Abstract
During this century, a number of reports have described the potential roles of thermophiles in the upper soil layers during high-temperature periods. This study evaluates the capabilities of these microorganisms and proposes some potential consequences and risks associated with the activity of soil thermophiles. They are active in organic matter mineralization, releasing inorganic nutrients (C, S, N, P) that otherwise remain trapped in the organic complexity of soil. To process complex organic compounds in soils, these thermophiles require extracellular enzymes to break down large polymers into simple compounds, which can be incorporated into the cells and processed. Soil thermophiles are able to adapt their extracellular enzyme activities to environmental conditions. These enzymes can present optimum activity under high temperatures and reduced water content. Consequently, these microorganisms have been shown to actively process and decompose substances (including pollutants) under extreme conditions (i.e., desiccation and heat) in soils. While nutrient cycling is a highly beneficial process to maintain soil service quality, progressive warming can lead to excessive activity of soil thermophiles and their extracellular enzymes. If this activity is too high, it may lead to reduction in soil organic matter, nutrient impoverishment and to an increased risk of aridity. This is a clear example of a potential effect of future predicted climate warming directly caused by soil microorganisms with major consequences for our understanding of ecosystem functioning, soil health and the risk of soil aridity.
1. Introduction
Soil health and function are strictly linked to microbial activity [1,2,3,4,5]. A large number of processes are carried out mainly or exclusively by microorganisms, and this broad range of activities represents a major asset for soil maintenance and response to multiple variables leading to changing conditions. One of the main factors influencing the functional redundancy of soil processes is microbial diversity [1,5,6,7,8]. Soils are highly heterogeneous, and they present a huge microbial diversity and abundance [9,10]. Current estimates suggest that 1 g of soil contains about 1010 prokaryotic cells and includes about 30,000 different microorganisms [7,9,11]. The duplicity of metabolic capabilities allows soils to preserve functionality, maintaining a stable environment, in spite of drastic changes. Otherwise, a significant decrease in microbial diversity would represent a serious handicap on soils being able to maintain current balances and activities, which would negatively affect soil health and productivity [5,7].
Within the research on the almost unmeasurable high microbial diversity existing in soils, most work has been carried out on their major components, while the low-abundance microorganisms have been poorly considered. This represents a significant limitation because a large number of microbial processes with high relevance to, for instance, the biogeochemical cycling of elements are performed by minority microorganisms. This is, for example, the case for ammonium oxidation, denitrification, metal reduction/oxidation, sulfur oxidation, sulfate reduction, methanogenesis and the decomposition of specific recalcitrant pollutants; these processes are generally carried out by groups represented within the minorities of the natural microbial communities [12].
Within the vast microbial diversity of soils, a permanent component consists in thermophilic bacteria. Although thermophiles are expected to inhabit high-temperature environments, such as hot springs, geothermal areas or compost piles, different reports have confirmed the ubiquitous presence of thermophiles in all examined soils from a wide range of latitudes [13,14,15,16,17,18]. Cold and temperate soils hold thermophiles including distinctive taxa, but the major representatives are Geobacillus-related genera [13,14,15,16,18]. This study will focus on the potential role and consequences of these soil thermophiles within a perspective of global climate warming.
A question has arose about the timing available for thermophiles to grow, assuming these microorganisms are inhabiting the upper soil layers of cold or temperate environments. Previously, an analysis of average number of hot days against latitude [15,18,19] suggested the occurrence of a significant number of hot days (e.g., above 100 hot days/year, around 37° N, in Seville, Spain) [18,19] when soil thermophiles would have an opportunity to grow and show significant activity. At higher latitudes (i.e., above 50° N), the number of hot days is generally low (e.g., around 1–2 hot days/year, around 52° N, at Cambridge, UK) [18,19], but this could be enough to provide time for maintaining thermophile populations and a minimum extracellular enzyme stock in the soil environment. These soil thermophiles survive well through low-temperature periods [20].
Another group of microorganisms that must be mentioned when considering microorganisms thriving under periods of increased temperatures are the thermophilic fungi [21] also present in soils. The role of these fungi in soils during high-temperature events has not been clearly defined yet [21], and additional research is required. Nevertheless, thermophilic fungi can have a role in compost piles, where high temperatures are maintained for much longer time periods than in upper soil layers. Compost piles typically contain a high organic matter load and generate, as a result of microbial growth, a high-temperature environment [22,23,24,25].
Soil organic matter is a major reservoir of C with the potential to greatly influence global climate [3,4]. Most organic carbon is present in the upper soil layers [26]. Rich soils contain a high content of organic matter, represented by a variety of complex compounds. Soil organic matter, besides its carbon content, also includes other elements, some of them critical for plant and microbial growth, such as nitrogen, sulfur and phosphorous, often required as major soil fertilizers [27,28]. Furthermore, complex organics such as humic acids can complex with those elements as well as with metals [29,30]. Within the soil organic matter, large polymers and humic acids need to be broken into smaller compounds or monomers for microorganisms to be able to be taken up and processed as sources of energy and/or biomass [31,32,33]. This breaking down of complex compounds into smaller ones is mediated by extracellular enzymes. In fact, the bottle-neck for soil organic matter mineralization is this step involving the extracellular enzymes [3,19,34]. Microbial extracellular enzyme activity is highly related to organic matter mineralization in soils and has been proposed as a major indicator to evaluate the sink-link issue with the soil–atmosphere C balance [3,4], a critical parameter for modeling climate predictions [5,35,36,37,38].
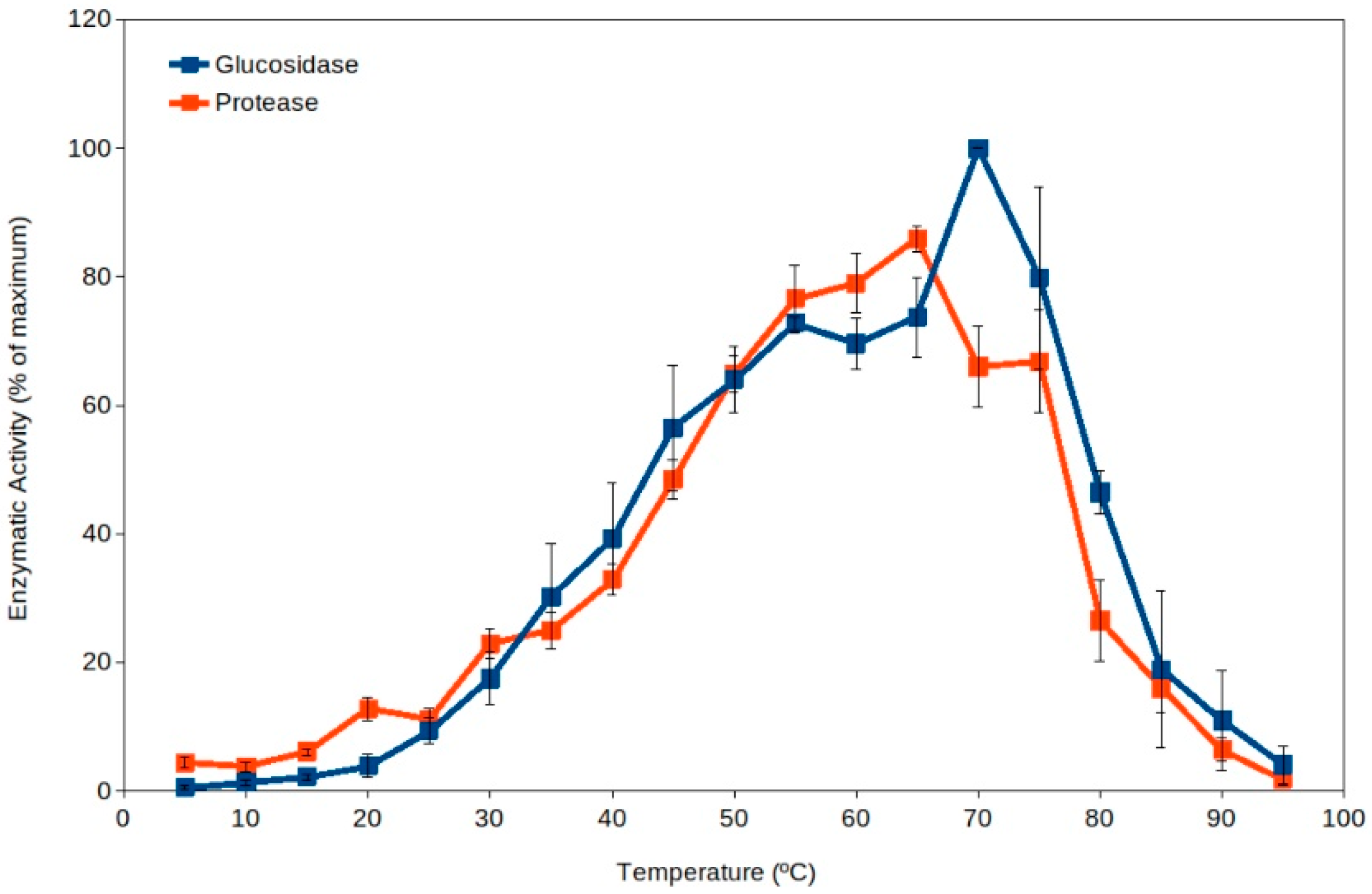
Extracellular enzyme activity has been proposed as an indicator of soil microbial activity [39,40,41], and it is commonly measured in ecological studies [37,40,41,42,43,44]. Soil thermophiles have been reported as a major source for extracellular enzymes dominating the pool of enzymes in soils [19] because they present higher total activity than the corresponding enzymes from mesophiles (Figure 1) [18,19,45]. Thus, high-temperature events are expected to enhance soil organic matter processing due to the activation of extracellular enzymes from thermophiles. In addition, the current scenario of global warming suggests an expected increase in frequency and duration of high-temperature events in the coming years [4,5,7,46]. As well, high temperature in soil upper layers implies an increased evaporation and therefore a decrease in water content in soils, leading to increased desiccation. Recent reports have also proposed that some soil microorganisms (including some thermophiles) have adapted to dryness by developing extracellular enzymes and metabolisms able to work optimally under dry conditions (at water activity, aw, between 0.3 and 0.8) [43,45]. The levels of desiccation showing maximum extracellular activity by soil thermophilic xerophiles can occur at values below the reported limit for microbial growth (aw 0.605) [47]. Besides extracellular enzyme activity by thermophiles, these cells have been reported to actively decompose recalcitrant pollutants at high temperature [17,48,49,50,51] and under dry conditions [43,45,48], suggesting that cell activity is significant under those extreme conditions in soil upper layers, where these microorganisms can be potential important bioremediation agents [17,48,49,50,51,52,53].
