Abstract
Meat from small ruminants is considered a high quality and delicacy product in many countries. Several benefits have been perceived from probiotics as dietary supplements, such as improved carcass weight, color, tenderness, flavor, muscle fiber structure, water-holding capacity, and healthy fatty acid profile of the meat. Thus, the present review focuses on the effect of probiotics on improving the quality of meat from small ruminants. Though many benefits have been associated with the use of probiotics, the findings of all the considered articles are not always consistent, and the mechanisms behind improving meat quality are not appropriately defined. This variability of findings could be due to the use of different probiotic strains, dosage rates, number of days of experiment, nutrition, breed, age, and health status of the animals. Therefore, future research should emphasize specific strains, optimal dose and days of administration, route, and mechanisms for the specific probiotic strains to host. This review provides a comprehensive overview of the use of probiotics for small ruminants and their impact on meat quality.
1. Introduction
The growing world population demand for animal proteins has been increasing substantially. A recent study showed that global meat production was 263 million tonnes in 2018 and is expected to rise to 445 million tonnes by 2050 to meet consumer demand [1]. The demand for small ruminant meat, such as mutton (sheep meat) and chevon (goat meat), consumption has increased significantly due to their nutritional values compared with other animal species [2]. As a result, production of sheep and goat meat with better quality, high nutritional value, and better taste have received special attention from researchers [3]. However, farmers have focused more on improving the health status, production, and feed efficiency of farm animals by using growth promoters in the animal feed [4]. Thus, the use of antibiotics for the treatment and as growth promoters for animals has augmented the creation of antibiotic-resistant microbes, which negatively impact human life and the environment. Recently, the scientific world has discussed avoiding the irrational use of antibiotics for treatment and restricting the use of antibiotics as growth-promoting additives to avoid the risks to meat consumers and the environment. Therefore, many investigators focused on antibiotics substitutes, namely feed additives or supplements as probiotics (as single or mixed strains) to the animal feed [5,6]. Probiotics have been shown to have positive effects on the microbial ecosystem [7], nutrient synthesis [4,8], growth performance [9], carcass weight [10,11], muscle production and meat quality [3,12,13,14], prevention of enteric diseases [15], and immunity [16]. A study showed that 2.5 months of dietary supplement of probiotics (Lacticasebacillus rhamnosus; Saccharomyces cerevisiae) could maintain microbial balance in the gastrointestinal tracts of weanling lambs [6]. Similarly, other studies revealed that probiotics can improve lambs’ nutrient synthesis and growth performance [8,9]. In addition, using probiotic (Lacticaseibacillus casei and Lactiplantibacillus plantarum) supplements in lambs exhibited improved meat tenderness and flavor [12]. Hernández-García et al. [9] reported that yeast supplementation showed improved carcass traits in lambs. In contrast, Gadekar et al. [17] reported that dietary supplements of Saccharomyces cerevisiae did not affect lamb carcass traits. However, Picard and Gagaoua [18] reported that probiotics could regulate muscle fiber properties, which are directly linked to carcass yield and meat quality traits. Yet, the molecular mechanisms of probiotics related to the development of muscle fiber and modification of the carcass traits are not well documented [19]. Thus, exploring the insight knowledge on the interaction between probiotics and muscle development will help to improve carcass yield and meat quality traits of small ruminants (sheep and goat). Therefore, this review article aims to explore the effects of specific probiotics to improve the carcass and meat quality traits of small ruminants.
2. Methodology
The objective of this review was to collect and uphold the critical insight information available on the use of probiotics use in small ruminant production and their effects on performance and meat quality parameters. An electronic search was conducted using some keywords relevant to this study. The keywords used for the search were: probiotics, performance, meat quality, goat, sheep, and small ruminants. The articles published until 2022 were considered in search results and duplicates were removed. Articles were retrieved from four databases: Google Scholar (https://scholar.google.com/, accessed on 30 November 2022), Scopus (https://www.elsevier.com/ja-jp/solutions/scopus, accessed on 5 December 2022), PubMed (https://pubmed.ncbi.nlm.nih.gov/, accessed on 14 December 2022), as well as Science Direct (https://www.sciencedirect.com/, accessed on 22 December 2022). Information from each selected source was compiled while accounting for the probiotic strain, dosage, host, period of use, and the effect of probiotics on performance and meat quality. Finally, general information, details of treatment, and variable outputs from these articles (19 articles) were summarized in registered in the database of review. The literature was selected based on the following criteria mentioned in Figure 1.
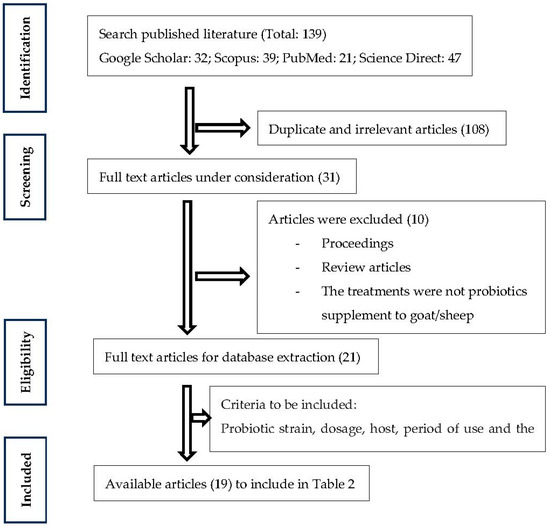
Figure 1.
Flowchart of scientific literature search and selection for this review.
