Phosphate-Solubilizing Bacteria with Low-Solubility Fertilizer Improve Soil P Availability and Yield of Kikuyu Grass
Abstract
1. Introduction
2. Materials and Methods
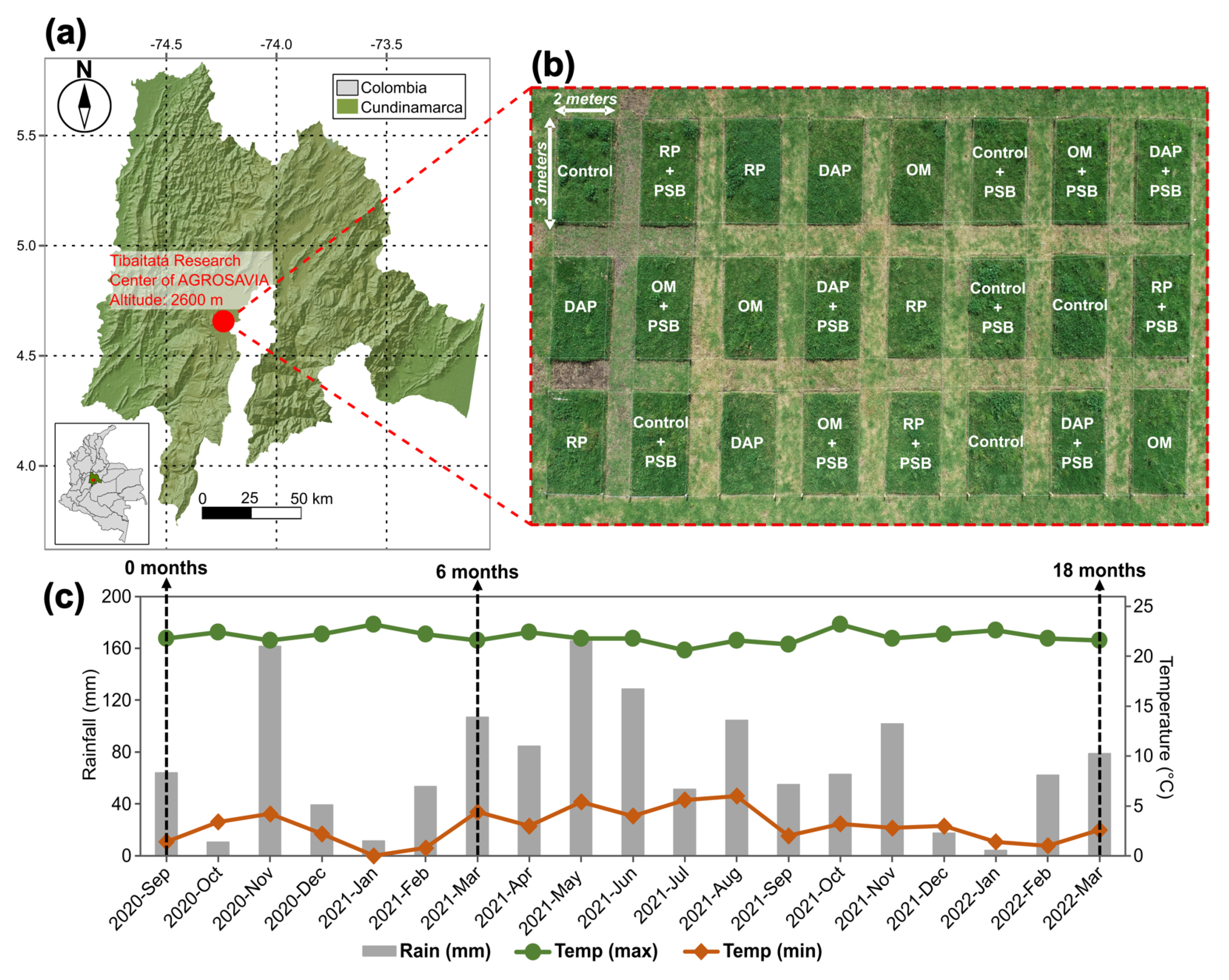
2.1. Description of the Experimental Site
2.2. Soil P Fractions
2.3. Enzymatic Activity
2.4. Productive Parameters
2.5. Statistical Analysis
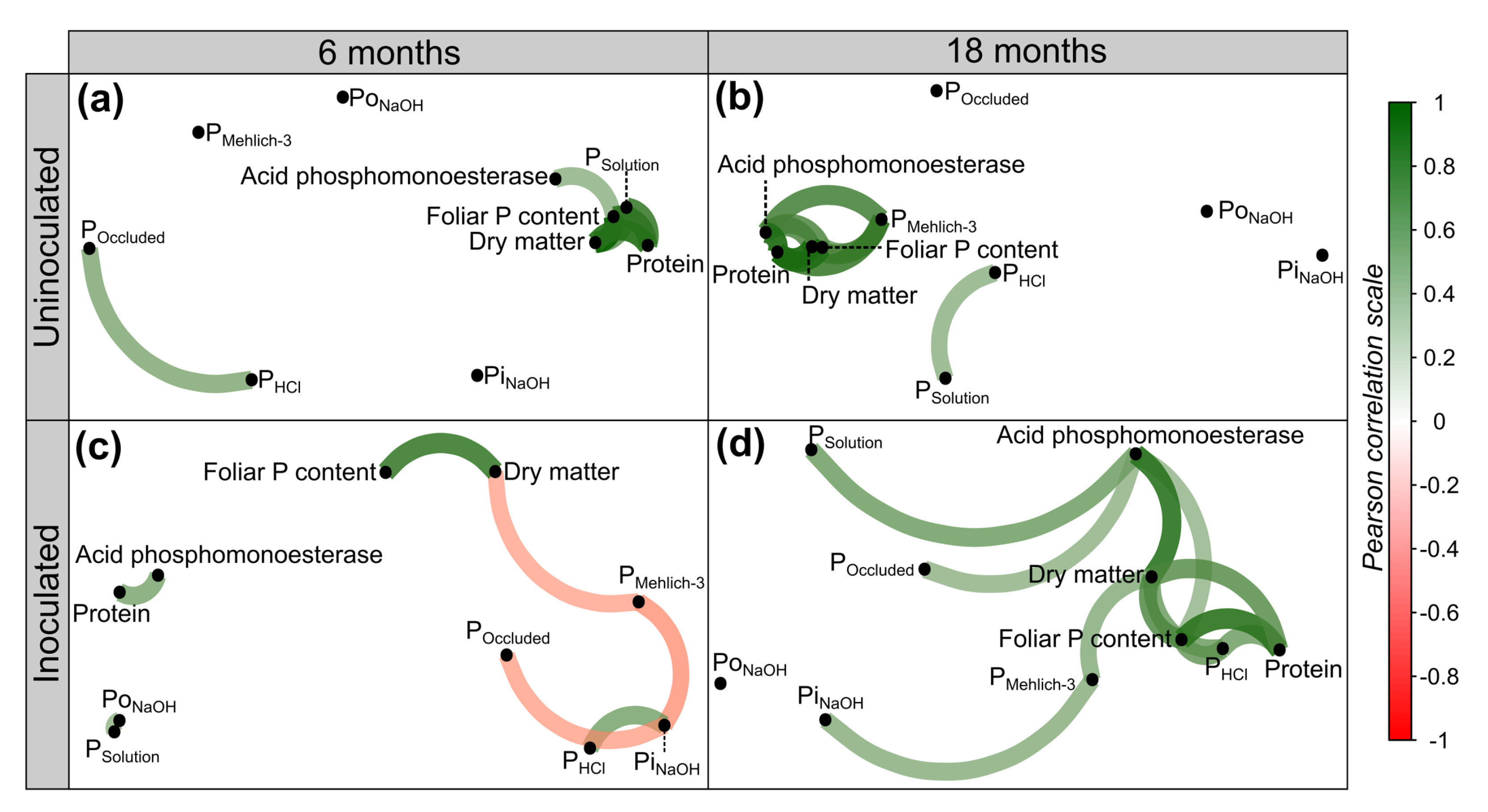
3. Results
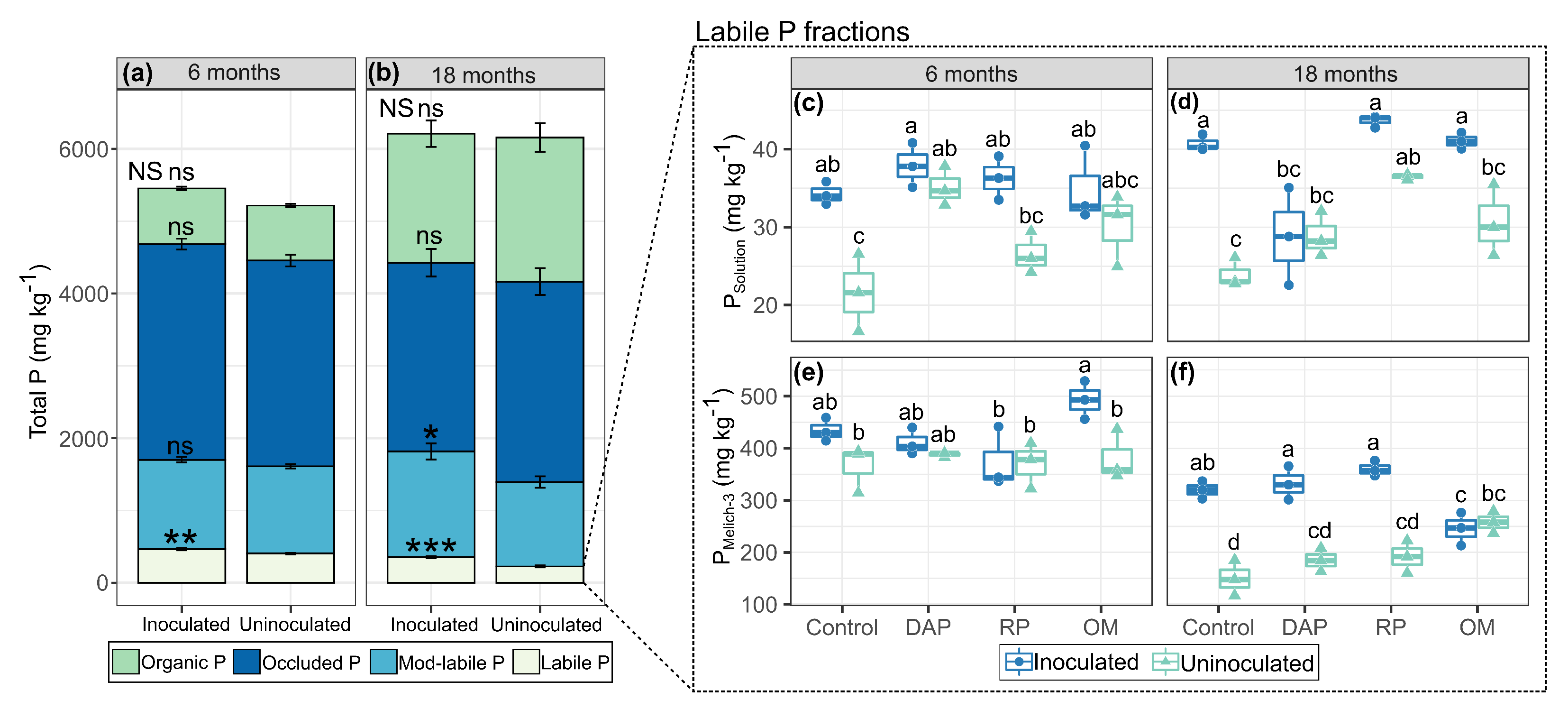
3.1. Dynamics of P Fractions
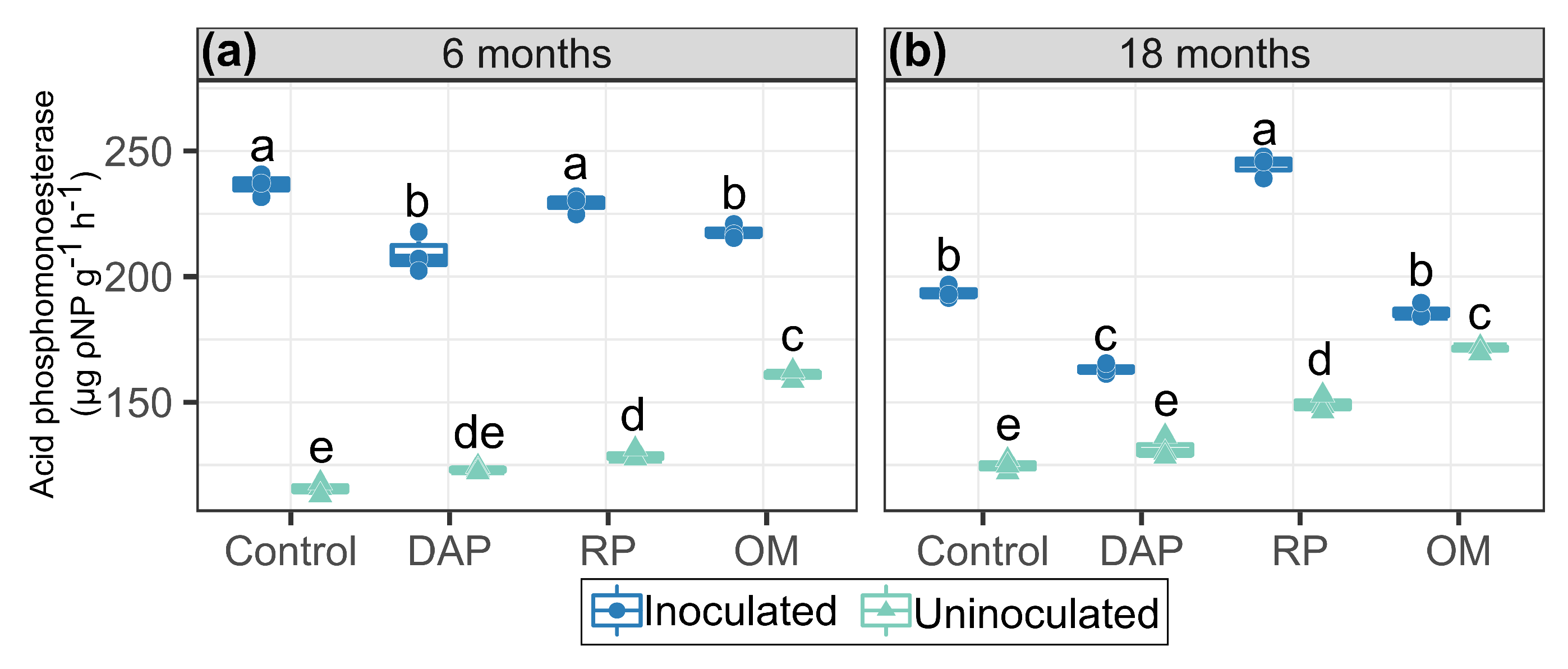
3.2. Phosphomonoesterase Activity in Response to PSB Inoculation and P Sources
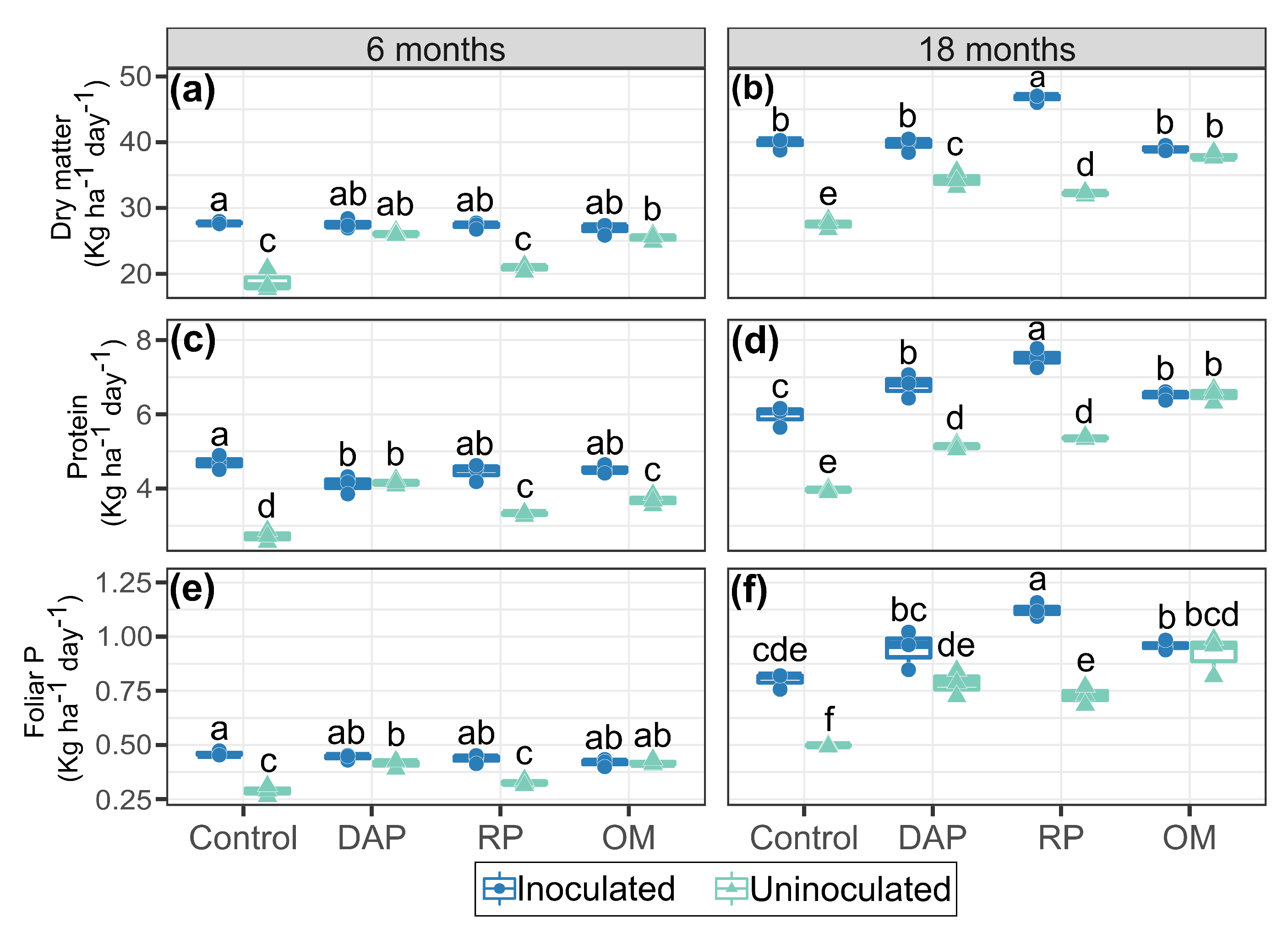
3.3. Productivity and Quality of Kikuyu Grass
4. Discussion
4.1. Soil P Dynamics
4.2. Acid Phosphomonoesterase Activity
4.3. Productive Parameters of Kikuyu Grass
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| P | Phosphorus |
| Pi | Inorganic phosphorus |
| Po | Organic phosphorus |
| PSB | Phosphate-solubilizing bacteria |
| OM | Organic matter |
| RP | Rock phosphate |
| DAP | Diammonium phosphate |
| pH | Hydrogen potential |
| Ca | Calcium |
| Mg | Magnesium |
| K | Potassium |
| NP | -nitrophenyl phosphate |
Appendix A
Appendix A.1
| P Source | PSB | Moderately Labile P | Organic P | Occluded P | |
|---|---|---|---|---|---|
| P | Pi | Po | |||
