Listeria monocytogenes Strains Persisting in a Meat Processing Plant in Central Italy: Use of Whole Genome Sequencing and In Vitro Adhesion and Invasion Assays to Decipher Their Virulence Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates
2.2. Whole Genome Sequencing (WGS) and Bioinformatic Analysis
2.2.1. Next Generation Sequencing and Genome Assembly
2.2.2. Multilocus Sequence Typing Analysis
2.2.3. Genetic Determinants Involved in Virulence Potential
2.3. In Vitro Adhesion and Invasion of Lm Isolates in Caco2 Cells
2.3.1. Epithelial Cell Line
2.3.2. Adhesion Assay
2.3.3. Invasion Assay
2.4. Statistical Analysis
3. Results
3.1. Whole Genome Sequencing (WGS) Data Analysis
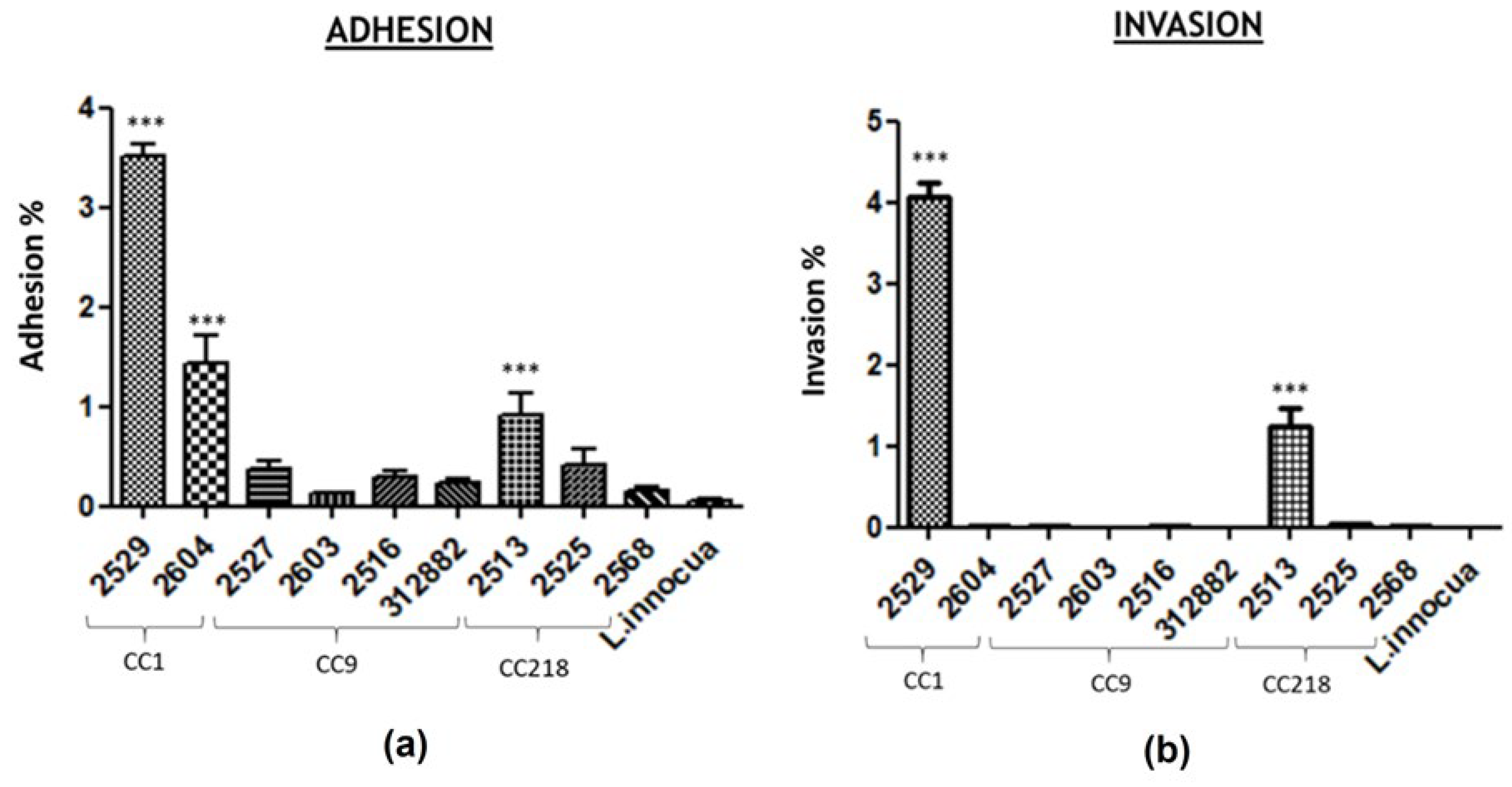
3.2. In Vitro Adhesion and Invasion of Lm Isolates in Caco2 Cells
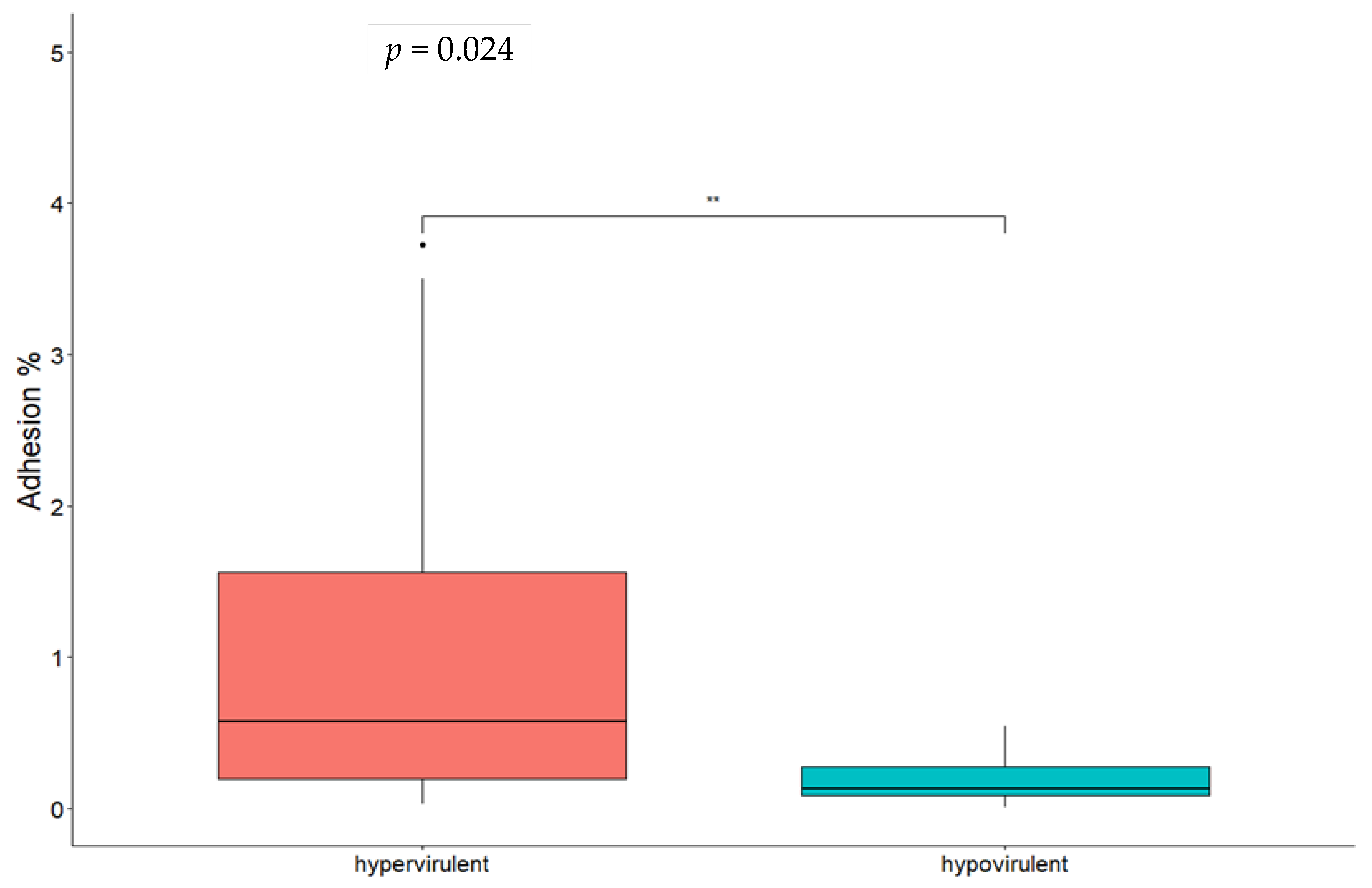
3.3. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EFSA; ECDC. The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, e07666. [Google Scholar] [CrossRef]
- De Castro, V.; Escudero, J.; Rodriguez, J.; Muniozguren, N.; Uribarri, J.; Saez, D.; Vazquez, J. Listeriosis outbreak caused by Latin-style fresh cheese, Bizkaia, Spain, August 2012. Eurosurveillance 2012, 17, 20298. [Google Scholar] [CrossRef] [PubMed]
- Lomonaco, S.; Verghese, B.; Gerner-Smidt, P.; Tarr, C.; Gladney, L.; Joseph, L.; Katz, L.; Turnsek, M.; Frace, M.; Chen, Y.; et al. Novel epidemic clones of Listeria monocytogenes, United States, 2011. Emerg. Infect. Dis. 2013, 19, 147–150. [Google Scholar] [CrossRef]
- Makino, S.-I.; Kawamoto, K.; Takeshi, K.; Okada, Y.; Yamasaki, M.; Yamamoto, S.; Igimi, S. An outbreak of food-borne listeriosis due to cheese in Japan, during 2001. Int. J. Food Microbiol. 2005, 104, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Tau, N.P.; Smouse, S.L.; Allam, M.; Ismail, A.; Ramalwa, N.R.; Disenyeng, B.; Ngomane, M.; Thomas, J. Outbreak of Listeria monocytogenes in South Africa, 2017–2018: Laboratory Activities and Experiences Associated with Whole-Genome Sequencing Analysis of Isolates. Foodborne Pathog. Dis. 2019, 16, 524–530. [Google Scholar] [CrossRef]