3. Probiotics: Definition, Characteristics, and Use of Microorganisms as Probiotics
Many ancient civilizations used microbes to prepare fermented food items. However, research on the use of microorganisms in food and feed products and their effects on health have been studied recently. The idea of probiotics was first evolved in 1908 by Élie Metchnikoff, a Nobel laureate at the Pasteur Institute, who established a link between health and longevity by consuming beneficial microbes from yogurt [20]. The word “probiotics” was first used in 1965 by Lilly and Stillwell [21] to refer to the substances secreted by microorganisms that stimulate the growth of other microorganisms. The “probiotic” word originated from the Greek word meaning “for life” and has various meanings over the years. During the following decade, the probiotic word was applied by Fujii and Cook [22] and indicated synthetic chemicals in mice that conferred protection against infection due to Staphylococcus aureus. Subsequently, in 1974, Parker [23] used the term “probiotic” in a broader sense to refer to microorganisms which have interactions with the host (animal or human), i.e., “organisms and substances, which contribute to intestinal microbial balance”. In 1989, the definition of probiotics was used by Fuller where probiotics refer to a live microbial feed supplement, including Lactobacillus species, Bifidobacterium species, Streptococcus species, yeasts, and molds [24]. Numerous studies concerning probiotics have been published since then. The most commonly used definition of “probiotics” was proposed by FAO and WHO [25]. According to these organizations, probiotics are live microorganisms when administered in an adequate amount to confer a health benefit to the host. Currently, the U.S. Food and Drug Administration declared that probiotics are also safe ingredients for animal use [26]. When probiotics are used in the animal industry, they must have common characteristics (Figure 2), such as the ability to colonize and be metabolically active in the host body; promotion of animal health; applicability to industrial use, and safety to the animal and human body [27].
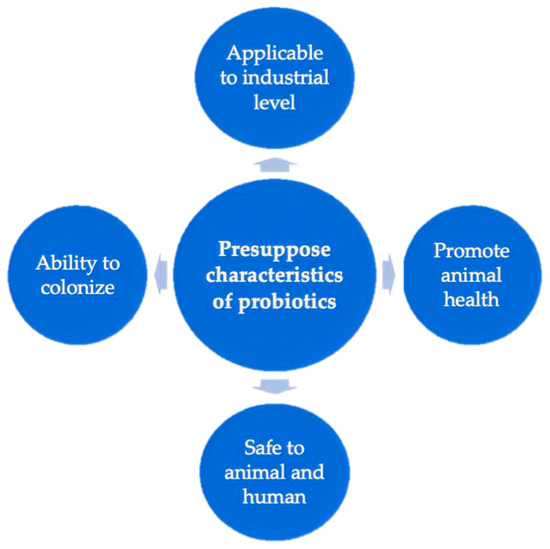
Figure 2.
Considerable characteristics of probiotics.
The microorganisms that are generally used as probiotics are presented in Table 1. The Lactobacillus and Bifidobacterium genera are reported as the bacterial groups found in the gastrointestinal tract (GIT) and commonly used in small ruminants’ nutrition [28]. Additionally, other genera, Enterococcus and Pediococcus, are normally commensal to the GIT and are widely used for livestock feeding. Bacillus sp. are Gram-positive spore-forming microorganisms; though some Bacillus species produce toxins to the animal body, some species have a probiotic effect on the host. The yeast, Saccharomyces cerevisiae, is widely present in nature and is used as probiotics in the livestock industry [29]. Other microorganisms are also used as probiotics in small ruminants’ nutrition as mentioned in Table 1. Probiotics are used in all stages of small ruminant production. However, the target of probiotic use depends on the situational demands, such as growth promotion, animal health promotion, treatment of the disease, or improvement of the product quality (meat, milk etc.).

Table 1.
List of microorganisms commonly used as probiotics in small ruminants 1.
4. Role of Probiotics on Animal Health and Nutrition
Using probiotics has become popular during the last two decades for their health benefits to animals and humans [31,32]. Probiotics have been used in ruminants as therapeutic [33] and dietary supplements [11] to reduce morbidity and mortality and increase production (meat and milk). Applying single or multi-strain microorganisms at the farm level may act complementarily or synergistically. However, the possible mechanisms of probiotics are not well defined. Several factors, such as strain specific mechanisms on the host and specific actions against certain diseases, influence the loose definition. Some proposed mechanisms by which probiotics act against unwanted microorganisms include contributing to colonizing the beneficiary microbes, reinforcing the intestinal barrier by secretion of mucus, producing antimicrobial compounds, modulating the immune system, and maintaining the beneficiary intestinal composition and their activity.
Like other organic nutrients in the intestine, probiotics are partly digested and broken down. Thus, only small portions are viable. Later, probiotics colonize the intestinal layer, competitively exclude the pathogen, and enhance the nutrient synthesis in the GIT. A study by Chen et al. [34] reported that dietary supplements of probiotics foster rumen microbial protein synthesis in lambs. It is also considered that probiotics compete with other non-beneficiary organisms for the endowment of nutrients to the host body [35]. For instance, Lactiplantibacillus plantarum breaches the carbohydrate to simpler compounds such as glucose, which provides energy to the animal [36]. Moreover, Lactobacillus can improve muscle development and meat quality by regulating the different mechanistic pathways associated with muscle development [3]. In addition, the use of Aspergillus oryzae as probiotics ameliorates the animal performance and body weight by producing different enzymes related to improving fiber digestion and nutrient absorption to the animal [37].
