The Slaughterhouse as Hotspot of CC1 and CC6 Listeria monocytogenes Strains with Hypervirulent Profiles in an Integrated Poultry Chain of Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Collection
2.2. Whole Genome Sequencing (WGS) Analysis
2.3. Multilocus Sequence Typing, Core Genome Multilocus Sequence Typing and Single-Nucleotide Polymorphism Analysis
2.4. Genetic Determinants Involved in Virulence Potential, Antimicrobial Resistance, Stress Adaptation and Heavy Metal and Disinfectant Resistance
3. Results
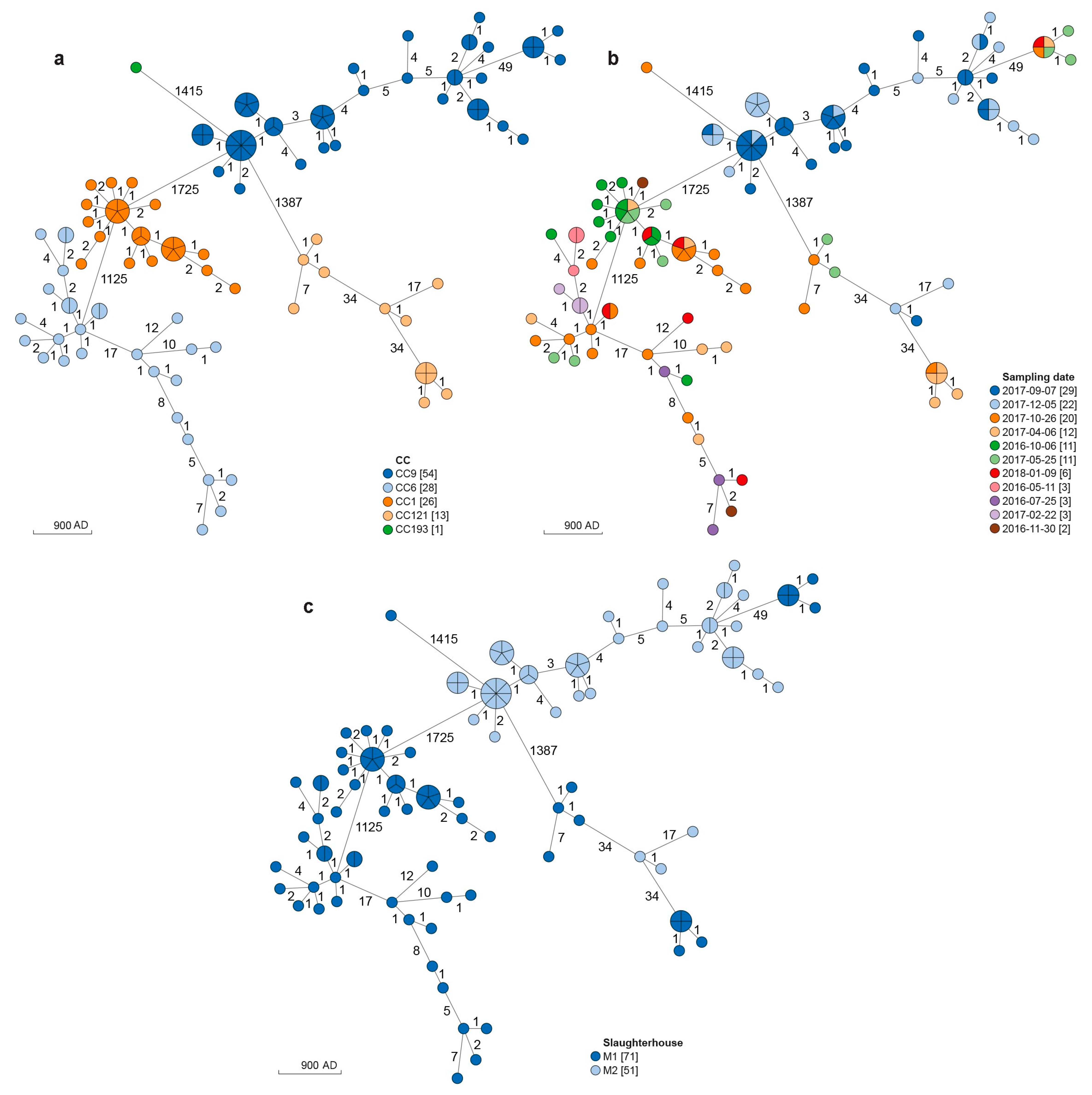
3.1. MLST Analysis
3.2. cgMLST and SNPs Analyses
3.3. Genetic Determinants Involved in Virulence Potential, Antimicrobial Resistance, Stress Adaptation and Heavy Metal and Disinfectant Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Available online: https://agriculture.ec.europa.eu/farming/animal-products/poultry_en (accessed on 15 February 2023).
