Effect of Probiotic Yogurt Supplementation(Bifidobacterium animalis ssp. lactis BB-12) on Gut Microbiota of Female Taekwondo Athletes and Its Relationship with Exercise-Related Psychological Fatigue
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
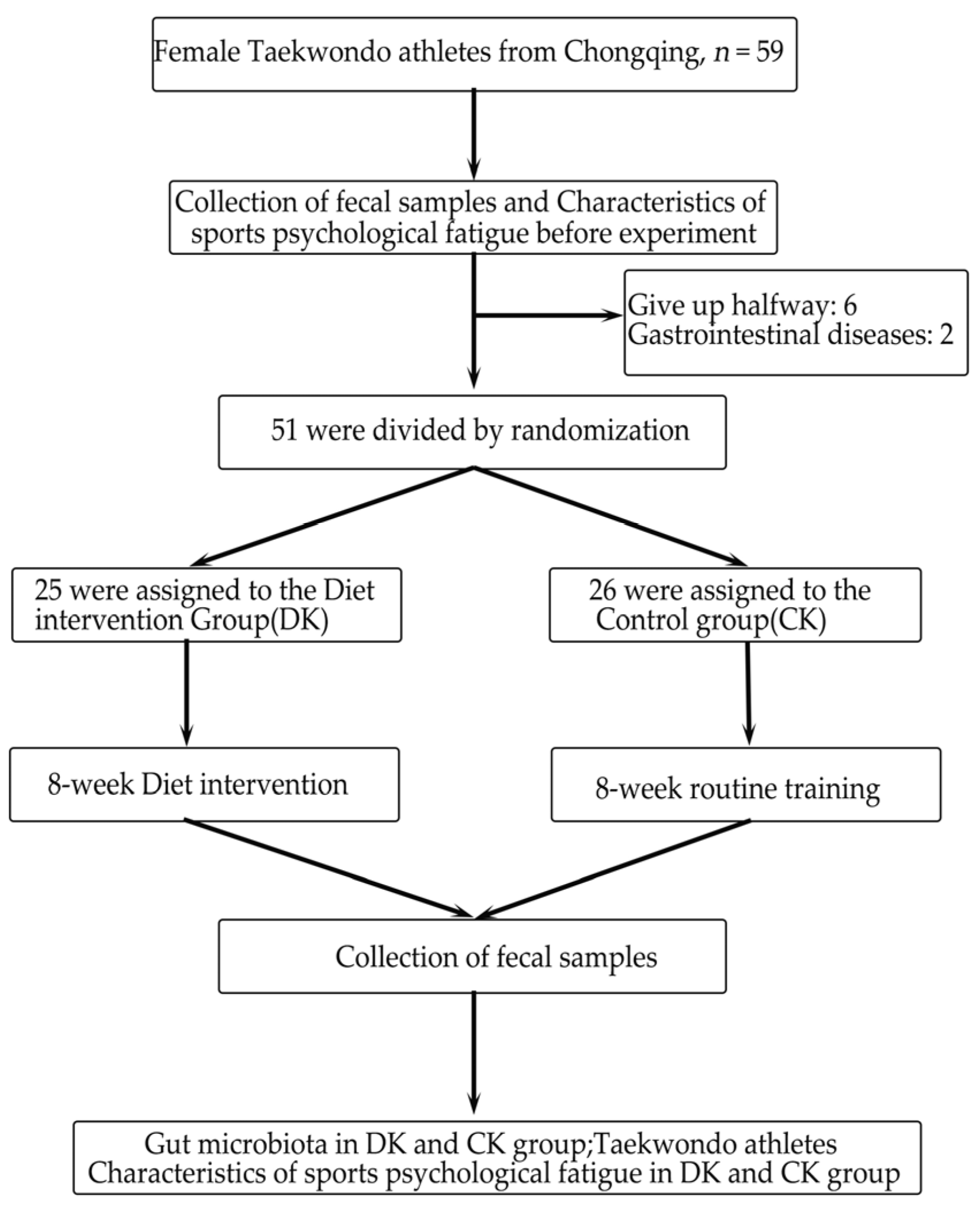
2.2. Experimental Design
2.3. Sample Collection
2.4. Yogurt
2.5. Athletes’ Mental Fatigue
2.6. Bioinformatics Analysis of the Gut Microbiota
2.7. Statistical Analysis
3. Results
3.1. Basic Characteristics of Taekwondo Athletes
3.2. Sports-Related Mental Fatigue Scores in Taekwondo Athletes
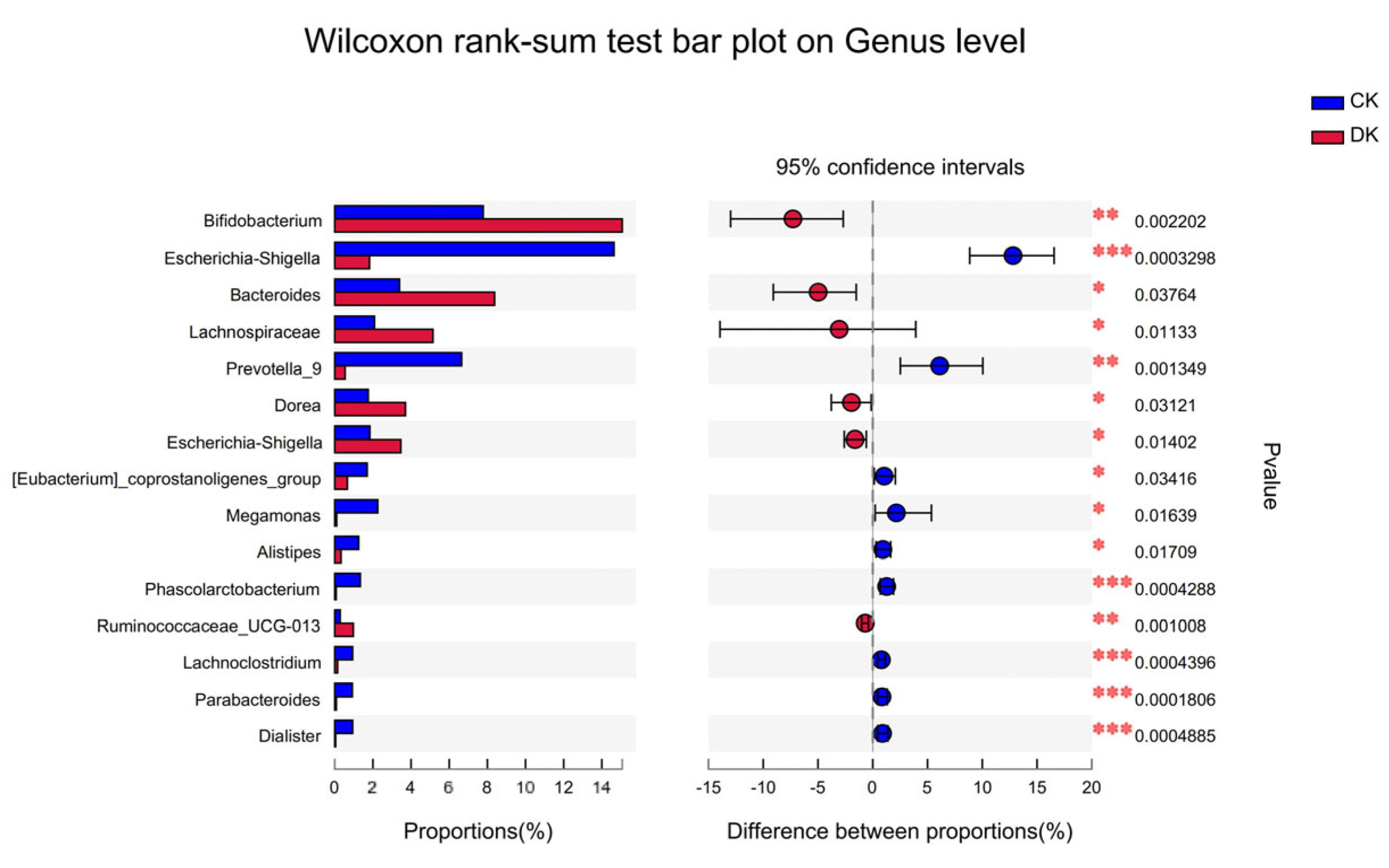
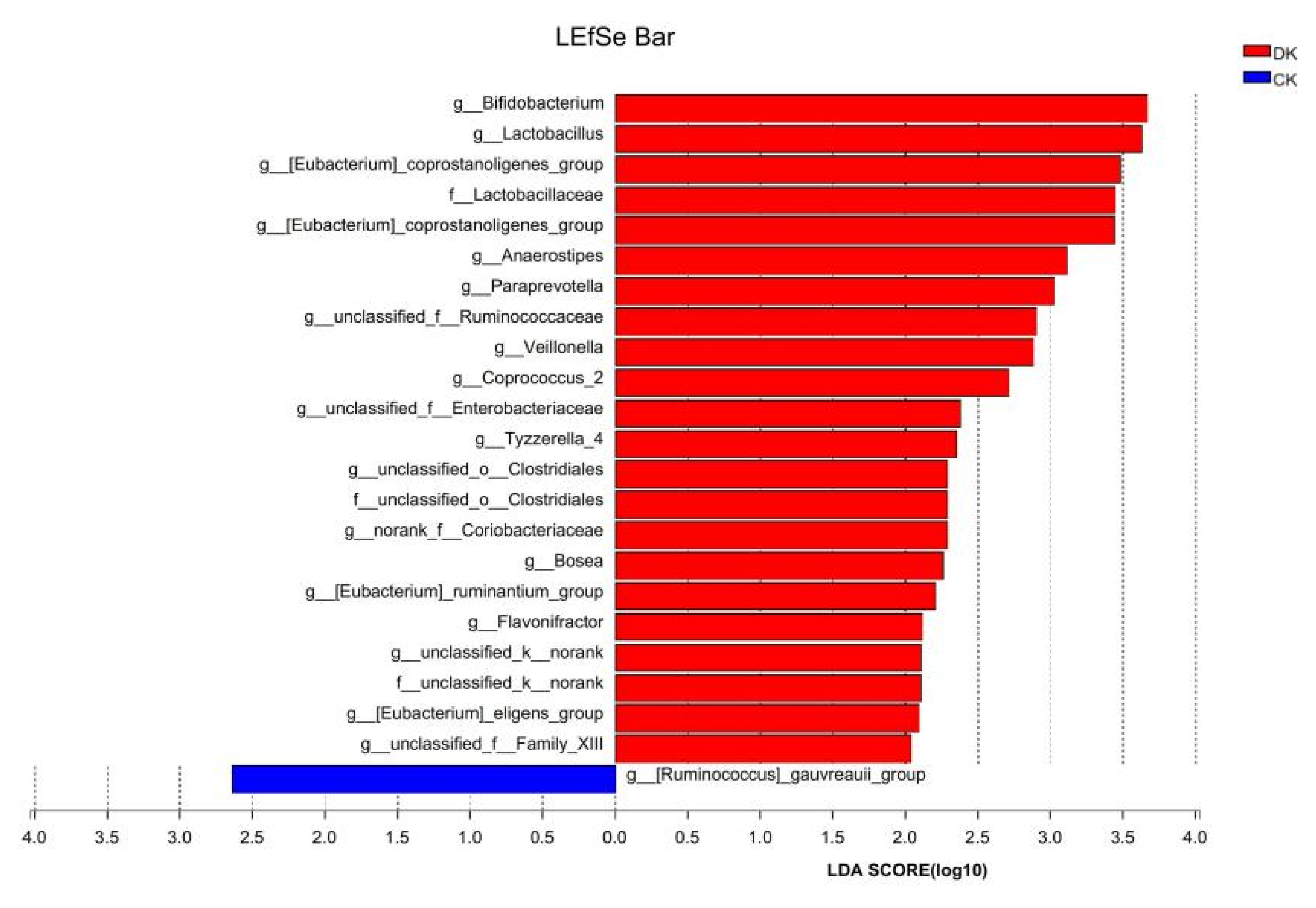
3.3. Differences in Intestinal Microbiota between the DK and CK Groups after Dietary Intervention
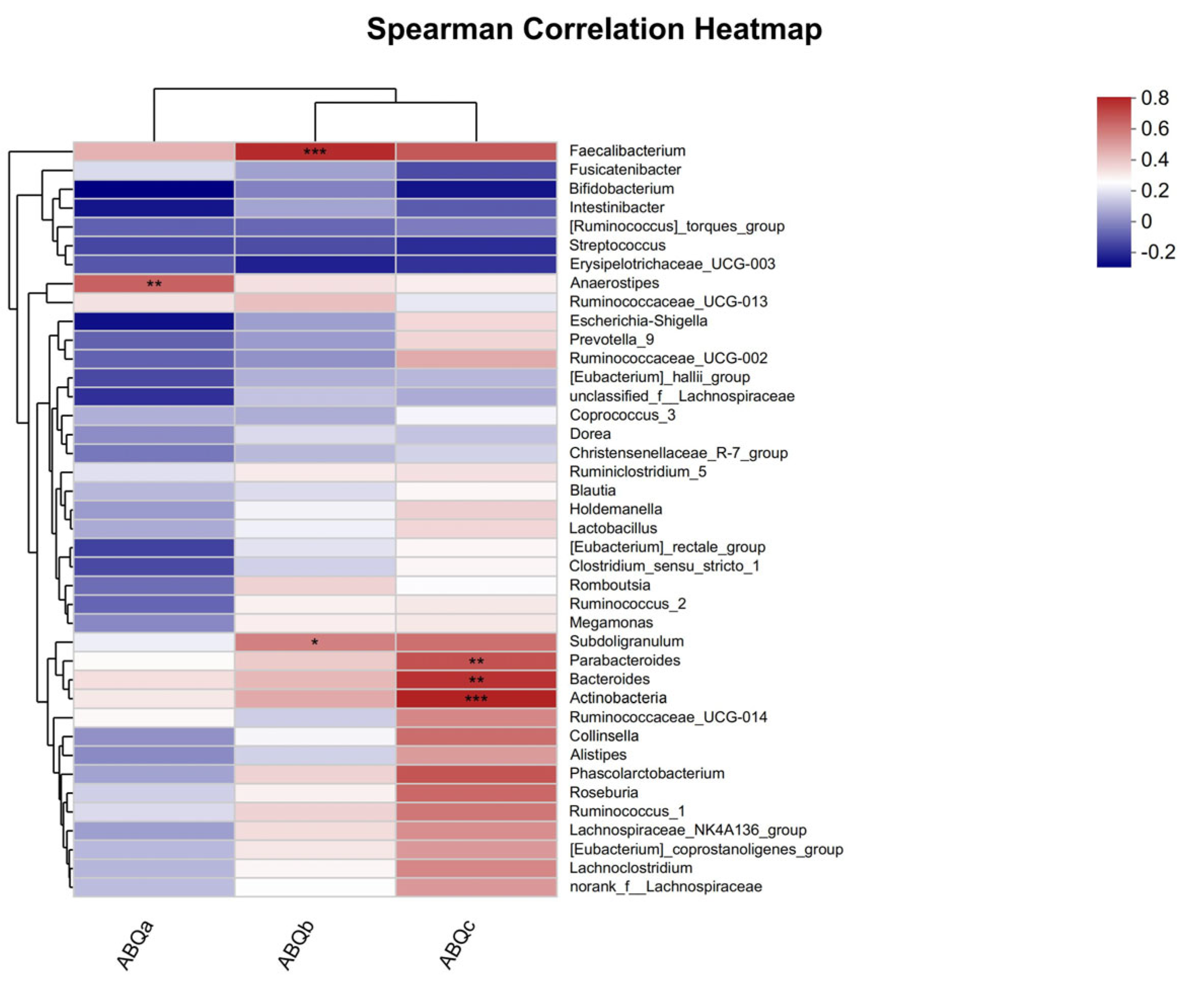
3.4. Relationship between Exercise Psychological Fatigue and Gut Microbiota after Dietary Intervention
3.5. Metabolic Pathways of the Gut Microbiota in the Dietary Intervention and Control Groups
4. Discussion
4.1. Effect of Bifidobacterium animalis ssp. lactis BB-12Supplementation on Motor Mental Fatigue Clearance in Athletes
4.2. Effect of Bifidobacterium animalis ssp. Lactis BB-12 Supplementation on the Intestinal Microflora of Athletes
4.3. The Relationship between Gut Microbiotaand Exercise Mental Fatigue
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, R.E. Toward a Cognitive-affective Model of Athletic Burnout. J. Sport Exerc. Psychol. 1986, 8, 36–50. [Google Scholar] [CrossRef]
- Penna, E.M.; Filho, E.; Wanner, S.P.; Campos, B.T.; Quinan, G.R.; Mendes, T.T.; Smith, M.R.; Prado, L.S. Mental Fatigue Impairs Physical Performance in Young Swimmers. Pediatr. Exerc. Sci. 2018, 30, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Soh, K.G.; Roslan, S.; Wazir, M.; Soh, K.L. Does mental fatigue affect skilled performance in athletes? A systematic review. PLoS ONE 2021, 16, e258307. [Google Scholar] [CrossRef] [PubMed]
