Abstract
Evidence of the effectiveness of the tests used to diagnose Helicobacter pylori (H. pylori) in primary healthcare is limited. This cross-sectional study aims to assess the accuracy of tests used for to diagnose H. pylori infection in primary care patients and its relationship with gastroduodenal pathologies. Over 12 months, 173 primary care patients with dyspeptic symptoms were referred for upper gastrointestinal endoscopy to obtain gastric biopsies, and venous blood was extracted from them. H. pylori infection was detected using a rapid urease test (RUT), real-time polymerase chain reaction (RT-PCR), H. pylori-IgG ELISA, and Western blot (WB). The culture and histological findings were used as the reference standard for H. pylori infection. H. pylori prevalence was 50%. There were no significant differences between men and women overall or by age group. The presence of H. pylori was associated with chronic moderate gastritis and its absence with chronic inactive gastritis, as well as the combination of gastritis and gastric lesions (p < 0.05). RUT and ELISA H. pylori -IgG tests showed the highest overall performance (accuracy 98.9% and 84.4%), followed by WB and RT-PCR (accuracy 79.3% and 73.9%). These findings support the notion that combined invasive and noninvasive methods, such as RUT and H. pylori-IgG ELISA, can be a primary diagnostic screening tool for detecting H. pylori among adult dyspeptic patients in Cuba’s primary care setting.
1. Introduction
Helicobacter pylori (H. pylori) is a common bacterium, infecting 50% of the human population worldwide and almost 90% in developing countries. It is considered one of the most prevalent chronic bacterial infections in humans [1], associated with gastritis, duodenal ulcer, gastric carcinoma, and mucosa-associated lymphoid tissue [2]. In 2014, the International Agency for Research on Cancer classified H. pylori infection as a class 1 carcinogen. In 2017, the World Health Organization raised an alert about H. pylori resistance to clarithromycin, the most crucial antibiotic in combined therapy for eradicating this microorganism [3,4].
H. pylori infection can be diagnosed by several invasive (e.g., histology, rapid urease test [RUT], bacterial culture from biopsy) and noninvasive (e.g., polymerase chain reaction, serological tests) techniques [3]. Among noninvasive techniques, the guidelines for detecting and managing H. pylori consider the urea breath test (UBT) and the stool antigen test (SAT) as valid options for initial screening in dyspeptic patients [5,6,7]. The choice of diagnostic test depends on the prevalence of H. pylori, the local incidence of age-related gastric cancer, the advantages and disadvantages of each method, the different clinical conditions for each patient, and the test costs [8].
Multiple factors, such as socioeconomic level, age, hygiene, and sanitary habits, can influence H. pylori infection rates in developing countries [1,6]. Some studies [9,10,11,12] have suggested that the mean prevalence of H. pylori infection in Cuban adults is similar to that reported in the Latin America and Caribbean region (59.3 %, 95% CI 52.9–65.6) [1]. About 1300 deaths per year in Cuba are attributed to peptic ulcer (n = 366) and stomach cancer (n = 865), and the overall death rate is higher for men older than 40 years of age [13]. Therefore, public health policies have been directed toward the early detection of gastroduodenal diseases associated with H. pylori by endoscopic and histopathological methods [9]. Although the biopsy-based direct methods have been accepted as reference standards for H. pylori infection diagnosis, these tests are considered inappropriate for routine screening. The requirement of mandatory biopsy implies more discomfort for the patient and demands skillfulness in the performance and interpretation [3,5]. In addition, the evaluation of other diagnostic tests remains poorly explored at the primary care level.
Because of all these reasons, strategies for community-based diagnoses should be established based on local epidemiology and the availability of diagnostic tools. Hence, this study assesses the accuracy of tests in diagnosing H. pylori infection, in agreement with the evidence-based diagnostic criteria [14] and its relationship with gastroduodenal pathologies. The aim is to determine which diagnostic tests are more accurate when applied to adult patients with dyspepsia in Cuba’s primary care setting.
