Epigenetic Induction of Secondary Metabolites Production in Endophytic Fungi Penicillium chrysogenum and GC-MS Analysis of Crude Metabolites with Anti-HIV-1 Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Endophytic Fungi Isolation
2.2. Treatment of Endophytic Fungi with Small Epigenetic Modifiers
2.3. Crude Extraction of Fungal Secondary Metabolites
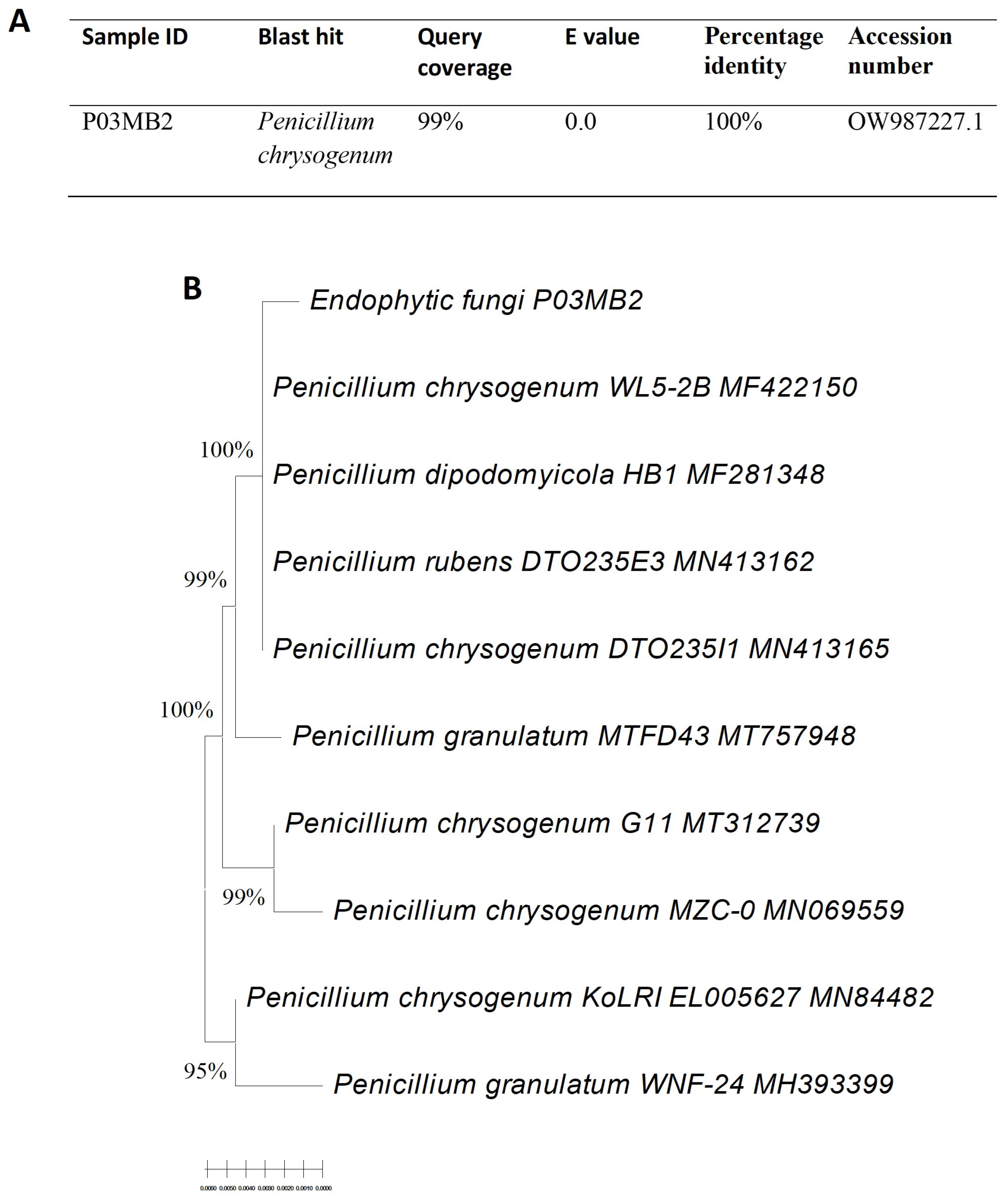
2.4. Molecular Identification of Endophytic Fungi of Active Fungal Extracts
2.4.1. Cultivation and DNA Isolation of Endophytic fungi
2.4.2. Amplification of Fungal DNA
2.4.3. Identification of Endophytic Fungi by ITS Sequencing
2.5. Cell Cultures
2.5.1. Transformation of XL1 Blue Super Competent Cells and Construction of Viral Plasmids
2.5.2. Plasmid DNA Purification
2.5.3. Generation of Pseudoviruses by Transfection
2.5.4. Titration of Virus (TCID Assay)
2.6. Evaluation of Cytotoxicity of Fungal Crude Extracts
2.7. Luciferase-Based Antiviral Assay
2.8. Bioassay-Guided Fractionation and Chemical Profiling of Secondary Metabolites Using Gas Chromatography-Mass Spectrometry (GC-MS)
2.8.1. Extraction of Secondary Metabolites through Large-Scale Fermentation
2.8.2. Solid-Phase Extraction (SPE) of Fungal Crude Extracts
2.8.3. Gas Chromatography-Mass Spectrometry Analysis
3. Results
3.1. Isolation and Molecular Identification of Endophytic Fungi
3.2. Cytotoxicity Effects of Crude Methanol Extracts
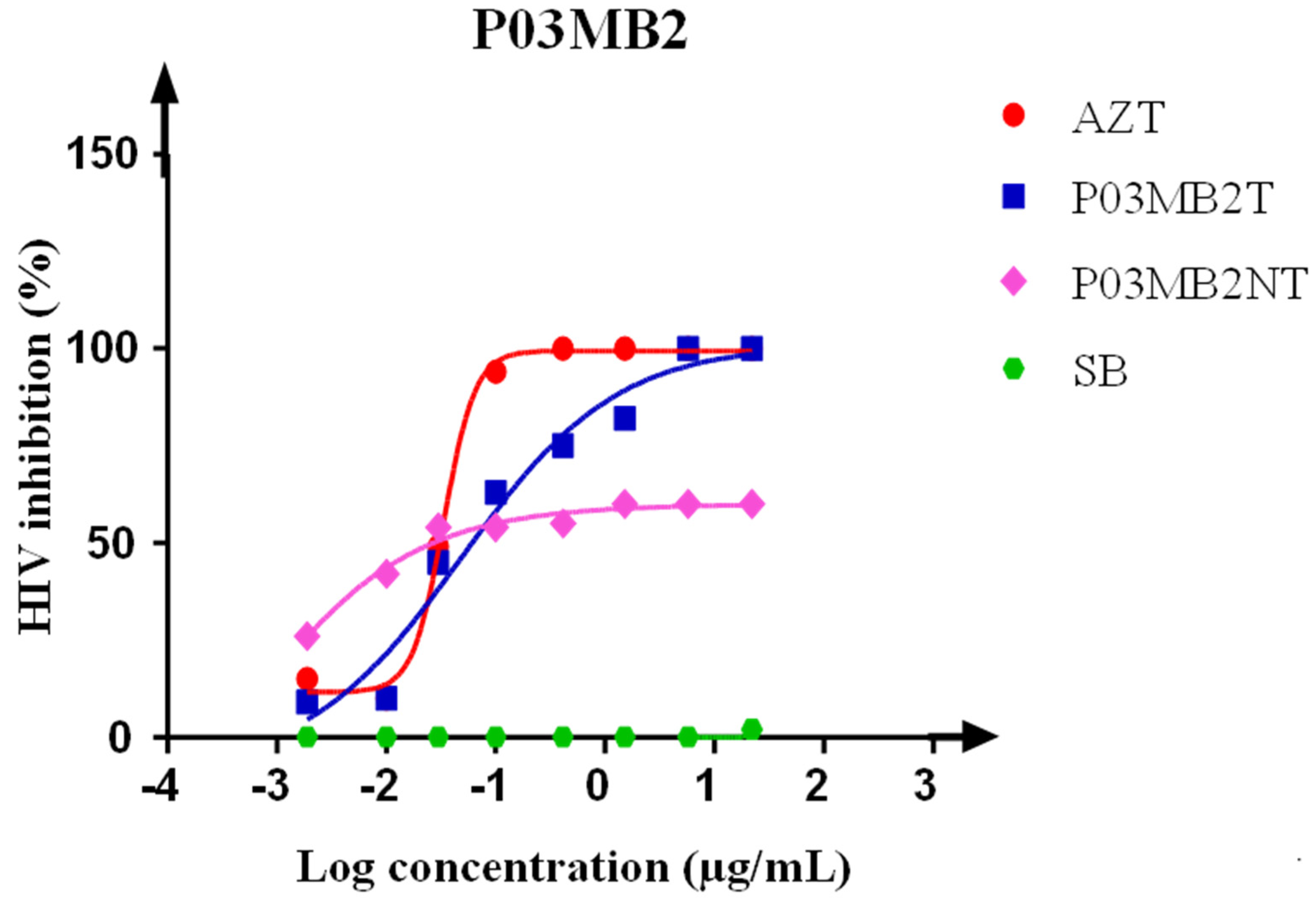
3.3. Luciferase-Based Antiviral Activity Assay on Crude Extracts
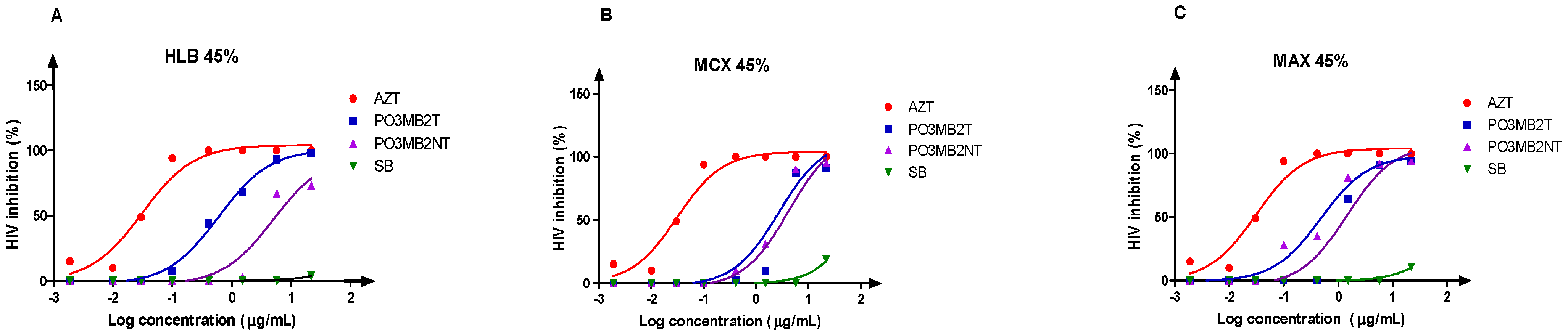
3.4. Bio-Assay Guided Fractionation Approach to the Anti-HIV Activity of Secondary Metabolite Fractions (Solid-Phase Extraction)
3.5. GC-MS Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Clercq, E. Antiretroviral drugs. Curr. Opin. Pharmacol. 2010, 10, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.H.; Venkatesh, K.K. Antiretroviral therapy as HIV prevention: Status and prospects. Am. J. Public Health 2010, 100, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Broder, S. The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antivir. Res. 2010, 85, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kurapati, K.R.V.; Atluri, V.S.; Samikkannu, T.; Garcia, G.; Nair, M.P. Natural products as anti-HIV agents and role in HIV-associated neurocognitive disorders (HAND): A brief overview. Front. Microbiol. 2016, 6, 1444. [Google Scholar] [CrossRef]
- Roy, B.G. Potential of small-molecule fungal metabolites in antiviral chemotherapy. Antivir. Chem. Chemother. 2017, 25, 20–52. [Google Scholar] [CrossRef]
- Bashyal, B.P.; Wellensiek, B.P.; Ramakrishnan, R.; Faeth, S.H.; Ahmad, N.; Gunatilaka, A.A. Altertoxins with potent anti-HIV activity from Alternaria tenuissima QUE1Se, a fungal endophyte of Quercus emoryi. Bioorg. Med. Chem. 2014, 22, 6112–6116. [Google Scholar] [CrossRef]