- Kaptchouang Tchatchouang, C.D.; Fri, J.; De Santi, M.; Brandi, G.; Schiavano, G.F.; Amagliani, G.; Ateba, C.N. Listeriosis Outbreak in South Africa: A Comparative Analysis with Previously Reported Cases Worldwide. Microorganisms 2020, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Gillesberg Lassen, S.; Ethelberg, S.; Björkman, J.T.; Jensen, T.; Sørensen, G.; Kvistholm Jensen, A.; Müller, L.; Nielsen, E.M.; Mølbak, K. Two listeria outbreaks caused by smoked fish consumption-using whole-genome sequencing for outbreak investigations. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2016, 22, 620–624. [Google Scholar] [CrossRef]
- Lüth, S.; Halbedel, S.; Rosner, B.; Wilking, H.; Holzer, A.; Roedel, A.; Dieckmann, R.; Vincze, S.; Prager, R.; Flieger, A.; et al. Backtracking and forward checking of human listeriosis clusters identified a multiclonal outbreak linked to Listeria monocytogenes in meat products of a single producer. Emerg. Microbes Infect. 2020, 9, 1600–1608. [Google Scholar] [CrossRef]
- Halbedel, S.; Wilking, H.; Holzer, A.; Kleta, S.; Fischer, M.A.; Lüth, S.; Pietzka, A.; Huhulescu, S.; Lachmann, R.; Krings, A.; et al. Large Nationwide Outbreak of Invasive Listeriosis Associated with Blood Sausage, Germany, 2018–2019. Emerg. Infect. Dis. 2020, 26, 1456–1464. [Google Scholar] [CrossRef]
- Allen, K.J.; Wałecka-Zacharska, E.; Chen, J.C.; Katarzyna, K.-P.; Devlieghere, F.; Van Meervenne, E.; Osek, J.; Wieczorek, K.; Bania, J. Listeria monocytogenes–An examination of food chain factors potentially contributing to antimicrobial resistance. Food Microbiol. 2016, 54, 178–189. [Google Scholar] [CrossRef]
- Iwu, C.D.; Okoh, A.I. Characterization of antibiogram fingerprints in Listeria monocytogenes recovered from irrigation water and agricultural soil samples. PLoS ONE 2020, 15, e0228956. [Google Scholar] [CrossRef] [PubMed]
- Radoshevich, L.; Cossart, P. Listeria monocytogenes: Towards a complete picture of its physiology and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 32–46. [Google Scholar] [CrossRef] [PubMed]
- EFSA_Panel_on_Biological_Hazards; Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernández Escámez, P.S.; Girones, R.; Herman, L.; Koutsoumanis, K.; et al. Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA J. 2018, 16, e05134. [Google Scholar] [PubMed]
- EU-Reference_Laboratory_for_Listeria_monocytogenes; ANSES-Food_Safety_Laboratory. EURL Lm GUIDANCE DOCUMENT to Evaluate the Competence of Laboratories Implementing Challenge Tests and Durability Studies Related to Listeria Monocytogenes in Ready-to-Eat Foods Version 3 10/02/2023; EU-Reference_Laboratory_for_Listeria_monocytogenes: Paris, France, 2023. [Google Scholar]