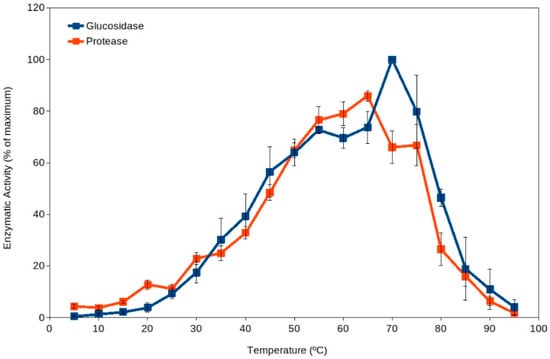
Figure 1.
Extracellular enzyme activity in soils over a broad range of temperatures (5 °C to 95 °C). Maximum activity was observed at temperatures in the thermophilic microorganisms range (55 °C–75 °C). Data averaged from Gonzalez et al. [19]. Blue squares, glucosidase; red squares, protease. Error bars, one standard deviation.
Current modeling of global warming shows important limitations on the incorporation of microbial parameters into model predictions [3,53,54,55]. Under these circumstances of novel microbial activities and microorganisms with previously under-studied capabilities, current previsions from global warming perspectives should be re-examined by incorporating these novel views and the current knowledge on the direct influence of previously underestimated microorganisms. This study will present some potential positive and negative consequences of the activity of soil thermophiles from the perspective of maintaining soil health and productivity.
2. Singularity of Soil Thermophiles and Thermophilic Extracellular Enzymes
Soil thermophiles represent a singularity in the microbial communities from cold and temperate soils. However, thermophiles are ubiquitous inhabitants of soils, and their presence as viable cells with an important role and capabilities to survive under those conditions have been reported [14,15]. Thus, thermophiles thriving in temperate environments, depending on periodic/sporadic high-temperature events, can show some growth and produce extracellular enzymes that are required to process organic matter [18,19]. These extracellular enzymes might persist in the environment [56] and become active under heat events. Consequently, these extracellular enzymes actively participate in soil organic matter decomposition [19,43,56].
Mesophilic microorganisms present extracellular enzymes with optimum activity at moderate temperatures (i.e., generally measured around 30 °C or below) [57,58,59], but the extracellular enzymes from thermophiles present optimum activity at temperatures above 50 °C [19,45]. Thus, the activity by extracellular enzymes from thermophiles can be easily detected by carrying out enzyme assays at high temperature (50 °C to 70 °C), and therefore, mesophilic and thermophilic activities can be differentiated. Results discriminating the activity over a range of temperatures (from 5 °C to 95 °C) in a variety of soil samples showed unexpected results [19]. The results clearly indicated that thermophilic activities were always higher than mesophilic ones in all soils tested (Figure 1) [18,19]. This suggests that thermophilic extracellular enzyme activities are dominant in soils. It is important to note that the pioneering work [19] and some subsequent studies [43,45] included samples from soils exposed to hot temperatures and others from cold environments. Additional studies have shown significant roles of thermophiles at higher latitudes [16,17,49,50,51,52], corroborating that soil thermophiles can also show significant environmental activity in relatively cold climate zones.
Because soil thermophiles represent a minority fraction of the total microbial community in cold and temperate soils [13,15,18,43], it is required to look for different potential scenarios to explain that large activity measured in the thermophile temperature range. An easy explanation would be that soil thermophiles show a very high production of extracellular enzymes and/or these enzymes present higher activity than their mesophilic counterparts. Although enzymes from thermophiles have been reported to present higher activity than those from mesophiles [60,61], the difference is not likely to be able to explain the much higher total activity measured in soils due to thermophiles (a minority group) than due to the total mesophilic microbial community, which presents a much higher abundance. According to previous estimates [15], the fraction of thermophiles in soils is generally below 1% of the total community. A potential justification for that large activity at high temperatures in soils could be that soil thermophiles could produce a large amount of extracellular enzymes during hot periods or extreme heat events. Soil thermophiles, such as Geobacillus related taxa, require extracellular enzymes to access complex organic matter in soils and grow, so a high production of enzymes is needed for their development. Nevertheless, the relatively low abundance of thermophiles suggests that the production of thermophilic extracellular enzymes could not be as high as needed—in relationship to the production by mesophiles showing equivalent metabolism—to explain the higher activity in the thermophile temperature range. The level of extracellular enzyme production that could be potentially needed to explain that scenario is likely to be out of reach for the soil thermophilic cells. Otherwise, during hot periods, thermophiles could respond to heat events by growing and producing a moderate amount of enzymes that would persist in the environment over time. This could result in a progressive accumulation of thermophilic enzymes in the environment. These enzymes should be able to persist in the environment at least until the next hot event, and, at that moment, the thermophilic enzymes will show their full activity. For this to be a reasonable explanation, the thermophilic extracellular enzymes should show longer persistence in the soil environment. The potential for accumulation of extracellular enzymes in soils could represent a singular strategy that would allow thermophilic cells to start growing rapidly when the right growth conditions arrive in the ecosystem (e.g., an extreme heat event or hot days during the summer period). A rapid response would take advantage of even the shortest periods at high temperature to recover and grow. During these hot periods, those thermophilic enzymes could decompose soil organic matter, allowing a variety of organisms (both microorganisms and plants) to profit from that release of smaller compounds (and monomers) readily available as substrates for growth and energy [62]. A mechanism facilitating a rapid growth response to high-temperature events (i.e., extracellular enzyme accumulation) represents an interesting adaptive feature for thermophiles to thrive in cold and temperate environments. This type of strategy has not been reported before for microorganisms.
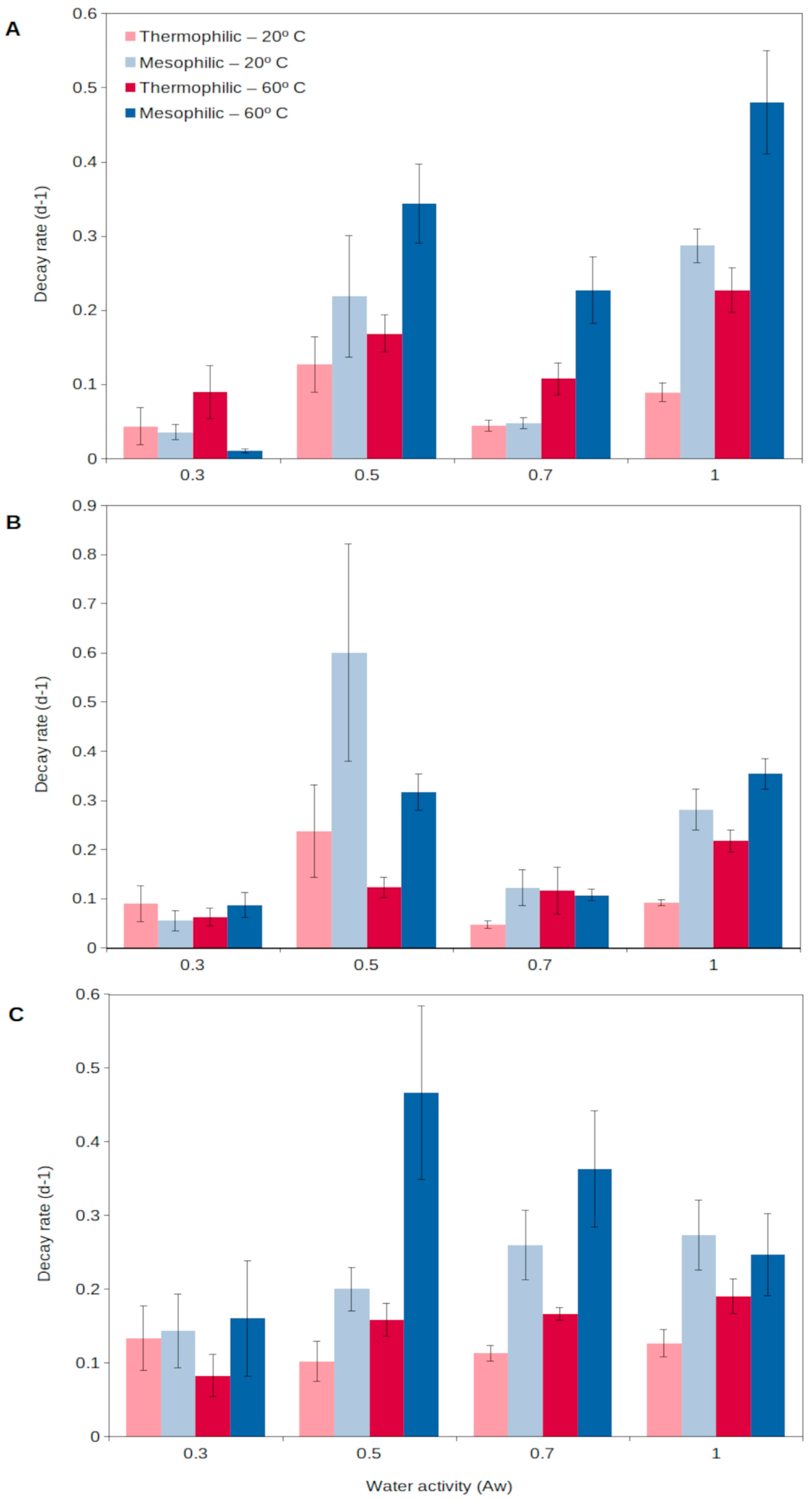
For extracellular enzymes to accumulate in the environment, two aspects need to be fulfilled: a relatively long production of extracellular enzymes and a long persistence in the environment. Generally, enzymes from thermophiles (as well as from other extremophiles) have been reported to present higher durability than those from mesophiles, resulting from a higher stability and resistance to external factors (detergents, denaturants, decomposition, etc.) [60]. Assuming enzymes are produced at a relatively high rate and then show a long persistence in the environment, they could progressively accumulate in soils [56]. In this way, thermophilic extracellular enzymes could generate an active enzymatic pool in soils, readily available to catalyze complex organic matter decomposition as soon as temperatures rise. A recent report [56] has shown that extracellular enzymes from thermophiles are able to persist for a longer time in soils than those from mesophiles (Figure 2). Thermophilic extracellular enzymes maintain their activity in soils even at the highest temperatures and desiccation levels reached in the upper soil layers (Figure 2). Mesophilic enzymes are rapidly denatured during extreme heat events, including summer periods, and their persistence is lower than that for soil thermophiles [56,63]. Thus, the extracellular enzymes from thermophiles are able to persist in the environment, representing a soil asset that could allow the rapid growth of microorganisms, both mesophiles and thermophiles, and so promote soil health and functioning. This unique strategy proposed for soil thermophiles represents a singular mechanism to survive in environments (i.e., cold and temperate soils) considered adverse for thermophiles.