Probiotics combat pathogens by producing different inhibitory substances, such as organic acids, hydrogen peroxide, and bacteriocins [33]. Furthermore, many antibiotic metabolites (aciolin, acidophilin, lactobacillin and lactolin) release from the probiotics, which have inhibitory activities against different pathogenic microorganisms (Salmonella, Shigella, Staphylococcus, Proteus, Klebsiella, Pseudomonas, and E. coli) [28]. Additionally, probiotic bacteria can regulate immunomodulatory stimulation of the immune system [38,39] and regenerate intestinal mucosa [40]. Probiotics can increase the concentration of immunoglobulin [41] and augment the activity of macrophages and natural killer cells [42]. Probiotics can modulate the anti and pro-inflammatory cytokine production to control inflammation in the host [43]. For instance, Lactobacillus strains can reduce inflammation by downregulating the proinflammatory cytokines (T lymphocytes, IL-8, TNF-α, IL-6) as well as upregulating the anti-inflammatory cytokines (IL-10, TGF-β, IL-1Ra) in the intestine of piglets [44].
5. Effects of Probiotics on Meat Quality
5.1. Effects of Probiotic Supplementation on General Eating Quality Traits
Reports on the effects of probiotic supplementation on the meat quality of small ruminants (sheep and goat) are scarce. Instead, most of the studies focused on growth performance and disease control. Table 2 presents the list of recent studies focused on the effects of probiotics on the meat quality of small ruminants (sheep and goat). Meat quality is a term which impels consumer purchasing decisions and eating experiences. In general, the quality of meat includes the attributes of color, tenderness, juiciness, flavor, and water-holding capacity (WHC) [45]. These attributes can be influenced by animal (animal, breed, sex, diet) and environmental factors (climate, slaughter hygiene and procedure, and preparation of final meat products). As a dietary supplement, probiotics also showed positive effects on carcass weight and meat quality [46,47]. Such effects on meat quality includes improvement of product quality and shelf life [3], upgrading the sensory qualities [12,13], and improving color and tenderness [5,12] and healthy fatty acid profiles [48,49]. The beneficial effects of probiotics supplements on carcass weight and meat quality are exhibited in Figure 3.

Table 2.
Effect of probiotics on carcass and meat quality of small ruminants.
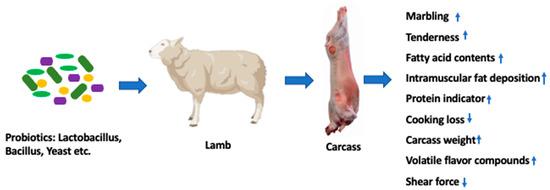
Figure 3.
Effect of dietary supplement of probiotics on meat quality of lambs. (Created with BioRender.com). Abbreviations: ↑, increased; ↓, decreased.
Meat color and sensory traits are considered important attributes and indicate consumer purchasing decisions. This is because consumers consider color and flavor for the freshness and wholesomeness of the meat [56]. Meat color and sensory traits depend on pre- and postmortem handling and relate to the amount of myoglobin pigment, hemoglobin, oxidation of lipids, and the pH level. Normally, myoglobin is responsible for the color of meat. Any oxidation or reduction of myoglobin can decrease the color intensity of the meat. A study by Nie et al. [5] found that feeding a mixture of probiotics (B. licheniformis, B. subtilis, and L. plantarum) for 60 days to lambs improved the meat color by reducing the oxidation of myoglobin. As probiotics, Lactobacillus sp. (L. casei and L. plantarum) have shown a positive effect on meat color and flavor [12]. Likewise, Lactobacillus and yeast (Sacchaomyces cerevisiae) supplements also improved the meat color [6]. In general, volatile compounds are responsible for producing odor and flavor in meat; supplementation of probiotics increases the antioxidant activity, which may reduce the lipid oxidation and thereby improve lamb meat flavor [12]. In recent years, researchers explored pH as an important trait for meat color and its effect on the glycolysis and lactic acid formation during the pre- and postmortem slaughtering stages. In general, pH gradually declines from an initial value of approximately 7.2 to an ultimate pH of about 5.6. However, a rapid decline of pH level in carcasses leads to the development of pale, soft, and exudative meat. In this case, myoglobin (a water-soluble protein) is lost along with water that exudates from the meat, which causes a pale, unsavory appearance in meat. Along with pH, the oxidation of muscle fiber relates to the meat’s color and lightness [12]. Oxidation of myoglobin reduces the redness of meat [5]. Considering the above facts, recently, researchers have explored the potential use of probiotics to improve meat color and stability. For example, Tian et al. [57] reported that dietary supplementation of Limosilactobacillus reuteri altered muscle fiber characteristics and can contribute to improving the meat color. Furthermore, feeding L. reuteri significantly improves the tenderness of meat by increasing water-holding capacity in muscle. The water-holding capacity of meat is responsible for the tenderness and juiciness of the meat [57]. Diets with probiotic supplements assist to keep the high pH in meat, which improves WHC in meat. Lambe et al. [58] revealed that probiotic supplements are positively impacted by intramuscular fat deposition, which is positively associated with marbling, tenderness, and meat flavor. We hypothesize that probiotic supplements induce adipocyte to develop and aid in depositing fat within the skeletal muscle. Liu et al. [12] reported that probiotic supplements increased the density of fibers in muscle, which causes softness and tenderness of meat. Several other studies reported that probiotic supplements improved meat color, flavor, and tenderness; thus, panelists preferred probiotic supplemented meat to meat without probiotic supplements [48,52]. In addition, dietary probiotic supplements might also alter the composition of volatile compounds, which is linked to the quality attributes of meat [5,12]. In addition, probiotics possess antioxidant properties [59] and aid in improving the poly unsaturated fatty acid profile in meat [60]. Some probiotic strains can produce bacteriocins that act as protective agents against lipid oxidation and keep positive organoleptic attributes in meat. It can be concluded that the use of probiotics improves the quality of meat.