- Bucur, F.I.; Grigore-Gurgu, L.; Crauwels, P.; Riedel, C.U.; Nicolau, A.I. Resistance of Listeria monocytogenes to stress conditions encountered in food and food processing environments. Front Microbiol. 2018, 9, 2700. [Google Scholar] [CrossRef]
- Jordan, K.; Hunt, K.; Lourenco, A.; Pennone, V. Listeria monocytogenes in the food processing environment. Curr. Clin. Microbiol. Rep. 2018, 5, 106–119. [Google Scholar] [CrossRef]
- Orsi, R.H.; den Bakker, H.C.; Wiedmann, M. Listeria monocytogenes lineages: Genomics, evolution, ecology, and phenotypic characteristics. Int. J. Med. Microbiol. 2011, 301, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Yao, H.; Doijad, S.; Kong, S.; Shen, Y.; Cai, X.; Tan, W.; Wang, Y.; Feng, Y.; Ling, Z.; et al. A hybrid sub-lineage of Listeria monocytogenes comprising hypervirulent isolates. Nat. Commun. 2019, 10, 4283. [Google Scholar] [CrossRef]
- Doumith, M.; Buchrieser, C.; Glaser, P.; Jacquet, C.; Martin, P. Differentiation of the major Listeria monocytogenes serovars by multiplex PCR. J. Clin. Microbiol. 2004, 42, 3819–3822. [Google Scholar] [CrossRef]
- Ragon, M.; Wirth, T.; Hollandt, F.; Lavenir, R.; Lecuit, M.; Le Monnier, A.; Brisse, S. A new perspective on Listeria monocytogenes evolution. PLoS Pathog. 2008, 4, e1000146. [Google Scholar] [CrossRef]
- Vázquez-Boland, J.A.; Kuhn, M.; Berche, P.; Chakraborty, T.; Domínguez-Bernal, G.; Goebel, W.; González-Zorn, B.; Wehland, J.r.; Kreft, J.r. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 2001, 14, 584–640. [Google Scholar] [CrossRef]
- Maury, M.M.; Tsai, Y.H.; Charlier, C.; Touchon, M.; Chenal-Francisque, V.; Leclercq, A.; Criscuolo, A.; Gaultier, C.; Roussel, S.; Brisabois, A.; et al. Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat. Genet 2016, 48, 308–313. [Google Scholar] [CrossRef]
- Moura, A.; Criscuolo, A.; Pouseele, H.; Maury, M.M.; Leclercq, A.; Tarr, C.; Björkman, J.T.; Dallman, T.; Reimer, A.; Enouf, V.; et al. Whole genome-based population biology and epidemiological surveillance of Listeria monocytogenes. Nat. Microbiol. 2016, 2, 16185. [Google Scholar] [CrossRef] [PubMed]
- Maury, M.M.; Bracq-Dieye, H.; Huang, L.; Vales, G.; Lavina, M.; Thouvenot, P.; Disson, O.; Leclercq, A.; Brisse, S.; Lecuit, M. Hypervirulent Listeria monocytogenes clones’ adaption to mammalian gut accounts for their association with dairy products. Nat. Commun 2019, 10, 2488. [Google Scholar] [CrossRef] [PubMed]
