The Survival of Psychobiotics in Fermented Food and the Gastrointestinal Tract: A Review
Abstract
1. Introduction
2. Characteristics of Psychobiotics and Their Impact on Human Health
3. Psychobiotics in Fermented Food
3.1. Food Fermentation
3.2. Effect of Fermented Foods on the Gut–Brain Axis and Brain Health
3.2.1. Animal Studies
3.2.2. Human Studies
4. Survival of Psychobiotics in Fermented Food
4.1. Survival of Psychobiotics in Dairy Products
4.2. Survival of Psychobiotics in Plant Products
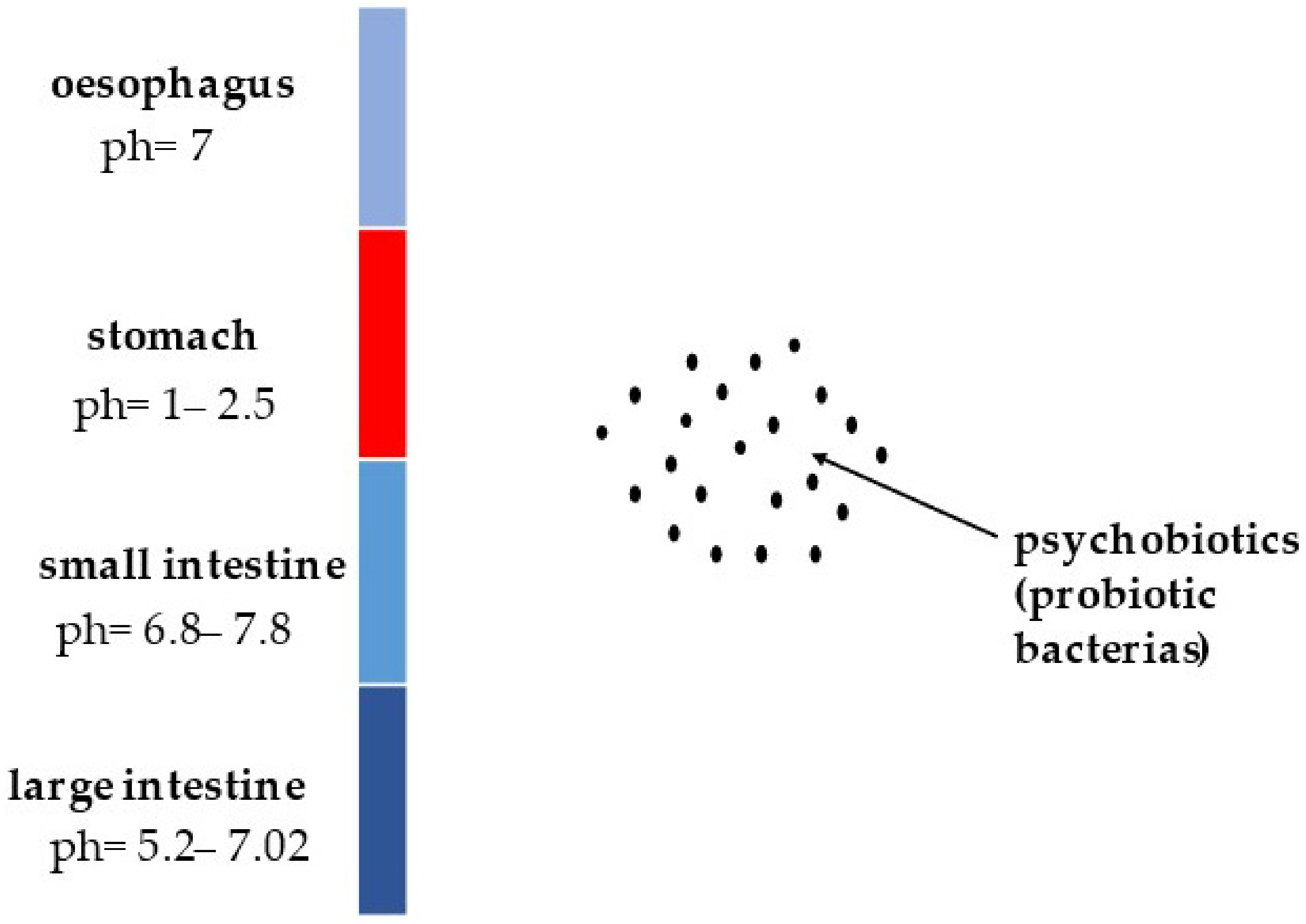
5. Survival of Psychobiotics in Human Gastrointestinal Tract
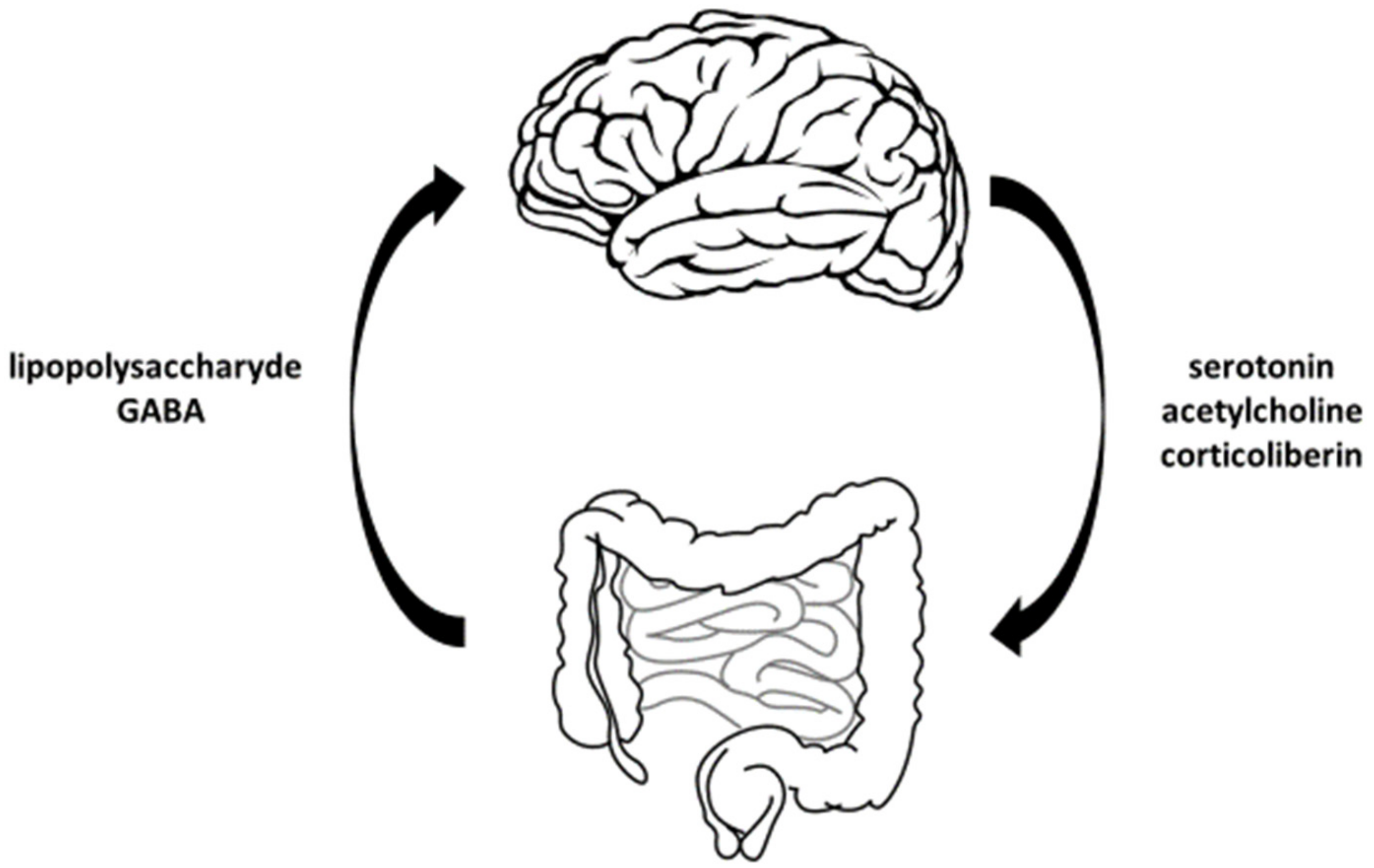
- lowers the concentration of cortisol in the blood,
- relieves stress,
- reduces anxiety, and
- soothes the symptoms of irritable bowel syndrome.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zagórska, A.; Marcinkowska, M.; Jamrozik, M.; Wiśniowska, B.; Paśko, P. From probiotics to psychobiotics—The gut-brain axis in psychiatric disorders. Benef. Microbes 2020, 11, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Bollwahn, W.; Bahr, K.H.; Hazem, A.S.; Amtsberg, G.; Schmidt, U. Investigations into the aetiology of moist eczema in pigs. DTW 1970, 77, 601–603. [Google Scholar]
- Food and Agriculture Organization of the United Nations and World Health Organization. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food; Food and Agriculture Organization of the United Nations: Rome, Italy; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Dinan, T.G.; Quigley, E.M. Probiotics in the Treatment of Depression: Science or Science Fiction? Aust. N.Z.J. Psychiatry 2011, 45, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- Zhou, L.; Foster, J. Psychobiotics and the gut–brain axis: In the pursuit of happiness. Neuropsychiatr. Dis. Treat. 2015, 11, 715–723. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W. Psychobiotics and the Manipulation of Bacteria–Gut–Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Martinez, T.M.; Meyer, R.K.; Duca, F.A. Therapeutic Potential of Various Plant-Based Fibers to Improve Energy Homeostasis via the Gut Microbiota. Nutrients 2021, 13, 3470. [Google Scholar] [CrossRef]
- Hamaker, B.R.; Tuncil, Y.E. A Perspective on the Complexity of Dietary Fiber Structures and Their Potential Effect on the Gut Microbiota. J. Mol. Biol. 2014, 426, 3838–3850. [Google Scholar] [CrossRef]
- Das, P.; Babaei, P.; Nielsen, J. Metagenomic analysis of microbe-mediated vitamin metabolism in the human gut microbiome. BMC Genom. 2019, 20, 208. [Google Scholar] [CrossRef]
- Wang, Z.-K.; Yang, Y.-S. Upper gastrointestinal microbiota and digestive diseases. World J. Gastroenterol. 2013, 19, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.J.; Guinane, C.M.; O’Toole, P.W.; Cotter, P.D. Beneficial modulation of the gut microbiota. FEBS Lett. 2014, 588, 4120–4130. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Gupta, D.; Mehrotra, R.; Mago, P. Psychobiotics: The Next-Generation Probiotics for the Brain. Curr. Microbiol. 2021, 78, 449–463. [Google Scholar] [CrossRef]
- Mossad, O.; Batut, B.; Yilmaz, B.; Dokalis, N.; Mezö, C.; Nent, E.; Nabavi, L.S.; Mayer, M.; Maron, F.J.M.; Buescher, J.M.; et al. Gut microbiota drives age-related oxidative stress and mitochondrial damage in microglia via the metabolite N6-carboxymethyllysine. Nat. Neurosci. 2022, 25, 295–305. [Google Scholar] [CrossRef]
- Chen, C.; Liao, J.; Xia, Y.; Liu, X.; Jones, R.; Haran, J.; McCormick, B.; Sampson, T.R.; Alam, A.; Ye, K. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut 2022, 71, 2233–2252. [Google Scholar] [CrossRef]
- Hwang, Y.-H.; Park, S.; Paik, J.-W.; Chae, S.-W.; Kim, D.-H.; Jeong, D.-G.; Ha, E.; Kim, M.; Hong, G.; Park, S.-H.; et al. Efficacy and Safety of Lactobacillus Plantarum C29-Fermented Soybean (DW2009) in Individuals with Mild Cognitive Impairment: A 12-Week, Multi-Center, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2019, 11, 305. [Google Scholar] [CrossRef]
- Chong, H.X.; Yusoff, N.A.A.; Hor, Y.Y.; Lew, L.C.; Jaafar, M.H.; Choi, S.-B.; Yusoff, M.S.B.; Wahid, N.; Abdullah, M.F.I.L.; Zakaria, N.; et al. Lactobacillus plantarum DR7 alleviates stress and anxiety in adults: A randomised, double-blind, placebo-controlled study. Benef. Microbes 2019, 10, 355–373. [Google Scholar] [CrossRef]
- Lew, L.-C.; Hor, Y.-Y.; Yusoff, N.A.A.; Choi, S.-B.; Yusoff, M.S.B.; Roslan, N.S.; Ahmad, A.; Mohammad, J.A.M.; Abdullah, M.F.I.L.; Zakaria, N.; et al. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: A randomised, double-blind, placebo-controlled study. Clin. Nutr. 2019, 38, 2053–2064. [Google Scholar] [CrossRef]
- Ma, T.; Jin, H.; Kwok, L.-Y.; Sun, Z.; Liong, M.-T.; Zhang, H. Probiotic consumption relieved human stress and anxiety symptoms possibly via modulating the neuroactive potential of the gut microbiota. Neurobiol. Stress 2021, 14, 100294. [Google Scholar] [CrossRef]
- Liao, J.-F.; Cheng, Y.-F.; You, S.-T.; Kuo, W.-C.; Huang, C.-W.; Chiou, J.-J.; Hsu, C.-C.; Hsieh-Li, H.-M.; Wang, S.; Tsai, Y.-C. Lactobacillus plantarum PS128 alleviates neurodegenerative progression in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse models of Parkinson’s disease. Brain, Behav. Immun. 2020, 90, 26–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-W.; Liong, M.T.; Chung, Y.-C.E.; Huang, H.-Y.; Peng, W.-S.; Cheng, Y.-F.; Lin, Y.-S.; Wu, Y.-Y.; Tsai, Y.-C. Effects of Lactobacillus plantarum PS128 on Children with Autism Spectrum Disorder in Taiwan: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 820. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-I.; Wu, C.-C.; Tsai, P.-J.; Cheng, L.-H.; Hsu, C.-C.; Shan, I.-K.; Chan, P.-Y.; Lin, T.-W.; Ko, C.-J.; Chen, W.-L.; et al. Psychobiotic Supplementation of PS128TM Improves Stress, Anxiety, and Insomnia in Highly Stressed Information Technology Specialists: A Pilot Study. Front. Nutr. 2021, 8, 614105. [Google Scholar] [CrossRef]
- Ho, Y.-T.; Tsai, Y.-C.; Kuo, T.B.; Yang, C.C.H. Effects of Lactobacillus plantarum PS128 on Depressive Symptoms and Sleep Quality in Self-Reported Insomniacs: A Randomized, Double-Blind, Placebo-Controlled Pilot Trial. Nutrients 2021, 13, 2820. [Google Scholar] [CrossRef] [PubMed]