- Habay, J.; Van Cutsem, J.; Verschueren, J.; De Bock, S.; Proost, M.; De Wachter, J.; Tassignon, B.; Meeusen, R.; Roelands, B. Mental Fatigue and Sport-Specific Psychomotor Performance: A Systematic Review. Sports Med. 2021, 51, 1527–1548. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.T.; Walsh, R.; Sheehan, A.E. Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials. Neurosci. Biobehav. Rev. 2019, 102, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.; Pourjafar, H.; Tabrizi, A.; Homayouni, A. The Effects of Probiotics and Prebiotics on Mental Disorders: A Review onDepression, Anxiety, Alzheimer, and Autism Spectrum Disorders. Curr. Pharm. Biotechnol. 2020, 21, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Morkl, S.; Butler, M.I.; Holl, A.; Cryan, J.F.; Dinan, T.G. Probiotics and the Microbiota-Gut-Brain Axis: Focus on Psychiatry. Curr. Nutr. Rep. 2020, 9, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Suganthy, N.; Kesika, P.; Chaiyasut, C. The Role of Microbiome, Dietary Supplements, and Probiotics in Autism SpectrumDisorder. Int. J. Environ. Res. Public. Health 2020, 17, 2647. [Google Scholar] [CrossRef] [PubMed]
- Roman, G.C.; Jackson, R.E.; Gadhia, R.; Roman, A.N.; Reis, J. Mediterranean diet: The role of long-chain omega-3 fatty acids in fish; polyphenolsin fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics andvitamins in prevention of stroke, age-related cognitive decline, and Alzheimerdisease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Salleh, R.M.; Kuan, G.; Aziz, M.; Rahim, M.; Rahayu, T.; Sulaiman, S.; Kusuma, D.; Adikari, A.; Razam, M.; Radhakrishnan, A.K.; et al. Effects of Probiotics on Anxiety, Stress, Mood and Fitness of Badminton Players. Nutrients 2021, 13, 1783. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: A systematic review for athletes. J. Int. Soc. Sports Nutr. 2016, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Carbuhn, A.F.; Reynolds, S.M.; Campbell, C.W.; Bradford, L.A.; Deckert, J.A.; Kreutzer, A.; Fry, A.C. Effects of Probiotic (Bifidobacterium longum 35624) Supplementation on ExercisePerformance, Immune Modulation, and Cognitive Outlook in Division I FemaleSwimmers. Sports 2018, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Marttinen, M.; Ala-Jaakkola, R.; Laitila, A.; Lehtinen, M.J. Gut Microbiota, Probiotics and Physical Performance in Athletes and Physically Active Individuals. Nutrients 2020, 12, 2936. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Wang, Y.; Liao, S.; Lai, M.; Peng, L.; Song, G. Reduction in the Choking Phenomenon in Elite Diving Athletes through Changes in Gut Microbiota Induced by Yogurt Containing Bifidobacterium animalis subsp. lactis BB-12: A Quasi Experimental Study. Microorganisms 2020, 8, 597. [Google Scholar] [CrossRef]
- Dong, W.; Wang, Y.; Liao, S.; Tang, W.; Peng, L.; Song, G. Bifidobacterium animalis subsp. lactis BB-12 Improves the State Anxiety and Sports Performance of Young Divers Under Stress Situations: A Single-Arm, Prospective Proof-of-Concept Study. Front. Psychol. 2020, 11, 570298. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Yang, X.; Yu, D.; Xue, L.; Li, H.; Du, J. Probiotics modulate the microbiota-gut-brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharm. Sin. B 2020, 10, 475–487. [Google Scholar] [CrossRef]
- Den, H.; Dong, X.; Chen, M.; Zou, Z. Efficacy of probiotics on cognition, and biomarkers of inflammation and oxidativestress in adults with Alzheimer’s disease or mild cognitive impairment—A meta-analysis of randomized controlled trials. Aging 2020, 12, 4010–4039. [Google Scholar] [CrossRef]
- Kim, C.S.; Cha, L.; Sim, M.; Jung, S.; Chun, W.Y.; Baik, H.W.; Shin, D.M. Probiotic Supplementation Improves Cognitive Function and Mood with Changes inGut Microbiota in Community-Dwelling Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 32–40. [Google Scholar] [CrossRef]
- Obrenovich, M.; Jaworski, H.; Tadimalla, T.; Mistry, A.; Sykes, L.; Perry, G.; Bonomo, R.A. The Role of the Microbiota-Gut-Brain Axis and Antibiotics in ALS and Neurodegenerative Diseases. Microorganisms 2020, 8, 784. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Bacque-Cazenave, J.; Bharatiya, R.; Barriere, G.; Delbecque, J.P.; Bouguiyoud, N.; Di Giovanni, G.; Cattaert, D.; De Deurwaerdere, P. Serotonin in Animal Cognition and Behavior. Int. J. Mol. Sci. 2020, 21, 1649. [Google Scholar] [CrossRef] [PubMed]