- Ma, X.; Li, L.; Zhu, T.; Ba, M.; Li, G.; Gu, Q.; Guo, Y.; Li, D. Phenylspirodrimanes with anti-HIV activity from the sponge-derived fungus Stachybotrys chartarum MXH-X73. J. Nat. Prod. 2013, 76, 2298–2306. [Google Scholar] [CrossRef]
- Hu, W.S.; Hughes, S.H. HIV-1 reverse transcription. Cold Spring Harb. Perspect. Med. 2012, 2, a006882. [Google Scholar] [CrossRef]
- Reeves, J.D.; Piefer, A.J. Emerging drug targets for antiretroviral therapy. Drugs 2005, 65, 1747–1766. [Google Scholar] [CrossRef]
- Singh, S.B.; Zink, D.L.; Guan, Z.; Collado, J.; Pelaez, F.; Felock, P.J.; Hazuda, D.J. Isolation, structure, and HIV-1 integrase inhibitory activity of Xanthoviridicatin E and F, two novel fungal metabolites produced by Penicillium chrysogenum. Helv. Chim. Acta 2003, 86, 3380–3385. [Google Scholar] [CrossRef]
- Henrich, T.J.; Kuritzkes, D.R. HIV-1 entry inhibitors: Recent development and clinical use. Curr. Opin. Virol. 2013, 3, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Kuritzkes, D.R. HIV-1 Entry inhbitors: An overview. Curr. Opin. HIV AIDS 2009, 4, 82. [Google Scholar] [CrossRef] [PubMed]
- Monini, P.; Sgadari, C.; Barillari, G.; Ensoli, B. HIV protease inhibitors: Antiretroviral agents with anti-inflammatory, anti-angiogenic and anti-tumour activity. J. Antimicrob. Chemother. 2003, 51, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Flexner, C. HIV-protease inhibitors. N. Engl. J. Med. 1998, 338, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Linnakoski, R.; Reshamwala, D.; Veteli, P.; Cortina-Escribano, M.; Vanhanen, H.; Marjomäki, V. Antiviral Agents From Fungi: Diversity, Mechanisms and Potential Applications. Front. Microbiol. 2018, 9, 2325. [Google Scholar] [CrossRef]
- Kjærbølling, I.; Mortensen, U.H.; Vesth, T.; Andersen, M.R. Strategies to establish the link between biosynthetic gene clusters and secondary metabolites. Fungal Genet. Biol. 2019, 130, 107–121. [Google Scholar] [CrossRef]
- Rutledge, P.J.; Challis, G.L. Discovery of microbial natural products by activation of silent biosynthetic gene clusters. Nat. Rev. Microbiol. 2015, 13, 509–523. [Google Scholar] [CrossRef]
- Reen, F.J.; Romano, S.; Dobson, A.D.; O’Gara, F. The Sound of Silence: Activating Silent Biosynthetic Gene Clusters in Marine Microorganisms. Mar. Drugs 2015, 13, 4754–4783. [Google Scholar] [CrossRef]
- Okada, B.K.; Seyedsayamdost, M.R. Antibiotic dialogues: Induction of silent biosynthetic gene clusters by exogenous small molecules. FEMS Microbiol. Rev. 2017, 41, 19–33. [Google Scholar] [CrossRef]