| 6 months | |||||
| Control | Uninoculated | 267.89 (15.37) b | 843.98 (9.16) a | 711.94 (55.78) a | 2711.70 (83.00) a |
| Inoculated | 254.04 (4.25) b | 900.85 (33.03) a | 696.29 (32.30) a | 3016.83 (142.43) a | |
| Uninoculated | 385.58 (24.17) a | 950.50 (18.96) a | 754.88 (70.00) a | 3134.90 (188.26) a | |
| Inoculated | 362.10 (20.35) a | 1028.49 (48.66) a | 785.36 (18.06) a | 3072.36 (60.44) a | |
| Uninoculated | 272.17 (2.35) b | 917.10 (48.35) a | 834.74 (22.63) a | 2726.90 (210.79) a | |
| Inoculated | 284.74 (19.24) b | 896.34 (79.98) a | 818.67 (86.39) a | 3015.48 (239.69) a | |
| Uninoculated | 267.49 (8.08) b | 918.91 (36.14) a | 735.96 (37.38) a | 2810.62 (33.54) a | |
| Inoculated | 277.82 (10.69) b | 944.18 (29.35) a | 781.28 (35.79) a | 2819.61 (151.99) a | |
| 18 months | |||||
| Control | Uninoculated | 264.64 (26.46) b | 684.94 (181.38) a | 1643.79 (635.96) a | 2676.70 (548.61) a |
| Inoculated | 362.26 (35.18) ab | 1075.97 (116.22) a | 1878.27 (338.83) a | 2868.20 (94.91) a | |
| Uninoculated | 439.35 (76.46) a | 899.05 (206.38) a | 2088.07 (560.02) a | 2690.64 (379.06) a | |
| Inoculated | 474.06 (33.30) a | 1148.12 (237.64) a | 1732.26 (519.53) a | 3162.63 (470.73) a | |
| Uninoculated | 374.73 (17.75) ab | 713.09 (172.02) a | 2162.92 (243.19) a | 3268.79 (232.59) a | |