- Painset, A.; Björkman, J.T.; Kiil, K.; Guillier, L.; Mariet, J.F.; Félix, B.; Amar, C.; Rotariu, O.; Roussel, S.; Perez-Reche, F.; et al. LiSEQ—Whole-genome sequencing of a cross-sectional survey of Listeria monocytogenes in ready-to-eat foods and human clinical cases in Europe. Microb. Genom. 2019, 5, e000257. [Google Scholar] [CrossRef]
- Shi, D.; Anwar, T.M.; Pan, H.; Chai, W.; Xu, S.; Yue, M. Genomic determinants of pathogenicity and antimicrobial resistance for 60 global Listeria monocytogenes isolates responsible for invasive infections. Front Cell Infect. Microbiol. 2021, 11, 718840. [Google Scholar] [CrossRef]
- Félix, B.; Sevellec, Y.; Palma, F.; Douarre, P.E.; Felten, A.; Radomski, N.; Mallet, L.; Blanchard, Y.; Leroux, A.; Soumet, C.; et al. A European-wide dataset to uncover adaptive traits of Listeria monocytogenes to diverse ecological niches. Sci. Data 2022, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Ireton, K.; Mortuza, R.; Gyanwali, G.C.; Gianfelice, A.; Hussain, M. Role of internalin proteins in the pathogenesis of Listeria monocytogenes. Mol. Microbiol. 2021, 116, 1407–1419. [Google Scholar] [CrossRef]
- Quereda, J.J.; Morel, C.; Lopez-Montero, N.; Ziveri, J.; Rolland, S.; Grenier, T.; Aulner, N.; Danckaert, A.; Charbit, A.; Enninga, J.; et al. A Role for Taok2 in Listeria monocytogenes Vacuolar Escape. J. Infect. Dis. 2022, 225, 1005–1010. [Google Scholar] [CrossRef]
- Quereda, J.J.; Meza-Torres, J.; Cossart, P.; Pizarro-Cerdá, J. Listeriolysin S: A bacteriocin from epidemic Listeria monocytogenes strains that targets the gut microbiota. Gut Microbes 2017, 8, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Loo, K.; Letchumanan, V.; Dhanoa, A.; Law, J.W.-F.; Pusparajah, P.; Goh, B.-H.; Ser, H.-L.; Wong, S.H.; Ab Mutalib, N.-S.; Chan, K.-G.; et al. Exploring the pathogenesis, clinical characteristics and therapeutic regimens of Listeria monocytogenes. Microbiology 2020, 3, 1–13. [Google Scholar]
- Disson, O.; Moura, A.; Lecuit, M. Making sense of the biodiversity and virulence of Listeria monocytogenes. Trends Microbiol. 2021, 29, 811–822. [Google Scholar] [CrossRef]
- Johansson, J.; Freitag, N.E. Regulation of Listeria monocytogenes virulence. Microbiol. Spectr. 2019, 7, 27. [Google Scholar] [CrossRef]