- Shamsipour, S.; Sharifi, G.; Taghian, F. An 8-Week Administration of Bifidobacterium bifidum and Lactobacillus plantarum Combined with Exercise Training Alleviates Neurotoxicity of Aβ and Spatial Learning via Acetylcholine in Alzheimer Rat Model. J. Mol. Neurosci. 2021, 71, 1495–1505. [Google Scholar] [CrossRef]
- Morsheedi, M.; Valenlia, K.B.; Saghafi-Asl, M.; Hadi, S.; Hadi, V.; Mirghazanfari, S.M.; Askari, G. Can psychobiotics administration influence behavioral responses and physiological stress in healthy rats? Pharm. Sci. 2022, 28, 541–551. [Google Scholar] [CrossRef]
- Andersson, H.; Tullberg, C.; Ahrné, S.; Hamberg, K.; Ahrén, I.L.; Molin, G.; Sonesson, M.; Håkansson, A. Oral Administration of Lactobacillus plantarum 299v Reduces Cortisol Levels in Human Saliva during Examination Induced Stress: A Randomized, Double-Blind Controlled Trial. Int. J. Microbiol. 2016, 2016, 8469018. [Google Scholar] [CrossRef]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef]
- He, B.; Hoang, T.K.; Tian, X.; Taylor, C.M.; Blanchard, E.; Luo, M.; Bhattacharjee, M.B.; Freeborn, J.; Park, S.; Couturier, J.; et al. Lactobacillus reuteri Reduces the Severity of Experimental Autoimmune Encephalomyelitis in Mice by Modulating Gut Microbiota. Front. Immunol. 2019, 10, 385. [Google Scholar] [CrossRef]
- Kong, Q.; Wang, B.; Tian, P.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Daily intake of Lactobacillus alleviates autistic-like behaviors by ameliorating the 5-hydroxytryptamine metabolic disorder in VPA-treated rats during weaning and sexual maturation. Food Funct. 2021, 12, 2591–2604. [Google Scholar] [CrossRef]
- Liang, S.; Wang, T.; Hu, X.; Luo, J.; Li, W.; Wu, X.; Duan, Y.; Jin, F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 2015, 310, 561–577. [Google Scholar] [CrossRef]
- Luo, J.; Wang, T.; Liang, S.; Hu, X.; Li, W.; Jin, F. Ingestion of Lactobacillus strain reduces anxiety and improves cognitive function in the hyperammonemia rat. Sci. China Life Sci. 2014, 57, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Chudzik, A.; Słowik, T.; Kochalska, K.; Pankowska, A.; Łazorczyk, A.; Andres-Mach, M.; Rola, R.; Stanisz, G.J.; Orzyłowska, A. Continuous Ingestion of Lacticaseibacillus rhamnosus JB-1 during Chronic Stress Ensures Neurometabolic and Behavioural Stability in Rats. Int. J. Mol. Sci. 2022, 23, 5173. [Google Scholar] [CrossRef] [PubMed]
- Kantak, P.A.; Bobrow, D.N.; Nyby, J.G. Obsessive–compulsive-like behaviors in house mice are attenuated by a probiotic (Lactobacillus rhamnosus GG). Behav. Pharmacol. 2014, 25, 71–79. [Google Scholar] [CrossRef]
- Sanborn, V.; Azcarate-Peril, M.A.; Updegraff, J.; Manderino, L.; Gunstad, J. Randomized Clinical Trial Examining the Impact of Lactobacillus rhamnosus GG Probiotic Supplementation on Cognitive Functioning in Middle-aged and Older Adults. Neuropsychiatr. Dis. Treat. 2020, 16, 2765–2777. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Jin, G.; Pang, X.; Mo, Q.; Bao, J.; Liu, T.; Wu, J.; Xie, R.; Liu, X.; Liu, J.; et al. Lactobacillus rhamnosus GG colonization in early life regulates gut-brain axis and relieves anxiety-like behavior in adulthood. Pharmacol. Res. 2022, 177, 106090. [Google Scholar] [CrossRef]
- Kumperscak, H.G.; Gricar, A.; Ülen, I.; Micetic-Turk, D. A Pilot Randomized Control Trial with the Probiotic Strain Lactobacillus rhamnosus GG (LGG) in ADHD: Children and Adolescents Report Better Health-Related Quality of Life. Front. Psychiatry 2020, 11, 181. [Google Scholar] [CrossRef]
- Sawada, D.; Kuwano, Y.; Tanaka, H.; Hara, S.; Uchiyama, Y.; Sugawara, T.; Fujiwara, S.; Rokutan, K.; Nishida, K. Daily intake of Lactobacillus gasseri CP2305 relieves fatigue and stress-related symptoms in male university Ekiden runners: A double-blind, randomized, and placebo-controlled clinical trial. J. Funct. Foods 2019, 57, 465–476. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Rokutan, K. Health Benefits of Lactobacillus gasseri CP2305 Tablets in Young Adults Exposed to Chronic Stress: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 1859. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Sugawara, T.; Aoki, Y.; Fujiwara, S.; Rokutan, K. Daily administration of paraprobiotic Lactobacillus gasseri CP2305 ameliorates chronic stress-associated symptoms in Japanese medical students. J. Funct. Foods 2017, 36, 112–121. [Google Scholar] [CrossRef]
- Adikari, A.M.; Appukutty, M.; Kuan, G. Effects of Daily Probiotics Supplementation on Anxiety Induced Physiological Parameters among Competitive Football Players. Nutrients 2020, 12, 1920. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Nishida, K.; Kataoka-Kato, A.; Gondo, Y.; Ishikawa, H.; Suda, K.; Kawai, M.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut–brain interaction in human and animal models. Neurogastroenterol. Motil. 2016, 28, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Otaka, M.; Kikuchi-Hayakawa, H.; Ogura, J.; Ishikawa, H.; Yomogida, Y.; Ota, M.; Hidese, S.; Ishida, I.; Aida, M.; Matsuda, K.; et al. Effect of Lacticaseibacillus paracasei Strain Shirota on Improvement in Depressive Symptoms, and Its Association with Abundance of Actinobacteria in Gut Microbiota. Microorganisms 2021, 9, 1026. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Sugahara, H.; Shimada, K.; Mitsuyama, E.; Kuhara, T.; Yasuoka, A.; Kondo, T.; Abe, K.; Xiao, J.-Z. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci. Rep. 2017, 7, 135. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kinoshita, T.; Matsumoto, A.; Yoshino, K.; Saito, I.; Xiao, J.Z. Bifidobacterium breve A1 supplementation improved cognitive decline in older adults with mild cognitive impairment: An open-label, single-arm study. J. Prev. Alzheimers Dis. 2019, 6, 1–75. [Google Scholar] [CrossRef]
- Okubo, R.; Koga, M.; Katsumata, N.; Odamaki, T.; Matsuyama, S.; Oka, M.; Narita, H.; Hashimoto, N.; Kusumi, I.; Xiao, J.; et al. Effect of Bifidobacterium breve A-1 on anxiety and depressive symptoms in schizophrenia: A proof-of-concept study. J. Affect. Disord. 2019, 245, 377–385. [Google Scholar] [CrossRef]
- Tian, P.; O’Riordan, K.J.; Lee, Y.-K.; Wang, G.; Zhao, J.; Zhang, H.; Cryan, J.F.; Chen, W. Towards a psychobiotic therapy for depression: Bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol. Stress 2020, 12, 100216. [Google Scholar] [CrossRef]