| Inoculated | 386.22 (10.15) ab | 794.34 (112.10) a | 1817.04 (318.03) a | 2247.60 (158.58) a | |
| Uninoculated | 328.67 (12.72) ab | 964.04 (6.25) a | 2074.74 (134.13) a | 2450.76 (264.51) a | |
| Inoculated | 415.19 (2.08) ab | 1198.73 (333.12) a | 1713.24 (491.06) a | 2156.32 (422.42) a | |
Appendix A.2
| P Source | PSB | pH | OM | S | Ca | Mg | K |
|---|---|---|---|---|---|---|---|
| - | |||||||
| 6 months | |||||||
| Control | Uninoculated | 6.020 (0.015) d | 10.890 (0.281) a | 16.930 (0.342) e | 15.590 (0.662) c | 7.100 (0.317) ab | 2.983 (0.121) b |
| Inoculated | 6.247 (0.012) b | 7.930 (0.253) c | 20.877 (0.292) d | 19.197 (0.838) a | 7.853 (0.417) ab | 4.187 (0.205) a | |
| RP | Uninoculated | 6.073 (0.035) cd | 10.847 (0.193) a | 16.677 (0.217) e | 18.950 (0.913) ab | 6.993 (0.432) ab | 3.387 (0.169) b |
| Inoculated | 6.387 (0.022) a | 8.980 (0.609) bc | 24.403(0.680) c | 18.763 (0.296) ab | 8.243 (0.347) a | 4.447 (0.168) a | |
| OM | Uninoculated | 6.130 (0.017) cd | 11.477 (0.322) a | 29.340 (0.809) b | 18.200 (0.578) abc | 8.770 (0.304) a | 4.503 (0.169) a |
| Inoculated | 6.460 (0.031) a | 10.450 (0.194) ab | 34.013 (0.194) a | 18.663 (0.436) ab | 8.390 (0.480) a | 4.627 (0.062) a | |