- Wiktorczyk-Kapischke, N.; Skowron, K.; Grudlewska-Buda, K.; Wałecka-Zacharska, E.; Korkus, J.; Gospodarek-Komkowska, E. Adaptive response of Listeria monocytogenes to the stress factors in the food processing environment. Front Microbiol. 2021, 12, 710085. [Google Scholar] [CrossRef]
- Sibanda, T.; Buys, E.M. Listeria monocytogenes Pathogenesis: The Role of Stress Adaptation. Microorganisms 2022, 10, 1522. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, B.; Barre, L. Guidelines on Sampling the Food Pprocessing Area and Equipment for the Detection of Listeria Monocytogenes Version 3–20/08/2012; EURL for Listeria monocytogenes, Maisons-Alfort Laboratory for Food Safety, ANSES: Paris, France, 2012. [Google Scholar]
- Guidi, F.; Lorenzetti, C.; Centorotola, G.; Torresi, M.; Camma, C.; Chiaverini, A.; Pomilio, F.; Blasi, G. Atypical serogroup IVb-v1 of Listeria monocytogenes assigned to new ST2801, widely spread and persistent in the environment of a porkmeat producing plant of Central Italy. Front Microbiol. 2022, 13, 2365. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.; Tourdjman, M.; Leclercq, A.; Hamelin, E.; Laurent, E.; Fredriksen, N.; Van Cauteren, D.; Bracq-Dieye, H.; Thouvenot, P.; Vales, G.; et al. Real-Time Whole-Genome Sequencing for Surveillance of Listeria monocytogenes, France. Emerg. Infect. Dis. 2017, 23, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Portmann, A.C.; Fournier, C.; Gimonet, J.; Ngom-Bru, C.; Barretto, C.; Baert, L. A Validation Approach of an End-to-End Whole Genome Sequencing Workflow for Source Tracking of Listeria monocytogenes and Salmonella enterica. Front Microbiol. 2018, 9, 446. [Google Scholar] [CrossRef]
- Cito, F.; Di Pasquale, A.; Cammà, C.; Cito, P. The Italian information system for the collection and analysis of complete genome sequence of pathogens isolated from animal, food and environment. Int. J. Infect. Dis. 2018, 73, 296–297. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Wingett, S.W.; Andrews, S. FastQ Screen: A tool for multi-genome mapping and quality control. F1000Res 2018, 7, 1338. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Timme, R.E.; Wolfgang, W.J.; Balkey, M.; Venkata, S.L.G.; Randolph, R.; Allard, M.; Strain, E. Optimizing open data to support one health: Best practices to ensure interoperability of genomic data from bacterial pathogens. One Health Outlook 2020, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Schiavano, G.F.; Ateba, C.N.; Petruzzelli, A.; Mele, V.; Amagliani, G.; Guidi, F.; De Santi, M.; Pomilio, F.; Blasi, G.; Gattuso, A.; et al. Whole-genome sequencing characterization of virulence profiles of Listeria monocytogenes food and human isolates and in vitro adhesion/invasion assessment. Microorganisms 2022, 10, 62. [Google Scholar] [CrossRef]
- Wagner, E.; Fagerlund, A.; Thalguter, S.; Jensen, M.R.; Heir, E.; Møretrø, T.; Moen, B.; Langsrud, S.; Rychli, K. Deciphering the virulence potential of Listeria monocytogenes in the Norwegian meat and salmon processing industry by combining whole genome sequencing and in vitro data. Int. J. Food Microbiol. 2022, 383, 109962. [Google Scholar] [CrossRef]
- Nightingale, K.K.; Windham, K.; Martin, K.E.; Yeung, M.; Wiedmann, M. Select Listeria monocytogenes subtypes commonly found in foods carry distinct nonsense mutations in inlA, leading to expression of truncated and secreted internalin A, and are associated with a reduced invasion phenotype for human intestinal epithelial cells. Appl. Environ. Microbiol. 2005, 71, 8764–8772. [Google Scholar] [CrossRef]
- Su, X.; Cao, G.; Zhang, J.; Pan, H.; Zhang, D.; Kuang, D.; Yang, X.; Xu, X.; Shi, X.; Meng, J. Characterization of internalin genes in Listeria monocytogenes from food and humans, and their association with the invasion of Caco-2 cells. Gut Pathog. 2019, 11, 30. [Google Scholar] [CrossRef]
- Cardenas-Alvarez, M.X.; Restrepo-Montoya, D.; Bergholz, T.M. Genome-Wide Association Study of Listeria monocytogenes Isolates Causing Three Different Clinical Outcomes. Microorganisms 2022, 10, 1934. [Google Scholar] [CrossRef]
- Raschle, S.; Stephan, R.; Stevens, M.J.A.; Cernela, N.; Zurfluh, K.; Muchaamba, F.; Nüesch-Inderbinen, M. Environmental dissemination of pathogenic Listeria monocytogenes in flowing surface waters in Switzerland. Sci. Rep. 2021, 11, 9066. [Google Scholar] [CrossRef]
- Gou, H.; Liu, Y.; Shi, W.; Nan, J.; Wang, C.; Sun, Y.; Cao, Q.; Wei, H.; Song, C.; Tian, C.; et al. The Characteristics and Function of Internalin G in Listeria monocytogenes. Pol. J. Microbiol. 2022, 71, 63–71. [Google Scholar] [CrossRef]