- Tian, P.; Thomaz, F.; Bastiaanssen, S.; Song, L.; Jiang, B.; Zhang, X.; Zhao, J.; Zhang, H.; Chen, W.; Cryan, J.F.; et al. Unraveling the Microbial Mechanisms Underlying the Psychobiotic Potential of a Bifidobacterium breve Strain. Mol. Nutr. Food Res. 2021, 65, e2000704. [Google Scholar] [CrossRef]
- Zhu, G.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Administration of Bifidobacterium breve Improves the Brain Function of Aβ1-42-Treated Mice via the Modulation of the Gut Microbiome. Nutrients 2021, 13, 1602. [Google Scholar] [CrossRef]
- Zhu, H.; Tian, P.; Qian, X.; Gu, L.; Zhao, J.; Wang, G.; Chen, W. Perinatal transmission of a probiotic Bifidobacterium strain protects against early life stress-induced mood and gastrointestinal motility disorders. Food Funct. 2022, 13, 7520–7528. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.-P.; Cominetti, O.; Welsh, C.; Rieder, A.; et al. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients with Irritable Bowel Syndrome. Gastroenterology 2017, 153, 448–459.e8. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.P.; Hutch, W.; Borre, Y.E.; Kennedy, P.J.; Temko, A.; Boylan, G.; Murphy, E.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Bifidobacterium longum 1714 as a translational psychobiotic: Modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry 2016, 6, 939. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Braun, C.; Murphy, E.F.; Enck, P. Bifidobacterium longum 1714™ Strain Modulates Brain Activity of Healthy Volunteers During Social Stress. Am. J. Gastroenterol. 2019, 114, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Nagabhushanam, K.; Arumugam, S.; Majeed, S.; Ali, F. Bacillus coagulans MTCC 5856 for the management of major depression with irritable bowel syndrome: A randomised, double-blind, placebo controlled, multi-centre, pilot clinical study. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Miyaoka, T.; Kanayama, M.; Wake, R.; Hashioka, S.; Hayashida, M.; Nagahama, M.; Okazaki, S.; Yamashita, S.; Miura, S.; Miki, H.; et al. Clostridium butyricum MIYAIRI 588 as Adjunctive Therapy for Treatment-Resistant Major Depressive Disorder: A Prospective Open-Label Trial. Clin. Neuropharmacol. 2018, 41, 151–155. [Google Scholar] [CrossRef]
- Dinan, T.G.; Butler, M.I.; Cryan, J.F. Psychobiotics: Evolution of Novel Antidepressants. Mod. Trends Psychiatry 2021, 32, 134–143. [Google Scholar] [CrossRef]
- Chang, L.; Wei, Y.; Hashimoto, K. Brain–gut–microbiota axis in depression: A historical overview and future directions. Brain Res. Bull. 2022, 182, 44–56. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Na, K.-S.; Myint, A.-M.; Leonard, B.E. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 277–284. [Google Scholar] [CrossRef]
- You, Z.; Luo, C.; Zhang, W.; Chen, Y.; He, J.; Zhao, Q.; Zuo, R.; Wu, Y. Pro- and anti-inflammatory cytokines expression in rat’s brain and spleen exposed to chronic mild stress: Involvement in depression. Behav. Brain Res. 2011, 225, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Hayley, S.; Scharf, J.; Anisman, H. Central administration of murine interferon-α induces depressive-like behavioral, brain cytokine and neurochemical alterations in mice: A mini-review and original experiments. Brain, Behav. Immun. 2013, 31, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, B.; Mazur-Bialy, A.; Pajdo, R.; Kwiecień, S.; Bilski, J.; Zwolinska-Wcislo, M.; Mach, T.; Brzozowski, T. Mechanisms by which Stress Affects the Experimental and Clinical Inflammatory Bowel Disease (IBD): Role of Brain-Gut Axis. Curr. Neuropharmacol. 2016, 14, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Karelakis, C.; Zevgitis, P.; Galanopoulos, K.; Mattas, K. Consumer Trends and Attitudes to Functional Foods. J. Int. Food Agribus. Mark. 2020, 32, 266–294. [Google Scholar] [CrossRef]
- Topolska, K.; Florkiewicz, A.; Filipiak-Florkiewicz, A. Functional Food—Consumer Motivations and Expectations. Int. J. Environ. Res. Public Health 2021, 18, 5327. [Google Scholar] [CrossRef]
- Goetzke, B.I.; Spiller, A. Health-improving lifestyles of organic and functional food consumers. Br. Food J. 2014, 116, 510–526. [Google Scholar] [CrossRef]
- Betoret, E.; Betoret, N.; Vidal, D.; Fito, P. Functional foods development: Trends and technologies. Trends Food Sci. Technol. 2011, 22, 498–508. [Google Scholar] [CrossRef]
- Bigliardi, B.; Galati, F. Innovation trends in the food industry: The case of functional foods. Trends Food Sci. Technol. 2013, 31, 118–129. [Google Scholar] [CrossRef]
- Granato, D.; Branco, G.F.; Nazzaro, F.; Cruz, A.G.; Faria, J.A.F. Functional Foods and Nondairy Probiotic Food Development: Trends, Concepts, and Products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 292–302. [Google Scholar] [CrossRef]
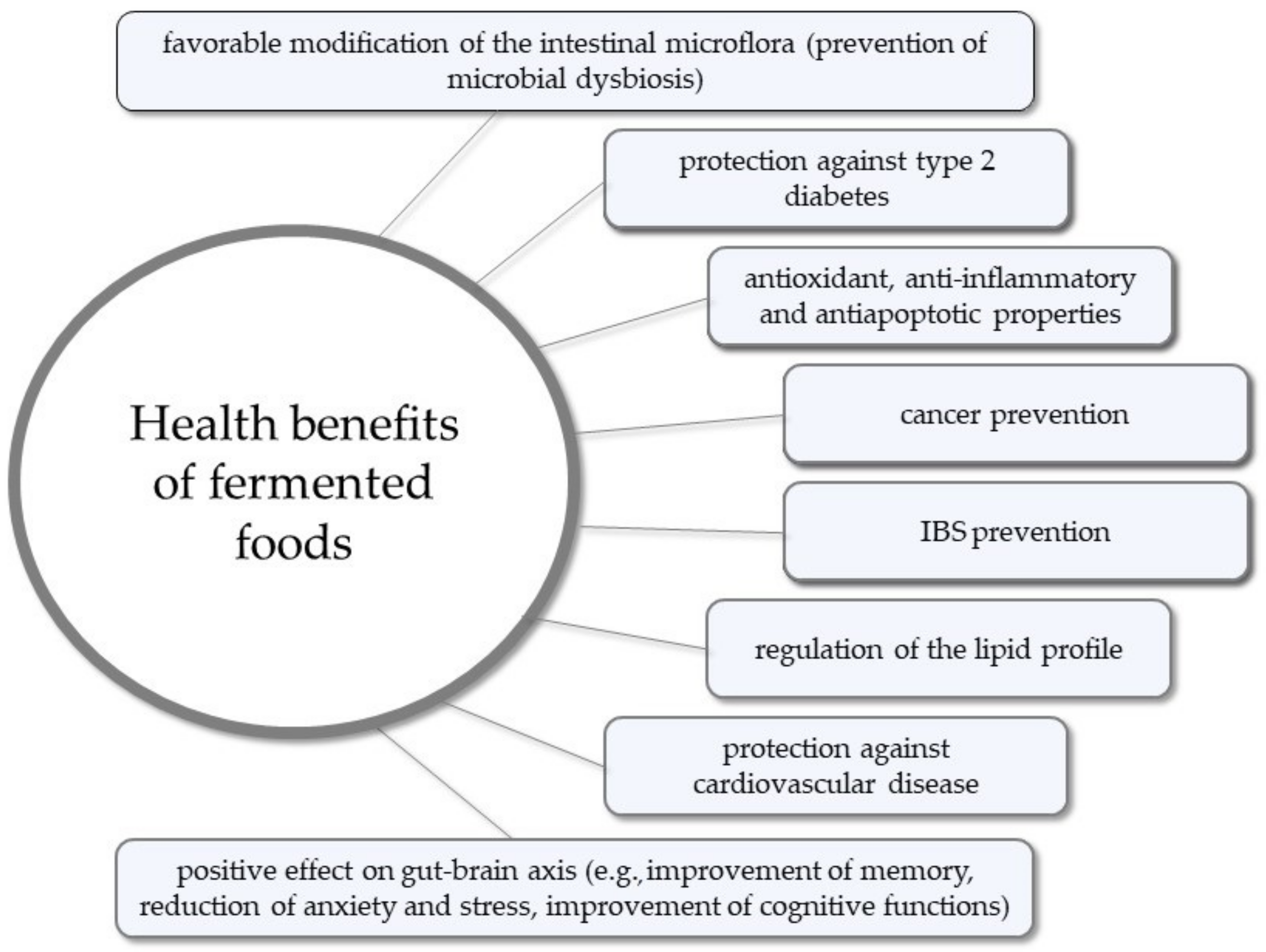
- Diez-Ozaeta, I.; Astiazaran, O.J. Fermented foods: An update on evidence-based health benefits and future perspectives. Food Res. Int. 2022, 156, 111133. [Google Scholar] [CrossRef]
- Bourdichon, F.; Casaregola, S.; Farrokh, C.; Frisvad, J.C.; Gerds, M.L.; Hammes, W.P.; Harnett, J.; Huys, G.; Laulund, S.; Ouwehand, A.; et al. Food fermentations: Microorganisms with technological beneficial use. Int. J. Food Microbiol. 2012, 154, 87–97. [Google Scholar] [CrossRef]
- Chilton, S.N.; Burton, J.P.; Reid, G. Inclusion of Fermented Foods in Food Guides around the World. Nutrients 2015, 7, 390–404. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.R.; Azizi, N.F.; Yeap, S.K.; Abdullah, J.O.; Khalid, M.; Omar, A.R.; Osman, M.A.; Leow, A.T.; Mortadza, S.A.; Alitheen, N.B. Clinical and Preclinical Studies of Fermented Foods and Their Effects on Alzheimer’s Disease. Antioxidants 2022, 11, 883. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yang, Z.; Jing, X.; Yu, P.; Zhang, Y.; Yi, H.; Zhang, L. Improvement of the Texture of Yogurt by Use of Exopolysaccharide Producing Lactic Acid Bacteria. BioMed Res. Int. 2016, 2016, 7945675. [Google Scholar] [CrossRef]
- Ravyts, F.; De Vuyst, L.; Leroy, F. Bacterial diversity and functionalities in food fermentations. Eng. Life Sci. 2012, 12, 356–367. [Google Scholar] [CrossRef]