| DAP | Uninoculated | 6.093 (0.023) cd | 10.517 (0.298) ab | 17.697 (0.472) e | 16.077 (0.164) bc | 6.327 (0.292) b | 3.360 (0.220) b |
| Inoculated | 6.133 (0.032) c | 8.337 (0.262) c | 22.160 (0.321) d | 19.060 (0.467) a | 7.947 (0.345) ab | 3.337 (0.107) b | |
| 18 months | |||||||
| Control | Uninoculated | 6.02 (0.006) de | 11.097 (0.339) c | 17.883 (0.189) e | 16.070 (0.468) d | 6.563 (0.230) ab | 2.530 (0.119) b |
| Inoculated | 6.303 (0.023) b | 7.453 (0.168) d | 21.240 (0.571) d | 20.143 (0.712) ab | 7.020 (0.185) a | 3.610 (0.108) a | |
| RP | Uninoculated | 6.007 (0.023) de | 12.617 (0.300) b | 17.810 (0.631) e | 19.370 (0.262) abc | 5.193 (0.116) c | 2.493 (0.273) b |
| Inoculated | 6.343 (0.012) ab | 8.280 (0.248) d | 25.917 (0.884) bc | 20.677 (0.428) a | a | a | |
| OM | Uninoculated | 6.177 (0.018) c | 13.957 (0.282) a | 28.047 (0.484) b | 20.290 (0.430) ab | 7.170 (0.078) a | 3.480 (0.191) ab |
| Inoculated | 6.423 (0.012) a | 11.110 (0.270) c | 35.403 (0.338) a | 21.040 (0.191) a | 7.507 (0.307) a | 4.280 (0.118) a | |
| DAP | Uninoculated | 5.983 (0.043) e | 11.377 (0.164) bc | 17.913 (0.515) e | 17.480 (0.450) cd | 5.557 (0.398) bc | 2.453 (0.171) b |
| Inoculated | 6.103 (0.009) cd | 8.157 (0.292) d | 24.760 (0.330) c | 18.277 (0.569) bcd | 6.653 (0.084) a | 3.707 (0.197) a | |