- Pillay, L.C.; Nekati, L.; Makhwitine, P.J.; Ndlovu, S.I. Epigenetic Activation of Silent Biosynthetic Gene Clusters in Endophytic Fungi Using Small Molecular Modifiers. Front. Microbiol. 2022, 13, 815008. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Elkhayat, E.S.; Mohamed, G.A.A.; Fat’hi, S.M.; Ross, S.A. Fusarithioamide A, a new antimicrobial and cytotoxic benzamide derivative from the endophytic fungus Fusarium chlamydosporium. Biochem. Biophys. Res. Commun. 2016, 479, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Magotra, A.; Kumar, M.; Kushwaha, M.; Awasthi, P.; Raina, C.; Gupta, A.P.; Shah, B.A.; Gandhi, S.G.; Chaubey, A. Epigenetic modifier induced enhancement of fumiquinazoline C production in Aspergillus fumigatus (GA-L7): An endophytic fungus from Grewia asiatica L. AMB Express 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.M.; Bradshaw, E.; Seipke, R.F.; Hutchings, M.I.; McArthur, M. Use and discovery of chemical elicitors that stimulate biosynthetic gene clusters in Streptomyces bacteria. Methods Enzymol. 2012, 517, 367–385. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Sandiford, S.K.; van Wezel, G.P. Triggers and cues that activate antibiotic production by actinomycetes. J. Ind. Microbiol. Biotechnol. 2014, 41, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kulkarni, M.G.; White, J.F.; Van Staden, J. Epigenetic-based developments in the field of plant endophytic fungi. S. Afr. J. Bot. 2020, 134, 394–400. [Google Scholar] [CrossRef]
- Akone, S.H. Secondary Metabolites from Fungi: Strategies of Activation of Silent Biosynthetic Pathways, Structure Elucidation and Bioactivity; Heinrich-Heine-Universität Düsseldorf: Düsseldorf, Germany, 2016. [Google Scholar]
- Henrikson, J.C.; Hoover, A.R.; Joyner, P.M.; Cichewicz, R.H. A chemical epigenetics approach for engineering the in situ biosynthesis of a cryptic natural product from Aspergillus niger. Org. Biomol. Chem. 2009, 7, 435–438. [Google Scholar] [CrossRef]
- Krämer, O.H.; Zhu, P.; Ostendorff, H.P.; Golebiewski, M.; Tiefenbach, J.; Peters, M.A.; Brill, B.; Groner, B.; Bach, I.; Heinzel, T. The histone deacetylase inhibitor valproic acid selectively induces proteasomal degradation of HDAC2. EMBO J. 2003, 22, 3411–3420. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-Z.; Liang, B.-W.; Li, X.-F.; Liu, Q. Induced production of new diterpenoids in the fungus Penicillium funiculosum. Nat. Prod. Commun. 2014, 9, 1934578X1400900502. [Google Scholar] [CrossRef]
- Shwab, E.K.; Bok, J.W.; Tribus, M.; Galehr, J.; Graessle, S.; Keller, N.P. Histone deacetylase activity regulates chemical diversity in Aspergillus. Eukaryot. Cell 2007, 6, 1656–1664. [Google Scholar] [CrossRef]
- Smith, W.L.; Edlind, T.D. Histone deacetylase inhibitors enhance Candida albicans sensitivity to azoles and related antifungals: Correlation with reduction in CDR and ERG upregulation. Antimicrob. Agents Chemother. 2002, 46, 3532–3539. [Google Scholar] [CrossRef]