- European Food Safety Authority. European Centre for Disease Prevention and Control the European Union One Health 2021 Zoonoses Report. EFSa J. 2022, 20, 07666. [Google Scholar] [CrossRef]
- Li, X.; Shi, X.; Song, Y.; Yao, S.; Li, K.; Shi, B.; Sun, J.; Liu, Z.; Zhao, W.; Zhao, C.; et al. Genetic Diversity, Antibiotic Resistance, and Virulence Profiles of Listeria Monocytogenes from Retail Meat and Meat Processing. Food Res. Int. 2022, 162, 112040. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-H.; Moura, A.; Gu, Z.-Q.; Chang, J.-H.; Liao, Y.-S.; Teng, R.-H.; Tseng, K.-Y.; Chang, D.-L.; Liu, W.-R.; Huang, Y.-T.; et al. Genomic Surveillance of Listeria Monocytogenes in Taiwan, 2014 to 2019. Microbiol. Spectr. 2022, 10, e01825-22. [Google Scholar] [CrossRef] [PubMed]
- López-Alonso, V.; Ortiz, S.; Corujo, A.; Martínez-Suárez, J.V. Analysis of Benzalkonium Chloride Resistance and Potential Virulence of Listeria Monocytogenes Isolates Obtained from Different Stages of a Poultry Production Chain in Spain. J. Food Prot. 2020, 83, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Coban, A.; Pennone, V.; Sudagidan, M.; Molva, C.; Jordan, K.; Aydin, A. Prevalence, Virulence Characterization, and Genetic Relatedness of Listeria Monocytogenes Isolated from Chicken Retail Points and Poultry Slaughterhouses in Turkey. Braz. J. Microbiol. 2019, 50, 1063–1073. [Google Scholar] [CrossRef]
- Iannetti, L.; Schirone, M.; Neri, D.; Visciano, P.; Acciari, V.A.; Centorotola, G.; Mangieri, M.S.; Torresi, M.; Santarelli, G.A.; Di Marzio, V.; et al. Listeria Monocytogenes in Poultry: Detection and Strain Characterization along an Integrated Production Chain in Italy. Food Microbiol. 2020, 91, 103533. [Google Scholar] [CrossRef]
- Agostinho Davanzo, E.F.; dos Santos, R.L.; Castro, V.H.d.L.; Palma, J.M.; Pribul, B.R.; Dallago, B.S.L.; Fuga, B.; Medeiros, M.; Titze de Almeida, S.S.; da Costa, H.M.B.; et al. Molecular Characterization of Salmonella Spp. and Listeria Monocytogenes Strains from Biofilms in Cattle and Poultry Slaughterhouses Located in the Federal District and State of Goiás, Brazil. PLoS ONE 2021, 16, e0259687. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Chang, X.; Qin, S.; Song, Y.; Tian, J.; Ma, A. Analysis of 90 Listeria Monocytogenes Contaminated in Poultry and Livestock Meat through Whole-Genome Sequencing. Food Res. Int. 2022, 159, 111641. [Google Scholar] [CrossRef]
- Marcus, R.; Hurd, S.; Mank, L.; Mshar, P.; Phan, Q.; Jackson, K.; Watarida, K.; Salfinger, Y.; Kim, S.; Ishida, M.L.; et al. Chicken Salad as the Source of a Case of Listeria Monocytogenes Infection in Connecticut. J. Food Prot. 2009, 72, 2602–2606. [Google Scholar] [CrossRef]
- Little, C.L.; Amar, C.F.L.; Awofisayo, A.; Grant, K.A. Hospital-Acquired Listeriosis Associated with Sandwiches in the UK: A Cause for Concern. J. Hosp. Infect. 2012, 82, 13–18. [Google Scholar] [CrossRef]
- Zwizwai, R. Infectious Disease Surveillance Update. Lancet Infect. Dis. 2019, 19, 699. [Google Scholar] [CrossRef]
- McLauchlin, J.; Aird, H.; Amar, C.; Barker, C.; Dallman, T.; Elviss, N.; Jørgensen, F.; Willis, C. Listeria Monocytogenes in Cooked Chicken: Detection of an Outbreak in the United Kingdom (2016 to 2017) and Analysis of L. Monocytogenes from Unrelated Monitoring of Foods (2013 to 2017). J. Food Prot. 2020, 83, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Maury, M.M.; Bracq-Dieye, H.; Huang, L.; Vales, G.; Lavina, M.; Thouvenot, P.; Disson, O.; Leclercq, A.; Brisse, S.; Lecuit, M. Hypervirulent Listeria Monocytogenes Clones’ Adaption to Mammalian Gut Accounts for Their Association with Dairy Products. Nat. Commun. 2019, 10, 2488. [Google Scholar] [CrossRef] [PubMed]
