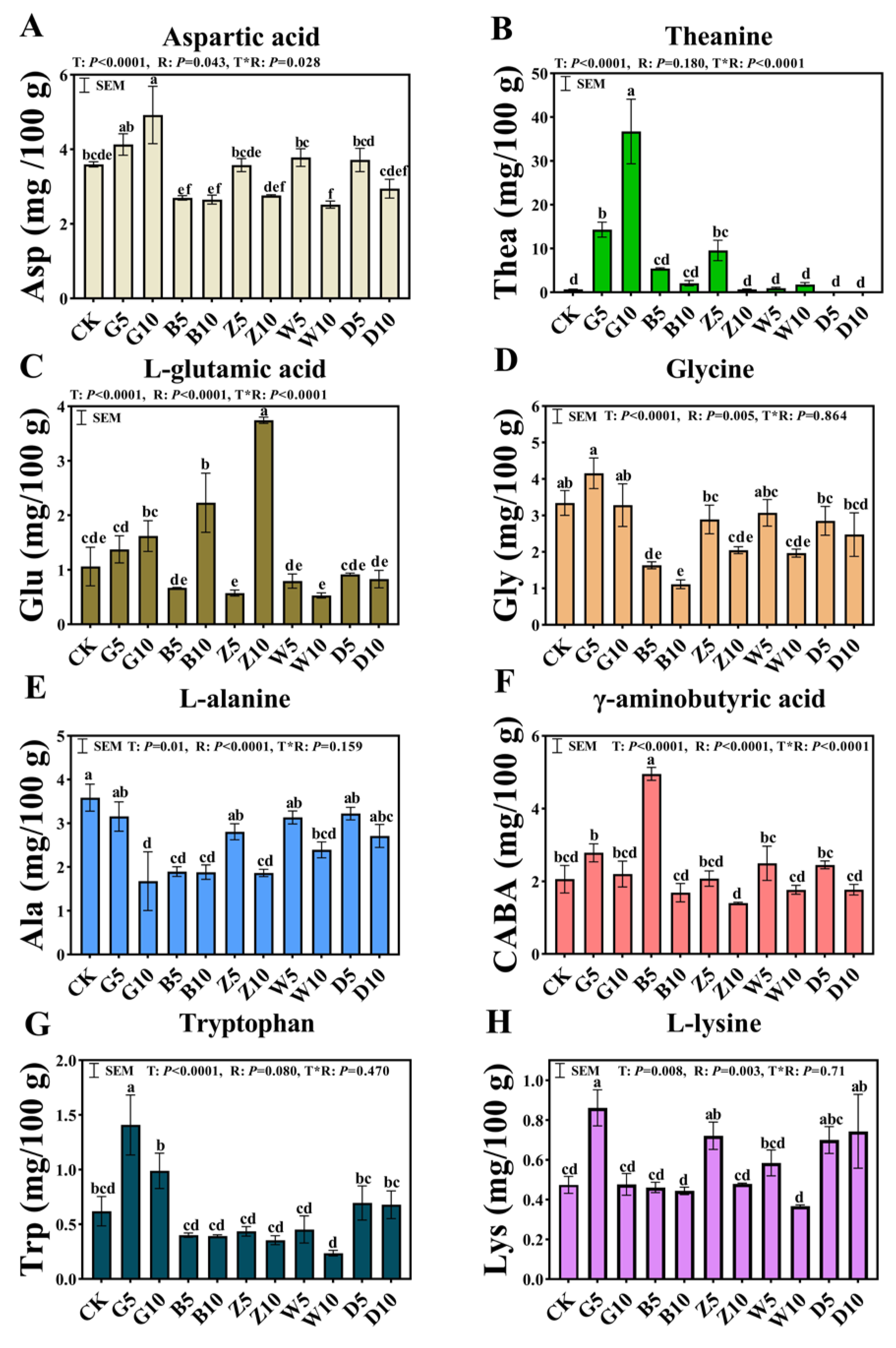
3.4. Free Amino Acids in Sweet Sorghum Silage with the Addition of Tea Residues
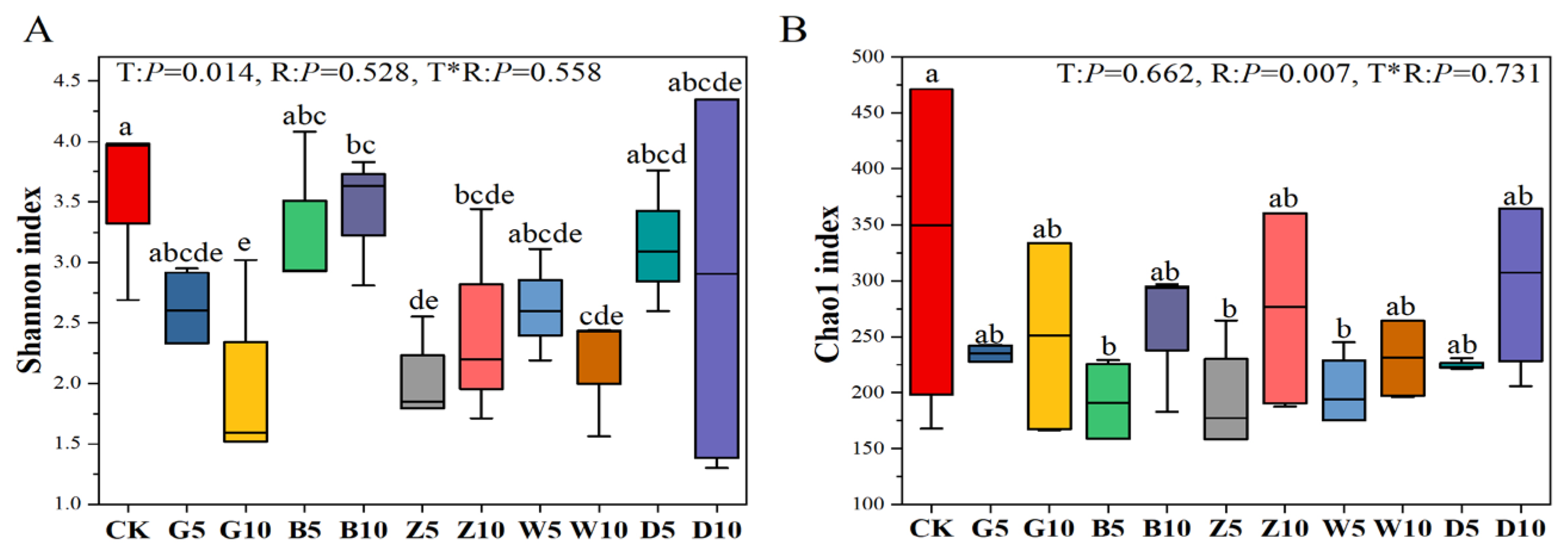
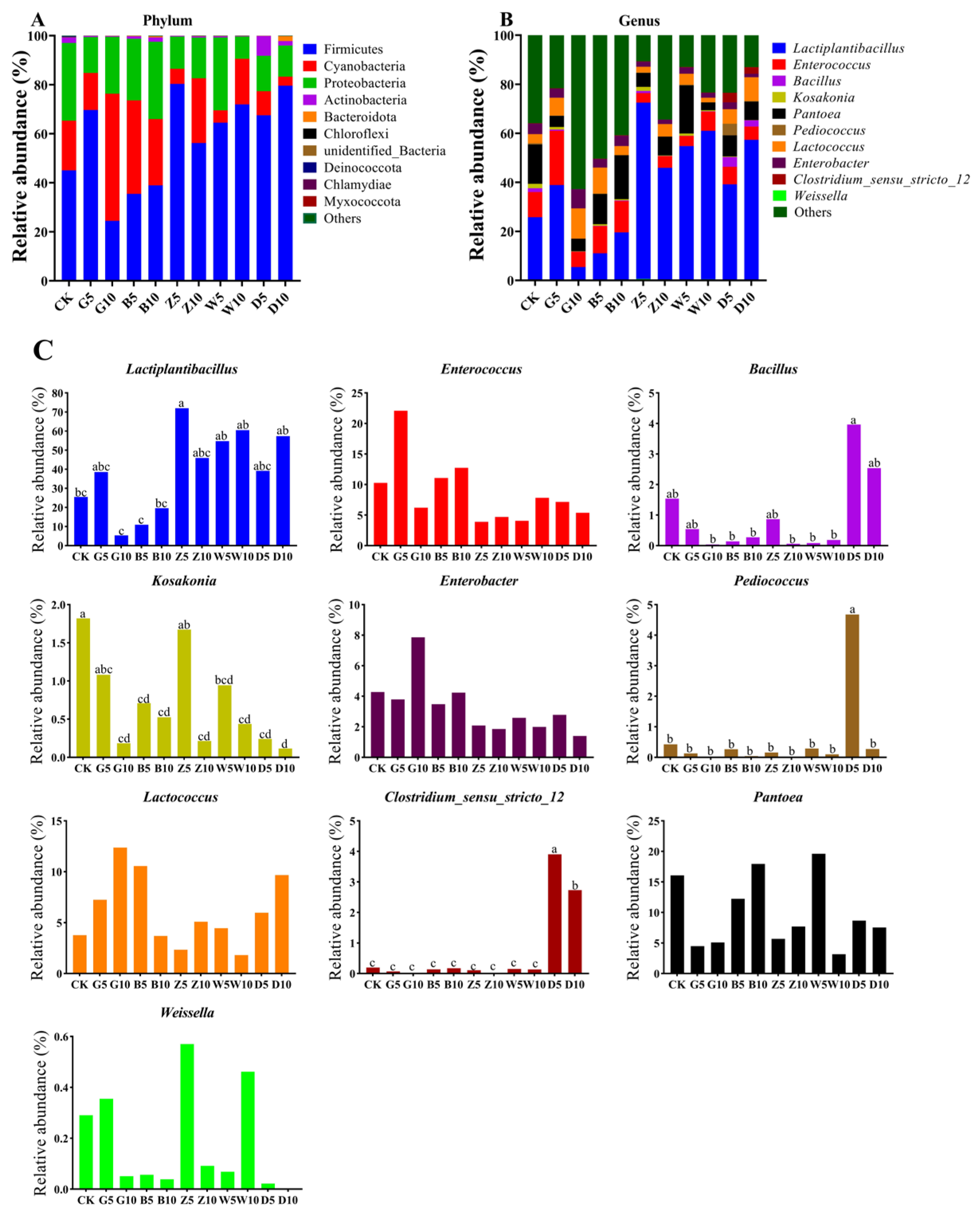
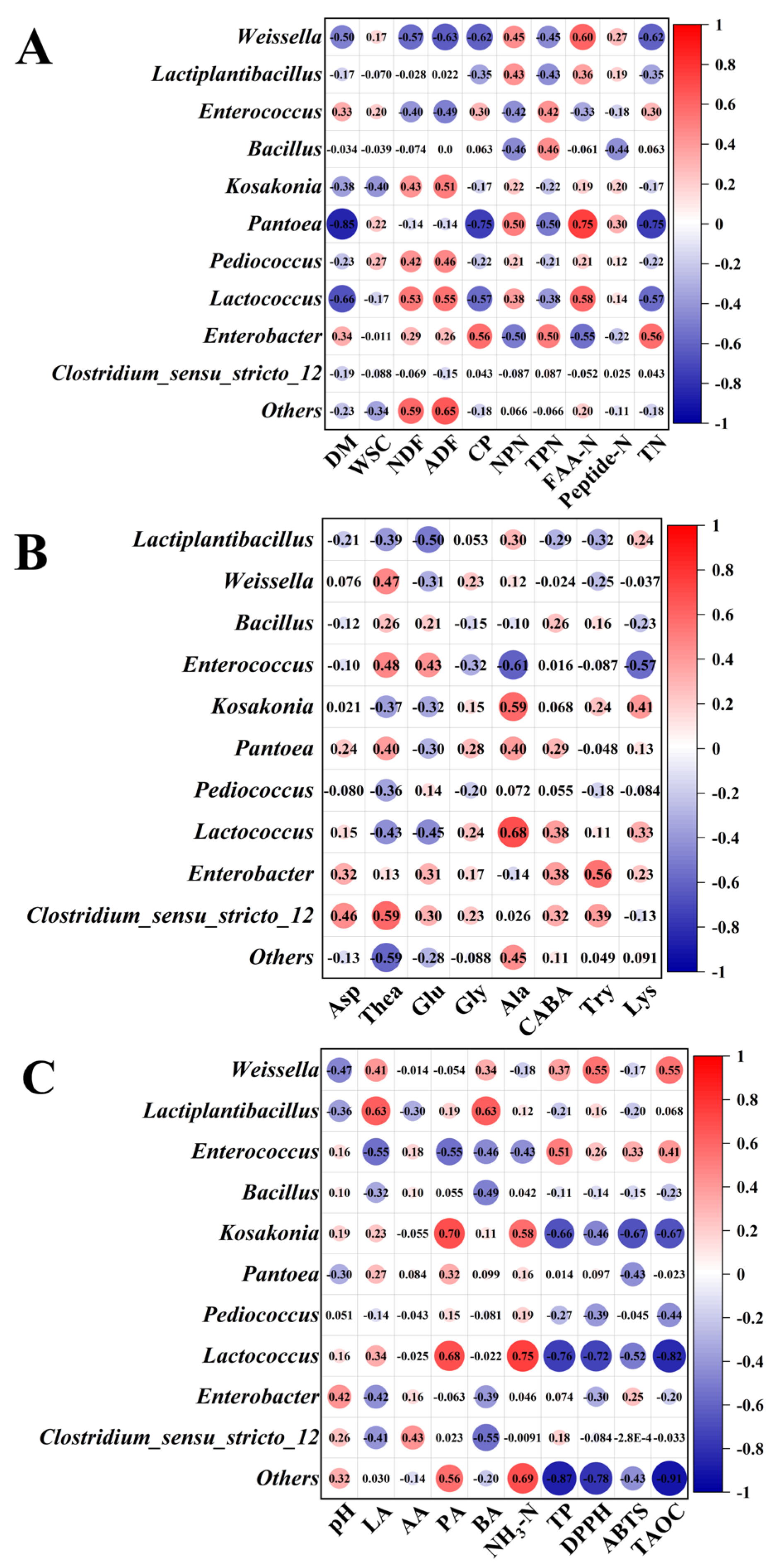
3.5. Bacterial Community Composition in Sweet Sorghum Silage with the Addition of Tea Residues
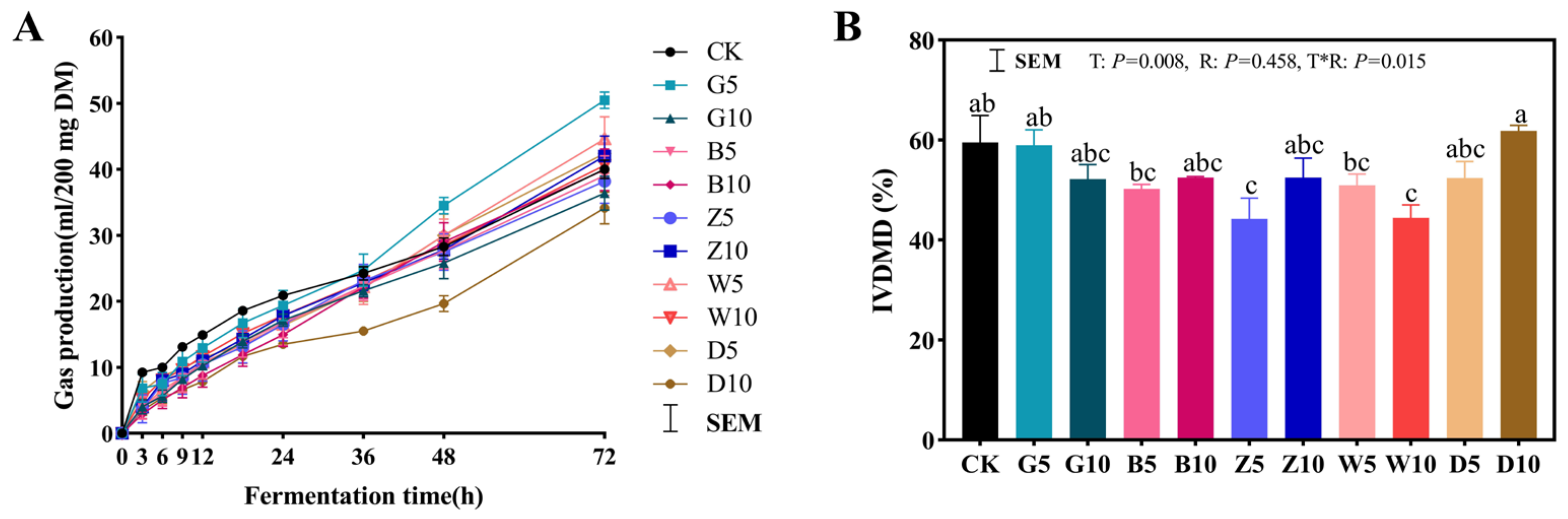
3.6. In Vitro Rumen Fermentation in Sweet Sorghum Silage with the Addition of Tea Residues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, S.Y.; Nie, Q.; Tai, H.C.; Song, X.L.; Tong, Y.F.; Zhang, L.J.; Liang, C. Tea and tea drinking: China’s outstanding contributions to the mankind. Chin. Med. 2022, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- National Bureau of Statistics of China. China Statistical Yearbook; China Statistical Publishing House: Beijing, China, 2023. [Google Scholar]
- Assad, M.; Ashaolu, T.J.; Khalifa, I.; Baky, M.H.; Farag, M.A. Dissecting the role of microorganisms in tea production of different fermentation levels: A multifaceted review of their action mechanisms, quality attributes and future perspectives. World J. Microbiol. Biotechnol. 2023, 39, 265. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, Z.; Chai, X.; Wang, Y.; Wang, W.; Zhang, M. Tea as a natural gift for discovering antiviral candidates. Acupunct. Herb. Med. 2022, 2, 211–220. [Google Scholar] [CrossRef]
- Hu, S.; Lin, Z.; Zhao, S.; Zhang, B.; Luo, L.; Zeng, L. Pu-erh tea alleviated colitis-mediated brain dysfunction by promoting butyric acid production. Food Chem. Toxicol. 2023, 172, 113594. [Google Scholar] [CrossRef]
- Jaziri, I.; Ben Slama, M.; Mhadhbi, H.; Urdaci, M.C.; Hamdi, M. Effect of green and black teas (Camellia sinensis L.) on the characteristic microflora of yogurt during fermentation and refrigerated storage. Food Chem. 2009, 112, 614–620. [Google Scholar] [CrossRef]
- Zhu, M.-Z.; Zhou, F.; Ouyang, J.; Wang, Q.-Y.; Li, Y.-L.; Wu, J.-L.; Liu, Z.H. Combined use of epigallocatechin-3-gallate (EGCG) and caffeine in low doses exhibits marked anti-obesity synergy through regulation of gut microbiota and bile acid metabolism. Food Funct. 2021, 12, 4105–4116. [Google Scholar] [CrossRef]