References
- Correa, H.; Pabón, M.; Carulla, J. Valor nutricional del pasto kikuyo (Pennisetum clandestinum Hoechst Ex Chiov.) para la producción de leche en Colombia (Una revisión): I-Composición química y digestibilidad ruminal y posruminal. Livest. Res. Rural. Dev. 2008, 20, 59. [Google Scholar]
- Vargas Martínez, J.d.J.; Sierra Alarcón, A.M.; Mancipe Muñoz, E.A.; Avellaneda Avellaneda, Y. El kikuyo, una gramínea presente en los sistemas de rumiantes en trópico alto colombiano. CES Med. Vet. Zootec. 2018, 13, 137–156. [Google Scholar] [CrossRef]
- Chaverra, H.; Dávila, V.; Villamizar, F.; Bernal, J. El Cultivo de los Pastos en la Sabana de Bogotá: Cursillo Sobre Manejo de Praderas y Cultivo de Pastos de Clima frío; Sociedad de Agricultores de Colombia. Aedita Editores Ltda., Bogotá, COL: Bogotá, Cundinamarca, 1967. [Google Scholar]
- Carulla, J.; Cárdenas, E.; Sánchez, N.; Riveros, C. Valor nutricional de los forrajes más usados en los sistemas de producción lechera especializada de la zona andina colombiana. Semin. Nac. Leche. Espec. Nutr. Impacto Product. MedelliN Septiembre 2004, 1, 21–38. [Google Scholar]
- Quintero, C.; Boschetti, N. Manejo del Fósforo en Pasturas; Misterio de Producción, Instituto Nacional de Tecnología Agropecuaria: Buenos Aires, Argentina, 2005.