- Stoller, A.; Stevens, M.; Stephan, R.; Guldimann, C. Characteristics of Listeria Monocytogenes Strains Persisting in a Meat Processing Facility over a 4-Year Period. Pathogens 2019, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.; Lee, S.; Kathariou, S. Dissemination and Conservation of Cadmium and Arsenic Resistance Determinants in Listeria and Other Gram-positive Bacteria. Mol. Microbiol. 2020, 113, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Palaiodimou, L.; Fanning, S.; Fox, E.M. Genomic Insights into Persistence of Listeria Species in the Food Processing Environment. J. Appl. Microbiol. 2021, 131, 2082–2094. [Google Scholar] [CrossRef]
- Gray, J.A.; Chandry, P.S.; Kaur, M.; Kocharunchitt, C.; Bowman, J.P.; Fox, E.M. Characterisation of Listeria Monocytogenes Food-Associated Isolates to Assess Environmental Fitness and Virulence Potential. Int. J. Food Microbiol. 2021, 350, 109247. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (EFSA BIOHAZ Panel); Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F.; et al. Whole Genome Sequencing and Metagenomics for Outbreak Investigation, Source Attribution and Risk Assessment of Food-borne Microorganisms. EFSa J. 2019, 17, 05898. [Google Scholar] [CrossRef]
- Lee, S.; Ryu, S. Draft Genome Sequence of Listeria Monocytogenes Clonal Complex 1 Strain SNU3 from South Korea. Microbiol. Resour. Announc. 2023, 12, e01226-22. [Google Scholar] [CrossRef]
- Brown, P.; Chen, Y.; Siletzky, R.; Parsons, C.; Jaykus, L.-A.; Eifert, J.; Ryser, E.; Logue, C.M.; Stam, C.; Brown, E.; et al. Harnessing Whole Genome Sequence Data for Facility-Specific Signatures for Listeria Monocytogenes: A Case Study with Turkey Processing Plants in the United States. Front. Sustain. Food Syst. 2021, 5, 742353. [Google Scholar] [CrossRef]
- Maury, M.M.; Tsai, Y.-H.; Charlier, C.; Touchon, M.; Chenal-Francisque, V.; Leclercq, A.; Criscuolo, A.; Gaultier, C.; Roussel, S.; Brisabois, A.; et al. Uncovering Listeria Monocytogenes Hypervirulence by Harnessing Its Biodiversity. Nat. Genet. 2016, 48, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.; Criscuolo, A.; Pouseele, H.; Maury, M.M.; Leclercq, A.; Tarr, C.; Björkman, J.T.; Dallman, T.; Reimer, A.; Enouf, V.; et al. Whole Genome-Based Population Biology and Epidemiological Surveillance of Listeria Monocytogenes. Nat. Microbiol. 2016, 2, 16185. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Boland, J.A.; Wagner, M.; Scortti, M. Why Are Some Listeria Monocytogenes Genotypes More Likely to Cause Invasive (Brain, Placental) Infection? mBio 2020, 11, e03126-20. [Google Scholar] [CrossRef]
- Shi, D.; Anwar, T.M.; Pan, H.; Chai, W.; Xu, S.; Yue, M. Genomic Determinants of Pathogenicity and Antimicrobial Resistance for 60 Global Listeria Monocytogenes Isolates Responsible for Invasive Infections. Front. Cell. Infect. Microbiol. 2021, 11, 718840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, W.; Wang, J.; Xu, B.; Liu, H.; Dong, Q.; Zhang, X. 10-Year Molecular Surveillance of Listeria Monocytogenes Using Whole-Genome Sequencing in Shanghai, China, 2009–2019. Front. Microbiol. 2020, 11, 551020. [Google Scholar] [CrossRef] [PubMed]
- Guidi, F.; Orsini, M.; Chiaverini, A.; Torresi, M.; Centorame, P.; Acciari, V.A.; Salini, R.; Palombo, B.; Brandi, G.; Amagliani, G.; et al. Hypo- and Hyper-Virulent Listeria Monocytogenes Clones Persisting in Two Different Food Processing Plants of Central Italy. Microorganisms 2021, 9, 376. [Google Scholar] [CrossRef] [PubMed]
- Portmann, A.-C.; Fournier, C.; Gimonet, J.; Ngom-Bru, C.; Barretto, C.; Baert, L. A Validation Approach of an End-to-End Whole Genome Sequencing Workflow for Source Tracking of Listeria Monocytogenes and Salmonella Enterica. Front. Microbiol. 2018, 9, 446. [Google Scholar] [CrossRef]