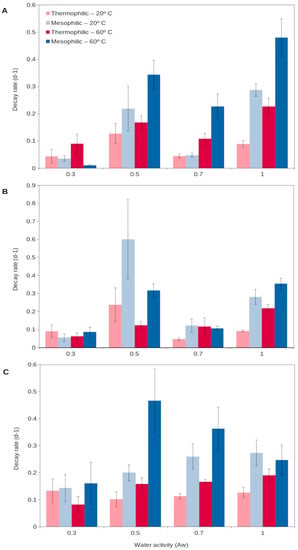
Figure 2.
Decay rate of natural extracellular enzymes in soils at different temperatures, 20 °C (light colors) and 60 °C (dark colors), corresponding to the mesophilic (blue) and thermophilic (red) microorganisms, respectively, over a wide spectrum of water contents (water activity, aw) for 3 different enzyme activities: (A), glucanase; (B), phosphatase; (C), protease. High decay rate indicates short persistence, and low decay rate shows long persistence. Data averaged from Gomez et al. [56]. Error bars, one standard deviation.
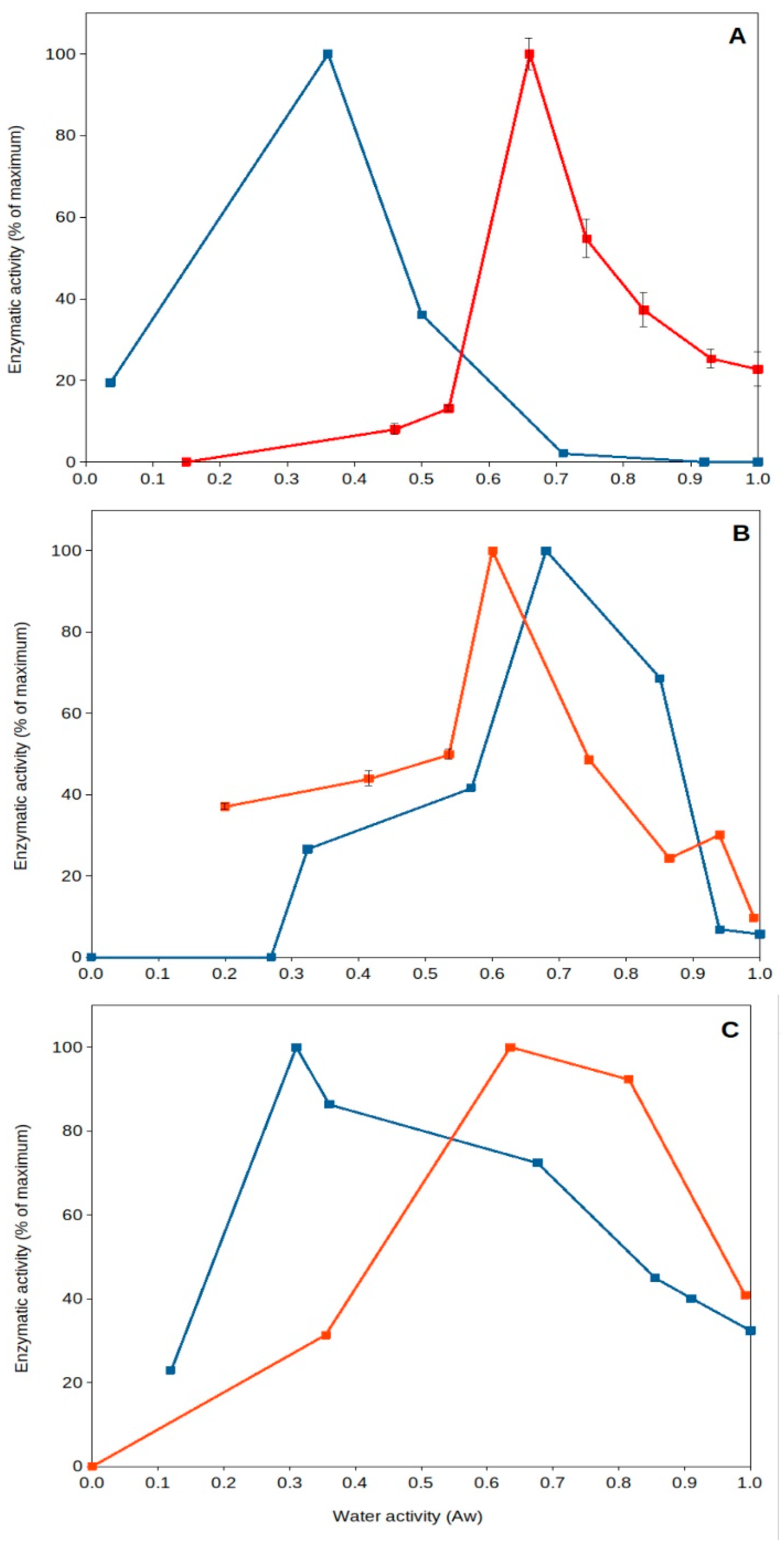
High temperature of upper soil layers implies increased evaporation, leading to a reduction in water content and desiccation. It has been generally thought that dried soils present poor or near-null biological activity. Nevertheless, recent work has shown that specific microorganisms present optimum extracellular enzyme activity under dry conditions [43,45,64,65]. This is the case for some soil thermophiles, among other mesophilic bacteria (i.e., Deinococcus). Deinococcus radiodurans, a wide spread soil bacterial species, has been reported to be a model microorganism for resistance to desiccation [66], and some of its extracellular enzymes can present optimum (maximum) activity under dried soil conditions (aw 0.40–0.55) (Figure 3) [45]. Soil thermophiles, those inhabitants of soils frequently exposed to high temperatures and droughts, exhibit an interesting feature: they present optimum extracellular enzyme activities at very low water content (aw 0.3–0.7). Some thermophilic enzymes from sites exposed to hot climate have been shown to reach optimum activities under dry conditions (aw < 0.7), but thermophiles from cooler locations present optima under wet conditions (aw > 0.9). Most mesophilic enzymes in those natural soil samples always presented optimum values in aqueous solutions (aw > 0.9). These results showed that soil thermophiles can adapt to thrive in a variety of environments and have the capacity to adapt their enzyme activities to extremely dry conditions [43,45].
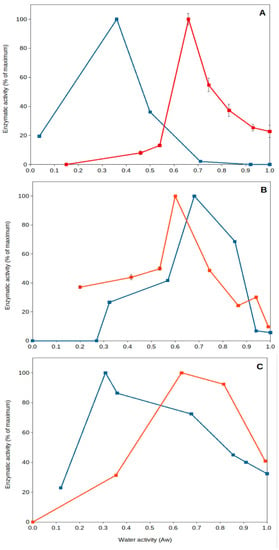
Figure 3.
Natural extracellular enzyme activity from Southeastern Spain soils versus water activity. A couple of examples showing optimum extracellular enzyme activity under severe desiccation. Red squares and lines, soil samples collected from Coria del Rio (Seville); blue squares and lines, soil samples collected from Benaocaz (Cadiz). (A), glucanase; (B), phosphatase; (C), protease. Data extracted from Gomez et al. [43]. Error bars, one standard deviation.
Furthermore, these thermophiles survive and remain viable under these dry conditions (aw 0.5), as shown by a comparative study on pollutant decomposition by Geobacillus (showing maximum pollutant decomposition at aw 0.5) compared to Rhodococcus spp. (showing maximum decomposition at aw > 0.9) [48]. This qualifies soil thermophiles from hot and dry environments as some of the most xerophilic cells reported on Earth. So far, the lowest water activity allowing growth is 0.6 for the fungus Xeromyces bisporus [47], and most microorganisms do not show growth below aw 0.8 [67]. Some soil thermophiles can show optimum extracellular enzymatic activity [43,45] and ability to decompose pollutants [48] at aw around 0.5. Consequently, singular features of soil thermophiles include their potential adaptability to extreme drought and high temperatures, which are valued because of their consequences for the environment [43,45,56], providing evidence of optimal activity under those extremes (Figure 3), as well as a great potential for biotechnological applications in high-temperature and non-aqueous treatments and processes [68,69,70,71].