- Goehler, L.E.; Gaykema, R.P.; Opitz, N.; Reddaway, R.; Badr, N.; Lyte, M. Activation in vagal afferents and central autonomic pathways: Early responses tointestinal infection with Campylobacter jejuni. Brain Behav. Immun. 2005, 19, 334–344. [Google Scholar] [CrossRef] [PubMed]
- van Bodegom, M.; Homberg, J.R.; Henckens, M. Modulation of the HypothalamicPituitary-Adrenal Axis by Early Life Stress Exposure. Front. Cell. Neurosci. 2017, 87, 2017. [Google Scholar]
- Raedeke, T.D.; Smith, A.L. Development and Preliminary Validation of an Athlete Burnout Measur. J. Sport Exerc. Psychol. 2001, 23, 281–306. [Google Scholar] [CrossRef]
- Beckmann, J.; Elbe, A.M. Sport Psychological Interventions in Competitive Sports; Cambridge Scholars Publishing: Newcastle, UK, 2015. [Google Scholar]
- Meeusen, R.; Duclos, M.; Foster, C.; Fry, A.; Gleeson, M.; Nieman, D.; Raglin, J.; Rietjens, G.; Steinacker, J.; Urhausen, A. Prevention, diagnosis, and treatment of the overtraining syndrome: Joint consensus statement of the European College of Sport Science and the American College of Sports Medicine. Med. Sci. Sports Exerc. 2013, 45, 186–205. [Google Scholar] [CrossRef]
- Hu, B.; Ning, X. The influence of lumbar extensor muscle fatigue on lumbar-pelvic coordination during weightlifting. Ergonomics 2015, 58, 1424–1432. [Google Scholar] [CrossRef]
- Gould, D.; Tuffey, S.; Udry, E.; Loehr, J. Burnout in competitive junior tennis players: I. A quantitative psychological assessment. Sport Psychol. 1996, 4, 322–340. [Google Scholar] [CrossRef]
- Gould, D.; Tuffey, S.; Udry, E.; Loehr, J. Burnout in competitive junior tennis players: II. Qualitative analysis. Sport Psychol. 1996, 4, 341–366. [Google Scholar] [CrossRef]
- Komano, Y.; Shimada, K.; Naito, H.; Fukao, K.; Ishihara, Y.; Fujii, T.; Kokubo, T.; Daida, H. Efficacy of heat-killed Lactococcus lactis JCM 5805 on immunity and fatigueduring consecutive high intensity exercise in male athletes: A randomized, placebo-controlled, double-blinded trial. J. Int. Soc. Sports Nutr. 2018, 15, 39. [Google Scholar] [CrossRef]
- Ho, Y.T.; Tsai, Y.C.; Kuo, T.; Yang, C. Effects of Lactobacillus plantarum PS128 on Depressive Symptoms and Sleep Quality in Self-Reported Insomniacs: A Randomized, Double-Blind, Placebo-Controlled Pilot Trial. Nutrients 2021, 13, 2820. [Google Scholar] [CrossRef] [PubMed]