2. Materials and Methods
2.1. Ethical Considerations
The ethical committee of the “Pedro Kourí” Institute of Tropical Medicine (IPK) approved this study (approval number 2014/08). The study was performed in compliance with ethical principles outlined in the Declaration of Helsinki and was consistent with Good Clinical Practice guidelines. All patients provided informed written consent for inclusion before they participated in the study, and the data obtained from each patient was kept confidential. The information in this study was used only for research purposes.
2.2. Study Area, Patients and Design
This is a cross-sectional diagnostic cohort study [15] aiming to evaluate the accuracy of invasive and noninvasive tests in samples from Cuban adults with dyspeptic symptoms. The study was carried out between November 2016 and November 2017 in the Health Area of Primary Care Polyclinic 19 de Abril, Plaza de la Revolución Municipality, Havana, Cuba. Health and academic institutions such as IPK, Teaching Surgical Clinical Hospital Manuel Fajardo, and Charité University also participated in this study.
One hundred and seventy-three patients of both sexes, aged >18 years, with uninvestigated dyspepsia (defined by any of the following symptoms: postprandial fullness; early satiety; epigastric pain; and epigastric burning [16]), and with the capacity to consent for themselves and interest in participating in the study were enrolled for routine upper gastrointestinal endoscopy and considered potentially eligible. Exclusion criteria comprised those who have received previous treatments with antimicrobials, proton pump inhibitors, non-steroidal anti-inflammatory drugs, and bismuth salts, as well as those with a history of digestive bleeding within three–four weeks before enrollment. Patients to whom a biological sample could not be obtained or with poor-quality samples were also excluded. All eligible’ patients were exposed to the same index tests and reference standards at the same time point.
The study is in agreement with the “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) statement guidelines [17]. The STROBE checklist is included in Supplementary Table S1.
2.3. Specimen Collection and Samples Treatment
Four biopsy samples of each patient were taken from the lesions and areas around the pyloric antrum and gastric body by experienced gastroenterologists using an Olympus fibroendoscope [9]. The examination was conducted under topical anesthesia following at least 8 h of fasting. The first biopsy sample was taken for histopathology; the second biopsy sample was taken for culture. Two additional biopsies samples were taken for RUT and real-time polymerase chain reaction (RT-PCR). In addition, 10 mL of venous blood was collected from the patients, processed according to the technical standards established for obtaining serum, and stored at −20 °C until its use for serological tests. Sample processing was performed by pathologists and microbiologists with >8 years of experience to avoid variability between observers. All patient information and the test outcome were withheld until after the completion of the study.
2.4. Histopathology
The biopsy samples were fixed in 10% formalin solution, cut into slides, and stained with hematoxylin and eosin (H&E) and giemsa. The Sydney and OLGA/OLGIM classifications systems were used for histological characterization of the pathologies: chronic gastritis, peptic ulcer, and gastric lesions (intestinal metaplasia, gastric dysplasia, atrophic gastritis, and gastric adenocarcinoma) [18]. This method was performed independently on all patients to ensure the reliability of the estimates. Two pathologists examined each glass slide blindly for the presence/absence of H. pylori. The results were corroborated by a third pathologist to control for reporting bias.
2.5. Culture
One biopsy sample was grown on Columbia agar plates with Columbia blood agar medium (Oxoid, Hampshire, UK), containing 10% sheep-defibrinated blood and 1% heat-inactivated fetal bovine serum, supplemented with Dent antibiotic supplement (Oxoid, Hampshire, UK). The plates were incubated at 37 °C under microaerophilic conditions (10% CO2, 5% O2, and 85% N2) (CampyGen Compact, Oxoid, Hamphire, UK) with saturated humidity for five days. Morphological identification by Gram staining (Gram-negative bacilli), and biochemical oxidase, catalase, and urease tests were performed as described by Llanes et al. [9].