5.2. Effects of Probiotic Supplementation on Lipid Oxidation
Beyond the benefits of using probiotics in meat attributes, researchers have also found that using probiotics in fermented meat products combat the growth of pathogenic and spoilage microorganisms. For instance, L. rhamnosus GG (105 and 107 colony-forming unit (CFU)/g) exhibited an inhibitory effect on the growth of Enterobacteriaceae during the fermentation process of meat [61]. In general, lipid oxidation is a major concern for fresh and fermented meat products, which negatively impacts the product’s sensory attributes and subsequently, its acceptability by the purchaser [62]. Free fatty acids are the main precursors for lipid oxidation in fresh and fermented meats [63,64]. Probiotics can act as protective agents against lipid oxidation because of their ability to produce bacteriocins that inhibit lipolytic microbes from forming free fatty acids [65,66]. Özer et al. [67] reported that the use of L. plantarum at 105 CFU/kg in fermented sucuk remarkedly reduced the levels of thiobarbituric acid reactive substances (TBARS), which is a marker of lipid peroxidation, compared to control samples. Similar findings were documented by Trabelsi et al. [68] in which lesser TBARS were measured in minced meat that was inoculated with L. plantarum. Additionally, adding L. acidophilus and B. lactis in fermented meat sausage reduced lipid oxidation and contributed to affirmative organoleptic properties [69,70,71].
Collectively, the findings reveal that dietary supplement of probiotics contributed to ameliorating the meat quality of small ruminants (sheep and goat).
6. Safety Concerns Relate to Probiotic Use
When microorganisms are used as probiotics, safety is a significant issue. Not only for the animal’s health but also for human health, probiotics should be nontoxic, non-pathogenic, and not related to antibiotic resistance gene transmission. Much research concerning probiotics are published mainly focusing on efficacy, capacities to improve gastrointestinal health, digestion, and growth performance rather than considering their safety and risks in practical usage. In general terms, there is greater number of articles describing the beneficial effects of the use of probiotics (>80%) rather than the negative effects [72]. However, we must consider the differences in experimental factors, such as animal age, dosage, dosing route/method, breed, sanitary status, probiotic use days, or diets, which are deemed generally safe for animal use. In most cases, probiotics are considered safe, but little evidence exists that probiotics are utterly safe and zero risk does not exist [73]. Safety issues related to probiotics use are mostly based on the Lactobacillus and Bifidobacterium bacteria [74,75]. These two probiotic genera are barely associated with any negative effect on the host. Any array of microorganisms could be used as probiotics, but uncertainty always exists about the safety issue. Thus, more research are needed on the safety issue of probiotics. Thus, probiotic developers or researchers should pay special attention to some issues related to probiotic safety (FAO, 2016) [76]:
- (i)
- Safety assessment of one probiotic or specific probiotic cannot be generalized to other probiotics. Each probiotic requires its own specific safety and risk assessment.
- (ii)
- The adverse and severe effects of the probiotics could be context specific, depending on the host’s susceptibility and physiological state.
- (iii)
- No probiotics can be issued as 100% safe or zero risk in the case of drug use.
- (iv)
- Public awareness related to probiotic safety and risk is limited. There is a need for risk-benefit analysis and information to the user or consumer.
Although microorganisms used as probiotics in animal feed are relatively safe, precautions should be considered to protect the animals from unsafe microorganisms associated with some risks that may occur when using microorganisms as probiotics [76,77]. Some are as follows:
- o Any infection to the animals when fed the probiotic.
- o Any infection to the consumers of animal products produced by animals fed probiotics.
- o Transfer of antibiotic resistance from probiotics to other pathogenic microorganisms.
- o Release of infectious and pernicious compounds to the environment from the animal production systems.
- o Chance of transfer infection to the animal and animal feed handlers.
- o Skin and/or eye and/or mucus membrane sensitization in the handler of probiotics.
- o Any detrimental metabolic or toxic effects in the host due to production of toxins by probiotics.
- o Hyper-stimulation of the immune system of the animal.
However, to fulfill the list of risks is very difficult for probiotic developers or researchers in practical aspects. Thus, some of them may consider a priority basis, such as the transfer of antibiotic resistance genes/determinants from some probiotic bacteria, the chance of infection, and the presence or production of toxins by probiotic bacteria [77]. Safety and risk assessments of a particular microorganism are considered when used as probiotics for animal or human use.
7. Conclusions
In this review, we summarized the previous studies to provide an overview of the effects of probiotics to improve the meat quality of small ruminants. Probiotics appear as promising feed additives. They are of natural origin and are generally regarded as safe for animals. Although probiotics are safe, it is better to consider further safety issues when used in animal trials and animal products. Using probiotics in the diet of small ruminants may improve the different attributes of meat and muscle fiber properties through different mechanisms in animals and carcasses. Moreover, many studies showed probiotics have positive impacts on improving meat quality, but in some cases, the effect was unclear. Thus, further studies characterizing specific strains, optimal dose, safety concerns, and understanding of the mechanisms to improve the particular traits of meat could help to use more effective probiotics to improve the quality of meat from small ruminants.