- Marotta, A.; Sarno, E.; Del, C.A.; Pane, M.; Mogna, L.; Amoruso, A.; Felis, G.E.; Fiorio, M. Effects of Probiotics on Cognitive Reactivity, Mood, and Sleep Quality. Front. Psychiatry 2019, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Braun, C.; Murphy, E.F.; Enck, P. Bifidobacterium longum 1714 Strain Modulates Brain Activity of Healthy Volunteers During Social Stress. Am. J. Gastroenterol. 2019, 114, 1152–1162. [Google Scholar] [CrossRef]
- Sanchez, B.; Delgado, S.; Blanco-Miguez, A.; Lourenco, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Wei, L.; Chiu, Y.S.; Hsu, Y.J.; Tsai, T.Y.; Wang, M.F.; Huang, C.C. Lactobacillus plantarum TWK10 Supplementation Improves Exercise Performance and Increases Muscle Mass in Mice. Nutrients 2016, 8, 205. [Google Scholar] [CrossRef]
- Mazani, M.; Nemati, A.; Amani, M.; Haedari, K.; Mogadam, R.A.; Baghi, A.N. The effect of probiotic yoghurt consumption on oxidative stress and inflammatoryfactors in young females after exhaustive exercise. J. Pak. Med. Assoc. 2018, 68, 1748–1754. [Google Scholar]
- Huang, W.C.; Wei, C.C.; Huang, C.C.; Chen, W.L.; Huang, H.Y. The Beneficial Effects of Lactobacillus plantarum PS128 on High-Intensity, Exercise-Induced Oxidative Stress, Inflammation, and Performance in Triathletes. Nutrients 2019, 11, 353. [Google Scholar] [CrossRef]
- Abate, K.H. Gender disparity in prevalence of depression among patient population: A systematic review. Ethiop. J. Health Sci. 2013, 23, 283–288. [Google Scholar] [CrossRef]
- Laws, K.R.; Irvine, K.; Gale, T.M. Sex differences in Alzheimer’s disease. Curr. Opin. Psychiatry 2018, 31, 133–139. [Google Scholar] [CrossRef] [PubMed]
- McLean, C.P.; Asnaani, A.; Litz, B.T.; Hofmann, S.G. Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res. 2011, 45, 1027–1035. [Google Scholar] [CrossRef]
- Seney, M.L.; Sibille, E. Sex differences in mood disorders: Perspectives from humans and rodent models. Biol. Sex. Differ. 2014, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Ganci, M.; Suleyman, E.; Butt, H.; Ball, M. Associations between self-reported psychological symptom severity and gut microbiota: Further support for the microgenderome. BMC Psychiatry 2022, 22, 307. [Google Scholar] [CrossRef] [PubMed]
