The Role of the Plant–Soil Relationship in Agricultural Production—With Particular Regard to PGPB Application and Phytoremediation
Abstract
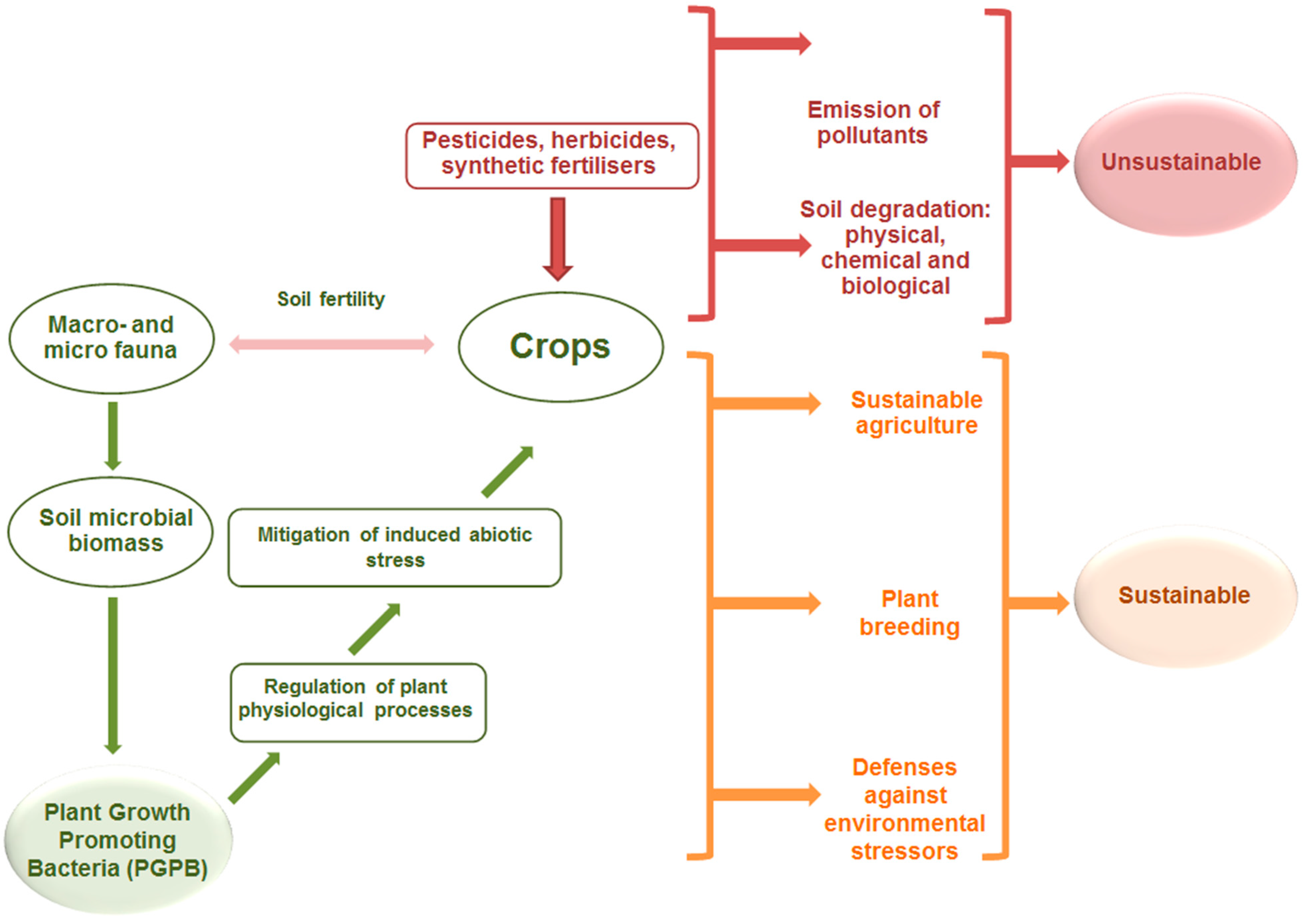
1. Introduction
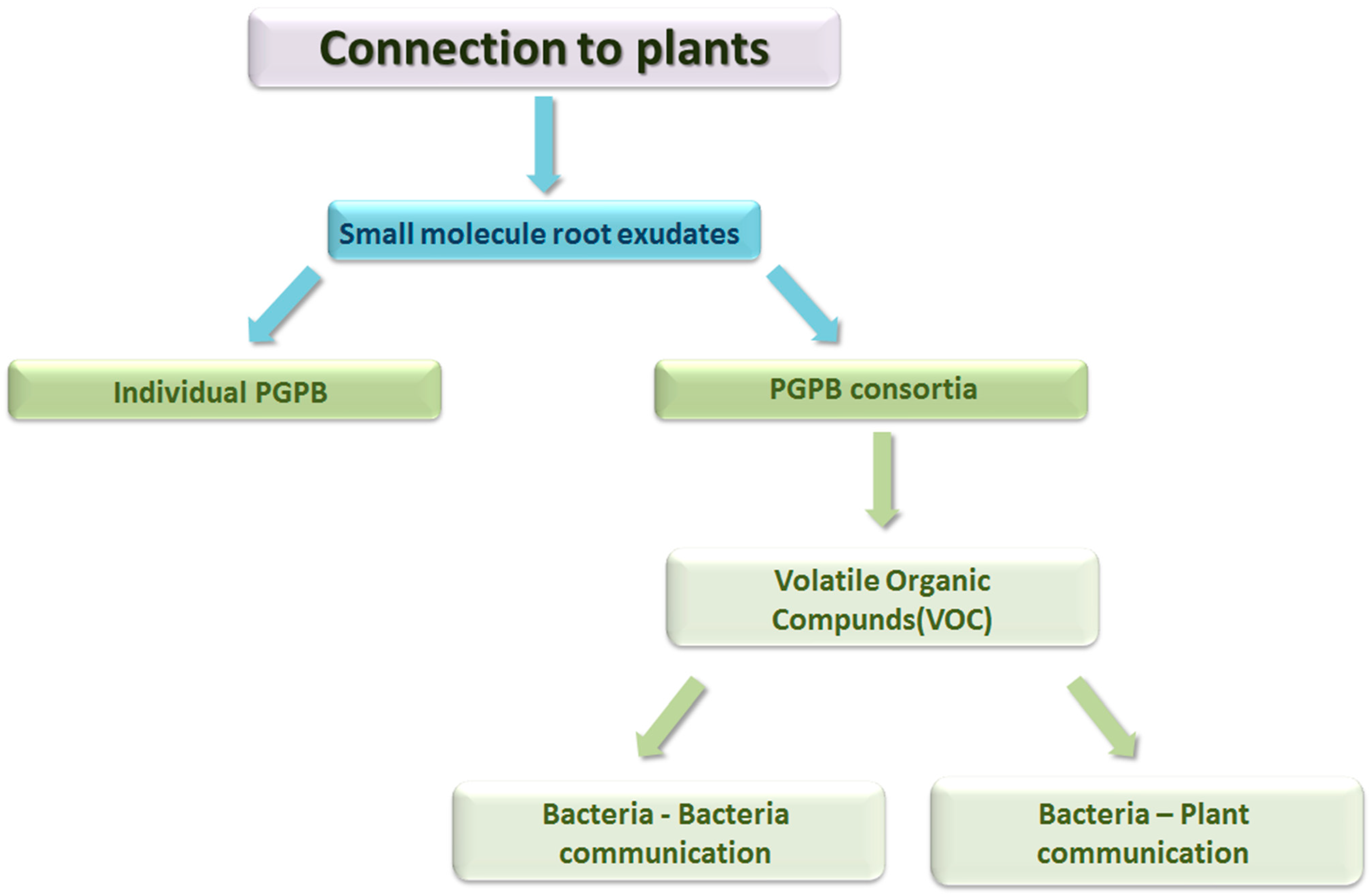
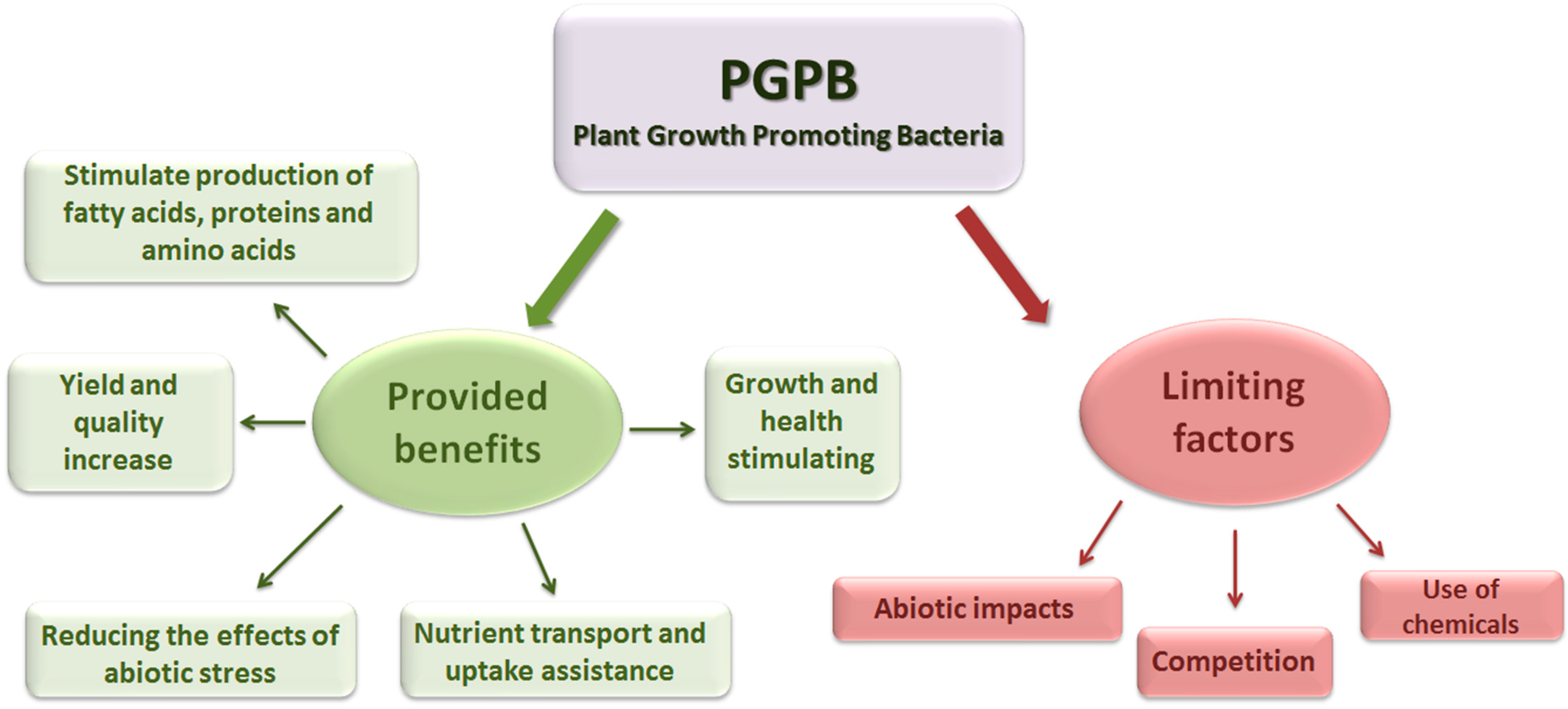
1.1. Functional Identification Technologies and Results
1.2. Role of PGPB in Phytoremediation
2. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, Y. Biotechnological potential of plant-microbe interactions in environmental decontamination. Front. Plant Sci. 2019, 10, 1519. [Google Scholar] [CrossRef]
- Chen, C.; Wang, M.; Zhu, J.; Tang, Y.; Zhang, H.; Zhao, Q.; Jing, M.; Chen, Y.; Xu, X.; Jiang, J.; et al. Long-term effect of epigenetic modification in plant–microbe interactions: Modification of DNA methylation induced by plant growth-promoting bacteria mediates promotion process. Microbiome 2022, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.T.; Shinwari, Z.K. Utilization of Plant Growth-Promoting Bacteria (PGPB) Against Phytopathogens. In Antifungal Metabolites of Rhizobacteria for Sustainable Agriculture; Springer International Publishing: Cham, Switzerland, 2022; pp. 53–63. [Google Scholar]
- Pellegrini, M.; Pagnani, G.; Bernardi, M.; Mattedi, A.; Spera, D.M.; Gallo, M.D. Cell-free supernatants of plant growth-promoting bacteria: A review of their use as biostimulant and microbial biocontrol agents in sustainable agriculture. Sustainability 2020, 12, 9917. [Google Scholar] [CrossRef]
- Rahman, A.N.S.N.; Hamid, A.N.W.; Nadarajah, K. Effects of abiotic stress on soil microbiome. Int. J. Mol. Sci. 2021, 22, 9036. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Yadav, R.; Li, K. Plant growth promoting bacteria in agriculture: Two sides of a coin. Appl. Soil Ecol. 2019, 138, 10–18. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, J. Biofilms Forming Microbes: Diversity and Potential Application in Plant–Microbe Interaction and Plant Growth. In Plant Microbiomes for Sustainable Agriculture; Yadav, A., Singh, J., Rastegari, A., Yadav, N., Eds.; Springer: Cham, Switzerland, 2020; Volume 25, pp. 173–197. [Google Scholar] [CrossRef]
- Gamalero, E.; Bona, E.; Glick, B.R. Current techniques to study beneficial plant-microbe interactions. Microorganisms 2022, 10, 1380. [Google Scholar] [CrossRef]
- Hatzenpichler, R.; Krukenberg, V.; Spietz, R.L.; Jay, Z.J. Next-generation physiology approaches to study microbiome function at single cell level. Nat. Rev. Microbiol. 2020, 18, 241–256. [Google Scholar] [CrossRef]
- Prosser, J.I. Putting science back into microbial ecology: A question of approach. Philos. Trans. R. Soc. B 2020, 375, 20190240. [Google Scholar] [CrossRef]
- Riva, V.; Mapelli, F.; Bagnasco, A.; Mengoni, A.; Borin, S. A meta-analysis approach to defining the culturable core of plant endophytic bacterial communities. Appl. Environ. Microbiol. 2022, 88, e02537-21. [Google Scholar] [CrossRef] [PubMed]