- Bierne, H.; Sabet, C.; Personnic, N.; Cossart, P. Internalins: A complex family of leucine-rich repeat-containing proteins in Listeria monocytogenes. Microbes Infect 2007, 9, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Popowska, M.; Krawczyk-Balska, A.; Ostrowski, R.; Desvaux, M. InlL from Listeria monocytogenes Is Involved in Biofilm Formation and Adhesion to Mucin. Front. Microbiol. 2017, 8, 660. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.; Disson, O.; Lavina, M.; Thouvenot, P.; Huang, L.; Leclercq, A.; Fredriksson-Ahomaa, M.; Eshwar, A.K.; Stephan, R.; Lecuit, M. Atypical Hemolytic Listeria innocua Isolates Are Virulent, albeit Less than Listeria monocytogenes. Infect. Immun. 2019, 87. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Draper, L.A.; Lawton, E.M.; Daly, K.M.; Groeger, D.S.; Casey, P.G.; Ross, R.P.; Hill, C. Listeriolysin S, a novel peptide haemolysin associated with a subset of lineage I Listeria monocytogenes. PLoS Pathog. 2008, 4, e1000144. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ye, Q.; Chen, M.; Zhang, J.; Xue, L.; Wang, J.; Wu, S.; Zeng, H.; Gu, Q.; Zhang, Y.; et al. Multiplex PCR for the Identification of Pathogenic Listeria in Flammulina velutipes Plant Based on Novel Specific Targets Revealed by Pan-Genome Analysis. Front. Microbiol. 2020, 11, 634255. [Google Scholar] [CrossRef]
- Martinez-Rios, V.; Gkogka, E.; Dalgaard, P. Predicting growth of Listeria monocytogenes at dynamic conditions during manufacturing, ripening and storage of cheeses—Evaluation and application of models. Food Microbiol. 2020, 92, 103578. [Google Scholar] [CrossRef]
- Tavares, R.M.; Silva, D.; Camargo, A.C.; Yamatogi, R.S.; Nero, L.A. Interference of the acid stress on the expression of llsX by Listeria monocytogenes pathogenic island 3 (LIPI-3) variants. Food Res. Int. 2020, 132, 109063. [Google Scholar] [CrossRef]

| Strain ID | CC | Cluster (Cl) | Sampling Date | Sampling Area | Surface |
|---|---|---|---|---|---|
| Lm_2529 | CC1 | Cl_CC1 | 16 July 2020 | Washing area | Floor grid |
| Lm_2604 | CC1 | 16 September 2021 | Cutting-processing room | Cold room—drain channel | |
| Lm_2527 | CC9 | Cl_CC9 | 16 July 2020 | Cutting-processing room | Drain channel |
| Lm_2603 | CC9 | 16 September 2021 | Cutting-processing room | Teflon table—legs and feet | |
| Lm_2516 | CC9 | 16 July 2020 | Cutting-processing room | Sausage table—legs and feet | |
| 2021.TE.312882.1.40 | CC9 | 13 May 2021 | Washing area | Shoe–cleaning machine—brushes and external part | |