- Taylor, B.C.; Lejzerowicz, F.; Poirel, M.; Shaffer, J.P.; Jiang, L.; Aksenov, A.; Litwin, N.; Humphrey, G.; Martino, C.; Miller-Montgomery, S.; et al. Consumption of Fermented Foods Is Associated with Systematic Differences in the Gut Microbiome and Metabolome. Msystems 2020, 5, 00901–00919. [Google Scholar] [CrossRef]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented Foods in a Global Age: East meets West. Compr. Rev. Food Sci. Food Saf. 2019, 19, 184–217. [Google Scholar] [CrossRef]
- Le Lay, C.; Coton, E.; Le Blay, G.; Chobert, J.; Haertlé, T.; Choiset, Y.; Van Long, N.N.; Meslet-Cladière, L.; Mounier, J. Identification and quantification of antifungal compounds produced by lactic acid bacteria and propionibacteria. Int. J. Food Microbiol. 2016, 239, 79–85. [Google Scholar] [CrossRef]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented Foods as a Dietary Source of Live Organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef]
- Shiby, V.K.; Mishra, H.N. Fermented Milks and Milk Products as Functional Foods—A Review. Crit. Rev. Food Sci. Nutr. 2013, 52, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.P.; Guimarães, J.T.; Esmerino, E.A.; Duarte, M.C.; Silva, M.C.; Silva, R.; Ferreira, B.M.; Sant’Ana, A.S.; Freitas, M.Q.; Cruz, A.G. Paraprobiotics and postbiotics: Concepts and potential applications in dairy products. Curr. Opin. Food Sci. 2020, 32, 1–8. [Google Scholar] [CrossRef]
- Yang, X.; Hu, W.; Xiu, Z.; Jiang, A.; Yang, X.; Ji, Y.; Guan, Y.; Feng, K. Comparison of northeast sauerkraut fermentation between single lactic acid bacteria strains and traditional fermentation. Food Res. Int. 2020, 137, 109553. [Google Scholar] [CrossRef]
- Park, K.-Y.; Jeong, J.-K.; Lee, Y.-E.; Daily, J.W. Health Benefits of Kimchi (Korean Fermented Vegetables) as a Probiotic Food. J. Med. Food 2014, 17, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zang, J.; Regenstein, J.M.; Xia, W. Technological roles of microorganisms in fish fermentation: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 1000–1012. [Google Scholar] [CrossRef]
- Barcenilla, C.; Ducic, M.; López, M.; Prieto, M.; Álvarez-Ordóñez, A. Application of lactic acid bacteria for the biopreservation of meat products: A systematic review. Meat Sci. 2022, 183, 108661. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Reale, A. A Holistic Review on Euro-Asian Lactic Acid Bacteria Fermented Cereals and Vegetables. Microorganisms 2020, 8, 1176. [Google Scholar] [CrossRef]
- Ziarno, M.; Cichońska, P. Lactic Acid Bacteria-Fermentable Cereal- and Pseudocereal-Based Beverages. Microorganisms 2021, 9, 2532. [Google Scholar] [CrossRef]
- Tamang, J.P.; Watanabe, K.; Holzapfel, W.H. Review: Diversity of Microorganisms in Global Fermented Foods and Beverages. Front. Microbiol. 2016, 7, 377. [Google Scholar] [CrossRef]
- Teshome, G. Review on lactic acid bacteria function in milk fermentation and preservation. Afr. J. Food Sci. 2015, 9, 170–175. [Google Scholar] [CrossRef]
- Cichońska, P.; Ziarno, M. Legumes and Legume-Based Beverages Fermented with Lactic Acid Bacteria as a Potential Carrier of Probiotics and Prebiotics. Microorganisms 2022, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Davydenko, S.; Meledina, T.; Mittenberg, A.; Shabelnikov, S.; Vonsky, M.; Morozov, A. Proteomics Answers Which Yeast Genes Are Specific for Baking, Brewing, and Ethanol Production. Bioengineering 2020, 7, 147. [Google Scholar] [CrossRef]
- Michel, M.; Meier-Dörnberg, T.; Jacob, F.; Methner, F.-J.; Wagner, R.S.; Hutzler, M. Review: Pure non- Saccharomyces starter cultures for beer fermentation with a focus on secondary metabolites and practical applications. J. Inst. Brew. 2016, 122, 569–587. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Benito, S. The Influence of Non-Saccharomyces Species on Wine Fermentation Quality Parameters. Fermentation 2019, 5, 54. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Lee, N.-K.; Yi, S.-H.; Hong, S.-P.; Paik, H.-D. Short communication: Physicochemical features and microbial community of milk kefir using a potential probiotic Saccharomyces cerevisiae KU200284. J. Dairy Sci. 2019, 102, 10845–10849. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Vidanarachchi, J.K.; Rocha, R.S.; Cruz, A.G.; Ajlouni, S. Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. Clinical Potential of Microbial Strains, Used in Fermentation for Probiotic Food, Beverages and in Synbiotic Supplements, as Psychobiotics for Cognitive Treatment through Gut–Brain Signaling. Microorganisms 2022, 10, 1687. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.-B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef]
- Xiang, H.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Cui, C.; Ruan, Z. Fermentation-enabled wellness foods: A fresh perspective. Food Sci. Hum. Wellness 2019, 8, 203–243. [Google Scholar] [CrossRef]
- Van Hylckama Vlieg, J.; Veiga, P.; Zhang, C.; Derrien, M.; Zhao, L. Impact of microbial transformation of food on health—From fermented foods to fermentation in the gastro-intestinal tract. Curr. Opin. Biotechnol. 2011, 22, 211–219. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.-C.; Choi, I.; Kim, G.-B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed]
- Rocks, T.; West, M.; Hockey, M.; Aslam, H.; Lane, M.; Loughman, A.; Jacka, F.N.; Ruusunen, A. Possible use of fermented foods in rehabilitation of anorexia nervosa: The gut microbiota as a modulator. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 107, 110201. [Google Scholar] [CrossRef]
- Selhub, E.M.; Logan, A.C.; Bested, A.C. Fermented foods, microbiota, and mental health: Ancient practice meets nutritional psychiatry. J. Physiol. Anthr. 2014, 33, 2. [Google Scholar] [CrossRef]
- Cenit, M.C.; Nuevo, I.C.; Codoñer-Franch, P.; Dinan, T.G.; Sanz, Y. Gut microbiota and attention deficit hyperactivity disorder: New perspectives for a challenging condition. Eur. Child Adolesc. Psychiatry 2017, 26, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Del Toro-Barbosa, M.; Hurtado-Romero, A.; Garcia-Amezquita, L.E.; García-Cayuela, T. Psychobiotics: Mechanisms of Action, Evaluation Methods and Effectiveness in Applications with Food Products. Nutrients 2020, 12, 3896. [Google Scholar] [CrossRef]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on intestine microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Ther. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Role of Probiotics and Diet in the Management of Neurological Diseases and Mood States: A Review. Microorganisms 2022, 10, 2268. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. North Am. 2017, 46, 77–89. [Google Scholar] [CrossRef]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Musa, N.H.; Mani, V.; Lim, S.M.; Vidyadaran, S.; Majeed, A.B.; Ramasamy, K. Lactobacilli-fermented cow’s milk attenuated lipopolysaccharide-induced neuroinflammation and memory impairment in vitro and in vivo. J. Dairy Res. 2017, 84, 488–495. [Google Scholar] [CrossRef] [PubMed]