- Vrba, J.; Trtkova, K.; Ulrichova, J. HDAC inhibitors sodium butyrate and sodium valproate do not affect human ncor1 and ncor2 gene expression in HL-60 cells. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2011, 155, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Arivudainambi, U.S.; Anand, T.D.; Shanmugaiah, V.; Karunakaran, C.; Rajendran, A. Novel bioactive metabolites producing endophytic fungus Colletotrichum gloeosporioides against multidrug-resistant Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 2011, 61, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Petrini, O. Taxonomy of endophytic fungi of aerial plant tissues. In Microbiology of the Phyllosphere; Cambridge University Press: Cambridge, UK, 1986. [Google Scholar]
- Gonzalez-Menendez, V.; Perez-Bonilla, M.; Perez-Victoria, I.; Martin, J.; Munoz, F.; Reyes, F.; Tormo, J.R.; Genilloud, O. Multicomponent Analysis of the Differential Induction of Secondary Metabolite Profiles in Fungal Endophytes. Molecules 2016, 21, 234. [Google Scholar] [CrossRef] [PubMed]
- Nzimande, B.; Kumalo, H.M.; Ndlovu, S.I.; Mkhwanazi, N.P. Secondary metabolites produced by endophytic fungi, Alternaria alternata, as potential inhibitors of the human immunodeficiency virus. Front. Genet. 2022, 13, 1077159. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Hsiang, S.-T. Embedded image coding using zeroblocks of subband/wavelet coefficients and context modeling. In Proceedings of the Proceedings DCC 2001, Data Compression Conference, Snowbird, UT, USA, 27–29 March 2001; pp. 83–92. [Google Scholar]
- Nutan, M.M.; Goel, T.; Das, T.; Malik, S.; Suri, S.; Rawat, A.K.S.; Srivastava, S.K.; Tuli, R.; Malhotra, S.; Gupta, S.K. Ellagic acid & gallic acid from Lagerstroemia speciosa L. inhibit HIV-1 infection through inhibition of HIV-1 protease & reverse transcriptase activity. Indian J. Med. Res. 2013, 137, 540. [Google Scholar]
- Wei, X.; Decker, J.M.; Liu, H.; Zhang, Z.; Arani, R.B.; Kilby, J.M.; Saag, M.S.; Wu, X.; Shaw, G.M.; Kappes, J.C. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 2002, 46, 1896–1905. [Google Scholar] [CrossRef]
- Derdeyn, C.A.; Decker, J.M.; Sfakianos, J.N.; Wu, X.; O’Brien, W.A.; Ratner, L.; Kappes, J.C.; Shaw, G.M.; Hunter, E. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J. Virol. 2000, 74, 8358–8367. [Google Scholar] [CrossRef]
- Platt, E.J.; Wehrly, K.; Kuhmann, S.E.; Chesebro, B.; Kabat, D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 1998, 72, 2855–2864. [Google Scholar] [CrossRef] [PubMed]
- Stoszko, M.; Al-Hatmi, A.M.S.; Skriba, A.; Roling, M.; Ne, E.; Crespo, R.; Mueller, Y.M.; Najafzadeh, M.J.; Kang, J.; Ptackova, R.; et al. Gliotoxin, identified from a screen of fungal metabolites, disrupts 7SK snRNP, releases P-TEFb, and reverses HIV-1 latency. Sci. Adv. 2020, 6, eaba6617. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Patnaik, S. GC-MS analysed phyto-chemicals and antibacterial activity of Withania somnifera (L.) Dunal extract in the context of treatment to liver cirrhosis. Biomed. Pharmacol. J. 2020, 13, 71–78. [Google Scholar] [CrossRef]
- Available online: https://www.nist.gov/nist-research-library (accessed on 12 October 2022).