Author Contributions
Conceptualization, S.S., K.N., M.D. and H.K.; writing—original draft preparation, S.S., K.F. and K.N.; writing—review and editing, S.S., F.N., M.D. and H.K.; supervision, S.S. and H.K.; funding acquisition, H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Grant-in-Aid for Scientific Research (A) (19H00965, 23H00354) and JSPS fellows (22F22080) from the Japan Society for the Promotion of Science (JSPS) to H.K.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Srutee, R.; Sowmya, R.S.; Annapure, U.S. Clean meat: Techniques for meat production and its upcoming challenges. Anim. Biotechnol. 2021, 33, 1721–1729. [Google Scholar] [CrossRef]
- Mazhangara, I.R.; Chivandi, E.; Mupangwa, J.F.; Muchenje, V. The potential of goat meat in the red meat industry. Sustainability 2019, 11, 3671. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, Y.; Su, R.; Corazzin, M.; Hou, R.; Xie, J.; Zhang, Y.; Zhao, L.; Jin, Y. Transcriptome analysis reveals the molecular regulatory network of muscle development and meat quality in Sunit lamb supplemented with dietary probiotic. Meat Sci. 2022, 194, 108996. [Google Scholar] [CrossRef]
- Saha, S.; Singha, S.; Ahmed, S.S.; Toledo-Alvarado, H.; Khan, M.M. Effects of yeast (Saccharomyces cerevisiae type boulardii CNCM I-1079) supplementation on growth performance and blood metabolites in Black Bengal goat kids. Vet. Arh. 2018, 88, 661–672. [Google Scholar] [CrossRef]
- Nie, C.; Hu, Y.; Chen, R.; Guo, B.; Li, L.; Chen, H.; Chen, H.; Song, X. Effect of probiotics and Chinese medicine polysaccharides on meat quality, muscle fibre type and intramuscular fat deposition in lambs. Ital. J. Anim. Sci. 2022, 21, 811–820. [Google Scholar] [CrossRef]
- Tekce, E.; Bayraktar, B.; Aksakal, V.; Dertli, E.; Kamíloglu, A.; Karaalp, M.; Timurkaan, S.; Mehmet, G.Ü.L. Response of probiotics and yeast added in diff erent doses to rations of Anatolian Merino lambs on fattening performance, meat quality, duodenum, and rumen histology. Kafkas Üniv. Vet. Fak. Derg. 2021, 27, 57–65. [Google Scholar]
- Musa, H.H.; Wu, S.L.; Zhu, C.H.; Seri, H.I.; Zhu, G.Q. The potential benefits of probiotics in animal production and health. J. Anim. Vet. Adv. 2009, 8, 313–321. [Google Scholar]
- Saleem, A.M.; Zanouny, A.I.; Singer, A.M. Growth performance, nutrients digestibility, and blood metabolites of lambs fed diets supplemented with probiotics during pre-and post-weaning period. Asian-Australas. J. Anim. Sci. 2017, 30, 523–527. [Google Scholar] [CrossRef]
- Hernández-García, P.A.; Lara-Bueno, A.; Mendoza-Martínez, G.D.; Bárcena-Gama, J.R.; Plata-Pérez, F.X.; López-Ordaz, R.; Martínez-García, J.A. Effects of feeding yeast (Saccharomyces cerevisiae), organic selenium and chromium mixed on growth performance and carcass traits of hair lambs. J. Integr. Agric. 2015, 14, 575–582. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Z.; Gao, Q.; Li, P.; Wang, J.; Cai, Y.; Wang, Z.; Li, D.; Li, H.; Wang, F.; et al. Combined Supplementation of Probiotics and Enzymes Improves Performance and Regulates Rumen Microbiota in Fattening Goats; Research Square: Durham, NC, USA, 2021; pp. 1–26. [Google Scholar]
- Muthuramalingam, T.; Jemimah, E.R.; Gnanaraj, P.T.; Pothiappan, P. Effect of feeding in the growth performance and carcass quality in native kids. Indian J. Vet. Anim. Sci. Res. 2019, 48, 21–30. [Google Scholar]
- Liu, C.; Hou, Y.; Su, R.; Luo, Y.; Dou, L.; Yang, Z.; Yao, D.; Wang, B.; Zhao, L.; Su, L.; et al. Effect of dietary probiotics supplementation on meat quality, volatile flavor compounds, muscle fiber characteristics, and antioxidant capacity in lambs. Food Sci. Nutr. 2022, 10, 2646–2658. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, T.; Zhang, Y.; Yang, L.; Duan, Y.; Su, L.; Tian, J.; Sun, L.; Wang, B.; Jin, Y. Impact of Feeding Probiotics on Blood Parameters, Tail Fat Metabolites, and Volatile Flavor Components of Sunit Sheep. Foods 2022, 11, 2644. [Google Scholar] [CrossRef]
- Inyang, U.A.; Akpan, I.P.; Enyenihi, G.E. Effect of probiotic fortified diets on physicochemical and sensory characteristics of wad goat (buck) meat. WJAS 2020, 11, 1852–1858. [Google Scholar]
- Ayala-Monter, M.A.; Hernandez-Sanchez, D.; Pinto-Ruiz, R.; Torres-Salado, N.; Martinez-Aispuro, J.A.; Barcena-Gama, J.R.; Caro-Hernandez, J.M. Effect of inulin and Lactobacillus casei on productive performance, ruminal variables and blood metabolites in weaned lambs. Agrociencia 2019, 53, 303–317. [Google Scholar]
- Li, A.; Wang, Y.; Li, Z.; Qamar, H.; Mehmood, K.; Zhang, L.; Liu, J.; Zhang, H.; Li, J. Probiotics isolated from yaks improves the growth performance, antioxidant activity, and cytokines related to immunity and inflammation in mice. Microb. Cell Factories 2019, 18, 112. [Google Scholar] [CrossRef]