- van de Wouw, M.; Walsh, A.M.; Crispie, F.; van Leuven, L.; Lyte, J.M.; Boehme, M.; Clarke, G.; Dinan, T.G.; Cotter, P.D.; Cryan, J.F. Distinct actions of the fermented beverage kefir on host behaviour, immunity and microbiome gut-brain modules in the mouse. Microbiome 2020, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.; Kim, M.J.; Song, Y.O. Bioactive Compounds in Kimchi Improve the Cognitive and Memory Functions Impaired by Amyloid Beta. Nutrients 2010, 10, 1554. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, P.; Pan, D.; Zeng, X.; Guo, Y.; Zhao, G. Effect of adzuki bean sprout fermented milk enriched in γ-aminobutyric acid on mild depression in a mouse model. J. Dairy Sci. 2021, 104, 78–91. [Google Scholar] [CrossRef]
- Go, J.; Kim, E.; Kwak, M.H.; Koh, E.K.; Song, S.H.; Sung, J.E.; Kim, D.S.; Hong, J.T.; Hwang, D.Y. Neuroprotective effects of fermented soybean products (Cheonggukjang) manufactured by mixed culture of Bacillus subtilis MC31 and Lactobacillus sakei 383 on trimethyltin-induced cognitive defects mice. Nutr. Neurosci. 2016, 19, 247–259. [Google Scholar] [CrossRef]
- Yoo, D.-H.; Kim, D.-H. Lactobacillus pentosus var. plantarum C29 increases the protective effect of soybean against scopolamine-induced memory impairment in mice. Int. J. Food Sci. Nutr. 2015, 66, 912–918. [Google Scholar] [CrossRef]
- Lee, H.-J.; Hwang, Y.-H.; Kim, D.-H. Lactobacillus plantarum C29-Fermented Soybean (DW2009) Alleviates Memory Impairment in 5XFAD Transgenic Mice by Regulating Microglia Activation and Gut Microbiota Composition. Mol. Nutr. Food Res. 2018, 62, 1800359. [Google Scholar] [CrossRef]
- Kim, C.-S.; Shin, D.-M. Probiotic food consumption is associated with lower severity and prevalence of depression: A nationwide cross-sectional study. Nutrition 2019, 63–64, 169–174. [Google Scholar] [CrossRef]
- Ohsawa, K.; Nakamura, F.; Uchida, N.; Mizuno, S.; Yokogoshi, H. Lactobacillus helveticus-fermented milk containing lactononadecapeptide (NIPPLTQTPVVVPPFLQPE) improves cognitive function in healthy middle-aged adults: A randomised, double-blind, placebo-controlled trial. Int. J. Food Sci. Nutr. 2017, 69, 369–376. [Google Scholar] [CrossRef]
- Mohammadi, A.A.; Jazayeri, S.; Khosravi-Darani, K.; Solati, Z.; Mohammadpour, N.; Asemi, Z.; Adab, Z.; Djalali, M.; Tehrani-Doost, M.; Hosseini, M.; et al. The effects of probiotics on mental health and hypothalamic–pituitary–adrenal axis: A randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr. Neurosci. 2016, 19, 387–395. [Google Scholar] [CrossRef]
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain-Raspaud, S.; Trotin, B.; Naliboff, B.; et al. Consumption of Fermented Milk Product with Probiotic Modulates Brain Activity. Gastroenterology 2013, 144, 1394–1401.e4. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Bastiaanssen, T.F.S.; Moloney, G.M.; Boscaini, S.; Strain, C.R.; Anesi, A.; Long-Smith, C.; Mattivi, F.; Stanton, C.; Clarke, G.; et al. Feed your microbes to deal with stress: A psychobiotic diet impacts microbial stability and perceived stress in a healthy adult population. Mol. Psychiatry 2023, 28, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Nishihira, J.; Kagami-Katsuyama, H.; Tanaka, A.; Nishimura, M.; Kobayashi, T.; Kawasaki, Y. Elevation of natural killer cell activity and alleviation of mental stress by the consumption of yogurt containing Lactobacillus gasseri SBT2055 and Bifidobacterium longum SBT2928 in a double-blind, placebo-controlled clinical trial. J. Funct. Foods 2014, 11, 261–268. [Google Scholar] [CrossRef]
- Kato-Kataoka, A.; Nishida, K.; Takada, M.; Suda, K.; Kawai, M.; Shimizu, K.; Kushiro, A.; Hoshi, R.; Watanabe, O.; Igarashi, T. Fermented milk containing Lactobacillus casei strain Shirota prevents the onset of physical symptoms in medical students under academic examination stress. Benef. Microbes 2016, 7, 153–156. [Google Scholar] [CrossRef]
- Márquez-Morales, L.; El-Kassis, E.G.; Cavazos-Arroyo, J.; Rocha-Rocha, V.; Martínez-Gutiérrez, F.; Pérez-Armendáriz, B. Effect of the Intake of a Traditional Mexican Beverage Fermented with Lactic Acid Bacteria on Academic Stress in Medical Students. Nutrients 2021, 13, 1551. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Probiotics for Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Foods 2021, 10, 1672. [Google Scholar] [CrossRef]
- Handajani, Y.S.; Turana, Y.; Yogiara, Y.; Widjaja, N.T.; Sani, T.P.; Christianto, G.A.M.; Suwanto, A. Tempeh Consumption and Cognitive Improvement in Mild Cognitive Impairment. Dement. Geriatr. Cogn. Disord. 2020, 49, 497–502. [Google Scholar] [CrossRef]
- Ton, A.M.M.; Campagnaro, B.P.; Alves, G.A.; Aires, R.; Côco, L.Z.; Arpini, C.M.; Guerra e Oliveira, T.; Campos-Toimil, M.; Meyrelles, S.S.; Pereira, T.M.C.; et al. Oxidative Stress and Dementia in Alzheimer’s Patients: Effects of Synbiotic Supplementation. Oxidative Med. Cell. Longev. 2020, 2020, 2638703. [Google Scholar] [CrossRef]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: A randomized, double-blind and controlled trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef]
- Zinöcker, M.K.; Lindseth, I.A. The Western Diet–Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Casertano, M.; Fogliano, V.; Ercolini, D. Psychobiotics, gut microbiota and fermented foods can help preserving mental health. Food Res. Int. 2022, 152, 110892. [Google Scholar] [CrossRef] [PubMed]
- Forssten, S.D.; Sindelar, C.W.; Ouwehand, A.C. Probiotics from an industrial perspective. Anaerobe 2011, 17, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.K.; Giri, S.K. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- Cichońska, P.; Domian, E.; Ziarno, M. Application of Optical and Rheological Techniques in Quality and Storage Assessment of the Newly Developed Colloidal-Suspension Products: Yogurt-Type Bean-Based Beverages. Sensors 2022, 22, 8348. [Google Scholar] [CrossRef]