- Verhaar, B.; Hendriksen, H.; de Leeuw, F.A.; Doorduijn, A.S.; van Leeuwenstijn, M.; Teunissen, C.E.; Barkhof, F.; Scheltens, P.; Kraaij, R.; van Duijn, C.M.; et al. Gut Microbiota Composition Is Related to AD Pathology. Front. Immunol. 2021, 12, 794519. [Google Scholar] [CrossRef] [PubMed]
- Bruning, J.; Chapp, A.; Kaurala, G.A.; Wang, R.; Techtmann, S.; Chen, Q.H. Gut Microbiota and Short Chain Fatty Acids: Influence on the Autonomic Nervous System. Neurosci. Bull. 2020, 36, 91–95. [Google Scholar] [CrossRef]
- Yang, L.L.; Millischer, V.; Rodin, S.; MacFabe, D.F.; Villaescusa, J.C.; Lavebratt, C. Enteric short-chain fatty acids promote proliferation of human neural progenitor cells. J. Neurochem. 2020, 154, 635–646. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.R.; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef]
- Huang, Y.; Shen, Z.; He, W. Identification of Gut Microbiome Signatures in Patients with Post-stroke Cognitive Impairment and Affective Disorder. Front. Aging Neurosci. 2021, 13, 706765. [Google Scholar] [CrossRef]
- Qian, Y.; Yang, X.; Xu, S.; Wu, C.; Song, Y.; Qin, N.; Chen, S.D.; Xiao, Q. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav. Immun. 2018, 70, 194–202. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Sikaroodi, M.; Fagan, A.; Heuman, D.; Gilles, H.; Gavis, E.A.; Fuchs, M.; Gonzalez-Maeso, J.; Nizam, S.; Gillevet, P.M.; et al. Posttraumatic stress disorder is associated with altered gut microbiota that modulates cognitive performance in veterans with cirrhosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G661–G669. [Google Scholar] [CrossRef]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef]