- Kondo, M.; Kita, K.; Yokota, H.-O. Feeding value to goats of whole-crop oat ensiled with green tea waste. Anim. Feed Sci. Technol. 2004, 113, 71–81. [Google Scholar] [CrossRef]
- Peng, T.-Q.; Yin, X.-L.; Gu, H.-W.; Sun, W.; Ding, B.; Hu, X.-C.; Ye, S.Y. HPLC-DAD fingerprints combined with chemometric techniques for the authentication of plucking seasons of Laoshan green tea. Food Chem. 2021, 347, 128959. [Google Scholar] [CrossRef]
- Debnath, B.; Haldar, D.; Purkait, M.K. Potential and sustainable utilization of tea waste: A review on present status and future trends. J. Environ. Chem. Eng. 2021, 9, 106179. [Google Scholar] [CrossRef]
- Sui, W.; Xiao, Y.; Liu, R.; Wu, T.; Zhang, M. Steam explosion modification on tea waste to enhance bioactive compounds’ extractability and antioxidant capacity of extracts. J. Food Eng. 2019, 261, 51–59. [Google Scholar] [CrossRef]
- Mandal, S.; Pu, S.; Shangguan, L.; Liu, S.; Ma, H.; Adhikari, S.; Hou, D. Synergistic construction of green tea biochar supported nZVI for immobilization of lead in soil: A mechanistic investigation. Environ. Int. 2020, 135, 105374. [Google Scholar] [CrossRef] [PubMed]
- Afdhol, M.K.; Lubis, H.Z.; Siregar, C.P. Bioethanol Production from Tea Waste as a Basic Ingredient in Renewable Energy Sources. J. Earth Energy Eng. 2019, 8, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Harvey, I. Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Tang, M.G.; Zhang, S.; Xiong, L.G.; Zhou, J.H.; Huang, J.A.; Zhao, A.Q.; Liu, A.L. A comprehensive review of polyphenol oxidase in tea (Camellia sinensis): Physiological characteristics, oxidation manufacturing, and biosynthesis of functional constituents. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2267–2291. [Google Scholar] [CrossRef]
- Kondo, M.; Hirano, Y.; Ikai, N.; Kita, K.; Jayanegara, A.; Yokota, H.O. Assessment of Anti-nutritive Activity of Tannins in Tea By-products Based on In vitro Rumen Fermentation. Asian-Australas. J. Anim. Sci. 2014, 27, 1571–1576. [Google Scholar] [CrossRef]
- Cao, Y.; Takahashi, T.; Horiguchi, K.-I. Effects of addition of food by-products on the fermentation quality of a total mixed ration with whole crop rice and its digestibility, preference, and rumen fermentation in sheep. Anim. Feed Sci. Technol. 2009, 151, 1–11. [Google Scholar] [CrossRef]