- Cito, F.; Di Pasquale, A.; Cammà, C.; Cito, P. The Italian Information System for the Collection and Analysis of Complete Genome Sequence of Pathogens Isolated from Animal, Food and Environment. Int. J. Infect. Dis. 2018, 73, 296–297. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Wingett, S.W.; Andrews, S. FastQ Screen: A Tool for Multi-Genome Mapping and Quality Control. F1000Research 2018, 7, 1338. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality Assessment Tool for Genome Assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Timme, R.E.; Wolfgang, W.J.; Balkey, M.; Venkata, S.L.G.; Randolph, R.; Allard, M.; Strain, E. Optimizing Open Data to Support One Health: Best Practices to Ensure Interoperability of Genomic Data from Bacterial Pathogens. One Health Outlook 2020, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Ragon, M.; Wirth, T.; Hollandt, F.; Lavenir, R.; Lecuit, M.; Le Monnier, A.; Brisse, S. A New Perspective on Listeria Monocytogenes Evolution. PLoS Pathog. 2008, 4, e1000146. [Google Scholar] [CrossRef]
- Silva, M.; Machado, M.P.; Silva, D.N.; Rossi, M.; Moran-Gilad, J.; Santos, S.; Ramirez, M.; Carriço, J.A. ChewBBACA: A Complete Suite for Gene-by-Gene Schema Creation and Strain Identification. Microb. Genom. 2018, 4, 000166. [Google Scholar] [CrossRef]
- Moura, A.; Tourdjman, M.; Leclercq, A.; Hamelin, E.; Laurent, E.; Fredriksen, N.; Van Cauteren, D.; Bracq-Dieye, H.; Thouvenot, P.; Vales, G.; et al. Real-Time Whole-Genome Sequencing for Surveillance of Listeria Monocytogenes, France. Emerg. Infect. Dis. 2017, 23, 1462–1470. [Google Scholar] [CrossRef]
- Zhou, Z.; Alikhan, N.-F.; Sergeant, M.J.; Luhmann, N.; Vaz, C.; Francisco, A.P.; Carriço, J.A.; Achtman, M. GrapeTree: Visualization of Core Genomic Relationships among 100,000 Bacterial Pathogens. Genome Res. 2018, 28, 1395–1404. [Google Scholar] [CrossRef]
- Davis, S.; Pettengill, J.B.; Luo, Y.; Payne, J.; Shpuntoff, A.; Rand, H.; Strain, E. CFSAN SNP Pipeline: An Automated Method for Constructing SNP Matrices from next-Generation Sequence Data. PeerJ Comput. Sci. 2015, 1, e20. [Google Scholar] [CrossRef]
- Yang, H.; Hoffmann, M.; Allard, M.W.; Brown, E.W.; Chen, Y. Microevolution and Gain or Loss of Mobile Genetic Elements of Outbreak-Related Listeria Monocytogenes in Food Processing Environments Identified by Whole Genome Sequencing Analysis. Front. Microbiol. 2020, 11, 866. [Google Scholar] [CrossRef]
- Pightling, A.W.; Pettengill, J.B.; Luo, Y.; Baugher, J.D.; Rand, H.; Strain, E. Interpreting Whole-Genome Sequence Analyses of Foodborne Bacteria for Regulatory Applications and Outbreak Investigations. Front. Microbiol. 2018, 9, 1482. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Calleja, C.; Gómez-Fernández, S.; Carballo, J.; Capita, R. Prevalence, Molecular Typing, and Determination of the Biofilm-Forming Ability of Listeria Monocytogenes Serotypes from Poultry Meat and Poultry Preparations in Spain. Microorganisms 2019, 7, 529. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, A.; Zeinali, T. Significance and Characteristics of Listeria Monocytogenes in Poultry Products. Int. J. Food Sci. 2019, 2019, 7835253. [Google Scholar] [CrossRef]
- Fagerlund, A.; Langsrud, S.; Schirmer, B.C.T.; Møretrø, T.; Heir, E. Genome Analysis of Listeria Monocytogenes Sequence Type 8 Strains Persisting in Salmon and Poultry Processing Environments and Comparison with Related Strains. PLoS ONE 2016, 11, e0151117. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Song, Z.; Luo, L.; Wang, Y.; Li, L.; Mao, P.; Ye, C.; Wang, Y. Whole-Genome Sequencing Reveals Genomic Characterization of Listeria Monocytogenes from Food in China. Front. Microbiol. 2023, 13, 1049843. [Google Scholar] [CrossRef]