- Marais, J. Factors affecting the nutritive value of kikuyu grass (Pennisetum clandestinum)—A review. Trop. Grasslands 2001, 35, 65–84. [Google Scholar]
- Walpola, B.C.; Yoon, M.H. Prospectus of phosphate solubilizing microorganisms and phosphorus availability in agricultural soils: A review. Afr. J. Microbiol. Res. 2012, 6, 6600–6605. [Google Scholar]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus 2013, 2, 1–14. [Google Scholar] [CrossRef]
- Huygens, D.; Boeckx, P.; Van Cleemput, O.; Oyarzun, C.; Godoy, R. Aggregate and soil organic carbon dynamics in South Chilean Andisols. Biogeosciences 2005, 2, 159–174. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Wang, Y.; Zhang, H.; Zhu, Q.; Yan, B.; Fei, J.; Xiangmin, R.; Peng, J.; Luo, G. Intercropping regulation of soil phosphorus composition and microbially-driven dynamics facilitates maize phosphorus uptake and productivity improvement. Field Crop. Res. 2022, 287, 108666. [Google Scholar] [CrossRef]
- Escudey, M.; Galindo, G.; Förster, J.E.; Briceño, M.; Diaz, P.; Chang, A. Chemical forms of phosphorus of volcanic ash-derived soils in Chile. Commun. Soil Sci. Plant Anal. 2001, 32, 601–616. [Google Scholar] [CrossRef]
- Borie, F.; Rubio, R. Total and organic phosphorus in chilean volcanic soils fosforo total y fosforo organico en suelos volcánicos de chile. Gayana Bot 2003, 60, 69–78. [Google Scholar] [CrossRef]
- Briceño, M.; Escudey, M.; Galindo, G.; Borchardt, D.; Chang, A. Characterization of chemical phosphorus forms in volcanic soils using 31P-NMR spectroscopy. Commun. Soil Sci. Plant Anal. 2004, 35, 1323–1337. [Google Scholar] [CrossRef]
- Redel, Y.; Staunton, S.; Durán, P.; Gianfreda, L.; Rumpel, C.; de La Luz Mora, M. Fertilizer P uptake determined by soil P fractionation and phosphatase activity. J. Soil Sci. Plant Nutr. 2019, 19, 166–174. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Baillie, I.; Jien, S.H.; Hallett, L.; Hallett, S. Sequestration of P fractions in the soils of an incipient ferralisation chronosequence on a humid tropical volcanic island. Bot. Stud. 2021, 62, 20. [Google Scholar] [CrossRef]
- Sattari, S.Z.; Bouwman, A.F.; Giller, K.E.; van Ittersum, M.K. Residual soil phosphorus as the missing piece in the global phosphorus crisis puzzle. Proc. Natl. Acad. Sci. USA 2012, 109, 6348–6353. [Google Scholar] [CrossRef]
- Silva, C.F.d.; Vitorino, L.; Mendonça, M.; Araújo, W.; Dourado, M.; Albuquerque, L.; Soares, M.; Souchie, E. Screening of plant growth-promoting endophytic bacteria from the roots of the medicinal plant Aloe vera. S. Afr. J. Bot. 2020, 134, 3–16. [Google Scholar] [CrossRef]
- Estrada-Bonilla, G.A.; Durrer, A.; Cardoso, E.J. Use of compost and phosphate-solubilizing bacteria affect sugarcane mineral nutrition, phosphorus availability, and the soil bacterial community. Appl. Soil Ecol. 2021, 157, 103760. [Google Scholar] [CrossRef]
- Romero-Perdomo, F.; Beltrán, I.; Mendoza-Labrador, J.; Estrada-Bonilla, G.; Bonilla, R. Phosphorus nutrition and growth of cotton plants inoculated with growth-promoting bacteria under low phosphate availability. Front. Sustain. Food Syst. 2021, 4, 618425. [Google Scholar] [CrossRef]
- Santos-Torres, M.; Romero-Perdomo, F.; Mendoza-Labrador, J.; Gutiérrez, A.Y.; Vargas, C.; Castro-Rincon, E.; Caro-Quintero, A.; Uribe-Velez, D.; Estrada-Bonilla, G.A. Genomic and phenotypic analysis of rock phosphate-solubilizing rhizobacteria. Rhizosphere 2021, 17, 100290. [Google Scholar] [CrossRef]
- Schneider, K.D.; Thiessen Martens, J.R.; Zvomuya, F.; Reid, D.K.; Fraser, T.D.; Lynch, D.H.; O’Halloran, I.P.; Wilson, H.F. Options for improved phosphorus cycling and use in agriculture at the field and regional scales. J. Environ. Qual. 2019, 48, 1247–1264. [Google Scholar] [CrossRef]
- Soltangheisi, A.; Rodrigues, M.; Coelho, M.J.A.; Gasperini, A.M.; Sartor, L.R.; Pavinato, P.S. Changes in soil phosphorus lability promoted by phosphate sources and cover crops. Soil Tillage Res. 2018, 179, 20–28. [Google Scholar] [CrossRef]
- Lopes, C.M.; Silva, A.M.M.; Estrada-Bonilla, G.A.; Ferraz-Almeida, R.; Vieira, J.L.V.; Otto, R.; Vitti, G.C.; Cardoso, E.J.B.N. Improving the fertilizer value of sugarcane wastes through phosphate rock amendment and phosphate-solubilizing bacteria inoculation. J. Clean. Prod. 2021, 298, 126821. [Google Scholar] [CrossRef]
- Cortés-Patiño, S.; Vargas, C.; Álvarez-Flórez, F.; Bonilla, R.; Estrada-Bonilla, G. Potential of Herbaspirillum and Azospirillum consortium to promote growth of perennial ryegrass under water deficit. Microorganisms 2021, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Patiño, S.; Vargas, C.D.; Alvarez-Flórez, F.; Estrada-Bonilla, G. Co-Inoculation of Plant-Growth-Promoting Bacteria Modulates Physiological and Biochemical Responses of Perennial Ryegrass to Water Deficit. Plants 2022, 11, 2543. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Díaz, S.; Romero-Perdomo, F.; Mendoza-Labrador, J.; Delgadillo-Duran, D.; Castro-Rincon, E.; Silva, A.M.; Rojas-Tapias, D.F.; Cardoso, E.J.; Estrada-Bonilla, G.A. Endophytic PGPB improves plant growth and quality, and modulates the bacterial community of an intercropping system. Front. Sustain. Food Syst. 2021, 5, 354. [Google Scholar] [CrossRef]
- Ministerio de Vivienda, Ciudad y Territorio Corporación Autónoma Regional de Cundinamarca—CAR. Levantamiento Detallado de Suelos en las áreas Planas de los 14 Municipios de la 436 Sabana de Bogotá, Departamento de Cundinamarca, Escala 1:10.000. 2012. Available online: https://sie.car.gov.co/handle/20.500.11786/37174 (accessed on 20 June 2023).