- Available online: https://www.npatlas.org/explore/compounds/NPA032343 (accessed on 15 April 2023).
- Available online: https://lotus.naturalproducts.net/ (accessed on 15 April 2023).
- Rutz, A.; Sorokina, M.; Galgonek, J.; Mietchen, D.; Willighagen, E.; Gaudry, A.; Graham, J.G.; Stephan, R.; Page, R.; Vondrášek, J.; et al. The LOTUS initiative for open knowledge management in natural products research. eLife 2022, 11, e70780. [Google Scholar] [CrossRef]
- Maiques-Diaz, A.; Somervaille, T.C. LSD1: Biologic roles and therapeutic targeting. Epigenomics 2016, 8, 1103–1116. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 18566, C.-, 2-Dicarbonitrile. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cyclobutane-1_2-dicarbonitrile (accessed on 22 April 2023).
- van der Kolk, M.R.; Janssen, M.A.; Rutjes, F.P.; Blanco-Ania, D. Cyclobutanes in small-molecule drug candidates. ChemMedChem 2022, 17, e202200020. [Google Scholar]
- Tatsumi, T.; Zhang, W.; Kida, T.; Nakatsuji, Y.; Ono, D.; Takeda, T.; Ikeda, I. Novel hydrolyzable and biodegradable cationic gemini surfactants: Bis (ester-ammonium) dichloride having a butenylene or a butynylene spacer. J. Surfactants Deterg. 2001, 4, 279–285. [Google Scholar] [CrossRef]
- Sikandar, A.; Zhang, M.; Wang, Y.; Zhu, X.; Liu, X.; Fan, H.; Xuan, Y.; Chen, L.; Duan, Y. Mycochemical Screening and Analysis, Antioxidant Activity, and Biochemical Composition of Fermentation Strain Snef1216 (Penicillium chrysogenum). J. Anal. Methods Chem. 2020, 2020, 3073906. [Google Scholar] [CrossRef]
- Priyanka, C.; Kumar, P.; Bankar, S.P.; Karthik, L. In vitro antibacterial activity and gas chromatography–mass spectroscopy analysis of Acacia karoo and Ziziphus mauritiana extracts. J. Taibah Univ. Sci. 2015, 9, 13–19. [Google Scholar] [CrossRef]
- Keskın, D.; Ceyhan, N.; Uğur, A.; Dbeys, A.D. Antimicrobial activity and chemical constitutions of West Anatolian olive (Olea europaea L.) leaves. J. Food Agric. Environ. 2012, 10, 99–102. [Google Scholar]
- Bhuyar, P.; Rahim, M.; Sundararaju, S.; Maniam, G.; Govindan, N. Antioxidant and antibacterial activity of red seaweed Kappaphycus alvarezii against pathogenic bacteria. Glob. J. Environ. Sci. Manag. 2020, 6, 47–58. [Google Scholar]
- Save, S.; Lokhande, R.; Chowdhary, A. Determination of 1, 2-Benzenedicarboxylic acid, bis (2-ethylhexyl) ester from the twigs of Thevetia peruviana as a Colwell Biomarker. J. Innov. Pharm. Biol. Sci. 2015, 2, 349–362. [Google Scholar]
- Rajeswari, G.; Murugan, M.; Mohan, V. GC-MS analysis of bioactive components of Hugonia mystax L.(Linaceae). Res. J. Pharm. Biol. Chem. Sci. 2012, 3, 301–308. [Google Scholar]
- Rahman, M.; Anwar, M.N. Fungitoxic and cytotoxic activity of a novel compound 1,2-benzenedicarboxylic acid, diisooctyl ester of Plumbago zeylanica linn. Asian J. Microbiol. Biotechnol. Environ. Sci. 2006, 8, 461–464. [Google Scholar]
- Li, M.; Zhou, L.; Yang, D.; Li, T.; Li, W. Biochemical composition and antioxidant capacity of extracts from Podophyllum hexandrum rhizome. BMC Complement. Altern. Med. 2012, 12, 263. [Google Scholar] [CrossRef]
- Kaviya, M.; Balasubramanian, B.; Bharathi, K.; Malaisamy, A.; Al-Dhabi, N.A.; Mariadhas, V.A.; Anand, A.V.; Liu, W. Evaluation of Nutritional Substances and Investigation of Antioxidant and Antimicrobial Potentials of Boerhavia diffusa with in Silico Molecular Docking. Molecules 2022, 27, 1280. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, E.; Balduque-Gil, J.; Barriuso-Vargas, J.J.; Casanova-Gascón, J.; González-García, V.; Cuchí-Oterino, J.A.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Holm Oak (Quercus ilex subsp. ballota (Desf.) Samp.) Bark Aqueous Ammonia Extract for the Control of Invasive Forest Pathogens. Int. J. Mol. Sci. 2022, 23, 11882. [Google Scholar] [CrossRef]
- Tajick Ghanbary, M.; Khani, H.; Babaeizad, V. Identification of some secondary metabolites produced by four Penicillium species. Mycol. Iran. 2014, 1, 107–113. [Google Scholar]