- Gadekar, Y.P.; Shinde, A.K.; Sahoo, A.; Karim, S.A. Effect of probiotic supplementation on carcass traits and meat quality of Malpura lambs. Indian J. Small Rumin. 2015, 2, 306–310. [Google Scholar] [CrossRef]
- Picard, B.; Gagaoua, M. Muscle fiber properties in cattle and their relationships with meat qualities: An overview. J. Agric. Food Chem. 2020, 68, 6021–6039. [Google Scholar] [CrossRef]
- Reuben, R.C.; Elghandour, M.M.; Alqaisi, O.; Cone, J.W.; Márquez, O.; Salem, A.Z. Influence of microbial probiotics on ruminant health and nutrition: Sources, mode of action and implications. J. Sci. Food Agric. 2022, 102, 1319–1340. [Google Scholar] [CrossRef]
- Espitia, P.J.; Batista, R.A.; Azeredo, H.M.; Otoni, C.G. Probiotics and their potential applications in active edible films and coatings. Food Res. Int. 2016, 90, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Lilly, D.M.; Stillwell, R.H. Probiotics: Growth-promoting factors produced by microorganisms. Science 1965, 147, 747–748. [Google Scholar] [CrossRef]
- Fujii, A.; Cook, E.S. Probiotics. Antistaphylococcal and antifibrinolytic activities of omega-guanidine acids andomega-guanidinoacyl-L-histidines. J. Med. Chem. 1973, 16, 1409–1411. [Google Scholar] [CrossRef]
- Parker, R.B. Probiotics, the other half of the antibiotic story. Anim. Nutr. Health 1974, 29, 4–8. [Google Scholar]
- Fuller, R. Probiotics in man and Animals. J. Appl. Bacteriol. 1989, 66, 365–378. [Google Scholar] [PubMed]
- Food and Agricultural Organization of the United Nations; World Health Organization. Probiotics in Food-Health and Nutritional Properties and Guidelines for Evaluation; Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food; Food and Agricultural Organization of the United Nations: Rome, Italy, 2002; Volume 85, Available online: https://www.fao.org/3/a0512e/a0512e.pdf (accessed on 11 January 2023).
- Bhogoju, S.; Nahashon, S. Recent advances in probiotic application in animal health and nutrition: A review. Agriculture 2022, 12, 304. [Google Scholar] [CrossRef]
- Barba-Vidal, E.; Martín-Orúe, S.M.; Castillejos, L. Practical aspects of the use of probiotics in pig production: A review. Livst. Sci. 2019, 223, 84–96. [Google Scholar] [CrossRef]
- Abd El-Trwab, M.M.; Youssef, I.I.; Bakr, H.A.; Fthenakis, G.C.; Giadinis, N.D. Role of probiotics in nutrition and health of small ruminants. Pol. J. Vet. Sci. 2016, 19, 893–906. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumar, S.; Verma, A.K.; Singh, P. Effect of live Saccharomyces cerevisiae on immune response in early weaned crossbred piglets. Indian J. Anim. Nutr. 2012, 29, 393–396. [Google Scholar]
- Seo, J.K.; Kim, S.W.; Kim, M.H.; Santi, D.; Kam, D.K.; Ha, J.K. Direct-fed microbials for ruminant animals. Asian Australas. J. Anim. Sci. 2010, 23, 1657–1667. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. Int. Sch. Res. Notices. 2013, 2013, 481651. [Google Scholar] [CrossRef]
- Ban, Y.; Guan, L.L. Implication and challenges of direct-fed microbial supplementation to improve ruminant production and health. J. Anim.l Sci. Biotechnol. 2021, 12, 109. [Google Scholar] [CrossRef]
- Kober, A.H.; Saha, S.; Islam, M.A.; Rajoka, M.S.R.; Fukuyama, K.; Aso, H.; Villena, J.; Kitazawa, H. Immunomodulatory Effects of Probiotics: A Novel Preventive Approach for the Control of Bovine Mastitis. Microorganisms 2022, 10, 2255. [Google Scholar] [CrossRef]
- Chen, H.; Guo, B.; Yang, M.; Luo, J.; Hu, Y.; Qu, M.; Song, X. Response of growth performance, blood biochemistry indices, and rumen bacterial diversity in lambs to diets containing supplemental probiotics and chinese medicine polysaccharides. Front. Vet. Sci. 2021, 8, 681389. [Google Scholar] [CrossRef]
- Rolfe, R.D. The role of probiotic cultures in the control of gastrointestinal health. J. Nutr. 2000, 130, 396S–402S. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, M.; Zheng, Y.; Miao, K.; Qu, X. The carbohydrate metabolism of Lactobacillus plantarum. Int. J. Mol. Sci. 2021, 22, 13452. [Google Scholar] [CrossRef]
- Khalid, M.F.; Shahzad, M.A.; Sarwar, M.; Rehman, A.U.; Sharif, M.; Mukhtar, N. Probiotics and lamb performance: A review. Afr. J. Agric. Res. 2011, 6, 5198–5203. [Google Scholar]
- Schierack, P.; Filter, M.; Scharek, L.; Toelke, C.; Taras, D.; Tedin, K.; Haverson, K.; Lübke-Becker, A.; Wieler, L.H. Effects of Bacillus cereus var. toyoi on immune parameters of pregnant sows. Vet. Immunol. Immunopathol. 2009, 127, 26–37. [Google Scholar] [CrossRef]
- Villena, J.; Kitazawa, H. The modulation of mucosal antiviral immunity by immunobiotics: Could they offer any benefit in the SARS-CoV-2 pandemic? Front. Physiol. 2020, 11, 699. [Google Scholar] [CrossRef]