2.6. RUT
A biopsy specimen from each patient was inoculated into a vial containing 0.3 mL of urease test broth (Stuart’s transport medium, BBL, Cockeysville, MD, USA) at room temperature. A positive test was considered by the color change from the original yellowish to fuchsia [19].
2.7. H. pylori-IgG ELISA
The detection of IgG-class antibodies to H. pylori was performed using a commercial ELISA test kit (IBL International, Hamburg, Germany), previously validated at the IPK [20]. 100 μL of negative control, positive control, and serum samples were prepared according to the manufacturer’s instructions and added to each well of the ELISA microplate. The optical density was determined using a microplate reader at 450 nm. Cutoff index values of >1.2 were considered positive, values <0.8 were considered negative, and values from 0.8 to 1.2 were considered indeterminate.
2.8. Western Blotting (WB)
WB qualitative assay validated at the IPK [21] was performed using the Helicoblot 2.1 kit (Genelabs Diagnostics, Singapore) following the manufacturer’s instructions [22]. Immunoreactive bands were scanned using a GS-800 Calibrated Densitometer; (BioRad Laboratories, Hercules, CA, USA) equipped with Quantity One software (version 4.6.2, Bio-Rad). The presence of H. pylori recombinant proteins (89 kDa [VacA], 116 kDa [CagA], 37 kDa, 35 kDa, 30 kDa, 19.5kDa) with or without the current infection marker (10 kDa) was considered a positive result.
2.9. RT-PCR
DNA from biopsy specimens was extracted using the Macherey-Nagel kit (GmbH & Co. KG, Germany). A 267 base-pair fragment of the 23S ribosomal RNA gene was amplified using a LightCycler 480 thermal cycler version LCS480 1.5.1.62 (Roche Diagnostics, France) [23]. Each run used 10−2–10−6 dilutions of DNA (45-mg/mL) of the Hp H37Rv reference strain as a positive control and sterile water as a negative control. RT-PCR program consisted of one cycle of 10 min at 95 °C, followed by 50 cycles of 10 s at 95 °C, 10 s at 60 °C, and a final cycle of 17 s at 72 °C. Cycle number threshold values between 17–33 were considered positive when the negative control was undetectable.
2.10. Definition of H. pylori Status
A diagnostic positive was defined based on culture and histopathology positives. If at least one of the tests was negative, the H. pylori infection status was considered negative.
2.11. Statistical Analysis
The sample size was estimated through paired design analysis based on the McNemar test using Epidat Software version 3.1 [24]. Sample recruitment was guided by an expected 59% prevalence of H. pylori infection in a screening cohort, based on previous national and regional published data [11,12,21,25]. Calculations were performed using an expected sensitivity of 80% to detect sensitivity differences between the pairs (18%). A minimum sample size of 84 participants was deemed sufficient for 80% power and a significance level of α = 0.05.
Personal data (age, sex), diagnostic test results, H. pylori status definition (Table S2), and endoscopic and histopathological reports were included in the patient’ inquiry form. Absolute and relative frequencies were determined for each variable studied. The association between H. pylori infection and each diagnosis was studied using Pearson’s chi-squared test of independence and a significance level α = 0.05. Sensitivity, specificity, positive and negative predictive values, positive and negative likelihood ratios, diagnostic accuracy with 95% confidence intervals (CIs), and the Youden index of each test were calculated using the Epidat program (Table S3). Kappa values over 0.61 were considered a good agreement.
3. Results
After excluding 81 patients who did not meet the criteria for eligibility, 92 patients were included in the diagnostic test analysis. They were grouped according to the H. pylori status definition (infected patients, n = 46; non-infected patients, n = 46) (Figure 1).