- Ghaly, N.S.; Mina, S.A.; Younis, N.N. Schistosomicidal and molluscicidal activities of two Junipers species cultivated in Egypt and the chemical composition of their essential oils. J. Med. Plants Res. 2016, 10, 47–53. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Sholkamy, E.N.; Bukhari, N.; Al-Enazi, N.M.; Alsamhary, K.I.; Al-Khiat, S.H.A.; Ibraheem, I.B.M. Bioremoval capacity of Co(+2) using Phormidium tenue and Chlorella vulgaris as biosorbents. Environ. Res. 2022, 204, 111630. [Google Scholar] [CrossRef]
- Kapoor, K. Coumarin analogues as a potential inhibitor of leishmaniasis: A multi-targeting protein inhibition approach by molecular docking. Univers. J. Pharm. Res. 2019, 4, 20–25. [Google Scholar] [CrossRef]
- Cherne, M.; Hall, J.; Kellner, A.; Chong, C.; Cole, A.; Cole, A. Avirulins, a Novel Class of HIV-1 Reverse Transcriptase Inhibitors Effective in the Female Reproductive Tract Mucosa. Viruses 2019, 11, 408. [Google Scholar] [CrossRef] [PubMed]
- Douka, M.D.; Litinas, K.E. An Overview on the Synthesis of Fused Pyridocoumarins with Biological Interest. Molecules 2022, 27, 7256. [Google Scholar] [CrossRef] [PubMed]
- Krátký, M.; Vinšová, J. Antifungal Activity of Salicylanilides and Their Esters with 4-(Trifluoromethyl)benzoic Acid. Molecules 2012, 17, 9426–9442. [Google Scholar] [CrossRef]
- Prakash, J.; Arora, N. Novel Metabolites Identified From Bacillus Safensis and Their Antifungal Property against Alternaria alternata. Antonie Van Leeuwenhoek 2021, 114, 1245–1258. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Sugawara, S.; Arihara, S. Phenylpropanoids and other secondary metabolites from fresh fruits of Picrasma quassioides. Phytochemistry 1995, 40, 253–256. [Google Scholar] [CrossRef]
- Tang, X.X.; Liu, S.Z.; Yan, X.; Tang, B.W.; Fang, M.J.; Wang, X.M.; Wu, Z.; Qiu, Y.K. Two New Cytotoxic Compounds from a Deep-Sea Penicillum citreonigrum XT20-134. Mar Drugs 2019, 17, 509. [Google Scholar] [CrossRef]
- Sharma, A.; Rai, P. Assessment of bioactive compounds in Brassica juncea using chromatographic techniques. J. Pharmacogn. Phytochem. 2018, 7, 1274–1277. [Google Scholar]
- Ying, W.; Zhao, Y.-C.; Fan, L.-L.; Xia, X.-D.; Li, Y.-H.; Zhou, J.-Z. Identification and characterization of Pichia membranifaciens Hmp-1 isolated from spoilage blackberry wine. J. Integr. Agric. 2018, 17, 2126–2136. [Google Scholar]
- Pradhan, S.; Dubey, R.C. GC–MS analysis and molecular docking of bioactive compounds of Camellia sinensis and Camellia assamica. Arch. Microbiol. 2021, 203, 2501–2510. [Google Scholar] [CrossRef]
- Pradhan, S.; Nautiyal, V.; Dubey, R.C. Antioxidant potential and molecular docking of bioactive compound of Camellia sinensis and Camellia assamica with cytochrome P450. Arch. Microbiol. 2022, 204, 350. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, F.; Zhang, R.; Vidyarthi, S.K.; Sun, X.; Pan, Z. Comparison of volatile compounds and fatty acids of jujubes (Ziziphus jujuba mill.) before and after blackening process. Int. J. Food Prop. 2022, 25, 1079–1098. [Google Scholar] [CrossRef]
- Jasim, H.; Hussein, A.O.; Hameed, I.H.; Kareem, M.A. Characterization of alkaloid constitution and evaluation of antimicrobial activity of Solanum nigrum using gas chromatography mass spectrometry (GC-MS). J. Pharmacogn. Phytother. 2015, 7, 56–72. [Google Scholar]
- Chraibi, M.; Farah, A.; Elamin, O.; Iraqui, H.M.; Fikri-Benbrahim, K. Characterization, antioxidant, antimycobacterial, antimicrobial effcts of Moroccan rosemary essential oil, and its synergistic antimicrobial potential with carvacrol. J. Adv. Pharm. Technol. Res. 2020, 11, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Li, X.Q.; Zhao, D.L.; Zhang, P. Antifungal Secondary Metabolites Produced by the Fungal Endophytes: Chemical Diversity and Potential Use in the Development of Biopesticides. Front. Microbiol. 2021, 12, 689527. [Google Scholar] [CrossRef] [PubMed]