3. Organic Matter Decomposition
Soil organic matter is largely processed by microorganisms and dependent on concentration, composition and a variety of biotic and abiotic factors [3,35,37,40,41,59]. Most research has been performed to understand microbial organic matter decomposition in soils during standard conditions (temperature and high-water-content conditions) [3,35,37,40,41,59] and after wetting events [72,73,74,75,76], but scarce information is available on the potential of microbial activity under extreme events (i.e., high temperature, desiccation) [15,19,43,77]. Soil thermophiles are able to thrive under periodic or sporadic hot and dry periods (and survive in the cold), unlike what is assumed for most microorganisms. The relevance of thermophiles in soil ecosystems during those extreme events must be evaluated so that researchers and technologists can incorporate the information into current local and global system management, models and predictions.
Microbial organic C processing in soils was thought to be inhibited during extreme events (i.e., high temperature and desiccation) [57,58,78,79,80]. In the last decade, researchers have started to understand that some microorganisms are able to occupy niches that are not able to support growth of others, leading to alternating feast–famine cycles in the environment [81,82,83]. As an example, different microorganisms packed tightly along a temperature gradient [84] and thedistinctive taxa naturally distributed according to the environmental conditions and outcomes of competition with other microorganisms. Soil thermophiles have been confirmed [13,15,18,43] to present optimum activity under extreme conditions in which other microorganisms do not grow. During the temperate and cold periods, those soil thermophiles persist under near-zero or maintenance growth stages [14,62,85]. In fact, periods of growth limitation and even inhibition have been reported to be the most common conditions for most microorganisms in nature [86,87]. Thus, growth limitation and the wait for appropriate conditions to arrive should be considered as general features for microorganisms in the environment and, specifically, a way of living for soil thermophiles [14,19].
Organic matter is a parameter of major importance to determine soil health and productivity [88,89]. Microorganisms are major participants in maintaining soil functioning [90,91,92], including the working of biogeochemical cycles of elements sustaining plant growth and interactions with plants [27,90,92,93,94]. During extreme events (i.e., extreme temperature and desiccation), soil thermophiles can perform some of these functions during periods when other microorganisms are inhibited. Soil thermophiles (as mentioned above) utilize extracellular enzymes to start the decomposition of complex organic matter in soils. This first step is generally the limiting bottle-neck for soil organic matter mineralization [3,19,34]. The decomposition of organic matter into smaller compounds or monomers that can directly be incorporated by cells fosters microbial growth. During growth, aerobic respiration generates CO2 as a result of complete organic matter mineralization [32], and the metabolism of those organic molecules leads to the release of other inorganic compounds (i.e., ammonium, sulfate, phosphates) [15,32,85,93]. Thus, the role of soil thermophiles, both those thriving at high temperatures and those adapted to thrive under dry conditions, should be accounted for when evaluating microbial activity and the consequences on local and global scales, in order to fully understand ecosystem functioning and the fate of C at the soil–atmosphere equilibrium of relevance and thereby achieve reasonable predictions on climate impact [4,5].
Soil organic matter, including humic acids, presents a significant content of nitrogen [29,30,95,96,97,98]. Proteinaceous compounds contain nitrogen, and the consumption of these compounds represents an important source of N for microorganisms. Excess N from this metabolism can be released into the environment, generally in the form of ammonium, and it can be a substrate for other bacteria directly involved in critical steps of the biogeochemical cycle of N (e.g., anammox, nitrifiers, denitrifiers) [99]. The inorganic N released, with synthesis of ammonium, nitrate and nitrite, represents a significant source of N for all soil organisms, specifically plants. Inorganic N is frequently a growth-limiting nutrient for plants [100]. Soil thermophiles have been cited to release N as ammonium at a rate similar to that reported for super-ammonifiers in the mesophile temperature range. Thus, this source of inorganic N can be maintained and potentially enhanced during extreme heat and dryness periods by the role of soil thermophiles and their decomposition of soil organic matter.
Processing of soil organic matter, besides C and N, affects other elements. One of the elements most relevant for plant and microbial life is sulfur. S is often required in plant cultures as a fertilizer because the availability of S in soils is frequently low. In this respect, most S in soils is part of soil organic matter (about 90% of total soil S) [101,102,103], for example, integrated in humic acids and proteinaceous compounds [29,30]. Nevertheless, the capacity of most soil bacteria (analyzed at 20 °C, in the mesophile temperature range) to mineralize organic S has been shown to be highly limited [101,102] and has been suggested to be frequently unable to support the S requirements for soil organisms (microorganisms and plants). Soil thermophiles, during extreme heat and desiccation periods, can process soil organic matter releasing inorganic S, mainly as sulfate [15,85,104]. A significant release of sulfate has been reported for soil thermophiles at a much higher rate than that by soil mesophiles [15]. A putative pathway involved in the release of inorganic S (i.e., sulphate) has been described [85,104], supporting the potential for S cycling in soils carried out mostly by thermophiles within the phylum Firmicutes, bacteria closely related to the Geobacillus genus. These soil thermophiles could reduce S limitation in soils during high-temperature and dry periods by increasing the recycling rate of organic S [85,104].
Another major microbial growth-limiting nutrient is P. Phosphates are relatively abundant in soils, but they are generally complexed into organic matter and minerals [105,106,107,108,109,110]. Numerous microorganisms are able to release phosphates, making P freely available for biological utilization and incorporation into the cell metabolism of both microorganisms and plants [32,107]. The role of phosphatases is important for the solubilization of phosphates in soils [32,43,107]. Some soil thermophiles (i.e., Geobacillus related strains) have been shown to solubilize phosphates during growth, which represents an additional feature of relevance for the working of soil ecosystems during high-temperature and desiccation periods.
Soil thermophiles metabolism, as shown above, is directly implicated in a number of features related to the cycling and processing of organic matter. The role of their extracellular enzymes is critical and limits the rate of the subsequent pathways and metabolism performed by the thermophilic cells. An additional aspect of interest is the capability of soil thermophiles to decompose recalcitrant pollutants under extreme conditions. This has been shown by various research teams. An example is the ability of microorganisms in natural soil samples and of Geobacillus strains isolated from soils to decompose halocompounds (e.g., chlorophenol) under high-temperature and low-water-activity conditions [48]. Another example was reported of the major role of thermophiles in the decomposition of bituminous hydrocarbons in soils in a relatively cold climatic zone (59 °N) [17]. The degradation of n-hexadecane in soil has been carried out by thermophiles [51]. Another report pointed out the role of thermophiles in crude oil degradation in soils [50]. The decomposition by thermophiles of oily food residues in soils has also been confirmed [49]. Thus, soil thermophiles expand the time when and conditions under which bioremediation could be carried out in soils [17,48,49,50,51,52], including periods of high temperature and drought in upper soil layers.
Herein, the role of thermophiles has been summarized in relationship to natural soil systems. However, similar roles are to be expected if these processes are incorporated into bioreactors [50,111] or composting [112,113,114,115]. Under these technological scenarios, thermophiles could be functioning under most defined and optimum conditions, showing increased relevance to organic matter processing, inorganic nutrient release and pollutant decomposition. Biotechnological processes can significantly profit from the capabilities of soil thermophiles.
4. Positive Implications (Services)
The use of soil thermophiles as potential biofertilizers has been suggested and is supported by the evidence shown above. Soil thermophiles could significantly contribute to C processing and the release of inorganic ammonium, sulfate and phosphate, which can be directly utilized by plants [62,85,93]. As a consequence of this active participation of soil thermophiles in nutrient cycling, one could suggest that periods of extreme climate events also contribute to maintaining soil health and productivity, for example, by enriching soil with available inorganic nutrients that can be readily used for plant growth.
Soil thermophiles’ activity during hot and dry periods can represent an additional level of redundancy that enhances the role of microbial diversity by providing pathways leading to nutrient cycling and sustainability during extreme conditions [1,94,104,116,117]. Soil health requires microbial diversity so that changes can be compensated through different alternative pathways and soil functioning can continue providing similar services [6,8,118].
In order to confirm soil thermophiles’ positive effects on plant growth, a series of studies have shown the potential capabilities of those soil thermophiles to enhance plant growth [62,85,93] and drought tolerance [62,94]. These reports showed that plants significantly benefit from soil thermophiles’ activities, which contribute to compensating nutrient deficits by increasing decomposition of soil organic matter and releasing essential plant nutrients.
In addition to the participation of soil thermophiles in the biogeochemical cycling of elements (C, N, S, P), these microorganisms can provide bioremediation services under high-temperature and dry conditions. Previously, the potential bacterial decomposition of pollutants during extreme heat and dry events had been considered to be highly limited [119,120]. Studies on soil thermophiles have shown the importance of soil thermophiles in these processes, contributing significantly to decomposing pollutants under these conditions [17,48,49,50,51]. In fact, high temperature, for example, can contribute to increasing solubility of some pollutants, increasing the accessibility to thermophilic cells [121]. Similarly, drought enhances evaporation, and this can lead to pollutant concentration, which can facilitate decomposition by overcoming affinity issues at low concentrations [122]. Thus, thermophiles offer enhanced potential as soil bioremediation tools [17,48,51].