- Villena, J.; Vizoso-Pinto, M.G.; Kitazawa, H. Intestinal innate antiviral immunity and immunobiotics: Beneficial effects against rotavirus infection. Front. Immunol. 2016, 7, 563. [Google Scholar] [CrossRef]
- Hussein, A.F. Effect of probiotics on growth, some plasma biochemical parameters and immunoglobulins of growing Najdi lambs. World’s Vet. J. 2018, 8, 80–89. [Google Scholar]
- Islam, M.A.; Hashiguchi, K.; Kober, A.H.; Morie, K.; Zhou, B.; Tomokiyo, M.; Shimazu, T.; Aso, H.; Villena, J.; Suda, Y.; et al. Effect of dietary supplementation of immunobiotic lactiplantibacillus plantarum n14 fermented rakkyo (Allium chinense) pickled juice on the immunocompetence and production performance of pigs. Animals 2021, 11, 752. [Google Scholar] [CrossRef]
- Mishra, S.; Acharya, S. A brief overview on probiotics: The health friendly microbes. Biomed. Pharmacol. J. 2021, 14, 1869–1880. [Google Scholar] [CrossRef]
- Ding, Y.H.; Qian, L.Y.; Pang, J.; Lin, J.Y.; Xu, Q.; Wang, L.H.; Huang, D.S.; Zou, H. The regulation of immune cells by Lactobacilli: A potential therapeutic target for anti-atherosclerosis therapy. Oncotarget 2017, 8, 59915. [Google Scholar] [CrossRef]
- Ha, M.; Warner, R.D.; King, C.; Wu, S.; Ponnampalam, E.N. Retail packaging affects colour, water holding capacity, texture and oxidation of sheep meat more than breed and finishing feed. Foods 2022, 11, 144. [Google Scholar] [CrossRef]
- Al-Shawi, S.G.; Dang, D.S.; Yousif, A.Y.; Al-Younis, Z.K.; Najm, T.A.; Matarneh, S.K. The potential use of probiotics to improve animal health, efficiency, and meat quality: A Review. Agriculture 2020, 10, 452. [Google Scholar] [CrossRef]
- Issakowicz, J.; Bueno, M.S.; Sampaio, A.C.K.; Duarte, K.M.R. Effect of concentrate level and live yeast (Saccharomyces cerevisiae) supplementation on Texel lamb performance and carcass characteristics. Livest. Sci. 2013, 155, 44–52. [Google Scholar] [CrossRef]
- Arilov, A.; Pogodaev, V.; Appaev, B.; Lidzhiev, E.; Mashtykov, S. Sheep productivity when probiotic feed additive “Amilocin” introduced into the diets. IOP Conf. Ser. Earth Environ. Sci. 2019, 403, 012109. [Google Scholar] [CrossRef]
- Paengkoum, P.; Yong, H.; Traiyakun, S.; Khotsakdee, J. Effect of blend probiotics on rumen fermentation and plasma fatty acid contents and plasma n6:n3 ratios of growing goats. J. Anim. Vet. Adv. 2011, 10, 3112–3117. [Google Scholar]
- Domínguez-Vara, I.A.; González-Muñoz, S.S.; Pinos-Rodríguez, J.M.; Bórquez-Gastelum, J.L.; Bárcena-Gama, R.; Mendoza-Martínez, G.; Zapata, L.E.; Landois-Palencia, L.L. Effects of feeding selenium-yeast and chromium-yeast to finishing lambs on growth, carcass characteristics, and blood hormones and metabolites. Anim. Feed Sci. Technol. 2009, 152, 42–49. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Karim, S.A. Effect of yeast cultures supplementation on live weight change, rumen fermentation, ciliate protozoa population, microbial hydrolytic enzymes status and slaughtering performance of growing lamb. Livest. Sci. 2011, 135, 17–25. [Google Scholar] [CrossRef]
- Guimarães, L.J.; Santiago, L.F.; Nicolau, J.P.; de Almeida Rego, F.C.; Castilho, C.; Cunha Filho, L.F.; Giotto, F.M.; Zundt, M. Effect of yeast (Saccharomyces cerevisiae) associated or not to micro minerals in chemical composition, tissue composition, lipid oxidation and quality of meat of feedlot lambs. Res. Soc. Dev. 2020, 9, e1539119563. [Google Scholar] [CrossRef]
- Raghebian, M.; Dabiri, N.; Yazdi, A.B.; Bahrani, M.J.; Shomeyzi, J.; Raghebian, A.; Hatami, P. Probiotic effect on meat quality and carcass parameters of Iranian Zandi lambs. J. Livest. Sci. 2007, 8, 163–168. [Google Scholar]
- Freitas, H.S.; Alcalde, C.R.; Lima, L.S.D.; Macedo, F.D.A.F.D.; Macedo, V.D.P.; Molina, B.S.D.L. Quantitative characteristics of carcass and meat quality of ¾ Boer+ ¼ Saanen and Saanen goat kids fed diets with dry yeast. Rev. Bras. Zootec. 2011, 40, 630–638. [Google Scholar] [CrossRef]
- Whitley, N.C.; Cazac, D.; Rude, B.J.; Jackson-O’Brien, D.; Parveen, S. Use of a commercial probiotic supplement in meat goats. J. Anim. Sci. 2009, 87, 723–728. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef]
- Tian, Z.; Cui, Y.; Lu, H.; Wang, G.; Ma, X. Effect of long-term dietary probiotic Lactobacillus reuteri 1 or antibiotics on meat quality, muscular amino acids and fatty acids in pigs. Meat Sci. 2021, 171, 108234. [Google Scholar] [CrossRef]
- Lambe, N.R.; McLean, K.A.; Gordon, J.; Evans, D.; Clelland, N.; Bunger, L. Prediction of intramuscular fat content using CT scanning of packaged lamb cuts and relationships with meat eating quality. Meat Sci. 2017, 123, 112–119. [Google Scholar] [CrossRef]
- Yu, L.; Peng, Z.; Dong, L.; Wang, H.; Shi, S. Enterococcus faecium NCIMB 10415 supplementation improves the meat quality and antioxidant capacity of muscle of broilers. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1099–1106. [Google Scholar] [CrossRef]
- Santillo, A.; Annicchiarico, G.; Caroprese, M.; Marino, R.; Sevi, A.; Albenzio, M. Probiotics in milk replacer influence lamb immune function and meat quality. Animal. 2012, 6, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Rubio, R.; Aymerich, T.; Bover-Cid, S.; Guàrdia, M.D.; Arnau, J.; Garriga, M. Probiotic strains Lactobacillus plantarum 299V and Lactobacillus rhamnosus GG as starter cultures for fermented sausages. LWT-Food Sci. Technol. 2013, 54, 51–56. [Google Scholar] [CrossRef]
- Love, J.D.; Pearson, A. Lipid oxidation in meat and meat products—A review. J. Am. Oil Chem. Soc. 1971, 48, 547–549. [Google Scholar] [CrossRef]
- Gianelli, M.P.; Salazar, V.; Mojica, L.; Friz, M. Volatile compounds present in traditional meat products (charqui and longaniza sausage) in Chile. Braz. Arch. Biol. Technol. 2012, 55, 603–612. [Google Scholar] [CrossRef]
- Slima, S.B.; Ktari, N.; Triki, M.; Trabelsi, I.; Abdeslam, A.; Moussa, H.; Makni, I.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. E ects of probiotic strains, Lactobacillus plantarum TN8 and Pediococcus acidilactici, on microbiological and physico-chemical characteristics of beef sausages. LWT-Food Sci. Technol. 2018, 92, 195–203. [Google Scholar] [CrossRef]
- Smaoui, S.; Ennouri, K.; Chakchouk-Mtibaa, A.; Karray-Rebai, I.; Hmidi, M.; Bouchaala, K.; Mellouli, L. Relationships between textural modifications, lipid and protein oxidation and sensory attributes of refrigerated turkey meat sausage treated with bacteriocin BacTN635. Food Bioprocess Technol. 2017, 10, 1655–1667. [Google Scholar] [CrossRef]
- Smaoui, S.; Elleuch, L.; Ben Salah, R.; Najah, S.; Chakchouk-Mtibaa, A.; Sellem, I.; Besbes, S.; Mellouli, L. Efficient role of BacTN635 on the safety properties, sensory attributes, and texture profile of raw minced meat beef and chicken breast. Food Addit. Contam. Part A 2014, 31, 218–225. [Google Scholar] [CrossRef]
- Özer, C.O.; Kılıç, B.; Kılıç, G.B. In-vitro microbial production of conjugated linoleic acid by probiotic L. plantarum strains: Utilization as a functional starter culture in sucuk fermentation. Meat Sci. 2016, 114, 24–31. [Google Scholar] [CrossRef]
- Trabelsi, I.; Slima, S.B.; Ktari, N.; Triki, M.; Abdehedi, R.; Abaza, W.; Moussa, H.; Abdeslam, A.; Salah, R.B. Incorporation of probiotic strain in raw minced beef meat: Study of textural modification, lipid and protein oxidation and color parameters during refrigerated storage. Meat Sci. 2019, 154, 29–36. [Google Scholar] [CrossRef]
- Amanatidou, A.; Smid, E.J.; Bennik, M.H.; Gorris, L.G. Antioxidative properties of Lactobacillus sake upon exposure to elevated oxygen concentrations. FEMS Microbiol. Lett. 2001, 203, 87–94. [Google Scholar] [CrossRef]
- Talon, R.; Leroy, S.; Lebert, I. Microbial ecosystems of traditional fermented meat products: The importance of indigenous starters. Meat Sci. 2007, 77, 55–62. [Google Scholar] [CrossRef]
- Simion, A.M.C.; Vizireanu, C.; Alexe, P.; Franco, I.; Carballo, J. Effect of the use of selected starter cultures on some quality, safety and sensorial properties of Dacia sausage, a traditional Romanian dry-sausage variety. Food Control. 2014, 35, 123–131. [Google Scholar] [CrossRef]
- Barba-Vidal, E.; Martín-Orúe, S.M.; Castillejos, L. Are we using probiotics correctly in post-weaning piglets? Animal 2018, 12, 2489–2498. [Google Scholar] [CrossRef]
- Marteau, P. Safety aspects of probiotic products. Näringsforskning 2001, 45, 22–24. [Google Scholar] [CrossRef][Green Version]
- Liao, S.F.; Nyachoti, M. Using probiotics to improve swine gut health and nutrient utilization. Anim. Nutr. 2017, 3, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, F. A commentary on the safety of probiotics. Gastroenterol. Clin. 2012, 41, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Food and Agricultural Organization of the United Nations; World Health Organization. Probiotics in Animal Nutrition: Production, Impact and Regulation; Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food; Food and Agricultural Organization of the United Nations: Rome, Italy, 2016; Volume 179, Available online: https://www.fao.org/3/i5933e/i5933e.pdf (accessed on 6 February 2023).
- Doron, S.; Snydman, D.R. Risk and safety of probiotics. Clin. Infect. Dis. 2015, 60, S129–S134. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).