- Stierle, A.; Stierle, D. Bioactive Secondary Metabolites Produced by the Fungal Endophytes of Conifers. Nat. Prod. Commun. 2015, 10, 1671–1682. [Google Scholar] [CrossRef]
- Zutz, C.; Bandian, D.; Neumayer, B.; Speringer, F.; Gorfer, M.; Wagner, M.; Strauss, J.; Rychli, K. Fungi treated with small chemicals exhibit increased antimicrobial activity against facultative bacterial and yeast pathogens. BioMed Res. Int. 2014, 2014, 540292. [Google Scholar] [CrossRef]
- Ameen, F.; Almansob, A.; Al Tami, M.; Al-Enazi, N.; Al-Sabri, A.; Orfali, R. Epigenetic Modifiers Affect the Bioactive Compounds Secreted by an Endophyte of the Tropical Plant Piper longum. Molecules 2021, 26, 29. [Google Scholar] [CrossRef]
- Xue, M.; Hou, X.; Fu, J.; Zhang, J.; Wang, J.; Zhao, Z.; Xu, D.; Lai, D.; Zhou, L. Recent Advances in Search of Bioactive Secondary Metabolites from Fungi Triggered by Chemical Epigenetic Modifiers. J. Fungi 2023, 9, 172. [Google Scholar] [CrossRef]
- Triastuti, A.; Vansteelandt, M.; Barakat, F.; Trinel, M.; Jargeat, P.; Fabre, N.; Amasifuen Guerra, C.A.; Mejia, K.; Valentin, A.; Haddad, M. How histone deacetylase inhibitors alter the secondary metabolites of Botryosphaeria mamane, an endophytic fungus isolated from Bixa orellana. Chem. Biodivers. 2019, 16, e1800485. [Google Scholar] [CrossRef] [PubMed]
- Ramesha, K.P.; Chandra Mohana, N.; Chandra Nayaka, S.; Satish, S. Epigenetic Modifiers Revamp Secondary Metabolite Production in Endophytic Nigrospora sphaerica. Front. Microbiol. 2021, 12, 730355. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.M.; Abd El-Ghany, M.N.; Rodríguez-Couto, S. Antifungal and anti-mycotoxin efficacy of biogenic silver nanoparticles produced by Fusarium chlamydosporum and Penicillium chrysogenum at non-cytotoxic doses. Chemosphere 2019, 218, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Puškárová, A.; Bučková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci. Rep. 2017, 7, 8211. [Google Scholar] [CrossRef]
- Rodino, S.; Butu, M.; Petrache, P.; Butu, A.; Cornea, C.P. Antifungal activity of four plants against Alternaria alternata. Sci. Bull. Ser. F. Biotechnol. 2014, 18, 60–65. [Google Scholar]

| Similarity Index | Retention Time (min) | Compound Name | Associated Biological Activity | Natural Product Sources | Cartridge | References |
|---|---|---|---|---|---|---|
| 95 | 3.643 | Propanenitrile | antimicrobial | Brassica rapa | MCX; MAX and HLB | [49,50] |
| 91 | 3.6625 | Propargylamine | Transcription repressing | Alternaria alternata | MCX; MAX and HLB | [36,51] |
| 89 | 3.540 | Cyclobutane carbonitrile | Anti-inflammatory; anti-depressant; anti-cancer and antiviral | Agelas sceptrum (sceptines) of the sea sponge | MCX; MAX and HLB | [52,53] |
| 89 | 6.625 | 2-Butyne | antimicrobial | NR | MCX; MAX and HLB | [54] |
| 64 | 4.070 | Cyclotrisiloxane, hexamethyl | Antioxidant properties; antibacterial activity | P. chrysogenum; Extract of Acacia karoo; Olea europaea L. West Anatolian olive leaves. Red seaweed Kappapjycus alvarezii | MCX; MAX and HLB | [55,56,57,58] |
| 92 | 11.24 | 1,2-Benzenedicarboxylic acid, butyl 2-ethylhexyl ester | Antioxidant, fungitoxic, cytotoxic activity, and antimicrobial agent drug development for arthritis and cancer | P. chrysogenum; twigs of Thevetia peruviana and root of Plumbago zeylanica; Podophyllum hexandrum rhizome Alternaria alternata | MCX; MAX | [55,59,60,61,62] |
| 93 | Phthalic acid, butyl hexyl ester | antimicrobial | Boerhavia diffusa, Podophyllum hexandrum rhizome | MCX; MAX | [62,63] | |
| 58 | 12,106 | Benzo[h]quinoline, 2,4-dimethyl-; 2,4-Dimethylbenzo[h]quinoline | Antibacterial agent by inhibiting protein synthesis Antiprotozoal activity | Lasiocarpa americana Holm oak (Quercus ilex subsp. ballota (Desf.) Samp.) bark Penicillium pusillum | MCX; MAX and HLB | [64,65] |
| 79 | 11.24 | Benzeneethanamine, N-[(pentafluorophenyl)methylene]-.beta.,3,4-tris[(trimethylsilyl)oxy]-; N-(Pentafluorobenzylidene)-beta.,3,4-tris(trimethylsiloxy | Antimicrobial and antioxidant | Boerhavia diffusa | MCX; MAX and HLB | [63] |