- Nordstedt, N.P.; Jones, M.L. Serratia plymuthica MBSA-MJ1 Increases Shoot Growth and Tissue Nutrient Concentration in Containerized Ornamentals Grown Under Low-Nutrient Conditions. Front. Microbiol. 2021, 12, 788198. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.; Otto-Pille, C.; Gupta, S.; Schillaci, M.; Roessner, U. Current perspectives and applications in plant probiotics. Microbiol. Aust. 2020, 41, 95–99. [Google Scholar] [CrossRef]
- Trash, J.C. Towards culturing the microbe of your choice. Environ. Microbiol. Rep. 2021, 13, 36–41. [Google Scholar] [CrossRef]
- Lee, K.S.; Palatinszky, M.; Pereira, F.C.; Nguyen, J.; Fernandez, V.I.; Mueller, A.J.; Menolascina, F.; Daims, H.; Berry, D.; Wagner, M.; et al. An automated Raman-based platform for the sorting of live cells by functional properties. Nat. Microbiol. 2019, 4, 1035–1048. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Qian, X.; Gu, J.; Chen, W.; Shen, X.; Tao, S.; Jiao, S.; Wei, G. Metagenomics insights into responses of rhizobacteria and their alleviation role in licorice allelopathy. Microbiome 2023, 11, 109. [Google Scholar] [CrossRef]
- Rilling, J.I.; Acuña, J.J.; Nannipieri, P.; Cassan, F.; Maruyama, F.; Jorquera, M.A. Current opinion and perspectives on the methods for tracking and monitoring plant growth–promoting bacteria. Soil Biol. Biochem. 2019, 130, 205–219. [Google Scholar] [CrossRef]
- Yu, J.; Tu, X.; Huang, A.C. Functions and biosynthesis of plant signaling metabolites mediating plant–microbe interactions. Nat. Prod. Rep. 2022, 39, 1393–1422. [Google Scholar] [CrossRef]
- Katsenios, N.; Andreou, V.; Sparangis, P.; Djordjevic, N.; Giannoglu, M.; Chainoti, S.; Kasimatis, C.N.; Kakabouki, I.; Leonidakis, D.; Danalatos, N.; et al. Assessment of plant growth promoting bacteria strains on growth, yield and quality of sweet corn. Sci. Rep. 2022, 12, 11598. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Chandra, D.; Sharma, A.K. Rhizosphere Plant–Microbe Interactions Under Abiotic Stress. In Rhizosphere Biology: Interactions Between Microbes and Plants; Gupta, V.V.S.R., Sharma, A.K., Eds.; Springer: Singapore, 2021; pp. 195–216. [Google Scholar] [CrossRef]
- Bashan, Y.; Prabhu, S.R.; de-Bashan, L.E.; Kloepper, J.W. Disclosure of exact protocols of fermentation, identity of microorganisms within consortia, formation of advanced consortia with microbe-based products. Biol. Fertil. Soils 2020, 56, 443–445. [Google Scholar] [CrossRef]
- Bukhat, S.; Imran, A.; Javaid, S.; Shahid, M.; Majeed, A.; Naqqash, T. Communication of plants with microbial world: Exploring the regulatory networks for PGPR mediated defense signaling. Microbiol. Res. 2020, 238, 126486. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Díaz, I.F.; Zelaya Molina, L.X.; Cruz Cárdenas, C.I.; Rojas Anaya, E.; Ruíz Ramírez, S.; Santos Villalobos, S.D.L. Consideraciones sobre el uso de biofertilizantes como alternativa agro-biotecnológica sostenible para la seguridad alimentaria en México. Rev. Mex. Cienc. Geológicas 2020, 11, 1423–1436. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Glick, B.R.; Rossi, M.J. Isolation and characterization of novel soil- and plant-associated bacteria with multiple phytohormone-degrading activities using a targeted methodology. Access Microbiol. 2019, 1, e000053. [Google Scholar] [CrossRef]