- Qiu, Q.; Wei, X.; Zhang, L.; Li, Y.; Qu, M.; Ouyang, K. Effect of dietary inclusion of tea residue and tea leaves on ruminal fermentation characteristics and methane production. Anim. Biotechnol. 2023, 34, 825–834. [Google Scholar] [CrossRef]
- Lv, X.K.; Chen, L.; Zhou, C.S.; Zhang, G.J.; Xie, J.J.; Kang, J.H.; Du, Z. Application of different proportions of sweet sorghum silage as a substitute for corn silage in dairy cows. Food Sci. Nutr. 2023, 11, 3575–3587. [Google Scholar] [CrossRef]
- Ren, H.W.; Sun, W.L.; Yan, Z.H.; Zhang, Y.C.; Wang, Z.Y.; Song, B.; Li, J. Bioaugmentation of sweet sorghum ensiling with rumen fluid: Fermentation characteristics, chemical composition, microbial community, and enzymatic digestibility of silages. J. Clean. Prod. 2021, 294, 126308. [Google Scholar] [CrossRef]
- Helrich, K.C. Official Methods of Analysis of the AOAC; Association of the Official Analytical Chemists: Rockville, MD, USA, 1990; Volume 1. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Thomas, T.A. An Automated Procedure for the Determination of Soluble Carbohydrate in Herbage; Wiley Online Library: Hoboken, NJ, USA, 1977. [Google Scholar]
- Wang, C.; He, L.; Xing, Y.; Zhou, W.; Yang, F.; Chen, X.; Zhang, Q. Fermentation quality and microbial community of alfalfa and stylo silage mixed with Moringa oleifera leaves. Bioresour. Technol. 2019, 284, 240–247. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Li, P.; Zhao, W.; Yan, L.; Chen, L.; Chen, Y.; Gou, W.; Chen, C. Inclusion of abandoned rhubarb stalk enhanced anaerobic fermentation of alfalfa on the Qinghai Tibetan Plateau. Bioresour. Technol. 2022, 347, 126347. [Google Scholar] [CrossRef]
- Zhang, L.Z.; Liu, R.H. Phenolic and carotenoid profiles and antiproliferative activity of foxtail millet. Food Chem. 2015, 174, 495–501. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, F.; Qin, L.; Zhang, N.; Cao, Q.; Schwab, W.; Song, C. Dynamic change in amino acids, catechins, alkaloids, and gallic acid in six types of tea processed from the same batch of fresh tea (Camellia sinensis L.) leaves. Subtrop. Plant Sci. 2019, 77, 28–38. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, L.; Liao, Y.; Li, J.; Zhou, B.; Yang, Z.; Tang, J. Enzymatic Reaction-Related Protein Degradation and Proteinaceous Amino Acid Metabolism during the Black Tea (Camellia sinensis) Manufacturing Process. Foods 2020, 9, 66. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, H.; Zhang, C.; Cheng, Q.; Zheng, Y.; Wang, C.; Chen, C. Ambient ultraviolet radiation: A new factor affecting anaerobic fermentation of oat and subsequent methane emissions. Bioresour. Technol. 2022, 355, 127243. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Ni, K.; Wang, F.; Zhu, B.; Yang, J.; Zhou, G.; Pan, Y.; Zhong, J. Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour. Technol. 2017, 238, 706–715. [Google Scholar] [CrossRef]
- Ma, C.; Zhou, B.; Wang, J.; Ma, B.; Lv, X.; Chen, X.; Li, X. Investigation and dynamic changes of phenolic compounds during a new-type fermentation for ripened Pu-erh tea processing. LWT-Food Sci. Technol. 2023, 180, 114683. [Google Scholar] [CrossRef]
- Li, X.; Chen, F.; Wang, X.; Xiong, Y.; Liu, Z.; Lin, Y.; Yang, F. Innovative utilization of herbal residues: Exploring the diversity of mechanisms beneficial to regulate anaerobic fermentation of alfalfa. Bioresour. Technol. 2022, 360, 127429. [Google Scholar] [CrossRef]
- Kung, L., Jr.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Guo, S.; Awasthi, M.K.; Wang, Y.; Xu, P. Current understanding in conversion and application of tea waste biomass: A review. Bioresour. Technol. 2021, 338, 125530. [Google Scholar] [CrossRef]
- He, L.; Lv, H.; Xing, Y.; Chen, X.; Zhang, Q. Intrinsic tannins affect ensiling characteristics and proteolysis of Neolamarckia cadamba leaf silage by largely altering bacterial community. Bioresour. Technol. 2020, 311, 123496. [Google Scholar] [CrossRef]
- Cheng, W.; Pian, R.; Chen, X.; Zhang, Q. Effects of polyphenol oxidases on proteolysis and lipolysis during ensiling of Moringa oleifera leaves with or without pyrocatechol. Anim. Feed Sci. Technol. 2021, 275, 114870. [Google Scholar] [CrossRef]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Abhari, F.M. The role of plant-derived natural antioxidants in reduction of oxidative stress. Biofactors 2022, 48, 611–633. [Google Scholar] [CrossRef]