- Yin, Y.; Tan, W.; Wang, G.; Kong, S.; Zhou, X.; Zhao, D.; Jia, Y.; Pan, Z.; Jiao, X. Geographical and Longitudinal Analysis of Listeria Monocytogenes Genetic Diversity Reveals Its Correlation with Virulence and Unique Evolution. Microbiol. Res. 2015, 175, 84–92. [Google Scholar] [CrossRef]
- Hilliard, A.; Leong, D.; O’Callaghan, A.; Culligan, E.; Morgan, C.; DeLappe, N.; Hill, C.; Jordan, K.; Cormican, M.; Gahan, C. Genomic Characterization of Listeria Monocytogenes Isolates Associated with Clinical Listeriosis and the Food Production Environment in Ireland. Genes 2018, 9, 171. [Google Scholar] [CrossRef]
- Lee, S.; Chen, Y.; Gorski, L.; Ward, T.J.; Osborne, J.; Kathariou, S. Listeria Monocytogenes Source Distribution Analysis Indicates Regional Heterogeneity and Ecological Niche Preference among Serotype 4b Clones. mBio 2018, 9, e00396-18. [Google Scholar] [CrossRef]
- Scaltriti, E.; Bolzoni, L.; Vocale, C.; Morganti, M.; Menozzi, I.; Re, M.C.; Pongolini, S. Population Structure of Listeria Monocytogenes in Emilia-Romagna (Italy) and Implications on Whole Genome Sequencing Surveillance of Listeriosis. Front. Public Health 2020, 8, 519293. [Google Scholar] [CrossRef]
- Lepe, J.A. Current Aspects of Listeriosis. Med. Clínica 2020, 154, 453–458. [Google Scholar] [CrossRef]
- Burnett, E.; Kucerova, Z.; Freeman, M.; Kathariou, S.; Chen, J.; Smikle, M. Whole-Genome Sequencing Reveals Multiple Subpopulations of Dominant and Persistent Lineage I Isolates of Listeria Monocytogenes in Two Meat Processing Facilities during 2011–2015. Microorganisms 2022, 10, 1070. [Google Scholar] [CrossRef] [PubMed]
- Nüesch-Inderbinen, M.; Bloemberg, G.V.; Müller, A.; Stevens, M.J.A.; Cernela, N.; Kollöffel, B.; Stephan, R. Listeriosis Caused by Persistence of Listeria Monocytogenes Serotype 4b Sequence Type 6 in Cheese Production Environment. Emerg. Infect. Dis. 2021, 27, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Cao, G.; Zhang, J.; Pan, H.; Zhang, D.; Kuang, D.; Yang, X.; Xu, X.; Shi, X.; Meng, J. Characterization of Internalin Genes in Listeria Monocytogenes from Food and Humans, and Their Association with the Invasion of Caco-2 Cells. Gut Pathog. 2019, 11, 30. [Google Scholar] [CrossRef]
- Tavares, R.d.M.; da Silva, D.A.L.; Camargo, A.C.; Yamatogi, R.S.; Nero, L.A. Interference of the Acid Stress on the Expression of LlsX by Listeria Monocytogenes Pathogenic Island 3 (LIPI-3) Variants. Food Res. Int. 2020, 132, 109063. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Draper, L.A.; Lawton, E.M.; Daly, K.M.; Groeger, D.S.; Casey, P.G.; Ross, R.P.; Hill, C. Listeriolysin S, a Novel Peptide Haemolysin Associated with a Subset of Lineage I Listeria Monocytogenes. PLoS Pathog. 2008, 4, e1000144. [Google Scholar] [CrossRef] [PubMed]
- Quereda, J.J.; Morón-García, A.; Palacios-Gorba, C.; Dessaux, C.; García-del Portillo, F.; Pucciarelli, M.G.; Ortega, A.D. Pathogenicity and Virulence of Listeria monocytogenes: A Trip from Environmental to Medical Microbiology. Virulence 2021, 12, 2509–2545. [Google Scholar] [CrossRef] [PubMed]
- Quereda, J.J.; Meza-Torres, J.; Cossart, P.; Pizarro-Cerdá, J. Listeriolysin S: A Bacteriocin from Epidemic Listeria monocytogenes Strains That Targets the Gut Microbiota. Gut Microbes 2017, 8, 384–391. [Google Scholar] [CrossRef]
- Gelbícová, T.; Kolácková, I.; Pantu, R. A Novel Mutation Leading to a Premature Stop Codon in InlA of Listeria Monocytogenes Isolated from Neonatal Listeriosis. New Microbiol. 2015, 38, 293–296. [Google Scholar]
- Van Stelten, A.; Simpson, J.M.; Ward, T.J.; Nightingale, K.K. Revelation by Single-Nucleotide Polymorphism Genotyping That Mutations Leading to a Premature Stop Codon in InlA Are Common among Listeria Monocytogenes Isolates from Ready-To-Eat Foods but Not Human Listeriosis Cases. Appl. Environ. Microbiol. 2010, 76, 2783–2790. [Google Scholar] [CrossRef]