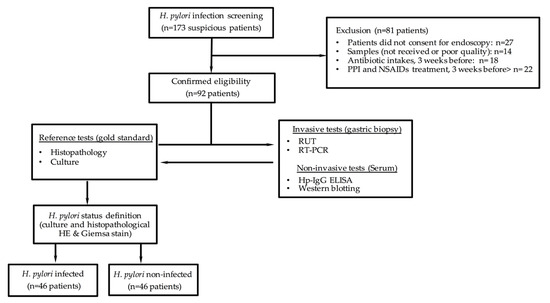
Figure 1.
Description of eligible, enrolled, and participating patients in the cross-sectional study cohort type to assess diagnostic accuracy.
Patients included in the study were, on average, 50 years old. The most frequent age range was 41–50 years (26.1%), followed by 60–87 (23.9%), and 51–60 (22.8%). Sixty-two percent of the participants (57) were females and 38% (35) were male, with a female/male ratio of 1.62:1.00 (Table 1).

Table 1.
Baseline characteristics of the participants.
According to the status definition, H. pylori infection prevalence was 50%, with a female predominance of 58.7%. The mean age of the patients who tested positive for H. pylori was 51.08 ± 15.49, with predominance in the age groups over 41 years (82.6%). There were no significant differences between men and women overall (p = 0.6676), or by age group (p = 0.1343) (Table 1).
Chronic gastritis (31.5%) and combined gastritis and duodenitis (29.3%) were the most frequent endoscopic and histopathological findings. The presence of H. pylori (12/26.1%) was associated with chronic moderate gastritis (p = 0.0007) and its absence was associated with chronic inactive gastritis (p = 0.0102) and the combined classification of gastritis and gastric lesions (p = 0.0352) (Table 2).

Table 2.
H. pylori infection status according to gastroduodenal diseases diagnosed by endoscopic and histopathological examination among the study population (n = 92).
RUT showed the highest overall performance with a sensitivity of 98.9%, specificity of 98.9%, accuracy of 98.9%, Youden index of 1.0, and kappa coefficient of 0.98, followed by Hp-IgG ELISA and WB. The RT-PCR showed the lowest performance with a sensitivity of 76.1%, specificity of 71.7%, total accuracy of 73.9%, Youden index of 0.5, and kappa coefficient of 0.48. RUT revealed the lowest false negative and false positive results and the best probability of correctly predicting the disease’s presence and/or absence (Table 3).

Table 3.
Precision indicators of diagnostic tests for detecting H. pylori in adult Primary Healthcare patients with the gastroduodenal disease compared to H. pylori status definition.
4. Discussion
H. pylori infection can be diagnosed by invasive and noninvasive techniques, such as RUT, culture, histopathology, RT-PCR, UBT, SAT, and serology. Each test has benefits and limitations; none is considered a perfect reference standard whose choice is decisive in assessing a diagnostic test’s accuracy [3,5,7]. Access to endoscopy for upper gastrointestinal tract diseases in Cuba is public at the primary healthcare level; however, some diagnostic tests are restricted to specialized hospitals and institutions. Moreover, the strategies for an effective diagnostic approach, particularly in primary care, depend on the clinical situation, local epidemiology, and cost effectiveness of diagnostic tests [8,26,27].
In the present study, we accurately evaluated noninvasive and invasive tests using samples from Cuban adult patients with dyspeptic symptoms. Consequently, RUT and H. pylori-IgG ELISA demonstrated the highest diagnostic performance for this infection. This information is particularly relevant for primary care services because the results support using these tools to monitor H. pylori-related diseases in susceptible populations.
The prevalence of H. pylori infection found in adults (50%) was superior to the current prevalence of H. pylori infection worldwide (44.3%) and similar to the H. pylori infection rate revealed in developing countries (50.8%) [1]. It is worth noting that a study by Galbán et al. showed an infection rate slightly higher (58.4%) in primary care facilities [10], comparable with the prevalence (59.3%) in Latin America and the Caribbean region [1].