- Ji, S.H.; Kim, J.S.; Lee, C.H.; Seo, H.S.; Chun, S.C.; Oh, J.; Choi, E.H.; Park, G. Enhancement of vitality and activity of a plant growth-promoting bacteria (PGPB) by atmospheric pressure non-thermal plasma. Sci. Rep. 2019, 9, 1044. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, R.K.; Singh, P.; Koyama, H.; Panda, S.K. Plant-microbe interactions for sustainable agriculture in the postgenomic era. Curr. Genom. 2020, 21, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Rigo, R.; Bazin, J.; Crespi, M.; Charon, C. Alternative Splicing in the Regulation of Plant-Microbe Interactions. Plant Cell Physiol. 2019, 60, 1906–1916. [Google Scholar] [CrossRef] [PubMed]
- Bertola, M.; Ferrarini, A.; Visioli, G. Improvement of soil microbial diversity through sustainable agricultural practices and its evaluation by-omics approaches: A perspective for the environment, food quality and human safety. Microorganisms 2021, 9, 1400. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front. Bioeng. Biotechnol. 2020, 8, 467. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Sharma, K. Microbial biodiversity and bioremediation assessment through omics approaches. Front. Environ. Chem. 2020, 1, 570326. [Google Scholar] [CrossRef]
- Li, H.P.; Gan, Y.N.; Yue, L.J.; Han, Q.Q.; Chen, J.; Liu, Q.M.; Zhao, Q.; Zhang, J.L. Newly isolated Paenibacillus monticola sp. nov., a novel plant growth-promoting rhizobacteria strain from high-altitude spruce forests in the Qilian Mountains, China. Front. Microbiol. 2022, 13, 123. [Google Scholar] [CrossRef]
- Gupta, R.; Anand, G.; Gaur, R.; Yadav, D. Plant–microbiome interactions for sustainable agriculture: A review. Physiol. Mol. Biol. Plants 2021, 27, 165–179. [Google Scholar] [CrossRef]
- Al Kahtani, M.D.F.; Fouda, A.; Attia, K.A.; Al-Otaibi, F.; Eid, A.M.; Ewais, E.E.-D.; Hijri, M.; St-Arnaud, M.; Hassan, S.E.-D.; Khan, N.; et al. Isolation and characterization of plant growth-promoting endophytic bacteria from desert plants and their use as bioinoculants in sustainable agriculture. Agronomy 2020, 10, 1325. [Google Scholar] [CrossRef]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Sheibani-Tezerji, R.; Rattei, T.; Sessitsch, A.; Trognitz, F.; Mitter, B. Transcriptome profiling of the endophyte Burkholderia phytofirmans PsJN indicates sensing of the plant environment and drought stress. mBio 2015, 6, e00621-15. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.J.; Alacid, V.; Tomas-Barberan, F.A.; Garcia, C.; Palazon, P. Untargeted metabolomics to explore the bacteria exo-metabolome related to plant biostimulants. Agronomy 2022, 12, 1926. [Google Scholar] [CrossRef]