- Nightingale, K.K.; Ivy, R.A.; Ho, A.J.; Fortes, E.D.; Njaa, B.L.; Peters, R.M.; Wiedmann, M. InlA Premature Stop Codons Are Common among Listeria Monocytogenes Isolates from Foods and Yield Virulence-Attenuated Strains That Confer Protection against Fully Virulent Strains. Appl. Environ. Microbiol. 2008, 74, 6570–6583. [Google Scholar] [CrossRef]
- Di Ciccio, P.; Rubiola, S.; Panebianco, F.; Lomonaco, S.; Allard, M.; Bianchi, D.M.; Civera, T.; Chiesa, F. Biofilm Formation and Genomic Features of Listeria Monocytogenes Strains Isolated from Meat and Dairy Industries Located in Piedmont (Italy). Int. J. Food Microbiol. 2022, 378, 109784. [Google Scholar] [CrossRef]
- Hurley, D.; Luque-Sastre, L.; Parker, C.T.; Huynh, S.; Eshwar, A.K.; Nguyen, S.V.; Andrews, N.; Moura, A.; Fox, E.M.; Jordan, K.; et al. Whole-Genome Sequencing-Based Characterization of 100 Listeria Monocytogenes Isolates Collected from Food Processing Environments over a Four-Year Period. mSphere 2019, 4, e00252-19. [Google Scholar] [CrossRef]
- Parsons, C.; Lee, S.; Kathariou, S. Heavy Metal Resistance Determinants of the Foodborne Pathogen Listeria Monocytogenes. Genes 2018, 10, 11. [Google Scholar] [CrossRef]
- Corbett, D.; Schuler, S.; Glenn, S.; Andrew, P.W.; Cavet, J.S.; Roberts, I.S. The Combined Actions of the Copper-Responsive Repressor CsoR and Copper-Metallochaperone CopZ Modulate CopA-Mediated Copper Efflux in the Intracellular Pathogen Listeria Monocytogenes. Mol. Microbiol. 2011, 81, 457–472. [Google Scholar] [CrossRef]
- Cotter, P.D.; Ryan, S.; Gahan, C.G.M.; Hill, C. Presence of GadD1 Glutamate Decarboxylase in Selected Listeria Monocytogenes Strains Is Associated with an Ability to Grow at Low PH. Appl. Environ. Microbiol. 2005, 71, 2832–2839. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.; Begley, M.; Hill, C.; Gahan, C.G.M. A Five-Gene Stress Survival Islet (SSI-1) That Contributes to the Growth of Listeria Monocytogenes in Suboptimal Conditions: Stress Survival Islet in L. Monocytogenes. J. Appl. Microbiol. 2010, 109, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Orsi, R.H.; Gaballa, A.; Wiedmann, M.; Boor, K.J.; Guariglia-Oropeza, V. Systematic Review of the Listeria Monocytogenes σ B Regulon Supports a Role in Stress Response, Virulence and Metabolism. Future Microbiol. 2019, 14, 801–828. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Motaal, H.; Meng, L.; Zhang, Z.; Abdelazez, A.H.; Shao, L.; Xu, T.; Meng, F.; Abozaed, S.; Zhang, R.; Jiang, J. An Uncharacterized Major Facilitator Superfamily Transporter From Planococcus Maritimus Exhibits Dual Functions as a Na+(Li+, K+)/H+ Antiporter and a Multidrug Efflux Pump. Front. Microbiol. 2018, 9, 1601. [Google Scholar] [CrossRef]
- Xu, N.; Zheng, Y.; Wang, X.; Krulwich, T.A.; Ma, Y.; Liu, J. The Lysine 299 Residue Endows the Multisubunit Mrp1 Antiporter with Dominant Roles in Na + Resistance and PH Homeostasis in Corynebacterium Glutamicum. Appl. Environ. Microbiol. 2018, 84, e00110-18. [Google Scholar] [CrossRef]
- Yan, L.; Wang, C.; Jiang, J.; Liu, S.; Zheng, Y.; Yang, M.; Zhang, Y. Nitrate Removal by Alkali-Resistant Pseudomonas Sp. XS-18 under Aerobic Conditions: Performance and Mechanism. Bioresour. Technol. 2022, 344, 126175. [Google Scholar] [CrossRef]
- Slipski, C.J.; Zhanel, G.G.; Bay, D.C. Biocide Selective TolC-Independent Efflux Pumps in Enterobacteriaceae. J. Membr. Biol. 2018, 251, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gupta, V.; Pathania, R. Efflux Pump Inhibitors for Bacterial Pathogens: From Bench to Bedside. Indian J. Med. Res. 2019, 149, 129. [Google Scholar] [CrossRef] [PubMed]