This study shows a female predominance in H. pylori infection compared to males as, in Cuba, they are more frequent visitors to endoscopy services than males. Despite the previous statement, Agah et al. [28] found an association between the female gender and this bacterial infection. However, some investigations reveal a discrepancy in susceptibility to H. pylori infection on behalf of male predominance [29,30].
Several investigations based on geographically defined Cuban populations indicated dissimilar sociodemographic characteristics and unhealthy lifestyles [9,20,21]. This fact could explain the increase in the greater number of patients with gastritis (29/92), the gastric disease most frequently encountered in primary care in Havana [10], and the most frequent cause of chronic gastroduodenal disease in Cuba [9,21]. Likewise, other observations also showed combined gastritis and peptic ulcer in H. pylori-positive cases. Previous studies have demonstrated the presence of the bacterium in 95–100% of duodenal ulcers and 85–95% of gastric ulcers, suggesting an improvement in these patients when they adopt lifestyle changes and use validated anti-H. pylori therapies [3,6,9,31].
The H. pylori-negative association was corroborated in patients with combined gastritis and gastric lesions because, in these cases, the bacterial load is lowest, which negatively influences H. pylori detection [31,32]. However, it is essential to have tests that allow an early diagnosis of gastric lesions due to their evolution into stomach cancer, whose incidence rate in Cuba in 2020 was 9.7 for men and 6.1 for women per 100,000 inhabitants [11].
A limitation of this study was the insufficient number of biopsies analyzed in the histopathological examination. Sydney’s protocol and international consensus on H. pylori diagnosis support taking five gastric biopsy punches in different locations to reduce sampling errors and increase the efficacy of invasive methods [5,31,32]. However, a single biopsy sampling from the lesser curvature near the incisura angularis or the greater curvature opposite the incisura angularis has been considered for H. pylori infection diagnosis [33]. In this investigation, the use of specific stains (H&E and giemsa) for H. pylori detection, the pathologist’s expertise, and concordance with a culture made it possible to establish the current H. pylori status definition. Combining two available invasive tests (culture and histopathology) produced a better indicator of disease status.
The results of the studies by Galbán et al. provided evidence that RUT might be useful for diagnosing H. pylori infection in adult patients with gastroduodenal symptoms [10]. The present study further extends these findings and highly recommends RUT as a valuable tool to use in the endoscopy department of all Cuban primary care services due to its simple execution and straightforward interpretation compared with other invasive tests [34,35].
Another test that showed good accuracy (>80%) compared to the H. pylori status definition was H. pylori-IgG ELISA. A prior evaluation through case-control analysis (109 adult dyspeptic patients and 277 healthy individuals) reported excellent performance of ELISA-IBL (91.2% sensitivity, 90.2% specificity, 93.9% positive predictive value, and 86% negative predictive value), recommending this technique to evaluate H. pylori infection prevalence in the population [20]. The anti H. pylori-IgG antibody levels correlate with high diagnostic precision [36,37], which could guide the physician when endoscopy is unavailable or unjustified. This test is considered suitable for initial screening of H. pylori infection in a population with a rate of infection greater than 30% and the absence of other noninvasive tests [32,38]. Hence, the IgG detection ELISA system could be a screening tool for diagnosing H. pylori infection at the community level.
The commercial WB assay Helicoblot 2.1 is an excellent test for identifying different H. pylori antigenic markers and providing valuable prognostic information [21,22,39]. However, this assay was used as a second-line serological method to evaluate H. pylori seropositivity because of the potential for a false positive result. Even though WB may be a valuable tool for the direct visualization of highly specific H. pylori antigens, the findings of this study do not sustain the use of the Helicoblot 2.1 system as an initial screening tool in primary care centers.
In the literature, there is a tendency towards better sensitivity for RT-PCR, essentially from gastric biopsy, as it has several advantages, such as short working time, direct detection of microorganism presence, and low risk of contamination [23,40]. However, RT-PCR used in the study is not valuable in routine practice because of the low accuracy. Ramírez et al. recommend the detection of two H. pylori genes for better sensitivity in diagnoses from biopsies [41]. Other studies support the use of two biopsies obtained from antrum and corpus to increase the accuracy in detecting H. pylori infection by RT-PCR [42,43].