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Gatiboni, L.C.; Condron, L.M. A rapid fractionation method for assessing key soil phosphorus parameters in agroecosystems. Geoderma 2021, 385, 114893. [Google Scholar] [CrossRef]
- Dick, W.; Tabatabai, M. An alkaline oxidation method for determination of total phosphorus in soils. Soil Sci. Soc. Am. J. 1977, 41, 511–514. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Tabatabai, M. Soil enzymes. In Methods of Soil Analysis: Part 2 Microbiological and Biochemical Properties; Wiley: Hoboken, NJ, USA, 1994; Volume 5, pp. 775–833. [Google Scholar]
- Nakayama, Y.; Wade, J.; Li, C.; Daughtridge, R.C.; Margenot, A.J. Quantifying the relative importance of controls and assay conditions for reliable measurement of soil enzyme activities with para-nitrophenol substrates. Geoderma 2023, 429, 116234. [Google Scholar] [CrossRef]
- Fonseca, C.; Balocchi, O.; Keim, J.; Rodríguez, C. Effect of defoliation frequency on yield and nutritional composition of Pennisetum clandestinum Hochst. ex Chiov. Agro Sur 2016, 44, 67–76. [Google Scholar]
- Ariza-Nieto, C.; Mayorga, O.L.; Mojica, B.; Parra, D.; Afanador-Tellez, G. Use of LOCAL algorithm with near infrared spectroscopy in forage resources for grazing systems in Colombia. J. Near Infrared Spectrosc. 2018, 26, 44–52. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Posit Team. RStudio: Integrated Development Environment for R; Posit Software, PBC: Boston, MA, USA, 2023. [Google Scholar]
- Beltran-Medina, I.; Romero-Perdomo, F.; Molano-Chavez, L.; Gutiérrez, A.Y.; Silva, A.M.; Estrada-Bonilla, G. Inoculation of phosphate-solubilizing bacteria improves soil phosphorus mobilization and maize productivity. Nutr. Cycl. Agroecosyst. 2023, 126, 21–34. [Google Scholar] [CrossRef]
- Billah, M.; Khan, M.; Bano, A.; Hassan, T.U.; Munir, A.; Gurmani, A.R. Phosphorus and phosphate solubilizing bacteria: Keys for sustainable agriculture. Geomicrobiol. J. 2019, 36, 904–916. [Google Scholar] [CrossRef]
- Kishore, N.; Pindi, P.K.; Ram Reddy, S. Phosphate-Solubilizing Microorganisms: A Critical Review. In Plant Biology and Biotechnology: Volume I: Plant Diversity, Organization, Function and Improvement; Springer: Berlin/Heidelberg, Germany, 2015; pp. 307–333. [Google Scholar]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S. Phosphate-solubilizing microorganisms: Mechanism and their role in phosphate solubilization and uptake. J. Soil Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Khan, I.; Zada, S.; Rafiq, M.; Sajjad, W.; Zaman, S.; Hasan, F. Phosphate solubilizing epilithic and endolithic bacteria isolated from clastic sedimentary rocks, Murree lower Himalaya, Pakistan. Arch. Microbiol. 2022, 204, 332. [Google Scholar] [CrossRef]
- Vistoso, E.; Iraira, S.; Sandaña, P. Phosphorus use efficiency in permanent pastures in Andisols. J. Soil Sci. Plant Nutr. 2021, 21, 2587–2599. [Google Scholar] [CrossRef]
- Biswas, S.S.; Biswas, D.; Ghosh, A.; Sarkar, A.; Das, A.; Roy, T. Phosphate solubilizing bacteria inoculated low-grade rock phosphate can supplement P fertilizer to grow wheat in sub-tropical inceptisol. Rhizosphere 2022, 23, 100556. [Google Scholar] [CrossRef]
- Chesworth, W. Encyclopedia of Soil Science; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Sánchez Espinosa, J.A.; Rubiano Sanabria, Y. Procesos específicos de formación en Andisoles, Alfisoles y Ultisoles en Colombia. Revista EIA 2015, 12, 85–97. [Google Scholar]
- Tiecher, T.; dos Santos, D.R.; Calegari, A. Soil organic phosphorus forms under different soil management systems and winter crops, in a long term experiment. Soil Tillage Res. 2012, 124, 57–67. [Google Scholar] [CrossRef]
- dos Santos Rheinheimer, D.; Fornari, M.R.; Bastos, M.C.; Fernandes, G.; Santanna, M.A.; Calegari, A.; dos Santos Canalli, L.B.; Caner, L.; Labanowski, J.; Tiecher, T. Phosphorus distribution after three decades of different soil management and cover crops in subtropical region. Soil Tillage Res. 2019, 192, 33–41. [Google Scholar] [CrossRef]
- Anda, M.; Kasno, A.; Ginting, C.; Barus, P.; Purwanto, S. Response of Andisols to intensive agricultural land use: Implication on changes in P accumulation and colloidal surface charge. IOP Conf. Ser. Earth Environ. Sci. 2021, 648, 012016. [Google Scholar] [CrossRef]
- Arifin, M.; Devnita, R.; Anda, M.; Goenadi, D.H.; Nugraha, A. Characteristics of Andisols developed from andesitic and basaltic volcanic ash in different agro-climatic zones. Soil Syst. 2022, 6, 78. [Google Scholar] [CrossRef]
- Conant, R.T.; Paustian, K.; Elliott, E.T. Grassland management and conversion into grassland: Effects on soil carbon. Ecol. Appl. 2001, 11, 343–355. [Google Scholar] [CrossRef]
- Hillel, D.; Hatfield, J.L. Encyclopedia of Soils in the Environment; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Motavalli, P.; Miles, R. Soil phosphorus fractions after 111 years of animal manure and fertilizer applications. Biol. Fertil. Soils 2002, 36, 35–42. [Google Scholar]