- Cherifi, T.; Arsenault, J.; Pagotto, F.; Quessy, S.; Côté, J.-C.; Neira, K.; Fournaise, S.; Bekal, S.; Fravalo, P. Distribution, Diversity and Persistence of Listeria Monocytogenes in Swine Slaughterhouses and Their Association with Food and Human Listeriosis Strains. PLoS ONE 2020, 15, e0236807. [Google Scholar] [CrossRef]
- Matereke, L.T.; Okoh, A.I. Listeria Monocytogenes Virulence, Antimicrobial Resistance and Environmental Persistence: A Review. Pathogens 2020, 9, 528. [Google Scholar] [CrossRef]
- Gorski, L.; Cooley, M.B.; Oryang, D.; Carychao, D.; Nguyen, K.; Luo, Y.; Weinstein, L.; Brown, E.; Allard, M.; Mandrell, R.E.; et al. Prevalence and Clonal Diversity of over 1200 Listeria Monocytogenes Isolates Collected from Public Access Waters near Produce Production Areas on the Central California Coast during 2011 to 2016. Appl Environ. Microbiol 2022, 88, e00357-22. [Google Scholar] [CrossRef] [PubMed]
- Muchaamba, F.; Stephan, R.; Tasara, T. Listeria Monocytogenes Cold Shock Proteins: Small Proteins with A Huge Impact. Microorganisms 2021, 9, 1061. [Google Scholar] [CrossRef]
- Harter, E.; Wagner, E.M.; Zaiser, A.; Halecker, S.; Wagner, M.; Rychli, K. Stress Survival Islet 2, Predominantly Present in Listeria Monocytogenes Strains of Sequence Type 121, Is Involved in the Alkaline and Oxidative Stress Responses. Appl. Environ. Microbiol. 2017, 83, e00827-17. [Google Scholar] [CrossRef]
- Müller, A.; Rychli, K.; Muhterem-Uyar, M.; Zaiser, A.; Stessl, B.; Guinane, C.M.; Cotter, P.D.; Wagner, M.; Schmitz-Esser, S. Tn6188—A Novel Transposon in Listeria Monocytogenes Responsible for Tolerance to Benzalkonium Chloride. PLoS ONE 2013, 8, e76835. [Google Scholar] [CrossRef]
- Cherifi, T.; Carrillo, C.; Lambert, D.; Miniaï, I.; Quessy, S.; Larivière-Gauthier, G.; Blais, B.; Fravalo, P. Genomic Characterization of Listeria Monocytogenes Isolates Reveals That Their Persistence in a Pig Slaughterhouse Is Linked to the Presence of Benzalkonium Chloride Resistance Genes. BMC Microbiol. 2018, 18, 220. [Google Scholar] [CrossRef]
- Parra-Flores, J.; Holý, O.; Bustamante, F.; Lepuschitz, S.; Pietzka, A.; Contreras-Fernández, A.; Castillo, C.; Ovalle, C.; Alarcón-Lavín, M.P.; Cruz-Córdova, A.; et al. Virulence and Antibiotic Resistance Genes in Listeria Monocytogenes Strains Isolated From Ready-to-Eat Foods in Chile. Front. Microbiol. 2022, 12, 796040. [Google Scholar] [CrossRef]
- Fredriksson-Ahomaa, M.; Sauvala, M.; Kurittu, P.; Heljanko, V.; Heikinheimo, A.; Paulsen, P. Characterisation of Listeria Monocytogenes Isolates from Hunted Game and Game Meat from Finland. Foods 2022, 11, 3679. [Google Scholar] [CrossRef] [PubMed]
- Mafuna, T.; Matle, I.; Magwedere, K.; Pierneef, R.E.; Reva, O.N. Whole Genome-Based Characterization of Listeria Monocytogenes Isolates Recovered from the Food Chain in South Africa. Front. Microbiol. 2021, 12, 669287. [Google Scholar] [CrossRef]
- Guidi, F.; Chiaverini, A.; Repetto, A.; Lorenzetti, C.; Centorotola, G.; Bazzucchi, V.; Palombo, B.; Gattuso, A.; Pomilio, F.; Blasi, G. Hyper-Virulent Listeria Monocytogenes Strains Associated With Respiratory Infections in Central Italy. Front. Cell. Infect. Microbiol. 2021, 11, 765540. [Google Scholar] [CrossRef] [PubMed]
- Hanes, R.M.; Huang, Z. Investigation of Antimicrobial Resistance Genes in Listeria Monocytogenes from 2010 through to 2021. Int. J. Environ. Res. Public Health 2022, 19, 5506. [Google Scholar] [CrossRef] [PubMed]
- Mpondo, L.; Ebomah, K.E.; Okoh, A.I. Multidrug-Resistant Listeria Species Shows Abundance in Environmental Waters of a Key District Municipality in South Africa. Int. J. Environ. Res. Public Health 2021, 18, 481. [Google Scholar] [CrossRef] [PubMed]

| Slaughterhouse | CC1-ST1 | CC6-ST6 | CC9-ST9 | CC121-ST121 | CC193-ST193 | Total |