- Zamanzadeh-Nasrabadi, S.M.; Mohammadiapanah, F.; Hosseini-Mazinani, M.; Sarikhan, S. Salinity stress endurance of the plants with the aid of bacterial genes. Front. Genet. 2023, 14, 1049608. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, L.; Palla, M.; Agnolucci, M.; Avio, L.; Sbrana, C.; Turrini, A.; Giovannetti, M. Arbuscular mycorrhizal fungi and associated microbiota as plant biostimulants: Research strategies for the selection of the best performing inocula. Agronomy 2020, 10, 106. [Google Scholar] [CrossRef]
- Vannini, C.; Domingo, G.; Fiorilli, V.; Seco, D.G.; Novero, M.; Marsoni, M.; Wisniewski-Dye, F.; Bracale, M.; Moilin, L.; Bonfante, P. Proteomic analysis reveals how pairing of a Mycorrhizal fungus with plant growth-promoting bacteria modulates growth and defense in wheat. Plant Cell Environ. 2021, 44, 1946–1960. [Google Scholar] [CrossRef]
- Hansda, A.; Kisku, P.C.; Kumar, V. Plant-microbe association to improve phytoremediation of heavy metal. In Advances in Microbe-Assisted Phytoremediation of Polluted Sites, 1st ed.; Bauddh, K., Ma, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 113–146. [Google Scholar]
- Xiang, L.; Harindintwali, J.D.; Wang, F.; Redmile-Gordon, M.; Chang, S.X.; Fu, Y.; He, C.; Muhoza, B.; Brahusi, F.; Bolan, N.; et al. Integrating biochar, bacteria, and plants for sustainable remediation of soils contaminated with organic pollutants. Environ. Sci. Technol. 2022, 56, 16546–16566. [Google Scholar] [CrossRef]
- Yadav, A.N. Beneficial plant-microbe interactions for agricultural sustainability. J. Appl. Biol. Biotechnol. 2021, 9, 1–4. [Google Scholar] [CrossRef]
- Bhateria, R.; Kumar, S. Role of Plant–Microbe Interactions in Combating Salinity Stress. In Plant Stress Mitigators: Action and Application; Springer Nature: Singapore, 2022; pp. 259–280. [Google Scholar]
- Dobrzyński, J.; Jakubowska, Z.; Dybek, B. Potential of Bacillus pumilus to directly promote plant growth. Front. Microbiol. 2022, 13, 1069053. [Google Scholar] [CrossRef]
- Olenska, E.; Malek, W.; Wojcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef]
- Ni, H.; Wu, Y.; Zong, R.; Ren, S.; Pan, D.; Yu, L.; Li, J.; Qu, Z.; Wang, Q.; Zhao, G.; et al. Combination of Aspergillus niger MJ1 with Pseudomonas stutzeri DSM4166 or mutant Pseudomonas fluorescens CHA0-nif improved crop quality, soil properties, and microbial communities in barrier soil. Front. Microbiol. 2023, 14, 3389. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I. An overview of metabolic activity, beneficial and pathogenic aspects of Burkholderia spp. Metabolites 2021, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J. Environ. Manag. 2020, 254, 109779. [Google Scholar] [CrossRef] [PubMed]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Factors affecting phytoextraction: A review. Pedosphere 2016, 26, 148–166. [Google Scholar] [CrossRef]
- Hou, D.; O’Connor, D.; Igalavithana, A.D.; Alessi, D.S.; Luo, J.; Tsang, D.C.W.; Sparks, D.L.; Yamauchi, Y.; Rinklebe, J.; Ok, Y.S. Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nat. Rev. Earth Environ. 2020, 1, 366–381. [Google Scholar] [CrossRef]