As mentioned above, soil thermophiles under moderate average conditions thrive in the environment when periodically or occasionally they are able to grow and show significant activity, thus contributing moderately to the sustainability and productivity of the soil ecosystem. Caution must be taken because uncontrolled increased exposure of soils to extreme climate events could lead to differential effects considered below.
5. Negative Implications (Risks)
Above, we have presented a number of positive implications of and services potentially provided by soil thermophiles during extreme heat and drought events. However, there are potential negative effects that need to be considered.
Excessive organic matter decomposition can lead to soil impoverishment [123,124]. Although additional research is required, Santana and Gonzalez [18] have suggested a potential correlation between the activity of soil thermophiles and soil organic content throughout Europe. Lower latitudes present higher temperatures, and it is expected that soil thermophiles show higher activity there; Southern Europe generally presents lower soil organic matter content than Northern Europe. Higher latitudes correspond to cooler climatic zones, so soil thermophiles and their extracellular enzymes will show limited activity.
Based on current climate change predictions, raising temperatures will likely result in a higher frequency and duration of extreme temperature events and droughts [3,4,124,125]. If so, the periods and duration of hot days and periods of drought will increase, leading to increased opportunities for growth of soil thermophiles and the enhanced activity of thermophilic extracellular enzymes. This potential increase in thermophilic activity could lead to excessive organic matter decomposition, with two major aspects to be considered.
A potential increased soil organic matter decomposition could lead to soil impoverishment due to a decrease in soil organic matter content. Extreme heat and dry periods could lead to increased activity derived from soil thermophiles. Future climatic scenarios and predictions suggest that the expected conditions will lead to an increase in soil thermophile-derived activities, which could potentially pose a significant risk of progressive soil impoverishment and CO2 release to the atmosphere, due to excessive organic matter mineralization. Increased extreme climatic conditions might lead to poor plant health and plant coverage reduction as a result of high temperature and reduced water availability [46,94,119,126,127]. Soils poorly covered with vegetation will be increasingly affected by radiation, heat and desiccation [128,129,130,131]. Thus, increased climate warming would lead to increased soil thermophile activity, which may result in an increased risk of soil aridity.
A second potential aspect that needs to be considered is that the above-mentioned enhanced soil organic matter decomposition should result in the increased release of inorganics (i.e., ammonium, sulfate, phosphate). Because plants under extreme heat and drought conditions might be under severe stress situations, plant uptake of inorganic nutrients might be poor, and so those available inorganic compounds might be easily lost, for example, in runoff water [132,133]. This might enhance nutrient loss and lead to increased risk of soil impoverishment and aridity.
Under the current global climate warming scenario, an increase in the activity of soil thermophiles is expected. If plants’ growth and potential adaptations are not supported by the warmer climatic conditions expected to occur in the future [125,134,135], a reduction in plant coverage of soil surface and an increased activity of soil thermophiles are suggested to result in a progressive soil impoverishment and risk of aridity. A large amount of thermophilic extracellular enzymes accumulated in soils [19,43,45] leads to the hypothesis that soils would experience a reduction in their organic matter content if temperatures and dryness increase through climate warming. These risks should be proportional to climate events, and these effects will be related to latitude and altitude, among other factors influencing environmental conditions in different soil systems [135,136,137]. Although moderate soil thermophile activity can result in positive benefits for soil sustainability and health, an excessive or enhanced activity of soil thermophiles could certainly have strong negative effects on soils. Evaluating these effects and potential consequences requires further investigation.
6. Conclusions
Within the huge microbial diversity existing in soils, some minority components could present a significant potential and certainly provide highly relevant value by expressing functional redundancy and warranting soil response to potential changes and drastic events, including climate changes predicted in a near future. In this study, we have focused on one minority group of soil communities, the soil thermophiles, and analyzed potential benefits and risks derived from expected climate changes.
Soil thermophiles can contribute positively to nutrient cycling by processing soil organic matter, including recalcitrant compounds and pollutants, and releasing inorganic nutrients complexed within the organic matter. During hot and dry periods, thermophiles can grow, consuming organic matter and producing extracellular enzymes to decompose organic complexes. Those enzymes can persist in the environment and show activity during hot periods. As a consequence of this organic matter mineralization, inorganic nutrients (ammonium, sulfate and phosphate) will be made available to soil organisms, including plants.
Nevertheless, poorly covered soils can be exposed to increased radiation. Increased soil temperature and droughts as a result of climate change will lead to increased activity of soil thermophiles, which might induce undesirable effects such as nutrient impoverishment (as a result of a decreased reduction in soil organic matter and potential runoff of soluble inorganics) and an increased risk of soil aridity.
At present, current previsions on climate warming barely incorporate direct effects of microbial activity on a global scale. Herein, we provide evidence and information suggesting that potentially significant effects of microorganisms can occur. Additional investigation is required to confirm the likelihood of those services and risks within future climate scenarios. A minority group in the microbial communities, the soil thermophiles, could potentially switch from positive (current/past scenario) to negative (potential future scenario) effects as a result of predicted climate changes. Climate change predictions foresee increased temperature and droughts, which could result in a spiral fostering negative effects and risks, decreasing soil health and productivity.
Author Contributions
Conceptualization, J.M.G.; writing—original draft preparation, J.M.G. and M.M.S.; analyses, writing—review and editing, all authors (J.M.G., E.J.G., J.A.D., M.M.S.). All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Spanish Ministry of Science and Innovation, project PID2020-119373GB-I00, funded through MCIN/AEI/10.13039/501100011033 and the European Union Next Generation (PRTR), and the Regional Government of Andalusia, project number P20_00774, with FEDER funds.
Acknowledgments
We thank Marta Lebrón López for technical support during the preparation of the manuscript and related experimental work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Whitman, W.B.; Coleman, D.C.; Wiebe, W.J. Prokaryotes: The unseen majority. Proc. Natl. Acad. Sci. USA 1998, 95, 6578–6583. [Google Scholar] [CrossRef] [PubMed]
- Uroz, S.; Calvaruso, C.; Turpault, M.-P.; Frey-Klett, P. Mineral weathering by bacteria: Ecology, actors and mechanisms. Trends Microbiol 2009, 17, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Conant, R.T.; Ryan, M.G.; Ågren, G.I.; Birge, H.E.; Davidson, E.A.; Eliasson, P.E.; Evans, S.E.; Frey, S.D.; Giardina, C.P.; Hopkins, F.; et al. Temperature and soil organic matter decomposition rates—Synthesis of current knowledge and a way forward. Glob. Chang. Biol. 2011, 17, 3392–3404. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientist’s warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Gallardo, A.; Bowker, M.A.; Wallenstein, M.D.; Quero, J.L.; Ochoa, V.; Gozalo, B.; García-Gómez, M.; Soliveres, S.; et al. Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature 2016, 502, 672–676. [Google Scholar] [CrossRef]
- Lladó, S.; López-Mondéjar, R.; Baldrian, P. Forest soil bacteria: Diversity, involvement in ecosystem processes, and response to global change. Microbiol. Mol. Biol. Rev. 2017, 81, e00063-16. [Google Scholar] [CrossRef]
- Maron, P.-A.; Sarr, A.; Kaisermann, A.; Lévêque, J.; Mathieu, O.; Guigue, J.; Karimi, B.; Bernard, L.; Dequiedt, S.; Terrat, S.; et al. High microbial diversity promotes soil ecosystem functioning. Appl. Environ. Microbiol. 2018, 84, e02738-17. [Google Scholar] [CrossRef]