- Wang, H.X.; Wang, Y.P. Gut Microbiota-brain Axis. Chin. Med. J. 2016, 129, 2373–2380. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.; Hu, X.; Wang, T.; Jin, F. Recognizing Depression from the Microbiota(-)Gut(-)Brain Axis. Int. J. Mol. Sci. 2018, 19, 1592. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.A.; Diaz-Arteche, C.; Eliby, D.; Schwartz, O.S.; Simmons, J.G.; Cowan, C. The gut microbiota in anxiety and depression—A systematic review. Clin. Psychol. Rev. 2021, 83, 101943. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.D.; Moazzami, K.; Wittbrodt, M.T.; Nye, J.A.; Lima, B.B.; Gillespie, C.F.; Rapaport, M.H.; Pearce, B.D.; Shah, A.J.; Vaccarino, V. Diet, Stress and Mental Health. Nutrients 2020, 12, 2428. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Su, Q.; Xie, B.; Duan, L.; Zhao, W.; Hu, D.; Wu, R.; Liu, H. Gut microbes in correlation with mood: Case study in a closed experimental human life support system. Neurogastroenterol. Motil. 2016, 28, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Barandouzi, Z.A.; Starkweather, A.R.; Henderson, W.A.; Gyamfi, A.; Cong, X.S. Altered Composition of Gut Microbiota in Depression: A Systematic Review. Front. Psychiatry 2020, 11, 541. [Google Scholar] [CrossRef]
- Gomez-Nguyen, A.; Basson, A.R.; Dark-Fleury, L.; Hsu, K.; Osme, A.; Menghini, P.; Pizarro, T.T.; Cominelli, F. Parabacteroides distasonis induces depressive-like behavior in a mouse model of Crohn’s disease. Brain Behav. Immun. 2021, 98, 245–250. [Google Scholar] [CrossRef]
- Zhuang, Z.Q.; Shen, L.L.; Li, W.W.; Fu, X.; Zeng, F.; Gui, L.; Lu, Y.; Cai, M.; Zhu, C.; Tan, Y.L.; et al. Gut Microbiota is Altered in Patients with Alzheimer’s Disease. J. Alzheimers Dis. 2018, 63, 1337–1346. [Google Scholar] [CrossRef]
- Tartar, J.L.; Kalman, D.; Hewlings, S. A Prospective Study Evaluating the Effects of a Nutritional Supplement Intervention on Cognition, Mood States, and Mental Performance in Video Gamers. Nutrients 2019, 11, 2326. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Konishi, T.; Nakamura, Y. L-Arginine Exerts Excellent Anti-Stress Effects on Stress-Induced Shortened Lifespan, Cognitive Decline and Depression. Int. J. Mol. Sci. 2021, 22, 508. [Google Scholar] [CrossRef]
- Xiao, C.; Fedirko, V.; Beitler, J.; Bai, J.; Peng, G.; Zhou, C.; Gu, J.; Zhao, H.; Lin, I.H.; Chico, C.E.; et al. The role of the gut microbiome in cancer-related fatigue: Pilot study on epigenetic mechanisms. Support. Care Cancer 2021, 29, 3173–3182. [Google Scholar] [CrossRef] [PubMed]