- Fernández, L.; Castaño, C.; García, P.; Saran, A.; Lorda, G.; Merini, L. Isolation and characterization of plant growth promoting bacteria (PGPB) from Larrea divaricata Cav., with potential use in phytoremediation of mining soils. Environ. Sustain. 2023, 117, 303–308. [Google Scholar] [CrossRef]
- Ahsan, N.; Marian, M.; Suga, H.; Shimizu, M. Lysinibacillus xylanilyticus strain GIC41 as a potential plant biostimulant. Microbes Environ. 2021, 36, ME21047. [Google Scholar] [CrossRef]
- Zuluaga, A.M.Y.; de Oliveira, M.A.L.; Valentinuzzi, F.; Tiziani, R.; Pii, Y.; Mimmo, T.; Cesco, S. Can inoculation with the bacterial biostimulant Enterobacter sp. strain 15S be an approach for the smarter P fertilization of maize and cucumber plants? Front. Plant Sci. 2021, 12, 719873. [Google Scholar] [CrossRef]
- Aviles, C.F.A.; Acosta, C.B.C.; de los Santos Villalobos, S.; Santoyo, G.; Cota, F.I.P. Caracterización de bacterias promotoras de crecimiento vegetal (BPCV) nativas y su efecto en el desarrollo del maíz (Zea mays L.). Biotecnia 2022, 24, 15–22. [Google Scholar]
- Fusco, G.M.; Nicastro, R.; Rouphael, Y.; Carillo, P. The Effects of the Microbial Biostimulants Approved by EU Regulation 2019/1009 on Yield and Quality of Vegetable Crops. Foods 2022, 11, 2656. [Google Scholar] [CrossRef]
- Ke, T.; Guo, G.; Liu, J.; Zhang, C.; Tao, Y.; Wang, P.; Xu, Y.; Chen, L. Improvement of the Cu and Cd phytostabilization efficiency of perennial ryegrass through the inoculation of three metal-resistant PGPR strains. Environ. Pollut. 2021, 271, 116314. [Google Scholar] [CrossRef]
- Phares, C.A.; Amoakwah, E.; Danquah, A.; Afrifa, A.; Beyaw, L.R.; Frimpong, K.A. Biochar and NPK fertilizer co-applied with plant growth promoting bacteria (PGPB) enhanced maize grain yield and nutrient use efficiency of inorganic fertilizer. J. Agric. Food Res. 2022, 10, 100434. [Google Scholar] [CrossRef]
- Ummara, U.; Noreen, S.; Afzal, M.; Zafar, Z.U.; Akhter, M.S.; Iqbal, S.; Hefft, D.I.; Kazi, M.; Ahmad, P. Induced systemic tolerance mediated by plant-microbe interaction in maize (Zea mays L.) plants under hydrocarbon contamination. Chemosphere 2022, 290, 133327. [Google Scholar] [CrossRef] [PubMed]
- Denaya, S.; Yulianti, R.; Pambudi, A.; Effendi, Y. Novel microbial consortium formulation as plant growth promoting bacteria (PGPB) agent. IOP Conf. Ser. Earth Environ. Sci. 2021, 637, 012030. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Shirokova, L.S.; Zabelina, S.A.; Jordan, G.; Bénézeth, P. Weak impact of microorganisms on Ca, Mg-bearing silicate weathering. NPJ Mater. Degrad. 2021, 5, 51. [Google Scholar] [CrossRef]
- Ngalimat, M.S.; Mohd Hata, E.; Zulperi, D.; Ismail, S.I.; Ismail, M.R.; Mohd Zainudin, N.A.I.; Saidi, N.B.; Yusof, M.T. Plant growth-promoting bacteria as an emerging tool to manage bacterial rice pathogens. Microorganisms 2021, 9, 682. [Google Scholar] [CrossRef]