| Lm_2513 | CC218 | Cl_CC218_A | 16 July 2020 | Cutting-processing room | Cold room—wall–floor connection |
| Lm_2525 | CC218 | Cl_CC218_B | 16 July 2020 | Cutting-processing room | Water puller |
| Lm_2568 | CC218 | Cl_CC218_C | 13 May 2021 | Cutting-processing room | Sausage table—legs and feet |
| Compared Clonal Complex | No. of Loci Having the Same Allele | Percentage of Equal Loci (n = 93) | % (95% C.I.) |
|---|---|---|---|
| CC218 vs. CC9 | 21 | 23% | 15.3–32.1% |
| CC1 vs. CC9 | 21 | 23% | 15.3–32.1% |
| CC1 vs. CC218 | 42 | 45% | 35.4–55.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiavano, G.F.; Guidi, F.; Pomilio, F.; Brandi, G.; Salini, R.; Amagliani, G.; Centorotola, G.; Palma, F.; Felici, M.; Lorenzetti, C.; et al. Listeria monocytogenes Strains Persisting in a Meat Processing Plant in Central Italy: Use of Whole Genome Sequencing and In Vitro Adhesion and Invasion Assays to Decipher Their Virulence Potential. Microorganisms 2023, 11, 1659. https://doi.org/10.3390/microorganisms11071659
Schiavano GF, Guidi F, Pomilio F, Brandi G, Salini R, Amagliani G, Centorotola G, Palma F, Felici M, Lorenzetti C, et al. Listeria monocytogenes Strains Persisting in a Meat Processing Plant in Central Italy: Use of Whole Genome Sequencing and In Vitro Adhesion and Invasion Assays to Decipher Their Virulence Potential. Microorganisms. 2023; 11(7):1659. https://doi.org/10.3390/microorganisms11071659
Chicago/Turabian StyleSchiavano, Giuditta Fiorella, Fabrizia Guidi, Francesco Pomilio, Giorgio Brandi, Romolo Salini, Giulia Amagliani, Gabriella Centorotola, Francesco Palma, Martina Felici, Cinzia Lorenzetti, and et al. 2023. "Listeria monocytogenes Strains Persisting in a Meat Processing Plant in Central Italy: Use of Whole Genome Sequencing and In Vitro Adhesion and Invasion Assays to Decipher Their Virulence Potential" Microorganisms 11, no. 7: 1659. https://doi.org/10.3390/microorganisms11071659
APA StyleSchiavano, G. F., Guidi, F., Pomilio, F., Brandi, G., Salini, R., Amagliani, G., Centorotola, G., Palma, F., Felici, M., Lorenzetti, C., & Blasi, G. (2023). Listeria monocytogenes Strains Persisting in a Meat Processing Plant in Central Italy: Use of Whole Genome Sequencing and In Vitro Adhesion and Invasion Assays to Decipher Their Virulence Potential. Microorganisms, 11(7), 1659. https://doi.org/10.3390/microorganisms11071659