- Misra, S.; Mohanty, D. Psychobiotics: A new approach for treating mental illness? Crit. Rev. Food Sci. Nutr. 2019, 59, 1230–1236. [Google Scholar] [CrossRef]
- Kowalska, E.; Ziarno, M. Characterization of Buckwheat Beverages Fermented with Lactic Acid Bacterial Cultures and Bifidobacteria. Foods 2020, 9, 1771. [Google Scholar] [CrossRef]
- Mirković, M.; Mirković, N.; Miočinović, J.; Radulović, A.; Paunović, D.; Ilić, M.; Radulović, Z. Probiotic yogurt and cheese from ultrafiltered milk: Sensory quality and viability of free-living and spray dried Lactiplantibacillus plantarum 564 and Lactiplantibacillus plantarum 299v. J. Food Process. Preserv. 2021, 45, 15713. [Google Scholar] [CrossRef]
- Hanafi, F.N.A.; Kamaruding, N.A.; Shaharuddin, S. Influence of coconut residue dietary fiber on physicochemical, probiotic (Lactobacillus plantarum ATCC 8014) survivability and sensory attributes of probiotic ice cream. LWT 2022, 154, 112725. [Google Scholar] [CrossRef]
- Sun, J.; Chen, H.; Qiao, Y.; Liu, G.; Leng, C.; Zhang, Y.; Lv, X.; Feng, Z. The nutrient requirements of Lactobacillus rhamnosus GG and their application to fermented milk. J. Dairy Sci. 2019, 102, 5971–5978. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Luo, J.; Wu, K.; Chen, Q.; Hao, L.; Zhou, X.; Wang, X.; Liu, C.; Zhou, H. Dendrobium candidum extract on the bioactive and fermentation properties of Lactobacillus rhamnosus GG in fermented milk. Food Biosci. 2021, 41, 100987. [Google Scholar] [CrossRef]
- Alvarado-Reveles, O.; Fernández-Michel, S.; Jiménez-Flores, R.; Cueto-Wong, C.; Vázquez-Moreno, L.; Montfort, G. Survival and Goat Milk Acidifying Activity of Lactobacillus rhamnosus GG Encapsulated with Agave Fructans in a Buttermilk Protein Matrix. Probiotics Antimicrob. Proteins 2019, 11, 1340–1347. [Google Scholar] [CrossRef]
- Sezer, E.; Ayar, A.; Yılmaz, S. Fermentation of Dietary Fibre-Added Milk with Yoghurt Bacteria and L. rhamnosus and Use in Ice Cream Production. Fermentation 2023, 9, 3. [Google Scholar] [CrossRef]
- Zamberlin, Š.; Samaržija, D. The effect of non-standard heat treatment of sheep’s milk on physico-chemical properties, sensory characteristics, and the bacterial viability of classical and probiotic yogurt. Food Chem. 2017, 225, 62–68. [Google Scholar] [CrossRef]
- Magariños, H.; Cartes, P.; Fraser, B.; Selaive, S.; Costa, M.; Figuerola, F.; Pizarro, O. Viability of probiotic micro-organisms (Lactobacillus casei Shirota and Bifidobacterium animalis subspp. lactis) in a milk-based dessert with cranberry sauce. J. Dairy Technol. 2008, 61, 96–101. [Google Scholar] [CrossRef]
- Sumalapao, D.E.; Mesina, J.A.; Cabrera, E.C.; Gloriani, N.G. Viability kinetics of Lactobacillus casei Shirota strain in a commercial fermented milk drink during refrigerated storage. Natl. J. Physiol. Pharm. Pharmacol. 2017, 7, 1242–1246. [Google Scholar] [CrossRef]
- Angmo, K.; Kumari, A.; Bhalla, T.C. Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. LWT 2016, 66, 428–435. [Google Scholar] [CrossRef]
- Gul, O. Microencapsulation of Lactobacillus casei Shirota by spray drying using different combinations of wall materials and application for probiotic dairy dessert. J. Food Process. Preserv. 2017, 41, 13198. [Google Scholar] [CrossRef]
- Lavrentev, F.V.; Ashikhmina, M.S.; Ulasevich, S.A.; Morozova, O.V.; Orlova, O.Y.; Skorb, E.V.; Iakovchenko, N.V. Perspectives of Bacillus coagulans MTCC 5856 in the production of fermented dairy products. LWT 2021, 148, 111623. [Google Scholar] [CrossRef]
- Amanda, E.; Choo, W.S. Effect of refrigerated storage on the physicochemical characteristics and viability of Lactobacillus plantarum in fermented watermelon juice with or without supplementation with inulin or fructooligosaccharide. J. Food Process. Preserv. 2018, 42, 13831. [Google Scholar] [CrossRef]
- Zoghi, A.; Khosravi-Darani, K.; Sohrabvandi, S.; Attar, H. Patulin removal from synbiotic apple juice using Lactobacillus plantarum ATCC 8014. J. Appl. Microbiol. 2018, 126, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-J.; Yeon, S.-J.; Park, W.-J.; Hong, G.-E.; Lee, C.-H. Production of sesaminol and antioxidative activity of fermented sesame with Lactobacillus plantarum P8, Lactobacillus acidophilus ATCC 4356, Streptococcus thermophilus S10. Food Sci. Biotechnol. 2016, 25, 199–204. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, Y.; Zhang, S.; Shang, W.; Gao, X.; Wang, H. Whole soybean as probiotic lactic acid bacteria carrier food in solid-state fermentation. Food Control. 2014, 41, 1–6. [Google Scholar] [CrossRef]
- Zhang, L.; Taal, M.A.; Boom, R.M.; Chen, X.D.; Schutyser, M.A. Effect of baking conditions and storage on the viability of Lactobacillus plantarum supplemented to bread. LWT 2018, 87, 318–325. [Google Scholar] [CrossRef]
- Supasil, R.; Suttisansanee, U.; Santivarangkna, C.; Tangsuphoom, N.; Khemthong, C.; Chupeerach, C.; On-Nom, N. Improvement of Sourdough and Bread Qualities by Fermented Water of Asian Pears and Assam Tea Leaves with Co-Cultures of Lactiplantibacillus plantarum and Saccharomyces cerevisiae. Foods 2022, 11, 2071. [Google Scholar] [CrossRef] [PubMed]
- Mirković, M.; Seratlić, S.; Kilcawley, K.; Mannion, D.; Mirković, N.; Radulović, Z. The Sensory Quality and Volatile Profile of Dark Chocolate Enriched with Encapsulated Probiotic Lactobacillus plantarum Bacteria. Sensors 2018, 18, 2570. [Google Scholar] [CrossRef] [PubMed]
- Giordano, I.; Abuqwider, J.; Altamimi, M.; Di Monaco, R.; Puleo, S.; Mauriello, G. Application of ultrasound and microencapsulation on Limosilactobacillus reuteri DSM 17938 as a metabolic attenuation strategy for tomato juice probiotication. Heliyon 2022, 8, 10969. [Google Scholar] [CrossRef] [PubMed]
- Mauro, C.S.I.; Garcia, S. Coconut milk beverage fermented by Lactobacillus reuteri: Optimization process and stability during refrigerated storage. J. Food Sci. Technol. 2019, 56, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, F.; Chai, Z.; Liu, M.; Battino, M.; Meng, X. Mixed fermentation of blueberry pomace with L. rhamnosus GG and L. plantarum-1: Enhance the active ingredient, antioxidant activity and health-promoting benefits. Food Chem. Toxicol. 2019, 131, 110541. [Google Scholar] [CrossRef]