- Tirry, N.; Kouchou, A.; El Omari, B.; Ferioun, M.; El Ghachtouli, N. Improved chromium tolerance of Medicago sativa by plant growth-promoting rhizobacteria (PGPR). J. Genet. Eng. Biotechnol. 2021, 19, 149. [Google Scholar] [CrossRef]
- Ren, X.M.; Guo, S.J.; Tian, W.; Chen, Y.; Han, H.; Chen, E.; Li, B.L.; Li, Y.Y.; Che, Z.J. Effects of plant growth-promoting bacteria (PGPB) inoculation on the growth, antioxidant activity, Cu uptake, and bacterial community structure of rape (Brassica napus L.) grown in Cu-contaminated agricultural soil. Microbiotechnology 2019, 10, 1455. [Google Scholar] [CrossRef]
- Ullah, I.; Mateen, A.; Ahmad, M.A.; Munir, I.; Iqbal, A.; Alghamdi, K.M.S.; Al-Solami, H.M.; Siddiqui, M.F. Heavy metal ATPase genes (HMAs) expression induced by endophytic bacteria, “AI001, and AI002” mediate cadmium translocation and phytoremediation. Environ. Pollut. 2022, 293, 118508. [Google Scholar] [CrossRef]
- Abedi, T.; Mojiri, A. Constructed wetland modified by biochar/zeolite addition for enhanced wastewater treatment. Environ. Technol. Innov. 2019, 16, 100472. [Google Scholar] [CrossRef]
- Ustiatik, R.; Nuraini, Y.; Suharjono, S.; Jeyakumar, P.; Anderson, C.W.; Handayanto, E. Mercury resistance and plant growth promoting traits of endophytic bacteria isolated from mercury-contaminated soil. Bioremed. J. 2022, 26, 208–227. [Google Scholar] [CrossRef]
- Margaryan, A.; Panosyan, H.; Birkeland, N.K. Heavy metal resistance in prokaryotes: Mechanism and application. In Microbial Communities and Their Interactions in the Extreme Environment, 2nd ed.; Dilfuza, E., Nils-Kare, B., Wen-Jun, L., Hovik, P., Eds.; Springer: Cham, Switzerland, 2021; Volume 32, pp. 273–313. [Google Scholar]
- Mishra, P.; Mishra, J.; Arora, N.K. Plant growth promoting bacteria for combating salinity stress in plants–Recent developments and prospects: A review. Microbiol. Res. 2021, 252, 126861. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Tufail, M.A.; Asghar, H.N.; Nazli, F.; Zahir, Z.A. Appraising the potential of EPS-producing rhizobacteria with ACC-deaminase activity to improve growth and physiology of maize under drought stress. Physiol. Plant. 2021, 172, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Murali, M.; Gowtham, H.G.; Singh, S.B.; Shilpa, N.; Aiyaz, M.; Niranjana, S.R.; Amruthesh, K.N. Bio-prospecting of ACC deaminase producing Rhizobacteria towards sustainable agriculture: A special emphasis on abiotic stress in plants. Appl. Soil Ecol. 2021, 168, 104142. [Google Scholar] [CrossRef]
- Moon, Y.S.; Ali, S. Possible mechanisms for the equilibrium of ACC and role of ACC deaminase-producing bacteria. Appl. Microbiol. Biotechnol. 2022, 106, 877–887. [Google Scholar] [CrossRef] [PubMed]
- del Carmen Orozco-Mosqueda, M.; Glick, B.R.; Santoyo, G. ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef]