- Curtis, T.P.; Sloan, W.T.; Scannell, J.W. Estimating prokaryotic diversity and its limits. Proc Natl. Acad. Sci. USA 2002, 99, 10494–10499. [Google Scholar] [CrossRef]
- Pedrós-Alió, C. Marine microbial diversity: Can it be determined? Trends Microbiol. 2006, 14, 257–263. [Google Scholar] [CrossRef]
- Roesch, L.F.; Fulthorpe, R.R.; Riva, A.; Casella, G.; Hadwin, A.K.; Kent, A.D.; Daroub, S.H.; Camargo, F.A.; Farmerie, W.G.; Triplett, E.W. Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J. 2007, 4, 283–290. [Google Scholar] [CrossRef]
- Saw, J.H.W. Characterizing the uncultivated microbial minority: Towards understanding the roles of the rare biosphere in microbial communities. mSystems 2021, 6, e00773-21. [Google Scholar] [CrossRef]
- Marchant, R.; Banat, I.M.; Rahman, T.J.; Berzano, M. The frequency and characteristics of highly thermophilic bacteria in cool soil environments. Environ. Microbiol. 2002, 4, 595–602. [Google Scholar] [CrossRef]
- Marchant, R.; Franzetti, A.; Pavlostathis, S.G.; Tas, D.O.; Erdbrugger, I.; Unyayar, A.; Mazmanci, M.A.; Banat, I.M. Thermophilic bacteria in cool temperate soils: Are they metabolically active or continually added by global atmospheric transport? Appl. Microbiol. Biotechnol 2008, 78, 841–852. [Google Scholar] [CrossRef]
- Portillo, M.C.; Santana, M.; Gonzalez, J.M. Presence and potencial role of thermophilic bacteria in temperate terrestrial environments. Naturwissenschaften 2012, 99, 43–53. [Google Scholar] [CrossRef]
- Cockell, C.S.; Cousins, C.; Wilkinson, P.T.; Olsson-Francis, K.; Rozitis, B. Are thermophilic microorganisms active in cold environments? Intl. J. Astrobiol. 2015, 14, 457–463. [Google Scholar] [CrossRef]
- Wong, M.-L.; An, D.; Caffrey, S.; Soh, J.; Dong, X.; Sensen, C.W.; Oldenburg, T.B.P.; Larter, S.R.; Voordouw, G. Roles of thermophiles and fungi in bitumen degradation in mostly cold oil sands outcrops. Appl. Environ. Microbiol. 2015, 81, 6825–6838. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.M.; González, J.M. High temperature microbial activity in upper soil layers. FEMS Microbiol. Lett. 2015, 362, fnv182. [Google Scholar] [CrossRef]
- Gonzalez, J.M.; Portillo, M.C.; Piñeiro-Vidal, M. Latitude-dependent underestimation of microbial extracellular enzyme activity in soils. Int. J. Environ. Sci. Technol. 2015, 12, 2427–2434. [Google Scholar] [CrossRef]
- Milojevic, T.; Cramm, M.A.; Hubert, C.R.J.; Westall, F. “Freezing” thermophiles: From one temperature extreme to another. Microorganisms 2022, 10, 2417. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, A.K.; Maheshwari, R. Thermophilic fungi: An assessment of their potential for growth in soil. J. Biosci. 1993, 18, 345–354. [Google Scholar] [CrossRef]
- Zak, J.C.; Howard, G.; Wildman, G. Fungi in stressful environments. In Biodiversity of Fungi. Inventory and Monitoring Methods; Mueller, G.M., Bills, G.F., Foster, M.S., Eds.; Elsevier Academy Press: Burlington, MA, USA, 2004; pp. 305–315. [Google Scholar]
- Neher, D.A.; Weicht, T.R.; Bates, S.T.; Leff, J.W.; Fierer, N. Changes in bacterial and fungal communities across compost recipes, preparation methods, and composting times. PLoS ONE 2013, 8, e79512. [Google Scholar] [CrossRef]
- Vigneswaran, S.; Kandasamy, J.; Johir, M.A.H. Sustainable operation of composting in solid waste management. Proc. Environ. Sci. 2016, 35, 408–415. [Google Scholar] [CrossRef]
- Diánez, F.; Marín, F.; Santos, M.; Gea, F.J.; Navarro, M.J.; Piñeiro, M.; González, J.M. Genetic analysis and in vitro enzymatic determination of bacterial community in compost teas from different sources. Compost. Sci. Util. 2018, 26, 256–270. [Google Scholar] [CrossRef]
- López-Bellido, R.J.; Lal, R.; Danneberger, T.K.; Street, J.R. Plant growth regulator and nitrogen fertilizer effects on soil organic carbon sequestration in creeping bentgrass fairway turf. Plant Soil 2010, 332, 247–255. [Google Scholar] [CrossRef]
- McGrath, J.M.; Spargo, J.; Penn, C.J. Soil fertility and plant nutrition. In Encyclopedia of Agriculture and Food; van Alfen, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 166–184. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Radowich, T.J.K.; Nguyen, H.V.; Uyeda, J.; Arakaki, A.; Cadby, J.; Paull, R.; Sugano, J.; Teves, G. Use of organic fertilizers to enhance soil fertility, plant growth, and yield in a tropical environment. In Organic Fertilizers; Larramendy, M.L., Soloneski, S., Eds.; IntechOpen: London, UK, 2016. [Google Scholar]
- Li, Y.; Fang, F.; Wei, J.; Wu, X.; Cui, R.; Li, G.; Zheng, F.; Tan, D. Humic acid fertilizer improved soil properties and soil microbial diversity of continuous cropping peanut: A three-year experiment. Sci. Rep. 2019, 9, 12014. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, N.; Li, J.; Wang, Y.; Liu, Y.; Cao, M.; Yan, Q. Characterization of humic acids from original coal and its oxidization production. Sci. Rep. 2021, 11, 15381. [Google Scholar] [CrossRef]
- Asmar, F.; Eiland, F.; Nielsen, N.E. Effect of extracellular-enzyme activities on solubilization rate of soil organic nitrogen. Biol. Fertil. Soils 1994, 17, 32–38. [Google Scholar] [CrossRef]
- Madigan, M.; Martinko, J.M.; Parker, J. Brock Biology of Microorganisms; Prentice Hall Inc.: Upper Saddle River, NJ, USA, 2003. [Google Scholar]
- Wallenstein, M.D.; Burns, R.G. Ecology of extracellular enzyme activities and organic matter degradationin soil: A complex community-driven process. In Methods in Soil Enzymology; Dick, R.P., Ed.; Soil Science Society of America Inc.: Madison, WI, USA, 2011; pp. 35–55. [Google Scholar]
- Cheng, L.; Zhang, N.; Yuan, M.; Xiao, J.; Qin, Y.; Deng, Y.; Tu, Q.; Xue, K.; van Nostrand, J.D.; Wu, L.; et al. Warming enhances old organic carbon decomposition through altering functional microbial communities. ISME J. 2017, 11, 1825–1835. [Google Scholar] [CrossRef]
- Sorouri, B.; Allison, S.D. Microbial extracellular enzyme activity with simulated climate change. Elem. Sci. Anth 2022, 10, 76. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Oppenheimer, M.; Warren, R.; Hallegatte, S.; Kopp, R.E.; Pörtner, H.O.; Scholes, R.; Birkmann, J.; Foden, W.; Licker, R.; et al. IPCC reasons for concern regarding climate change risks. Nat. Clim. Chang. 2017, 7, 28–37. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, X.; Jing, X.; Zhu, B. A meta-analysis of soil extracellular enzyme activities in response to global change. Soil Biol. Biochem. 2018, 123, 21–32. [Google Scholar] [CrossRef]
- Ye, J.S.; Bradford, M.A.; Dacal, M.; Maestre, F.T.; García-Palacios, P. Increasing microbial carbon use efficiency with warming predicts soil heterotrophic respiration globally. Glob. Chang. Biol. 2019, 25, 3354–3364. [Google Scholar] [CrossRef] [PubMed]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Chen, J.; Sinsabaugh, R.L. Linking microbial functional gene abundance and soil extracellular enzyme activity: Implications for soil carbon dynamics. Glob. Chang. Biol. 2021, 27, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
- Ndabankulu, K.; Egbewale, S.O.; Tcvuura, Z.; Magadlela, A. Soil microbes and associated extracellular enzymes largely impact nutrient bioavailability in acidic and nutrient poor grassland ecosystem soils. Sci. Rep. 2022, 12, 12601. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Biro, A.; Wong, M.Y.; Batterman, S.A.; Staver, A.C. Fire decreases soil enzyme activities and reorganizes microbially mediated nutrient cycles: A meta-analysis. Ecology 2022, 103, e3807. [Google Scholar] [CrossRef] [PubMed]
- Gomez, E.J.; Delgado, J.A.; Gonzalez, J.M. Environmental factors affect the response of microbial extracellular enzyme activity in soils when determined as a function of water availability and temperature. Ecol. Evol. 2020, 10, 10105–10115. [Google Scholar] [CrossRef]
- Jian, S.; Li, J.; Chen, J.; Wang, G.; Mayes, M.A.; Dzantor, K.E.; Hui, D.; Luo, Y. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta-analysis. Soil Biol. Biochem. 2016, 101, 32–43. [Google Scholar] [CrossRef]
- Gomez, E.J.; Delgado, J.A.; Gonzalez, J.M. Influence of water availability and temperature on estimates of microbial extracellular enzyme activity. PeerJ 2021, 9, e10994. [Google Scholar] [CrossRef]
- Smith, P.; Fang, C.M.; Dawson, J.J.C.; Moncrieff, J.B. Impact of global warming on soil organic carbon. Adv. Agron 2008, 97, 1–43. [Google Scholar] [CrossRef]
- Stevenson, A.; Burkhardt, J.; Cockell, C.S.; Cray, J.A.; Dijksterhuis, J.; Fox-Powell, M.; Kee, T.P.; Kminek, G.; McGenity, T.J.; Timmis, K.N.; et al. Multiplication of microbes below 0.690 water activity: Implications for terrestrial and extraterrestrial life. Environ. Microbiol. 2015, 17, 257–277. [Google Scholar] [CrossRef]
- Moxley, E.; Puerta-Fernández, E.; Gómez, E.J.; González, J.M. Influence of abiotic factors temperature and water content on bacterial 2-chlorophenol biodegradation in soils. Front. Environ. Sci. 2019, 7, 41. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Selvam, A.; Chan, M.T.; Wong, J.W.C. Bio-degradation of oily food waste employing thermophilic bacterial strains. Biores. Technol 2018, 248, 141–147. [Google Scholar] [CrossRef]