- Bernat, N.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Hazelnut milk fermentation using probiotic Lactobacillus rhamnosus GG and inulin. Int. J. Food Sci. Technol. 2014, 29, 2552–2562. [Google Scholar] [CrossRef]
- de Almeida Bianchini Campos, R.C.; Martins, E.M.; de Andare Pires, B.; do Carmo Gouveia Peluzio, M.; da Rocha Campos, A.; Ramos, A.M.; de Castro Leite Júnior, B.; de Oliveira Martins, A.D.; da Silva, R.R.; Martins, M.L. In vitro and in vivo resistance of Lactobacillus rhamnosus GG carried by a mixed pineapple (Ananas comosus L. Merril) and jussara (Euterpe edulis Martius) juice to the gastrointestinal tract. Food Res. Int. 2019, 116, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Alemneh, S.T.; Emire, S.A.; Jekle, M.; Hitzmann, B. Effect of refrigerated storage on some physicochemical characteristics of a teff-based fermented beverage and the viability of the fermenting Lactiplantibacillus plantarum and Lacticaseibacillus rhamnosus used. J. Food Process. Preserv. 2022, 46, 17034. [Google Scholar] [CrossRef]
- Chan, M.Z.; Toh, M.; Liu, S.-Q. Growth, survival, and metabolic activities of probiotics Lactobacillus rhamnosus GG and Saccharomyces cerevisiae var. boulardii CNCM-I745 in fermented coffee brews. Int. J. Food Microbiol. 2021, 350, 109229. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, A.S.; Bhardwaj, A.; Sharma, V. Fermentation of tender coconut water by probiotic bacteria Bacillus coagulans. Int. J. Food Stud. 2018, 7, 100–110. [Google Scholar] [CrossRef]
- Soccol, C.R.; de Souza Vandenberghe, L.P.; Spier, M.R.; Medeiros, A.B.; Yamaguishi, C.T.; De Dea Lindner, J.; Pandey, A.; Thomaz-Soccol, V. The Potential of Probiotics: A Review. Food Technol. Biotechnol. 2010, 48, 413–434. [Google Scholar]
- Granato, D.; Branco, G.F.; Cruz, A.G.; Faria, J.A.F.; Shah, N.P. Probiotic Dairy Products as Functional Foods. Compr. Rev. Food Sci. Food Saf. 2010, 9, 455–470. [Google Scholar] [CrossRef]
- Kumar, B.V.; Vijayendra, S.V.; Reddy, O.V. Trends in dairy and non-dairy probiotic products—A review. J. Food Sci. Technol. 2015, 52, 6112–6124. [Google Scholar] [CrossRef]
- Cichońska, P.; Ziębicka, A.; Ziarno, M. Properties of Rice-Based Beverages Fermented with Lactic Acid Bacteria and Propionibacterium. Molecules 2022, 27, 2558. [Google Scholar] [CrossRef]
- Shori, A.B. Influence of food matrix on the viability of probiotic bacteria: A review based on dairy and non-dairy beverages. Food Biosci. 2016, 13, 1–8. [Google Scholar] [CrossRef]
- Reque, P.M.; Brandelli, A. Encapsulation of probiotics and nutraceuticals: Applications in functional food industry. Trends Food Sci. Technol. 2021, 114, 1–10. [Google Scholar] [CrossRef]
- Panghal, A.; Janghu, S.; Virkar, K.; Gat, Y.; Kumar, V.; Chhikara, N. Potential non-dairy probiotic products—A healthy approach. Food Biosci. 2018, 21, 80–89. [Google Scholar] [CrossRef]
- Ziarno, M.; Zaręba, D.; Maciejak, M.; Veber., A.L. The impact of dairy starter cultures on selected qualitative properties of functional fermented beverage prepared from germinated White Kidney Beans. J. Food Nutr. Res. 2019, 2, 167–176. [Google Scholar]
- Mousavi, R.; Mottawea, W.; Audet, M.-C.; Hammami, R. Survival and Interplay of γ-Aminobutyric Acid-Producing Psychobiotic Candidates with the Gut Microbiota in a Continuous Model of the Human Colon. Biology 2022, 11, 1311. [Google Scholar] [CrossRef] [PubMed]
- Nordström, E.A.; Teixeira, C.; Montelius, C.; Jeppsson, B.; Larsson, N. Lactiplantibacillus plantarum 299v (LP299V®): Three decades of research. Benef. Microbes 2021, 12, 441–465. [Google Scholar] [CrossRef]
- Goossens, D.; Jonkers, D.; Russel, M.; Thijs, A.; Van den Bogaard, A.; Stobberingh, E.; Stockbrügger, R. Survival of the probiotic, L. plantarum 299v and its effects on the faecal bacterial flora, with and without gastric acid inhibition. Dig. Liver Dis. 2005, 37, 44–50. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Y.; Polk, D.B.; Tomasula, P.M.; Yan, F.; Liu, L. Preserving viability of Lactobacillus rhamnosus GG in vitro and in vivo by a new encapsulation system. J. Control. Release 2016, 230, 79–87. [Google Scholar] [CrossRef]
- Paniágua, A.L.; Correia, A.F.; Pereira, L.C.; de Alencar, B.M.; Silva, F.B.A.; Almeida, R.M.; de Madeiros Nóbrega, Y.K. Inhibitory effects of Lactobacillus casei Shirota against both Candida auris and Candida spp. isolates that cause vulvovaginal candidiasis and are resistant to antifungals. BMC Complement. Med. Ther. 2021, 21, 237. [Google Scholar] [CrossRef]
- Wang, R.; Chen, S.; Jin, J.; Ren, F.; Li, Y.; Qiao, Z.; Wang, Y.; Zhao, L. Survival of Lactobacillus casei strain Shirota in the intestines of healthy Chinese adults. Microbiol. Immunol. 2015, 59, 268–276. [Google Scholar] [CrossRef]
- Salonen, A.; de Vos, W.M. Impact of Diet on Human Intestinal Microbiota and Health. Annu. Rev. Food Sci. Technol. 2014, 5, 239–262. [Google Scholar] [CrossRef]
- Yuki, N.; Watanabe, K.; Mike, A.; Tagami, Y.; Tanaka, R.; Ohwaki, M.; Morotomi, M. Survival of a probiotic, Lactobacillus casei strain Shirota, in the gastrointestinal tract: Selective isolation from faeces and identification using monoclonal antibodies. Int. J. Food Microbiol. 1999, 48, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Katsumata, N.; Bernier, F.; Ohno, K.; Yamauchi, Y.; Odamaki, T.; Yoshikawa, K.; Ito, K.; Kaneko, T. Probiotic Bifidobacterium breve in Improving Cognitive Functions of Older Adults with Suspected Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Alzheimer’s Dis. 2020, 77, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Adamberg, S.; Sumeri, I.; Uusna, R.; Ambalam, P.; Kondepudi, K.K.; Adamberg, K.; Wadström, T.; Ljungh, Å. Survival and synergistic growth of mixed cultures of bifidobacteria and lactobacilli combined with prebiotic oligosaccharides in a gastrointestinal tract simulator. Microb. Ecol. Health Dis. 2014, 25, 23062. [Google Scholar] [CrossRef] [PubMed]