- Gowtham, H.G.; Singh, B.; Murali, M.; Shilpa, N.; Prasad, M.; Aiyaz, M.; Amruthesh, K.N.; Niranjana, S.R. Induction of drought tolerance in tomato upon the application of ACC deaminase producing plant growth promoting rhizobacterium Bacillus subtilis Rhizo SF 48. Microbiol. Res. 2020, 234, 126422. [Google Scholar] [CrossRef]
- Mukhtar, T.; Rehman, S.U.; Smith, D.; Sultan, T.; Seleiman, M.F.; Alsadon, A.A.; Amna; Ali, S.; Chaudhary, H.J.; Solieman, T.H.I.; et al. Mitigation of heat stress in Solanum lycopersicum L. by ACC-deaminase and exopolysaccharide producing Bacillus cereus: Effects on biochemical profiling. Sustainability 2020, 12, 2159. [Google Scholar] [CrossRef]
- Danish, S.; Zafar-ul-Hye, M.; Fahad, S.; Saud, S.; Brtnicky, M.; Hammerschmiedt, T.; Datta, R. Drought stress alleviation by ACC deaminase producing Achromobacter xylosoxidans and Enterobacter cloacae, with and without timber waste biochar in maize. Sustainability 2020, 12, 6286. [Google Scholar] [CrossRef]
- Zheng, W.; Wu, Q.; Rao, C.; Chen, X.; Wang, E.; Liang, X.; Yan, W. Characteristics and interactions of soil bacteria, phytocommunity and soil properties in rocky desertification ecosystems of Southwest China. CATENA 2023, 220, 106731. [Google Scholar] [CrossRef]
- Stringlis, I.A.; Jonge, R.; Pieterse, C.M.J. The Age of Coumarins in Plant-Microbe Interactions. Plant Cell Physiol. 2019, 60, 1405–1419. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Ye, J.; Zeng, J.; Chen, L.; Korpelainen, H.; Li, C. Selenium species transforming along soil–plant continuum and their beneficial roles for horticultural crops. Hortic. Res. 2023, 10, 270. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a plant biostimulant in crops and during post-harvest: A new approach is needed. J. Sci. Food Agric. 2021, 101, 5297–5304. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.R.A.; Yin, Q.; Oliveria, R.S.; Silva, E.F.; Novo, L.A.B. Plant growth promoting bacteria in phytoremediation of metal-polluted soils: Current knowledge and future directions. Sci. Total Environ. 2022, 838, 156435. [Google Scholar] [CrossRef]
| Plant Species | Effects | PGPB | Ref. |
|---|---|---|---|
| Arabidopsis thaliana (L.) Heynh. | Affected root nutrient delivery system. Induce the genes related to nitrate and ammonium uptake and transfer. | Bacillus spp. | [45] |
| Brassica napus L. | Increased biomass and total Cu uptake. Growth-promoting. Reduce oxidative stress by heavy metals. | JYC17, Y1-3-9, J62 | [63] |
| Cucumis sativus L. | Growth-promoting. | Aspergillus niger MJ1, Pseudomonas stutzeri DSM4166, P. fluorescens CHA0-nif | [46] |
| Cucumis sativus L. | Support for phosphorus supply. | Enterobacter 15S | [53] |
| Festuca spp. L. | Phosphorus solubilization. | Bacilus sp. EhS7 strain | [56] |
| Impatiens walleriana Hook.f. | Stimulated production of fatty acids, proteins, and amino acids. Promoted the formation of a higher proportion of oxidative proteins. | Bacillus pumilus W8, B. pumilus LZP02, B. pumilus JPVS11, B. pumilus TUAT-1, B. pumilus TRS-3, B. pumilus EU927414 | [44] |
| Lactuca sativa L. | Growth-promoting. | Aspergillus niger MJ1, Pseudomonas stutzeri DSM4166 és P. fluorescens CHA0-nif | [46] |