- Lv, R.; Dong, Y.; Bao, Z.; Zhang, S.; Lin, S.; Sun, N. Advances in the activity evaluation and cellular regulation pathways of food-derived antioxidant peptides. Trends Food Sci. Technol. 2022, 122, 171–186. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Kiani, A.; Santhiravel, S.; Holman, B.W.B.; Lauridsen, C.; Dunshea, F.R. The Importance of Dietary Antioxidants on Oxidative Stress, Meat and Milk Production, and Their Preservative Aspects in Farm Animals: Antioxidant Action, Animal Health, and Product Quality-Invited Review. Animals 2022, 12, 3279. [Google Scholar] [CrossRef]
- Lindemann, B. Receptors and transduction in taste. Nature 2001, 413, 219–225. [Google Scholar] [CrossRef]
- Karolkowski, A.; Guichard, E.; Briand, L.; Salles, C. Volatile Compounds in Pulses: A Review. Foods 2021, 10, 3140. [Google Scholar] [CrossRef]
- Ren, F.; He, R.; Zhou, X.; Gu, Q.; Xia, Z.; Liang, M.; Zou, C. Dynamic changes in fermentation profiles and bacterial community composition during sugarcane top silage fermentation: A preliminary study. Bioresour. Technol. 2019, 285, 121315. [Google Scholar] [CrossRef]
- Guo, L.; Wang, X.; Chen, H.; Li, X.; Xiong, Y.; Zhou, H.; Ni, K. Exploring the fermentation quality, bacterial community and metabolites of alfalfa ensiled with mugwort residues and Lactiplantibacillus pentosus. Chem. Biol. Technol. Agric. 2023, 10, 107. [Google Scholar] [CrossRef]
- Ogunade, I.M.; Jiang, Y.; Cervantes, A.P.; Kim, D.H.; Oliveira, A.S.; Vyas, D.; Adesogan, A.T. Bacterial diversity and composition of alfalfa silage as analyzed by Illumina MiSeq sequencing: Effects of Escherichia coli O157:H7 and silage additives. J. Dairy Sci. 2018, 101, 2048–2059. [Google Scholar] [CrossRef]
- Xue, J.; Yang, L.; Yang, Y.; Yan, J.; Ye, Y.; Hu, C.; Meng, Y. Contrasting microbiomes of raw and ripened Pu-erh tea associated with distinct chemical profiles. LWT-Food Sci. Technol. 2020, 124, 109147. [Google Scholar] [CrossRef]
- Alvarez, A.L.; Weyers, S.L.; Goemann, H.M.; Peyton, B.M.; Gardner, R.D. Microalgae, soil and plants: A critical review of microalgae as renewable resources for agriculture. Algal Res. Biomass Biofuels Bioprod. 2021, 54, 102200. [Google Scholar] [CrossRef]
- Lin, S.; Huang, H.; Zheng, J.; Lin, H.; Wang, Y.; Xu, P. Microbial enrichment evaluation during the fermentation of ensiling pruned branches from tea plants. Int. J. Food Microbiol. 2022, 374, 109742. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, H.-Z.; Gao, Y.; Wang, N.-W.; Liu, H.; Yang, F.-Y.; Ni, K.K. Ensiling vine tea (Ampelopsis grossedentata) residue with Lactobacillus plantarum inoculant as an animal unconventional fodder. J. Integr. Agric. 2023, 22, 1172–1183. [Google Scholar] [CrossRef]
- Chen, L.; Guo, G.; Yuan, X.; Zhang, J.; Li, J.; Shao, T. Effects of applying molasses, lactic acid bacteria and propionic acid on fermentation quality, aerobic stability and in vitro gas production of total mixed ration silage prepared with oat-common vetch intercrop on the Tibetan Plateau. J. Sci. Food Agric. 2016, 96, 1678–1685. [Google Scholar] [CrossRef]
- Ramdani, D.; Jayanegara, A.; Chaudhry, A.S. Biochemical Properties of Black and Green Teas and Their Insoluble Residues as Natural Dietary Additives to Optimize In Vitro Rumen Degradability and Fermentation but Reduce Methane in Sheep. Animals 2022, 12, 305. [Google Scholar] [CrossRef]
- Zhou, R.; Wu, J.; Lang, X.; Liu, L.; Casper, D.P.; Wang, C.; Wei, S. Effects of oregano essential oil on in vitro ruminal fermentation, methane production, and ruminal microbial community. J. Dairy Sci. 2020, 103, 2303–2314. [Google Scholar] [CrossRef] [PubMed]
- Ala, M.S.; Pirmohammadi, R.; Khalilvandi-Behroozyar, H.; Anassori, E. Changes in vitro rumen fermentation, methane production and microbial populations in response to green tea extract. Ital. J. Anim. Sci. 2021, 20, 1114–1125. [Google Scholar] [CrossRef]
| Items | Fresh Forage | Tea Residue Materials | ||||
|---|---|---|---|---|---|---|
| G | B | W | Z | D | ||
| Dry Matter (% FM) | 28.0 | - | - | - | - | - |
| Crude Protein (% DM) | 5.76 | 33.8 | 29.8 | 14.5 | 27.2 | 30.9 |
| Water Soluble Carbohydrate (% DM) | 15.5 | 4.18 | 7.80 | 8.49 | 4.47 | 0.44 |
| Nonprotein-N (% TN) | 0.29 | 9.06 | 7.65 | 4.05 | 8.17 | 6.51 |
| Neutral Detergent Fiber (% DM) | 55.7 | 33.6 | 33.8 | 33.9 | 26.0 | 44.8 |
| Acid Detergent Fiber (% DM) | 36.3 | 8.13 | 14.0 | 18.6 | 7.74 | 36.8 |
| DPPH Scavenging Rate (%) | 50.4 | 91.5 | 95.3 | 94.1 | 91.1 | 43.4 |
| ABTS Scavenging Rate (%) | 89.9 | 92.5 | 99.3 | 90.4 | 92.3 | 91.4 |
| Total Antioxidant Capacity (%) | 5.60 | 18.8 | 18.3 | 17.6 | 19.3 | 4.47 |
| Treatment (T) | Ratio (R) | DM (% FM) | CP (% DM) | WSCs (% DM) | NDF (% DM) | ADF (% DM) |
|---|---|---|---|---|---|---|
| CK | 0% | 22.1 e | 5.86 h | 3.36 de | 47.8 abc | 26.5 bc |
| G | 5% | 25.7 cd | 11.1 d | 3.98 cd | 46.7 abc | 27.3 b |
| 10% | 30.3 a | 16.4 a | 3.64 de | 44.9 cd | 24.3 cd | |
| B | 5% | 26.7 c | 10.7 d | 6.22 a | 45.6 bcd | 26.4 bc |
| 10% | 29.7 a | 14.0 bc | 4.41 bc | 47.2 abc | 28.0 b | |
| Z | 5% | 24.7 d | 8.94 e | 4.80 bc | 46.4 abcd | 26.0 bc |
| 10% | 29.8 a | 13.4 c | 5.06 de | 45.1 cd | 25.6 bcd | |
| W | 5% | 25.2 d | 6.91 g | 4.53 b | 49.1 abc | 28.2 b |
| 10% | 28.4 b | 7.87 f | 3.28 b | 40.7 d | 23.2 d | |
| D | 5% | 25.7 cd | 10.9 d | 3.35 de | 51.9 a | 32.2 a |
| 10% | 29.6 a | 14.4 b | 3.15 e | 51.6 ab | 32.3 a | |
| SEM | 0.557 | 0.293 | 0.355 | 2.647 | 1.155 | |
| p-value | T | 0.009 | <0.001 | <0.001 | <0.001 | <0.001 |
| R | <0.001 | <0.001 | <0.001 | 0.091 | 0.016 | |
| T*R | 0.06 | <0.001 | 0.002 | 0.108 | 0.002 |
| Treatment (T) | Ratio (R) | TN (% DM) | Composition of Total N (% TN) | ||||
|---|---|---|---|---|---|---|---|
| TP-N | NPN | NH3-N | FAA-N | Peptide-N | |||
| CK | 0% | 0.938 h | 38.8 i | 61.2 c | 2.94 a | 20.8 a | 37.4 a |
| G | 5% | 1.78 d | 63.9 de | 36.1 bc | 1.16 bcd | 5.79 e | 29.13 c |
| 10% | 2.62 a | 68.6 ab | 31.4 ef | 1.03 bcd | 2.71 f | 27.68 c | |
| B | 5% | 1.71 d | 71.7 a | 28.3 f | 1.40 abc | 6.14 e | 20.7 d |
| 10% | 2.23 bc | 68.9 ab | 31.1 ef | 0.881 cd | 3.55 f | 26.6 c | |
| Z | 5% | 1.43 e | 55.7 f | 44.3 a | 1.13 bcd | 8.68 d | 34.5 ab |
| 10% | 2.15 c | 61.0 e | 39.0 b | 0.721 d | 3.90 f | 34.3 ab | |
| W | 5% | 1.11 g | 56.2 f | 43.8 a | 1.60 ab | 14.2 b | 27.9 c |
| 10% | 1.26 f | 64.8 cd | 35.2 cd | 1.40 abc | 11.3 c | 22.6 d | |
| D | 5% | 1.74 d | 62.0 de | 38.0 bc | 1.84 a | 6.35 e | 29.8 bc |
| 10% | 2.30 b | 67.4 bc | 32.6 de | 1.99 a | 3.59 f | 27.0 c | |
| SEM | 0.0457 | 1.623 | 1.623 | 0.268 | 0.762 | 2.283 | |
| p-value | T | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| R | 0.073 | <0.001 | <0.001 | 0.038 | <0.001 | 0.367 | |
| T*R | <0.449 | <0.001 | <0.001 | 0.293 | 0.021 | 0.004 | |
| Treatments | Ratio | b (mL) | c (mL/h) | CH4 (mL) |
|---|---|---|---|---|
| CK | 40.0 bc | 0.556 bc | 3.06 bc | |
| G | 5% | 50.5 a | 0.701 a | 4.96 a |
| 10% | 36.4 bc | 0.506 bc | 2.11 c | |
| B | 5% | 39.1 bc | 0.543 bc | 2.52 bc |
| 10% | 39.9 bc | 0.555 bc | 3.55 bc | |
| Z | 5% | 38.2 bc | 0.530 bc | 2.67 bc |
| 10% | 42.0 bc | 0.583 bc | 3.61 b | |
| W | 5% | 44.6 ab | 0.619 ab | 3.16 bc |
| 10% | 40.6 bc | 0.564 bc | 2.52 bc | |
| D | 5% | 42.4 bc | 0.589 bc | 3.07 bc |
| 10% | 34.2 c | 0.475 c | 2.79 bc | |
| SEM | 2.533 | 0.035 | 0.438 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Na, B.; Lei, X.; Qian, Y.; Xie, Y.; Zheng, Y.; Cheng, Q.; Li, P.; Chen, C.; Yang, F.; et al. Effects of Different Types and Ratios of Dry Tea Residues on Nutrient Content, In Vitro Rumen Fermentation, and the Bacterial Community of Ensiled Sweet Sorghum. Microorganisms 2024, 12, 2178. https://doi.org/10.3390/microorganisms12112178
Zhou T, Na B, Lei X, Qian Y, Xie Y, Zheng Y, Cheng Q, Li P, Chen C, Yang F, et al. Effects of Different Types and Ratios of Dry Tea Residues on Nutrient Content, In Vitro Rumen Fermentation, and the Bacterial Community of Ensiled Sweet Sorghum. Microorganisms. 2024; 12(11):2178. https://doi.org/10.3390/microorganisms12112178
Chicago/Turabian StyleZhou, Tong, Binbin Na, Xingcheng Lei, Yuangan Qian, Yixiao Xie, Yulong Zheng, Qiming Cheng, Ping Li, Chao Chen, Fuyu Yang, and et al. 2024. "Effects of Different Types and Ratios of Dry Tea Residues on Nutrient Content, In Vitro Rumen Fermentation, and the Bacterial Community of Ensiled Sweet Sorghum" Microorganisms 12, no. 11: 2178. https://doi.org/10.3390/microorganisms12112178
APA StyleZhou, T., Na, B., Lei, X., Qian, Y., Xie, Y., Zheng, Y., Cheng, Q., Li, P., Chen, C., Yang, F., & Sun, H. (2024). Effects of Different Types and Ratios of Dry Tea Residues on Nutrient Content, In Vitro Rumen Fermentation, and the Bacterial Community of Ensiled Sweet Sorghum. Microorganisms, 12(11), 2178. https://doi.org/10.3390/microorganisms12112178