- Elumalai, P.; Parthipan, P.; Narenkumar, J.; Anandakumar, B.; Madhavan, J.; Oh, B.-T.; Rajasekar, A. Role of thermophilic bacteria (Bacillus and Geobacillus) on crude oil degradation and biocorrosion in oil reservoir environment. Biotech 2019, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Marchant, R.; Sharkey, F.H.; Banat, I.M.; Rahman, T.J.; Perfumo, A. The degradation of n-hexadecane in soil by thermophilic geobacilli. FEMS Microbiol. Ecol. 2006, 56, 44–54. [Google Scholar] [CrossRef] [PubMed]
- DePoy, A.N.; King, G.M. Distribution and diversity of anaerobic thermophiles and putative anaerobic nickel-dependent carbon monoxide-oxidizing thermophiles in mesothermal soils and sediments. Front. Microbiol. 2023, 13, 1096186. [Google Scholar] [CrossRef]
- Hutchins, D.A.; Jansson, J.K.; Remains, J.V.; Rich, V.I.; Singh, B.K.; Trivedi, P. Climate change microbiology—Problems and perspectives. Nat. Rev. Microbiol. 2019, 17, 391–396. [Google Scholar] [CrossRef]
- Cruz-Paredes, C.; Tájmel, D.; Rousk, J. Variation in temperature dependences across Europe reveals the climate sensitivity of soil microbial decomposers. Appl. Environ. Microbiol. 2023, 89, e0209022. [Google Scholar] [CrossRef]
- Steinweg, J.M.; Dukes, J.S.; Wallenstein, M.D. Modeling the effects of temperature and moisture on soil enzyme activity: Linking laboratory assays to continuous field data. Soil Biol. Biochem. 2012, 55, 85–92. [Google Scholar] [CrossRef]
- Gomez, E.J.; Delgado, J.A.; Gonzalez, J.M. Persistence of microbial extracellular enzymes in soils under different temperatures and water availabilities. Ecol. Evol. 2020, 10, 10167–10176. [Google Scholar] [CrossRef]
- Townsend, A.; Vitousek, P.M.; Holland, E.A. Tropical soils could dominate the short-term carbon cycle feedbacks to increased global temperatures. Clim. Chang. 1992, 22, 293–303. [Google Scholar] [CrossRef]
- Chróst, R.J. Significance of bacterial ectoenzymes in aquatic environments. Hydrobiologia 1992, 244, 61–70. [Google Scholar] [CrossRef]
- Bai, X.; Dippold, M.A.; An, S.; Wang, B.; Zhang, H.; Loeppmann, S. Extracellular enzyme activity and stoichiometry: The effect of soil microbial element limitation during leaf litter decomposition. Ecol. Indic. 2021, 121, 107200. [Google Scholar] [CrossRef]
- Vieille, C.; Zeikus, G.J. Hyperthermophilic enzymes: Sources, uses, and molecular mechanisms for thermostability. Microbiol. Mol. Biol. Rev. 2001, 65, 1–43. [Google Scholar] [CrossRef]
- Li, G.; Maria-Solano, M.A.; Romero-Riva, A.; Osuna, S.; Reetz, M.T. Inducing high activity of a thermophilic enzyme at ambient temperatures by directed evolution. Chem. Comm. 2017, 53, 9454. [Google Scholar] [CrossRef]
- Santana, M.M.; Carvalho, L.; Melo, J.; Araújo, M.E.; Cruz, C. Unveiling the hidden interaction between thermophiles and plant crops: Wheat and soil thermophilic bacteria. J. Plant Interact. 2020, 15, 127–138. [Google Scholar] [CrossRef]
- Renella, G.; Szukics, U.; Landi, L.; Nannipieri, P. Quantitative assessment of hydrolase production and persistence in soil. Biol. Fertil. Soils 2007, 44, 321–329. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; Weintraub, M.N. Emerging tools for measuring and modeling the in situ activity of soil extracellular enzymes. Soil Biol. Biochem 2008, 40, 2098–2106. [Google Scholar] [CrossRef]
- Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlandan, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Comm. 2018, 9, 3033. [Google Scholar] [CrossRef] [PubMed]
- Slade, D.; Radman, M. Oxidative stress resistance in Deinococcus radiodurans. Microbiol Mol. Biol. Rev 2011, 75, 133–191. [Google Scholar] [CrossRef]
- Grant, W.D. Life at low water activity. Phil. Trans Royal Soc. London Ser. B Biol. Sci 2004, 359, 1249–1267. [Google Scholar] [CrossRef]
- Hudson, E.P.; Eppler, R.K.; Clark, D.S. Biocatalysis in semi-aqueous and nearly anhydrous conditions. Curr. Opinion Biotechnol. 2005, 16, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.K.; Tripathi, A.K.; Upadhyay, S.N. Exploration of soil bacterial communities for their potential as bioresource. Bioresour. Technol. 2006, 97, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Singhal, P.; Singh, H.; Damle, D.; Sharma, A.K. Insight into thermophiles and their wide-spectrum applications. Biotech 2016, 6, 81. [Google Scholar] [CrossRef]
- Lyer, P.V.; Ananthanarayan, L. Enzyme stability and stabilization—Aqueous and non-aqueous environment. Process Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Zhou, X.; Fornara, D.; Ikenaga, M.; Akagi, I.; Zhang, R.; Jia. Zhongjun. The resilience of microbial community under drying and rewetting cycles of three forest soils. Front. Microbiol. 2016, 7, 1101. [Google Scholar] [CrossRef]
- Székely, A.J.; Langenheder, S. Dispersal timing and drought history influence the response of bacterioplankton to drying–rewetting stress. ISME J. 2017, 11, 1764–1776. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Kim, S.; Han, S.H.; Chang, H.; Du, D.; Son, Y. Precipitation affects soil microbial and extracellular enzymatic activity responses to warming. Soil Biol. Biochem. 2018, 120, 212–221. [Google Scholar] [CrossRef]
- Nijs, E.A.; Hicks, L.C.; Leizeaga, A.; Tietema, A.; Rousk, J. Soil microbial moisture dependences and responses to drying-rewetting: The legacy of 18 years drought. Glob. Chang. Biol 2018, 25, 1005–1015. [Google Scholar] [CrossRef]
- Andrews, H.M.; Krichels, A.H.; Homyak, P.M.; Piper, S.; Aronson, A.L.; Botthoff, J.; Greene, A.C.; Jenerette, G.D. Wetting-induced soil CO2 emission pulses are driven by interactions among soil temperature, carbon, and nitrogen limitation in the Colorado Desert. Glob. Chang. Biol. 2023, 29, 3205–3220. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.M.; Okal, E.J.; Idris, A.L.; Qian, Z.; Xu, W.; Karanja, J.K.; Wani, S.H.; Yuan, W. Rhizosphere microbiomes can regulate plant drought tolerance. Pedosphere 2022, 32, 61–74. [Google Scholar] [CrossRef]
- Allison, S.D.; Treseder, K.K. Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Glob. Chang. Biol. 2008, 14, 2898–2909. [Google Scholar] [CrossRef]
- Weintraub, S.R.; Wieder, W.R.; Cleveland, C.C.; Townsend, A.R. Organic matter inputs shift soil enzyme activity and allocation patterns in a wet tropical forest. Biogeochemistry 2013, 114, 313–326. [Google Scholar] [CrossRef]
- Buscardo, E.; Souza, R.C.; Meir, P.; Geml, J.; Schmidt, S.K.; Costa, A.C.L.; Nagy, L. Effects of natural and experimental drought on soil fungi and biogeochemistry in an Amazon rain forest. Comm. Earth Environ. 2021, 2, 55. [Google Scholar] [CrossRef]
- Morita, R.Y. Bacteria in Oligotrophic Environments: Starvation-Survival Lifestyle; Chapman & Hall: New York, NY, USA, 1997. [Google Scholar]
- Himeoka, Y.; Mitarai, N. Dynamics of bacterial populations under the feast-famine cycles. Phys. Rev. Res. 2020, 2, 013372. [Google Scholar] [CrossRef]
- Zhu, M.; Dai, X. Stringent response ensures the timely adaptation of bacterial growth to nutrient downshift. Nat. Comm. 2023, 14, 467. [Google Scholar] [CrossRef]
- Cuecas, A.; Portillo, M.C.; Kanoksilapatham, W.; Gonzalez, J.M. Bacterial distribution along a 50ºC temperature gradient reveals a parceled out hot spring environment. Microb. Ecol. 2014, 68, 729–739. [Google Scholar] [CrossRef]
- Santana, M.M.; Dias, T.; Gonzalez, J.M.; Cruz, C. Transformation of organic and inorganic sulfur-adding perspectives to new players in soil and rhizosphere. Soil Biol. Biochem. 2021, 160, 108306. [Google Scholar] [CrossRef]
- Lempp, M.; Lubrano, P.; Bange, G.; Link, H. Metabolism of non-growing bacteria. Biol. Chem. 2020, 401, 1479–1485. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Kong, L.; Liu, T.; Yi, L.; Wang, H.; Huang, W.E.; Zheng, C. Raman-deuterium isotope probing to study metabolic activities of single bacterial cells in human intestinal microbiota. Microb. Biotechnol. 2020, 13, 572–583. [Google Scholar] [CrossRef]
- Weil, R.R.; Magdoff, F. Significance of soil organic matter to soil quality and health. In Soil Organic Matter in Sustainable Agriculture; Weil, R.R., Magdoff, F., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 1–43. [Google Scholar]
- Doran, J.W.; Zeiss, M.R. Soil health and sustainability: Managing the biotic component of soil quality. Appl. Soil Ecol 2000, 15, 3–11. [Google Scholar] [CrossRef]
- Hermans, S.M.; Buckley, H.L.; Case, B.S.; Curran-Cournane, F.; Taylor, M.; Lear, G. Bacteria as emerging indicators of soil condition. Appl Environ. Microbiol. 2016, 83, e02826-16. [Google Scholar] [CrossRef] [PubMed]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; van der Heijden, M.G.A. Soil microbiomes and one health. Nat. Rev. Microbiol. 2023, 21, 6–20. [Google Scholar] [CrossRef]
- Santana, M.M.; Portillo, M.C.; Gonzalez, J.M.; Clara, M.I. Characterization of new soil thermophilic bacteria potentially involved in soil fertilization. J. Plant Nutr. Soil Sci. 2013, 176, 47–56. [Google Scholar] [CrossRef]