| Lolium spp. L. | Phosphorus solubilization. | Bacilus sp. EhS7 strain | [56] |
| Medicago sativa L. | Growth-promoting. | Pseudomonas sp. | [62] |
| Oryza sativa L. | Improved and maintained health. | Bacillus, Pseudomonas, Enterobacter, Streptomyces | [61] |
| Petunia x hybrida (Sweet) D. Don ex W. H. Baxter | Stimulated production of fatty acids, proteins, and amino acids. Promoted the formation of higher proportion of oxidative proteins. | Bacillus pumilus W8, B. pumilus LZP02, B. pumilus JPVS11, B. pumilus TUAT-1, B. pumilus TRS-3, B. pumilus EU927414 | [44] |
| Solanum tuberosum L. | Effect on cadmium uptake. | Serratia sp. AI001, Klebsiella sp. AI002 strain | [64] |
| Spinacia oleracea L. | Increased dry weight of the shoot. Strengthened vitality. | Lysinibacillus GIC41 | [52] |
| Viola x wittrockiana Gams ex Nauenb. and Buttler | Stimulated production of fatty acids, proteins, and amino acids. Promoted the formation of higher proportion of oxidative proteins. | Bacillus pumilus W8, B. pumilus LZP02, B. pumilus JPVS11, B. pumilus TUAT-1, B. pumilus TRS-3, B. pumilus EU927414 | [44] |
| Zea mays L. | Support for phosphorus supply. | Enterobacter 15S | [53] |
| Yield increase. Higher protein and fiber content in the crop. | Bacillus mojavensis, Bacillus subtilis, Bacillus pumilus, Bacillus pseudomycoides | [19] | |
| Increased height, dry shoot, and root mass, and SPAD values. | Bacillus sp. (13B41), Advenella incenata (22A67), Pantoea dispersa (22B45), Rhizobium pusense (31B11) | [54] | |
| NPK fertilizer usage reduced. Increased diesel oil resistance. | Pseudomonas spp. | [57,58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kisvarga, S.; Hamar-Farkas, D.; Ördögh, M.; Horotán, K.; Neményi, A.; Kovács, D.; Orlóci, L. The Role of the Plant–Soil Relationship in Agricultural Production—With Particular Regard to PGPB Application and Phytoremediation. Microorganisms 2023, 11, 1616. https://doi.org/10.3390/microorganisms11061616
Kisvarga S, Hamar-Farkas D, Ördögh M, Horotán K, Neményi A, Kovács D, Orlóci L. The Role of the Plant–Soil Relationship in Agricultural Production—With Particular Regard to PGPB Application and Phytoremediation. Microorganisms. 2023; 11(6):1616. https://doi.org/10.3390/microorganisms11061616
Chicago/Turabian StyleKisvarga, Szilvia, Dóra Hamar-Farkas, Máté Ördögh, Katalin Horotán, András Neményi, Dezső Kovács, and László Orlóci. 2023. "The Role of the Plant–Soil Relationship in Agricultural Production—With Particular Regard to PGPB Application and Phytoremediation" Microorganisms 11, no. 6: 1616. https://doi.org/10.3390/microorganisms11061616
APA StyleKisvarga, S., Hamar-Farkas, D., Ördögh, M., Horotán, K., Neményi, A., Kovács, D., & Orlóci, L. (2023). The Role of the Plant–Soil Relationship in Agricultural Production—With Particular Regard to PGPB Application and Phytoremediation. Microorganisms, 11(6), 1616. https://doi.org/10.3390/microorganisms11061616