| 79 | 16.33 | N-(Trifluoroacetyl)-O,O’,O’’-tris(trimethylsilyl)norepinephrine; N-(2-(3,4-Bis[(trimethylsilyl)oxy]phenyl)-2-[(trimethylsilyl)oxy]ethyl)-2,2,2-trifluoro | Schistosomicidal and molluscicidal activities | Extracts Juniperus horizontalis and uniperus communis L. | MCX; MAX | [66] |
| 58 | 9.24 | 2,5-di-tert-Butyl-1,4-benzoquinone; 2,5-di-tert-Butyl-p-quinone; 2,5-Cyclohexadiene-1,4-dione, 2,5-bis(1,1-dimethylethyl)-; p-Benzoquinone | Not identified | Phormidium tenue and Leptolyngbya | MCX; MAX | [49,50,67] |
| 61 | 13.499 | Coumarin, 3,4-dihydro-4,5,7-trimethyl-; 4,5,7-Trimethyl-2-chromanone | anti-leishmaniasis agents; bacteriostatic and anti-tumor activity; anti-HIV; antifungal; antidiabetic | Calophyllum lanigerum; Alternaria alternata | MAX | [68,69,70] |
| 66 | 24.10 | 2-Fluoro-5-trifluoromethylbenzoic acid, 3-hexadonic | antifungal | P. chrosgenum | HLB | [55,71] |
| 70 | 32.64 | L-Proline, N-valeryl-, heptadecyl ester | Antifungal against A. alternata | Bacillus siamensis, Bacillus amyloliquefaciens, and Bacillus nakamurai Bacillus safensis STJP | HLB | [72] |
| 45 | 13.76 | 3,5-Dimethoxycinnamic acid; 2-Propenoic acid, 3-(3,5-dimethoxyphenyl)-; Cinnamic acid, 3,5-dimethoxy-(coumaric acid derivates) | Cytotoxic effect to human pepatoma tumor cells | Wasebia japônica, Picrasma quassioides, Penicillium citreonigrum XT20-134, Sibiraea angustata, Verbesina gigantea | HLB | [49,50,73,74] |
| 77 | 18.18 | 1,2-Benzisothiazol-3-amine tbdms | Antioxidant and antimicrobial; antibacterial | Plants of Thevetia neriifolia Acacia karoo root extract | HLB | [56] |
| 76 | 11.24 | 1,4-Bis(trimethylsilyl)benzene | Antimicrobial | Acacia karoo root | HLB | [56] |
| 73 | 5.76 | Oxime-, methoxy-phenyl | Antidotes for nerve agents, reactivate acetylcholinesterase | Brassica juncea | HLB | [75] |
| 69 | 17.14 | 4-Ethylbenzoic acid, cyclopentyl ester | Not identified | Pichia membranifaciens Hmp-1 isolated from blackberry wine | HLB | [76] |
| 76 | 16.25 | 2′,6′-Dihydroxyacetophenone,bis(trimethylsilyl) ether | Antimicrobial and antioxidant activity | Camellia sinensis and Camellia assamica | HLB | [77,78] |
| 61 | 12.33 | 1,1,3,3,5,5,7,7-Octamethyl-7-(2-methylpropoxy) tetrasiloxan-1-ol | Not identified | Ziziphus jujuba mill. | HLB | [79] |
| 58 | 10.68 | Pentasiloxane, dodecamethyl; Dodecamethylpentasiloxane; 1,1,1,3,3,5,5,7,7,9,9,9-Dodecamethylpentasiloxane | Antimicrobial activity | Solanum nigrum | HLB | [80] |
| 75 | 11.24 | l-Leucine, N-cyclopropylcarbonyl-, heptadecyl ester | Antimicrobial activities | Bacillus species | HLB | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makhwitine, J.P.; Kumalo, H.M.; Ndlovu, S.I.; Mkhwanazi, N.P. Epigenetic Induction of Secondary Metabolites Production in Endophytic Fungi Penicillium chrysogenum and GC-MS Analysis of Crude Metabolites with Anti-HIV-1 Activity. Microorganisms 2023, 11, 1404. https://doi.org/10.3390/microorganisms11061404
Makhwitine JP, Kumalo HM, Ndlovu SI, Mkhwanazi NP. Epigenetic Induction of Secondary Metabolites Production in Endophytic Fungi Penicillium chrysogenum and GC-MS Analysis of Crude Metabolites with Anti-HIV-1 Activity. Microorganisms. 2023; 11(6):1404. https://doi.org/10.3390/microorganisms11061404
Chicago/Turabian StyleMakhwitine, John P., Hezekiel M. Kumalo, Sizwe I. Ndlovu, and Nompumelelo P. Mkhwanazi. 2023. "Epigenetic Induction of Secondary Metabolites Production in Endophytic Fungi Penicillium chrysogenum and GC-MS Analysis of Crude Metabolites with Anti-HIV-1 Activity" Microorganisms 11, no. 6: 1404. https://doi.org/10.3390/microorganisms11061404
APA StyleMakhwitine, J. P., Kumalo, H. M., Ndlovu, S. I., & Mkhwanazi, N. P. (2023). Epigenetic Induction of Secondary Metabolites Production in Endophytic Fungi Penicillium chrysogenum and GC-MS Analysis of Crude Metabolites with Anti-HIV-1 Activity. Microorganisms, 11(6), 1404. https://doi.org/10.3390/microorganisms11061404