- Rosa, A.P.; Dias, T.; Mouazen, A.M.; Cruz, C.; Santana, M.M. Finding optimal microorganisms to increase crop productivity and sustainability under drought—A structured reflection. J. Plant Interact. 2023, 18, 2178680. [Google Scholar] [CrossRef]
- Gonzalez-Prieto, S.J.; Carballas, T. Composition of organic N in temperate humid region soils (NW Spain). Soil Biol. Biochem. 1991, 23, 887–895. [Google Scholar] [CrossRef]
- Eschenlauer, S.C.P.; McKain, N.; Walker, N.A.; McEwan, N.R.; Newbold, C.J.; Wallace, R.J. Ammonia production by ruminal microorganisms and enumeration, isolation, and characterization of bacteria capable of growth on peptides and amino acids from the sheep rumen. Appl. Environ. Microbiol. 2002, 68, 4925–4931. [Google Scholar] [CrossRef]
- Sutton, R.; Sposito, G. Molecular structure in soil humic substances: The new view. Environ. Sci. Technol. 2005, 39, 9009–9015. [Google Scholar] [CrossRef] [PubMed]
- Sposito, G. The Chemistry of Soils, 2nd ed.; Oxford University Press: New York, NY, USA, 2008. [Google Scholar]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Gruber, N.; Galloway, J.N. An earth-system perspective of the global nitrogen cycle. Nature 2008, 451, 293–296. [Google Scholar] [CrossRef]
- Ghani, A.; McLaren, R.G.; Swift, R.S. Mobilization of recently formed soil organic sulphur. Soil Biol. Biochem. 1993, 25, 1739–1744. [Google Scholar] [CrossRef]
- Eriksen, J. Soil sulfur cycling in temperate agricultural systems. In Sulfur: A Missing Link between Soils, Crops and Nutrition; Jez, J., Ed.; American Society of Agronomy Inc.: Madison, WI, USA, 2008; pp. 25–44. [Google Scholar]
- Kertesz, M.A.; Mirleau, P. The role of soil microbes in plant sulphur nutrition. J. Exp. Bot. 2004, 55, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.M.; Gonzalez, J.M.; Clara, M.I. Inferring pathways leading to organic-sulfur mineralization in the Bacillales. Crit. Rev. Microbiol. 2016, 42, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.L.; Condron, L.M.; Wells, A.; Andersen, K.M. Soil nutrient dynamics during podzol development under lowland temperate rain forest in New Zealand. Catena 2012, 97, 50–62. [Google Scholar] [CrossRef]
- Alewell, C.; Ringeval, B.; Ballabio, C.; Robinson, D.A.; Panagos, P.; Borrelli, P. Global phosphorus shortage will be aggravated by soil erosion. Nat. Comm. 2020, 11, 4546. [Google Scholar] [CrossRef]
- Margalef, O.; Sardans, J.; Maspons, J.; Molowny-Horas, R.; Fernández-Martínez, M.; Janssens, I.A.; Richter, A.; Ciais, P.; Obersteiner, M.; Peñuelas, J. The effect of global change on soil phosphatase activity. Glob. Change Biol. 2021, 27, 5989–6003. [Google Scholar] [CrossRef] [PubMed]
- Langhans, C.; Beusen, A.H.W.; Mogollón, J.M.; Bouwman, A.F. Phosphorus for sustainable development goal target of doubling smallholder productivity. Nat. Sustain. 2022, 5, 57–63. [Google Scholar] [CrossRef]
- Basilio, F.; Dias, T.; Santana, M.M. Multiple modes of action are needed to unlock soil phosphorus fractions unavailable for plants: The example of bacteria- and fungi-based fertilizers. Appl. Soil Ecol. 2022, 178, 104550. [Google Scholar] [CrossRef]
- Spohn, M.; Diáková, K.; Aburto, F.; Doetterl, S.; Borovec, J. Sorption and desorption of organic matter in soils as affected by phosphate. Geoderma 2022, 405, 115377. [Google Scholar] [CrossRef]
- Duncan, J.; Bokhary, A.; Fatehi, P.; Kong, F.; Lin, H.; Liao, B. Thermophilic membrane bioreactors: A review. Bioresour. Technol 2017, 243, 1180–1193. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Pletschke, B.I. Thermophilic Bacilli and their enzymes in composting. In Composting for Sustainable Agriculture; Maheshwari, D., Ed.; Springer: New York, NY, USA, 2014; pp. 103–124. [Google Scholar]
- Lin, C.; Cheruiyot, N.K.; Bui, X.-T.; Ngo, H.N. Composting and its application in bioremediation of organic contaminants. Bioengineered 2022, 13, 1073–1089. [Google Scholar] [CrossRef] [PubMed]
- Nemet, F.; Perić, K.; Lončarić, Z. Microbiological activities in the composting process: A review. J. Agric. Environ. Sci. 2021, 8, 41–53. [Google Scholar] [CrossRef]
- Finore, I.; Feola, A.; Russo, L.; Cattaneo, A.; Donato, P.D.; Nicolaus, B.; Poli, A.; Romano, I. Thermophilic bacteria and their thermozymes in composting processes: A review. Chem. Biol. Technol. Agric. 2023, 10, 1–22. [Google Scholar] [CrossRef]
- Saccá, M.L.; Caraccciolo, A.B.; Lenola, M.D.; Grenni, P. Ecosystem services provided by soil microorganisms. Chapter 2. In Soil Biological Communities and Ecosystem Resilience; Lukac, M., Grenni, P., Gamboni, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 9–24. [Google Scholar]
- Castro, S.P.; Cleland, E.E.; Wagner, R.; Al Sawad, R.; Lipson, D.A. Soil microbial responses to drought and exotic plants shift carbon metabolism. ISME J. 2019, 13, 1776–1787. [Google Scholar] [CrossRef]
- Balvanera, P.; Pfisterer, A.B.; Buchmann, N.; He, J.-S.; Nakashizuka, T.; Raffaelli, D.; Schmid, B. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol. Lett. 2006, 9, 1146–1156. [Google Scholar] [CrossRef]
- Vries, F.; Lau, J.; Hawkes, C.; Semchenko, M. Plant-soil feedback under drought: Does history shape the future? Trends Ecol. Evol. 2023, 3131, 1–11. [Google Scholar] [CrossRef]
- Malik, A.A.; Bouskill, N.J. Dorught impacts on microbial trait distribution and feedback to soil carbon cycling. Funct. Ecol. 2022, 36, 1442–1456. [Google Scholar] [CrossRef]
- Hefnet, G.T.; Tomkins, R.P.T. The Experimental Determination of Solubilities; John Wiley & Sons, Ltd.: New York, NY, USA, 2003. [Google Scholar]
- Mounir, S.H.; Feidt, M.; Vasse, C. Thermoeconomic study of a system for pollutant concentration with mechanical vapour compression. Appl. Thermal Eng. 2005, 25, 473–484. [Google Scholar] [CrossRef]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils; Pearson Press: Upper Saddle River, NJ, USA, 2017. [Google Scholar]
- Bragazza, L.; Buttler, A.; Robroek, B.J.M.; Albrecht, R.; Zaccone, C.; Jassey, V.E.J.; Signarbieux, C. Persistent high temperature and low precipitation reduce peat carbon accumulation. Glob. Change Biol. 2016, 22, 4114–4123. [Google Scholar] [CrossRef] [PubMed]
- Battisti, D.S.; Naylor, R.L. Historical warnings of future food insecurity with unprecedented seasonal heat. Science 2009, 323, 240–244. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Biederman, J.A.; Scott, R.L.; Goulden, M.L.; Vargas, R.; Litvak, M.E.; Kolb, T.E.; Yepez, E.A.; Oechel, W.C.; Blanken, P.D.; Bell, T.W.; et al. Terrestrial carbon balance in a drier world: The effects of water availability in southwestern North America. Glob. Chang. Biol. 2016, 22, 1867–1879. [Google Scholar] [CrossRef]
- Takeshima, A.; Kim, H.; Shiogama, H.; Lierhammer, L.; Scinocca, J.F.; Seland, O.; Mitchell, D. Global aridity changes due to differences in surface energy and water balance between 1.5 °C and 2 °C warming. Environ. Res. Lett. 2020, 15, 0940a7. [Google Scholar] [CrossRef]
- Chai, R.; Mao, J.; Chen, H.; Wang, Y.; Shi, X.; Jin, M.; Zhao, T.; Hoffman, F.M.; Ricciuto, D.M.; Wullschleger, S.D. Human-caused long-term changes in global aridity. Clim. Atmosph. Sci. 2021, 4, 65. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J. Impact of temperature on the biological properties of soil. Int. Agrophys. 2016, 30, 1–8. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J. Soil moisture as a factor affecting the microbiological and biochemical activity of soil. Plant Soil Environ. 2016, 62, 250–255. [Google Scholar] [CrossRef]
- Huffman, R.L.; Fangmeier, D.D.; Elliot, W.J.; Workman, S.R. Infiltration and Runoff. In Soil and Water Conservation Engineering, 7th ed.; Schwab, G.O., Fangmeier, D.D., Elliot, W.J., Frevert, R.K., Eds.; American Society of Agricultural Engineers: St. Joseph, MI, USA, 2013; pp. 81–113. [Google Scholar]
- Abraham, S.; Huynh, C.; Vu, H. Classification of soils into hydrologic groups using machine learning. Data 2020, 5, 2. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effects on plant growth and development. Weather. Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Merow, C.; Bois, S.T.; Allen, J.M.; Xie, Y.; Silander, J.A., Jr. Climate change both facilitates nd inhibits invasive plant ranges in New England. Proc. Natl. Acad. Sci. USA 2017, 114, E3276–E3284. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, E.; Yigini, Y.; Montanarella, L. Combining soil datasets for topsoil organic carbon mapping in Europe. PLoS ONE 2016, 11, e0152098. [Google Scholar] [CrossRef] [PubMed]
- Sleeter, B.M.; Loveland, T.; Domke, G.; Herold, N.; Wickham, J.; Wood, N. Land Cover and Land-Use Change. In Impacts, Risks, and Adaptation in the United States: Fourth National Climate Assessment; Reidmiller, D.R., Avery, C.W., Easterling, D.R., Kunkel, K.E., Lewis, K.L.M., Maycock, T.K., Stewart, B.C., Eds.; U.S. Global Change Research Program: Washington, DC, USA, 2018; Volume II, pp. 202–231. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).