- Andrews, E.B.; Eaton, S.C.; Hollis, K.A.; Hopkins, J.S.; Ameen, V.; Hamm, L.R.; Cook, S.F.; Tennis, P.; Mangel, A.W. Prevalence and demographics of irritable bowel syndrome: Results from a large web-based survey. Aliment. Pharmacol. Ther. 2005, 22, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, D.; Gibb, R.D.; Quigley, E.M. Fecal excretion of Bifidobacterium infantis 35624 and changes in fecal microbiota after eight weeks of oral supplementation with encapsulated probiotic. Gut Microbes 2013, 4, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.; Meriluoto, J.; Salminen, S. Adhesion and aggregation properties of probiotic and pathogen strains. Eur. Food Res. Technol. 2008, 226, 1065–1073. [Google Scholar] [CrossRef]
- Velez, M.P.; De Keersmaecker, S.C.; Vanderleyden, J. Adherence factors of Lactobacillus in the human gastrointestinal tract. FEMS Microbiol. Lett. 2007, 276, 140–148. [Google Scholar] [CrossRef]
- Rong, J.; Zheng, H.; Liu, M.; Hu, X.; Wang, T.; Zhang, X.; Jin, F.; Wang, L. Probiotic and anti-inflammatory attributes of an isolate Lactobacillus helveticus NS8 from Mongolian fermented koumiss. BMC Microbiol. 2015, 15, 196. [Google Scholar] [CrossRef]
| Bacterial Strain | Study Model | Potential Psychobiotic Effects | References |
|---|---|---|---|
| Lactiplantibacillus plantarum C29 | humans with mild cognitive impairment |
| [18] |
| L. plantarum DR7 | stressed adults |
| [19] |
| L. plantarum P8 | stressed adults |
| [20] |
| stressed adults |
| [21] | |
| L. plantarum PS128 | mouse with 1-methyl-4-phenyl-1,2,3,6-tetrathydropyridine-induced Parkinson’s disease |
| [22] |
| male children with autism spectrum disorder |
| [23] | |
| adult IT specialists |
| [24] | |
| patients with self-reported insomnia |
| [25] | |
| L. plantarum ATCC 8014TM | Wistar rats |
| [26] |
| male Wistar rats |
| [27] | |
| L. plantarum 299v | young adults under examination stress |
| [28] |
| patients with major depressive disorder (MDD) |
| [29] | |
| Limosilactobacillus reuteri DSM 17938 | mice |
| [30] |
| Lactobacillus helveticus CCFM1076 | rats withvalproic acid-induced autism |
| [31] |
| L helveticus NS8 | chronic stress rats |
| [32] |
| rats with hyperammonemia |
| [33] | |
| Lacticaseibacillus rhamnosus JB-1 | mice |
| [34] |
| Wistar rats subjected to chronic unpredictable mild stress protocol |
| [35] | |
| L. rhamnosus GG | mice with induced obsessive-compulsive disorder (OCD)-like behavior |
| [36] |
| middle-aged and older adults |
| [37] | |
| female mice |
| [38] | |
| drug-naive children and adolescents with a diagnosis of attention-deficit/hyperactivity disorder (ADHD) |
| [39] | |
| Lactobacillus gasseri CP2305 | male university Ekiden runners |
| [40] |
| young adults exposed to chronic stress |
| [41] | |
| Japanese medical students |
| [42] | |
| Lacticaseibacillus casei Shirota | male football players |
| [43] |
| healthy medical students under academic examination stress and rats with water avoidance stress |
| [44] | |
| patients with MDD or bipolar disorder (BD) |
| [45] | |
| Bifidobacterium breve A1 | AD mice |
| [46] |
| elderly with mild cognitive impairment |
| [47] | |
| patients with schizophrenia |
| [48] | |
| B. breve CCFM1025 | C57BL/6J mice |
| [49] |
| C57BL/6J mice |
| [50] | |
| Mice with AD |
| [51] | |
| pregnant mice |
| [52] | |
| B. longum NCC3001 | adults with irritable bowel syndrome (IBS) |
| [53] |
| B. longum 1714TM | healthy volunteers |
| [54] |
| healthy volunteers |
| [55] | |
| B. infantis 35624 | adult rats |
| [56] |
| B. bifidum ATCCVR 29521 | Wistar rats |
| [26] |
| Bacillus coagulans MTCC 5856 | patients diagnosed for major depressive disorder with IBS |
| [57] |
| Clostridium butyricum MIYAIRI 588 | adult patients diagnosed with treatment-resistant major depressive disorder |
| [58] |
| Food Category | Type of Fermented Food Product | Micro-Organisms Used in Fermentation | Viability of Micro-Organisms after Fermentation [log CFU/mL] | Viability of Micro-Organisms after Storage | References | |
|---|---|---|---|---|---|---|
| Storage Time [Days] | Population Viability [log CFU/mL] | |||||
| Dairy products | Yogurt | L. plantarum 299v | 8.0 | 56 | 7.5 | [140] |
| Cheese | 9.5 | 56 | 9.0 | [140] | ||
| Ice cream | L. plantarum ATCC 8014 | 7.5 | 60 | 7.6 | [141] | |
| Cow milk | L. rhamnosus GG | 9.0 | - | - | [142] | |
| Cow milk | 8.0 | - | - | [143] | ||
| Goat milk | 9.5 | - | - | [144] | ||
| Ice cream | 8.3 | 90 | 7.3 | [145] | ||
| Sheep milk yogurt | 7.5–8.0 | 21 | 7.4–7.8 | [146] | ||
| Milk-based dessert with cranberry sauce | L. casei Shirota | 8.0 | 21 | 7.3 | [147] | |
| Cow Milk | 8.0 | 31 | 8.0 | [148] | ||
| Cow Milk | 8.8 | 28 | 7.8 | [149] | ||
| Pudding | 7.3 | 20 | 9.0 | [150] | ||
| Skimmed milk with milk protein concentrate | B. coagulans MTCC 5856 | 8.4 | 60 | 8.1 | [151] | |
| Plant products | Watermelon juice | L. plantarum ATCC 8014 | 8.8 | 14 | 11.0 | [152] |
| Apple juice | 11.0–11.5 | 42 | 7.7–8.6 | [153] | ||
| Sesame | L. plantarum P8 | 8.6 | - | - | [154] | |
| Whole soybeans | 10.5 | - | - | [155] | ||
| Bread | 5.0 | 5 | 8.0 | [156] | ||
| Sourdough | L. plantarum 299v | 7.9 | - | - | [157] | |
| Dark chocolate | 8.2 | 360 | 5.5 | [158] | ||
| Tomato juice | L. reuteri DSM 17938 | 7.1 | 28 | 5.7 | [159] | |
| Coconut milk | 8.6 | 30 | 8.6 | [160] | ||
| Blueberry pomace | L. rhamnosus GG | 11.6 | - | - | [161] | |
| Hazelnut milk | 7.9 | 28 | 8.3 | [162] | ||
| Pineapple and jussarajuice | 7.2 | 28 | 7.7 | [163] | ||
| Teff-based beverage | 8.1 | 25 | 7.8 | [164] | ||
| Coffee brews | 7.8 | 49 | 7.0 | [165] | ||
| Coconut water | B. coagulans MTCC 5856 | 9.73 | - | - | [166] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cichońska, P.; Kowalska, E.; Ziarno, M. The Survival of Psychobiotics in Fermented Food and the Gastrointestinal Tract: A Review. Microorganisms 2023, 11, 996. https://doi.org/10.3390/microorganisms11040996
Cichońska P, Kowalska E, Ziarno M. The Survival of Psychobiotics in Fermented Food and the Gastrointestinal Tract: A Review. Microorganisms. 2023; 11(4):996. https://doi.org/10.3390/microorganisms11040996
Chicago/Turabian StyleCichońska, Patrycja, Ewa Kowalska, and Małgorzata Ziarno. 2023. "The Survival of Psychobiotics in Fermented Food and the Gastrointestinal Tract: A Review" Microorganisms 11, no. 4: 996. https://doi.org/10.3390/microorganisms11040996
APA StyleCichońska, P., Kowalska, E., & Ziarno, M. (2023). The Survival of Psychobiotics in Fermented Food and the Gastrointestinal Tract: A Review. Microorganisms, 11(4), 996. https://doi.org/10.3390/microorganisms11040996