Based on these findings, we recommend using antibody testing (H. pylori-IgG ELISA) in any adult with dyspepsia <40 years old without any previous diagnosis of H. pylori infection, use of proton pump inhibitors and antimicrobials, or elevated risk of gastric cancer. RUT could be considered together with histology as the best approach for H. pylori diagnosis in adults >40 years old with an indication for upper endoscopy and a family history of gastric cancer in a first-degree relative.
In the Cuban context, the assessment of diagnosis accuracy carried out with different invasive and noninvasive tests offers an alternative to the recommended tests by international consensus. Using H. pylori-IgG ELISA and RUT at the primary care level would help focus available resources and yield future diagnostic evidence-based investigations that impact national guidelines for diagnosing and managing gastroduodenal diseases with H. pylori infection in adult patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11040997/s1, Table S1: STROBE Statement–Checklist of items that are included in the cross-sectional study, Table S2: Report of Diagnostic Tests versus H. pylori status for each patient enrolled in the study, Table S3: Statistical measures for diagnostic accuracy assessment of index tests using Epidat 3.1 program. References [44,45,46] are cited in the supplementary materials.
Author Contributions
Conceptualization: A.D., R.F., B.G., O.F., O.G., W.B., M.C.F., R.L. and L.S.; methodology, A.D., R.F., B.G., O.F. and W.B.; validation, and formal analysis, A.D., R.F., B.G., W.B., M.C.F. and R.L.; investigation, data curation, and writing—original draft preparation, A.D., R.F., B.G., O.F. and O.G.; Writing—review and editing, A.D., R.F., B.G., O.F., O.G., W.B., M.C.F. and L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the “Pedro Kourí” Institute of Tropical Medicine (approval number 2014/08).
Informed Consent Statement
All subjects gave their informed consent for inclusion before they participated in the study. The data were anonymized and did not include details that can identify patients.
Data Availability Statement
The data presented in this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors are grateful for the contribution of Armando Orellana Molina, Odalys Borrego of the endoscopy service of the Primary Care Polyclinic 19 de Abril and Technician Tatiana Almaguer of the IPK for the realization of this study.
Conflicts of Interest
All authors have declared that no financial support was received from any organization for the submitted work. All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might be interested in the submitted work. All authors have declared that no other relationships or activities could appear to have influenced the submitted work.
References
- Zamani, M.; Ebrahimtabar, F.; Zamani, V.; Miller, W.H.; Alizadeh-Navaei, R.; Shokri-Shirvani, J.; Derakhshan, M.H. Systematic review with meta-analysis: The worldwide prevalence of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2018, 47, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Fong, I. Helicobacter pylori Infection: When Should It Be Treated? In Current Trends and Concerns in Infectious Diseases; Emerging Infectious Diseases of the 21st Century Ed.; Springer Nature: Gewerbesrasse, Switzerland, 2020; Chapter 4; pp. 81–102. [Google Scholar] [CrossRef]
- Lee, Y.C.; Dore, M.P.; Graham, D.Y. Diagnosis and Treatment of Helicobacter pylori Infection. Annu. Rev. Med. 2022, 73, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Dore, M.P.; Pes, G.M. What is new in Helicobacter pylori diagnosis. An overview. J. Clin. Med. 2021, 10, 2091. [Google Scholar] [CrossRef]
- Liu, W.Z.; Xie, Y.; Lu, H.; Cheng, H.; Zeng, Z.R.; Zhou, L.Y.; Chen, Y.; Wang, J.B.; Du, Y.Q.; Lu, N.H.; et al. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter 2018, 23, e12475. [Google Scholar] [CrossRef]
- Cardos, A.I.; