Research on Pachymaran to Ameliorate CsA-Induced Immunosuppressive Lung Injury by Regulating Microflora Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Establishment of Animal Model
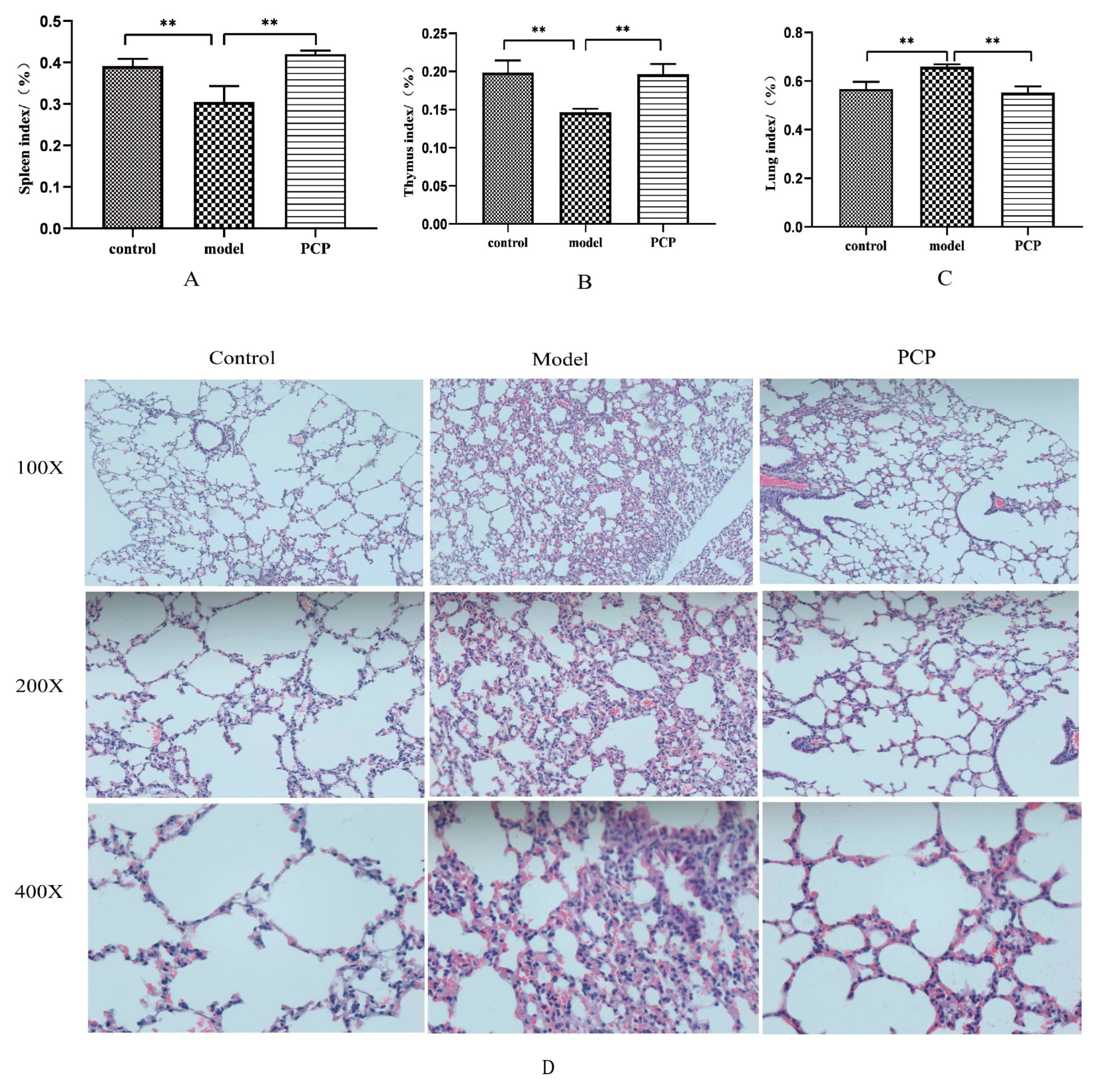
2.2. Each Organ Index
2.3. Analysis of the Hematoxylin–Eosin Staining of the Lungs
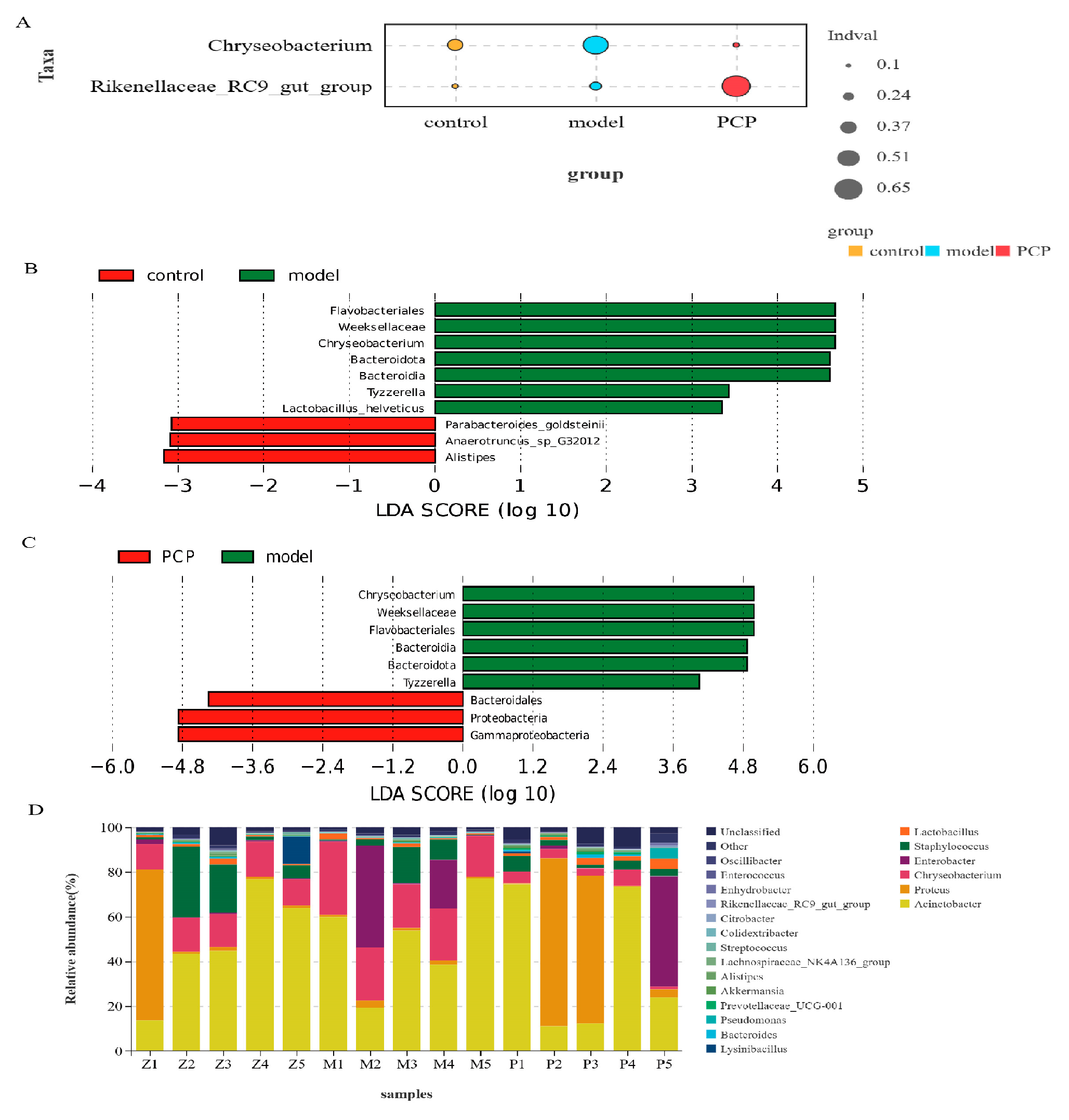
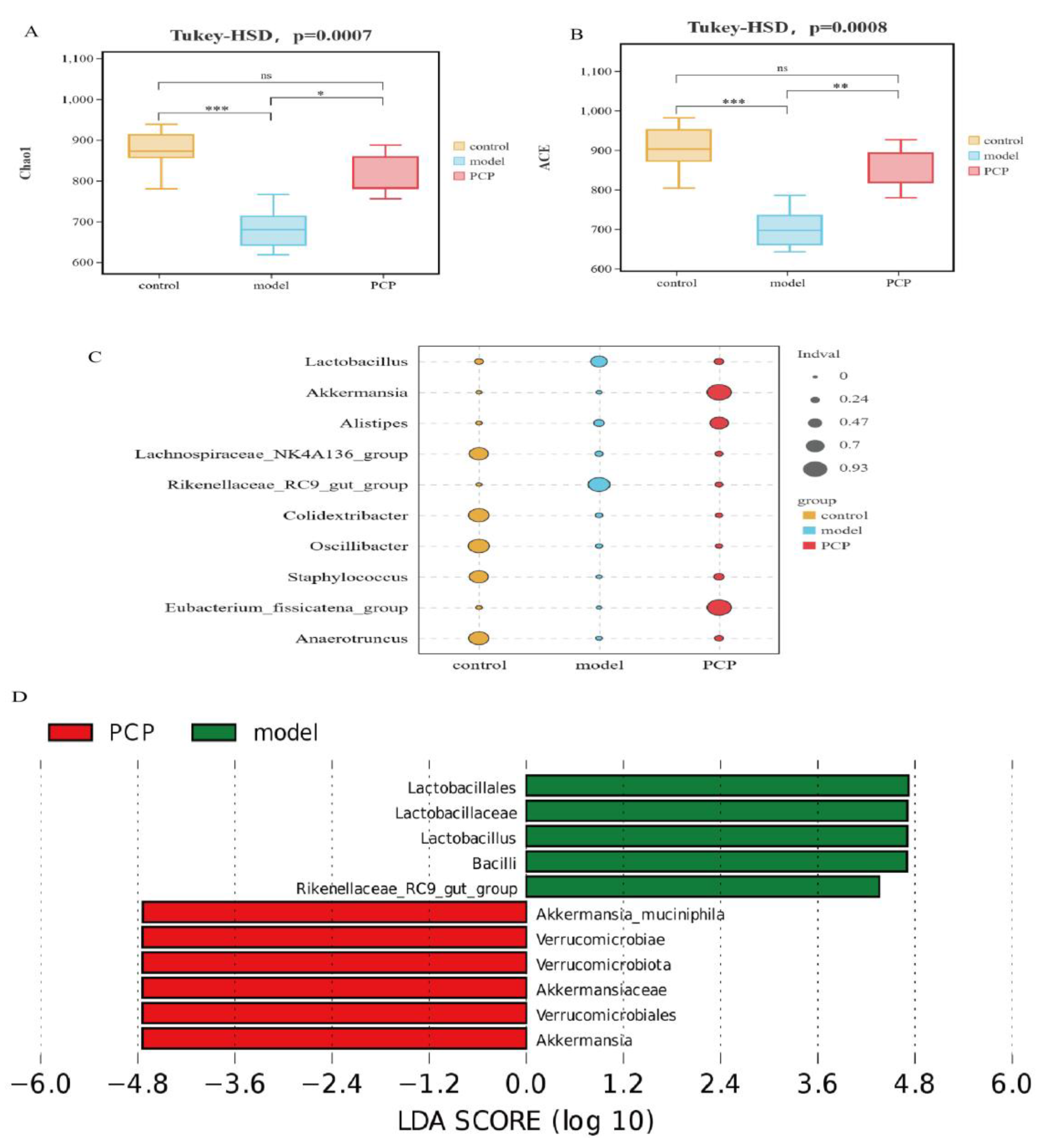
2.4. Detection of Colonic Fecal Matter and Lung Tissue via 16S rRNA Sequencing
2.5. Determination of Serum Metabolites via LC-MS
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Analysis of mRNA Expression via Quantitative PCR (qPCR)
2.8. Data Processing
3. Results
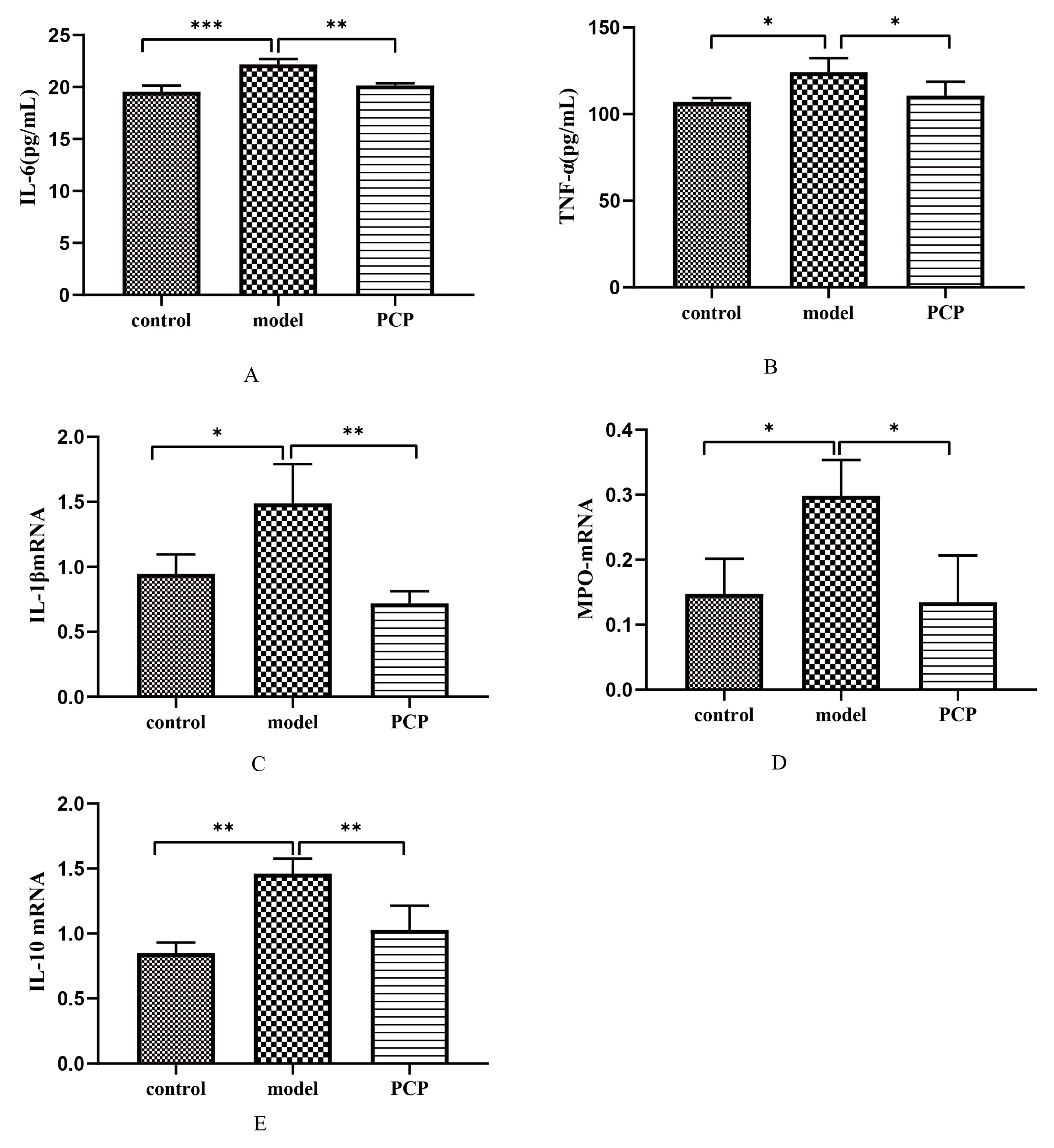
3.1. PCP Alleviates Immunosuppressive Lung Injury Caused by CsA
3.2. PCP Regulates the Microecological Diversity and Composition of Lung Tissue
3.3. PCP Regulates the Diversity and Composition of the Gut Microbiota
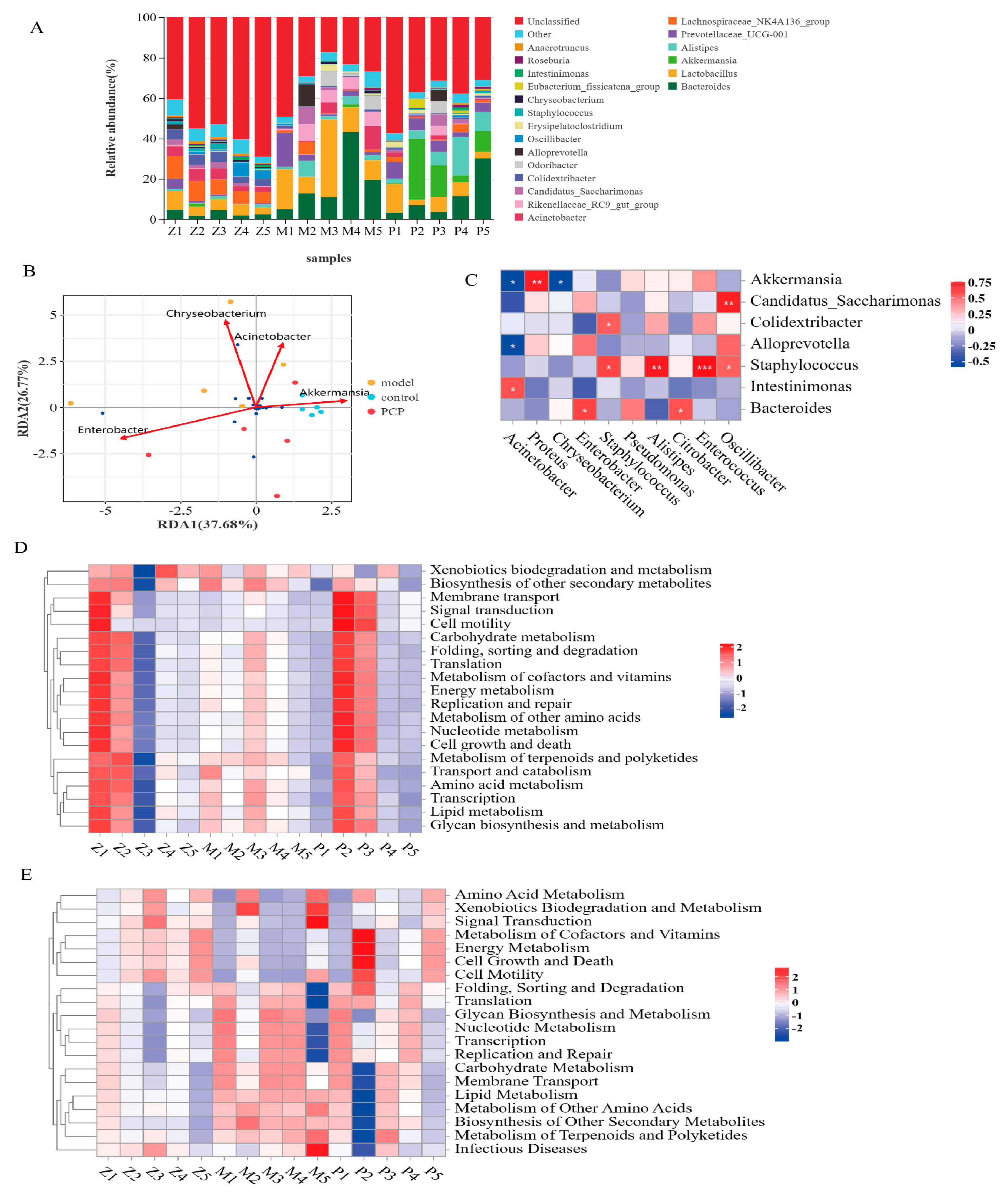
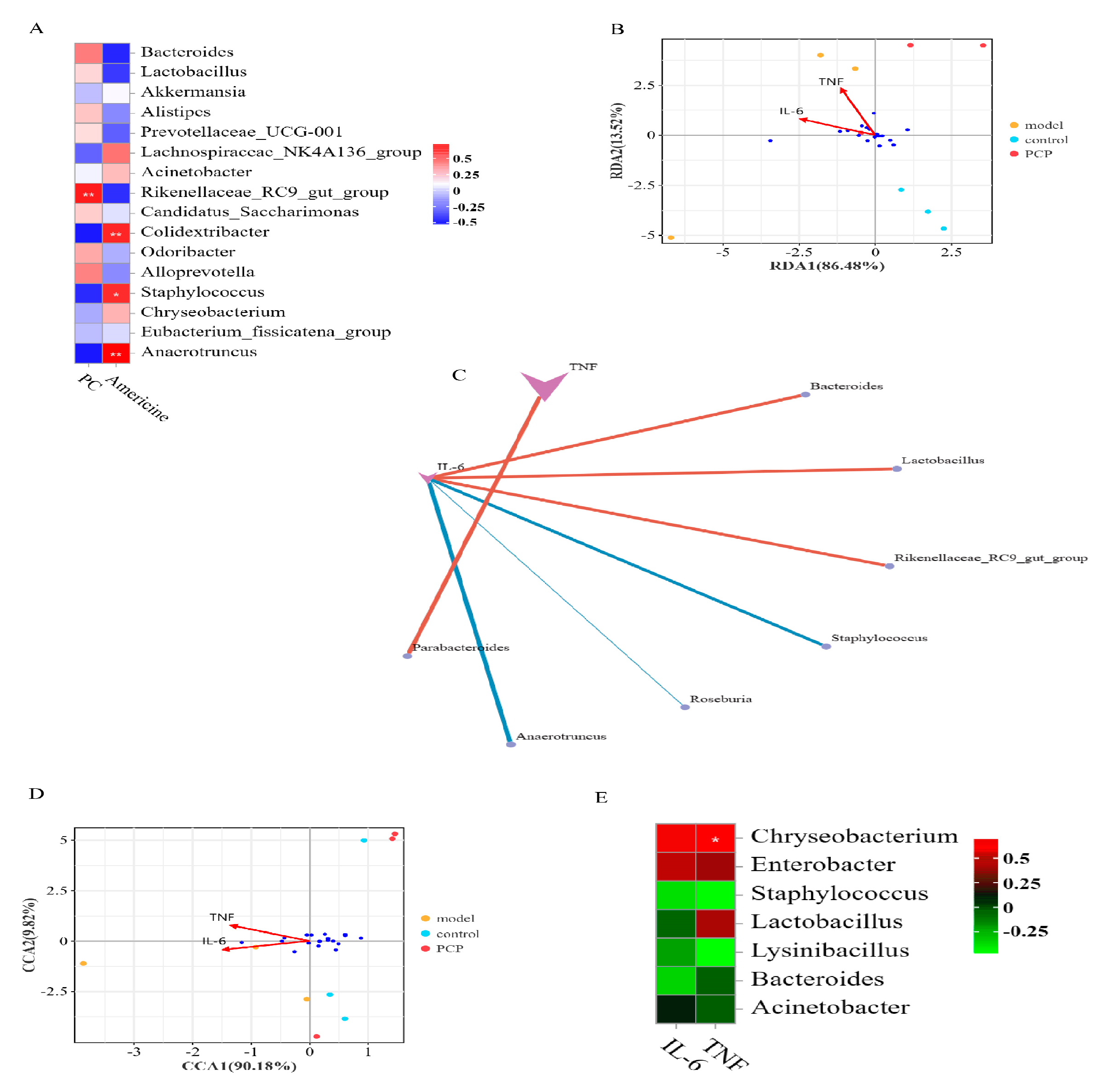
3.4. Analysis of the Relationship between Intestinal Flora and Lung Flora
3.5. Prediction and Analysis of Metabolic Function of Pulmonary Microflora
3.6. Prediction and Analysis of the Metabolic Function of the Intestinal Flora
3.7. Multivariate Statistical Analysis of the Distribution of Serum Metabolites and the Identification of Their Metabolites
3.8. Correlation Analysis of Microbiome and Metabolic Group
3.9. Analysis of the Relationship between Gut and Lung Flora and Cytokines
3.10. Effect of PCP on Cytokine Levels and Gene Expression in CsA-Treated Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, V. Pulmonary Innate Immune Response Determines the Outcome of Inflammation During Pneumonia and Sepsis-Associated Acute Lung Injury. Front. Immunol. 2020, 11, 1722. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Jiang, H.; Fang, J. Regulation of Immune Function by Polyphenols. J. Immunol. Res. 2018, 2018, 1264074. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiang, D.; Gao, F.; Yao, H.; Ye, Q.; Wang, Y. The Inhibition of P-Selectin Reduced Severe Acute Lung Injury in Immunocompromised Mice. Oxidative Med. Cell. Longev. 2020, 2020, 8430465. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Shishi, Z.; Bo, W.; Huiqing, L. Targeting immunometabolism against acute lung injury. Clin. Immunol. 2023, 249, 109289. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Zhang, J.; Zhao, Y.-L.; Li, T.; Shen, T.; Li, J.-Q.; Li, W.-Y.; Liu, H.-G. Mycology, cultivation, traditional uses, phytochemistry and pharmacology of Wolfiporia cocos (Schwein.) Ryvarden et Gilb.: A review. J. Ethnopharmacol. 2013, 147, 265–276. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Xu, X.; Zhang, X. Purification, antitumor and anti-inflammation activities of an alkali-soluble and carboxymethyl polysaccharide CMP33 from Poria cocos. Int. J. Biol. Macromol. 2019, 127, 39–47. [Google Scholar] [CrossRef]
- Wang, J.; Wang, A.; He, H.; She, X.; He, Y.; Li, S.; Liu, L.; Luo, T.; Huang, N.; Zou, K.; et al. Trametenolic acid B protects against cerebral ischemia and reperfusion injury through modulation of microRNA-10a and PI3K/Akt/mTOR signaling pathways. Biomed. Pharmacother. 2019, 112, 108692. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, M.; Xie, S.; Peng, D.; An, M.; Chen, Y.; Li, P.; Du, B. Effect of steam explosion pretreatment on polysaccharide isolated from Poria cocos: Structure and immunostimulatory activity. J. Food Biochem. 2022, 46, e14355. [Google Scholar] [CrossRef]
- Nataraj, A.; Govindan, S.; Ramani, P.; Subbaiah, K.A.; Sathianarayanan, S.; Venkidasamy, B.; Thiruvengadam, M.; Rebezov, M.; Shariati, M.A.; Lorenzo, J.M.; et al. Antioxidant, Anti-Tumour, and Anticoagulant Activities of Polysaccharide from Calocybe indica (APK2). Antioxidants 2022, 11, 1694. [Google Scholar] [CrossRef]
- Wu, Y.; Li, D.; Wang, H.; Wan, X. Protective Effect of Poria Cocos Polysaccharides on Fecal Peritonitis-Induced Sepsis in Mice through Inhibition of Oxidative Stress, Inflammation, Apoptosis, and Reduction of Treg Cells. Front. Microbiol. 2022, 13, 887949. [Google Scholar]
- Mousa, W.K.; Chehadeh, F.; Husband, S. Microbial dysbiosis in the gut drives systemic autoimmune diseases. Front. Immunol. 2022, 13, 906258. [Google Scholar] [CrossRef]
- Mjösberg, J.; Rao, A. Lung inflammation originating in the gut. Science 2018, 359, 36–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xie, J.; Yang, Y.; Zhang, F.; Wang, S.; Wu, T.; Shen, M.; Xie, M. Sulfated Cyclocarya paliurus polysaccharides markedly attenuates inflammation and oxidative damage in lipopolysaccharide-treated macrophage cells and mice. Sci. Rep. 2017, 7, 40402. [Google Scholar] [CrossRef] [PubMed]
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, M.; Corbera, J.A.; Suárez-Bonnet, A.; Tejedor-Junco, M.T. Virulence factors in coagulase-positive staphylococci of veterinary interest other than Staphylococcus aureus. Veter-Q. 2020, 40, 118–131. [Google Scholar] [CrossRef]
- Stopińska, K.; Radziwoń-Zaleska, M.; Domitrz, I. The Microbiota-Gut-Brain Axis as a Key to Neuropsychiatric Disorders: A Mini Review. J. Clin. Med. 2021, 10, 4640. [Google Scholar] [CrossRef]
- Lukasova, M.; Malaval, C.; Gille, A.; Kero, J.; Offermanns, S. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J. Clin. Investig. 2011, 121, 1163–1173. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Q.; Cheng, L.; Buch, H.; Zhang, F. Akkermansia muciniphila is a promising probiotic. Microb. Biotechnol. 2019, 12, 1109–1125. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Cani, P.D.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Yang, B.; Li, M.; Wang, S.; Ross, R.P.; Stanton, C.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus ruminis Alleviates DSS-Induced Colitis by Inflammatory Cytokines and Gut Microbiota Modulation. Foods 2021, 10, 1349. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Shu-Guang, H.E. Distribution and drug resistance of 216 strains of Chryseobacterium causing nosocomial infection. Chin. J. Nosocomiology 2012, 22, 3663. [Google Scholar]
- Cong, S.; Wang, L.; Meng, Y.; Cai, X.; Zhang, C.; Gu, Y.; Ma, X.; Luo, L. Saussurea involucrata oral liquid regulates gut microbiota and serum metabolism during alleviation of collagen-induced arthritis in rats. Phytother. Res. 2023, 37, 1242–1259. [Google Scholar] [CrossRef]
- Zong, S.; Ye, H.; Ye, Z.; He, Y.; Zhang, X.; Ye, M. Polysaccharides from Lachnum sp. Inhibited colitis-associated colon tumorigenesis in mice by modulating fecal microbiota and metabolites. Int. Immunopharmacol. 2022, 108, 108656. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, X.; Maruyama, D.; Arjomandi, M.; Prakash, A. Lung immune tone via gut-lung axis: Gut-derived LPS and short-chain fatty acids’ immunometabolic regulation of lung IL-1β, FFAR2, and FFAR3 expression. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2021, 321, L65–L78. [Google Scholar] [CrossRef] [PubMed]
- Ebeid, T.A.; Al-Homidan, I.H. Organic acids and their potential role for modulating the gastrointestinal tract, antioxidative status, immune response, and performance in poultry. World’s Poult. Sci. J. 2022, 78, 83–101. [Google Scholar] [CrossRef]
- Li, L.; Fu, W.W.; Wu, R.T.; Song, Y.H.; Wu, W.Y.; Yin, S.H.; Li, W.J.; Xie, M.Y. Protective effect of Ganoderma atrum polysaccharides in acute lung injury rats and its metabolomics. Int. J. Biol. Macromol. 2020, 142, 693–704. [Google Scholar] [CrossRef]
- Murakami, Y.; Hoshi, M.; Hara, A.; Takemura, M.; Arioka, Y.; Yamamoto, Y.; Matsunami, H.; Funato, T.; Seishima, M.; Saito, K. Inhibition of increased indoleamine 2,3-dioxygenase activity attenuates Toxoplasma gondii replication in the lung during acute infection. Cytokine 2012, 59, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Swolana, D.; Wojtyczka, R.D. Activity of Silver Nanoparticles against Staphylococcus spp. Int. J. Mol. Sci. 2022, 23, 4298. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhang, J.; Zhang, X.; Li, C.; Wang, Q.; Meng, G.; Kan, X.; Zhang, J.; Jia, Y. Oxypeucedanin relieves LPS-induced acute lung injury by inhibiting the inflammation and maintaining the integrity of the lung air-blood barrier. Aging 2022, 14, 6626–6641. [Google Scholar] [CrossRef]
- Fujishima, S.; Aikawa, N. Neutrophil-mediated tissue injury and its modulation. Intensive Care Med. 1995, 21, 277–285. [Google Scholar] [CrossRef]
- Meng Yun, L.B. The Correlation Between the Levels of Blood Uric Acid IL-2 IL-6 and TNF-αand Scores of Depressive Symptoms and Somatiization Sympton in Patiens with Depression. Hebei Med. 2021, 27, 1647–1652. [Google Scholar]
- Wang, H.; Zhou, C.; Huang, J.; Kuai, X.; Shao, X. The potential therapeutic role of Lactobacillus reuteri for treatment of inflammatory bowel disease. Am. J. Transl. Res. 2020, 12, 1569–1583. [Google Scholar] [PubMed]

| Gene | Upstream Primer | Downstream Primer |
|---|---|---|
| IL-1β | 5′-AAAGCTCTCCACCTCAATGG-3′ | 5′-CCCAAGGCCACAGGTATTT-3′ |
| MPO | 5′-AGATCCGGGAGCGACTATTT-3′ | 5′-GCGTCTCCAGGCATTGTATC-3′ |
| IL-10 | 5′-TTGAATTCCCTGGGTGAGAAG-3′ | 5′-TCCACTGCCTTGCTCTTATTT-3′ |
| Group | Weight | Group | Weight | Group | Weight |
|---|---|---|---|---|---|
| Z1 | 23 | M1 | 25 | P1 | 25 |
| Z2 | 22 | M2 | 22 | P2 | 25 |
| Z3 | 21 | M3 | 23 | P3 | 24 |
| Z4 | 24 | M4 | 23 | P4 | 24 |
| Z5 | 25 | M5 | 22 | P5 | 24 |
| Z6 | 22 | M6 | 24 | P6 | 25 |
| Z7 | 24.6 | M7 | 23 | P7 | 25 |
| Z8 | 22 | M8 | 23 | P8 | 26 |
| Z9 | 24 | M9 | 24 | P9 | 24 |
| Z10 | 23 | M10 | 24 | P10 | 25 |
| Z11 | 24 | M11 | 22 | P11 | 26 |
| Z12 | 22 | M12 | 24 | P12 | 24 |
| Z13 | 24 | M13 | 23 | P13 | 24 |
| Z14 | 24 | M14 | 21 | P14 | 23 |
| Z15 | 25 | M14 | 22 | P15 | 24 |
| Group | Shannon | Simpson | Chao | ACE |
|---|---|---|---|---|
| Control | 2.40 ± 0.78 | 0.62 ± 0.16 | 415.40 ± 52.30 | 434.39 ± 61.49 |
| Model | 2.28 ± 0.40 | 0.68 ± 0.08 | 395.63 ± 69.93 | 414.80 ± 76.38 |
| PCP | 2.56 ± 0.60 | 0.58 ± 0.14 | 500.82 ± 158.90 | 515.91 ± 168.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, C.; Gao, Z.-H.; Chen, K.-Q.; Lu, F.-G.; Wei, K. Research on Pachymaran to Ameliorate CsA-Induced Immunosuppressive Lung Injury by Regulating Microflora Metabolism. Microorganisms 2023, 11, 2249. https://doi.org/10.3390/microorganisms11092249
Ye C, Gao Z-H, Chen K-Q, Lu F-G, Wei K. Research on Pachymaran to Ameliorate CsA-Induced Immunosuppressive Lung Injury by Regulating Microflora Metabolism. Microorganisms. 2023; 11(9):2249. https://doi.org/10.3390/microorganisms11092249
Chicago/Turabian StyleYe, Chun, Zi-Han Gao, Kai-Qin Chen, Fang-Guo Lu, and Ke Wei. 2023. "Research on Pachymaran to Ameliorate CsA-Induced Immunosuppressive Lung Injury by Regulating Microflora Metabolism" Microorganisms 11, no. 9: 2249. https://doi.org/10.3390/microorganisms11092249
APA StyleYe, C., Gao, Z.-H., Chen, K.-Q., Lu, F.-G., & Wei, K. (2023). Research on Pachymaran to Ameliorate CsA-Induced Immunosuppressive Lung Injury by Regulating Microflora Metabolism. Microorganisms, 11(9), 2249. https://doi.org/10.3390/microorganisms11092249