|---|---|---|---|---|---|---|
| M1 | 26 | 28 | 6 | 10 | 1 | 71 |
| M2 | 0 | 0 | 48 | 3 | 0 | 51 |
| Total | 26 | 28 | 54 | 13 | 1 | 122 |
| CC | Cluster | N° of Strains | Slaughterhouse | Timeframe | AD | SNPs |
|---|---|---|---|---|---|---|
| CC1 | I | 26 | M1 | October 2016–January 2018 | 0–7 | 4–10 |
| CC6 | I | 16 | M1 | May 2016–January 2018 | 0–7 | 1–17 |
| II | 4 | M1 | July 2016–January 2018 | 1–7 | 6–12 | |
| III | 3 | M1 | July 2016–January 2018 | 0–1 | 1–3 | |
| IV | 2 | M1 | April–October 2017 | 1 | 0 | |
| V | 2 | M1 | April 2017 | 1 | 2 | |
| CC9 | I | 30 | M2 | September–December 2017 | 0–7 | 0–16 |
| II | 15 | M2 | September–December 2017 | 0–5 | 1–20 | |
| III | 2 | M2 | September 2017 | 1 | 0 | |
| IV | 6 | M1 | April 2017–January 2018 | 0–1 | 1–6 | |
| CC121 | I | 4 | M1 | May–October 2017 | 0–7 | 1–19 |
| II | 6 | M1 | April–October 2017 | 0–1 | 0–5 | |
| III | 2 | M2 | September–December 2017 | 1 | 1 |
| Main function | Gene/Island | Clonal Complex | |
|---|---|---|---|
| Virulence | LIPI-3 | CC1, CC6 | |
| gltA-gltB | CC1, CC6 | ||
| aut_IVb | CC1, CC6 | ||
| lapB | CC1, CC6, CC9, CC121 | ||
| ami | CC9, CC121, CC193 | ||
| tagB | CC9, CC121, CC193 | ||
| inlF | CC9, CC193 | ||
| inlG | CC9 | ||
| inlL | CC9 | ||
| Metal resistance | Cadmium | cadA, cadC | CC1 |
| Arsenic | arsB, arsC | CC1, CC6, CC9, CC121, CC193 | |
| arsA, arsD | CC9 | ||
| Copper | CsoR-copA-copZ | CC1, CC6, CC9, CC121, CC193 | |
| copB | CC9, CC193 | ||
| Stress response | Acid Tolerance | gadB, gadC | CC1, CC6, CC9, CC121, CC193 |
| SSI-1 | CC9 | ||
| Alkali response | mrpA, mrpB, mrpC, mrpE, mrpF | CC1, CC6, CC9, CC121, CC193 | |
| SSI-2 | CC121 | ||
| Low pH, high salt concentration, refrigeration | SSI-1 | CC9 | |
| Oxidative stress | SSI-2 | CC121 | |
| Cold-shock | cspD | CC1, CC6, CC9, CC121, CC193 | |
| Biocides resistance | EmrB, mepA, bmrA, bmr3, norM | CC1, CC6, CC9, CC121, CC193 | |
| QACs | Tn6188_qacH | CC121 | |
| Biofilm production | Lmo0673, lmo2504, luxS, recO | CC1, CC6, CC9, CC121, CC193 | |
| inlL | CC9 | ||
| PMSC inlA | CC9, CC121, CC193 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guidi, F.; Centorotola, G.; Chiaverini, A.; Iannetti, L.; Schirone, M.; Visciano, P.; Cornacchia, A.; Scattolini, S.; Pomilio, F.; D’Alterio, N.; et al. The Slaughterhouse as Hotspot of CC1 and CC6 Listeria monocytogenes Strains with Hypervirulent Profiles in an Integrated Poultry Chain of Italy. Microorganisms 2023, 11, 1543. https://doi.org/10.3390/microorganisms11061543
Guidi F, Centorotola G, Chiaverini A, Iannetti L, Schirone M, Visciano P, Cornacchia A, Scattolini S, Pomilio F, D’Alterio N, et al. The Slaughterhouse as Hotspot of CC1 and CC6 Listeria monocytogenes Strains with Hypervirulent Profiles in an Integrated Poultry Chain of Italy. Microorganisms. 2023; 11(6):1543. https://doi.org/10.3390/microorganisms11061543
Chicago/Turabian StyleGuidi, Fabrizia, Gabriella Centorotola, Alexandra Chiaverini, Luigi Iannetti, Maria Schirone, Pierina Visciano, Alessandra Cornacchia, Silvia Scattolini, Francesco Pomilio, Nicola D’Alterio, and et al. 2023. "The Slaughterhouse as Hotspot of CC1 and CC6 Listeria monocytogenes Strains with Hypervirulent Profiles in an Integrated Poultry Chain of Italy" Microorganisms 11, no. 6: 1543. https://doi.org/10.3390/microorganisms11061543
APA StyleGuidi, F., Centorotola, G., Chiaverini, A., Iannetti, L., Schirone, M., Visciano, P., Cornacchia, A., Scattolini, S., Pomilio, F., D’Alterio, N., & Torresi, M. (2023). The Slaughterhouse as Hotspot of CC1 and CC6 Listeria monocytogenes Strains with Hypervirulent Profiles in an Integrated Poultry Chain of Italy. Microorganisms, 11(6), 1543. https://doi.org/10.3390/microorganisms11061543