- Crews, T.E.; Brookes, P.C. Changes in soil phosphorus forms through time in perennial versus annual agroecosystems. Agric. Ecosyst. Environ. 2014, 184, 168–181. [Google Scholar] [CrossRef]
- Yang, Y.; Tilman, D.; Furey, G.; Lehman, C. Soil carbon sequestration accelerated by restoration of grassland biodiversity. Nat. Commun. 2019, 10, 718. [Google Scholar] [CrossRef] [PubMed]
- Chavarro-Bermeo, J.P.; Arruda, B.; Mora-Motta, D.A.; Bejarano-Herrera, W.; Ortiz-Morea, F.A.; Somenahally, A.; Silva-Olaya, A.M. Responses of soil phosphorus fractions to land-use change in Colombian Amazon. Sustainability 2022, 14, 2285. [Google Scholar] [CrossRef]
- Wei, Z.; Zuo, H.; Li, J.; Ding, G.; Zhan, Y.; Zhang, L.; Wu, W.; Su, L.; Wei, Y. Insight into the mechanisms of insoluble phosphate transformation driven by the interactions of compound microbes during composting. Environ. Sci. Pollut. Res. 2021, 28, 32844–32855. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of phosphatase enzymes in soil. In Phosphorus in Action: Biological Processes in Soil Phosphorus Cycling; Springer: Berlin/Heidelberg, Germany, 2011; pp. 215–243. [Google Scholar]
- Thomas, L.; Hodgson, D.A.; Wentzel, A.; Nieselt, K.; Ellingsen, T.E.; Moore, J.; Morrissey, E.R.; Legaie, R.; Wohlleben, W.; Rodríguez-García, A.; et al. Metabolic switches and adaptations deduced from the proteomes of Streptomyces coelicolor wild type and phoP mutant grown in batch culture. Mol. Cell. Proteom. 2012, 11, M111.013797. [Google Scholar] [CrossRef]
- Janes-Bassett, V.; Blackwell, M.S.; Blair, G.; Davies, J.; Haygarth, P.M.; Mezeli, M.M.; Stewart, G. A meta-analysis of phosphatase activity in agricultural settings in response to phosphorus deficiency. Soil Biol. Biochem. 2022, 165, 108537. [Google Scholar] [CrossRef]
- Buch, A.; Archana, G.; Kumar, G.N. Metabolic channeling of glucose towards gluconate in phosphate-solubilizing Pseudomonas aeruginosa P4 under phosphorus deficiency. Res. Microbiol. 2008, 159, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.F.; Schlesinger, W.H. A literature review and evaluation of the. Hedley fractionation: Applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma 1995, 64, 197–214. [Google Scholar] [CrossRef]
- Nash, D.M.; Haygarth, P.M.; Turner, B.L.; Condron, L.M.; McDowell, R.W.; Richardson, A.E.; Watkins, M.; Heaven, M.W. Using organic phosphorus to sustain pasture productivity: A perspective. Geoderma 2014, 221, 11–19. [Google Scholar] [CrossRef]
- Waldrip, H.M.; He, Z.; Erich, M.S. Effects of poultry manure amendment on phosphorus uptake by ryegrass, soil phosphorus fractions and phosphatase activity. Biol. Fertil. Soils 2011, 47, 407–418. [Google Scholar] [CrossRef]
- Davidson, J.; Milthorpe, F. Leaf Growth in Dactylis glomerate following Defoliation. Ann. Bot. 1966, 30, 173–184. [Google Scholar] [CrossRef]
- Kavanová, M.; Lattanzi, F.A.; Grimoldi, A.A.; Schnyder, H. Phosphorus deficiency decreases cell division and elongation in grass leaves. Plant Physiol. 2006, 141, 766–775. [Google Scholar] [CrossRef]
- Fageria, V. Nutrient interactions in crop plants. J. Plant Nutr. 2001, 24, 1269–1290. [Google Scholar] [CrossRef]
- Estrada, G.A.; Baldani, V.L.D.; de Oliveira, D.M.; Urquiaga, S.; Baldani, J.I. Selection of phosphate-solubilizing diazotrophic Herbaspirillum and Burkholderia strains and their effect on rice crop yield and nutrient uptake. Plant Soil 2013, 369, 115–129. [Google Scholar] [CrossRef]

| Sand | Silt | Clay | H | Bulk Density | OM | K | S | Ca | Mg |
|---|---|---|---|---|---|---|---|---|---|
| (%) | (cmol ) | ||||||||
| 32.9 | 433 | 23.8 | 60 | 0.44 | 11.2 | 4.2 | 27.4 | 18 | 7.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Cuesta, D.; Mora-Motta, D.; Chavarro-Bermeo, J.P.; Olaya-Montes, A.; Vargas-Garcia, C.; Bonilla, R.; Estrada-Bonilla, G. Phosphate-Solubilizing Bacteria with Low-Solubility Fertilizer Improve Soil P Availability and Yield of Kikuyu Grass. Microorganisms 2023, 11, 1748. https://doi.org/10.3390/microorganisms11071748
Torres-Cuesta D, Mora-Motta D, Chavarro-Bermeo JP, Olaya-Montes A, Vargas-Garcia C, Bonilla R, Estrada-Bonilla G. Phosphate-Solubilizing Bacteria with Low-Solubility Fertilizer Improve Soil P Availability and Yield of Kikuyu Grass. Microorganisms. 2023; 11(7):1748. https://doi.org/10.3390/microorganisms11071748
Chicago/Turabian StyleTorres-Cuesta, Daniel, Duber Mora-Motta, Juan P. Chavarro-Bermeo, Andres Olaya-Montes, Cesar Vargas-Garcia, Ruth Bonilla, and German Estrada-Bonilla. 2023. "Phosphate-Solubilizing Bacteria with Low-Solubility Fertilizer Improve Soil P Availability and Yield of Kikuyu Grass" Microorganisms 11, no. 7: 1748. https://doi.org/10.3390/microorganisms11071748
APA StyleTorres-Cuesta, D., Mora-Motta, D., Chavarro-Bermeo, J. P., Olaya-Montes, A., Vargas-Garcia, C., Bonilla, R., & Estrada-Bonilla, G. (2023). Phosphate-Solubilizing Bacteria with Low-Solubility Fertilizer Improve Soil P Availability and Yield of Kikuyu Grass. Microorganisms, 11(7), 1748. https://doi.org/10.3390/microorganisms11071748