- Solis-Ortiz, S.; Arriaga-Avila, V.; Trejo-Bahena, A.; Guevara-Guzman, R. Deficiency in the Essential Amino Acids l-Isoleucine, l-Leucine and l-Histidine and Clinical Measures as Predictors of Moderate Depression in Elderly Women: A Discriminant Analysis Study. Nutrients 2021, 13, 3875. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia anddepression. Nat. Rev. Neurosci. 2016, 17, 524–532. [Google Scholar] [CrossRef] [PubMed]


| ± S) | CK | DK | P Sig. (Two-Tail) |
|---|---|---|---|
| Age (year) | 22.10 ± 0.88 | 22.50 ± 0.83 | 0.412 |
| Training years (year) | 4.82 ± 0.52 | 5.02 ± 0.64 | 0.660 |
| Sports level (grade) | 2.05 ± 0.36 | 2.14 ± 0.28 | 0.538 |
| Food intake rate (%) | 98.2 ± 6.12 | 97.8 ± 4.29 | 0.410 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Zhu, Y.; Song, G. Effect of Probiotic Yogurt Supplementation(Bifidobacterium animalis ssp. lactis BB-12) on Gut Microbiota of Female Taekwondo Athletes and Its Relationship with Exercise-Related Psychological Fatigue. Microorganisms 2023, 11, 1403. https://doi.org/10.3390/microorganisms11061403
Zhu J, Zhu Y, Song G. Effect of Probiotic Yogurt Supplementation(Bifidobacterium animalis ssp. lactis BB-12) on Gut Microbiota of Female Taekwondo Athletes and Its Relationship with Exercise-Related Psychological Fatigue. Microorganisms. 2023; 11(6):1403. https://doi.org/10.3390/microorganisms11061403
Chicago/Turabian StyleZhu, Jiang, Yuping Zhu, and Gang Song. 2023. "Effect of Probiotic Yogurt Supplementation(Bifidobacterium animalis ssp. lactis BB-12) on Gut Microbiota of Female Taekwondo Athletes and Its Relationship with Exercise-Related Psychological Fatigue" Microorganisms 11, no. 6: 1403. https://doi.org/10.3390/microorganisms11061403
APA StyleZhu, J., Zhu, Y., & Song, G. (2023). Effect of Probiotic Yogurt Supplementation(Bifidobacterium animalis ssp. lactis BB-12) on Gut Microbiota of Female Taekwondo Athletes and Its Relationship with Exercise-Related Psychological Fatigue. Microorganisms, 11(6), 1403. https://doi.org/10.3390/microorganisms11061403






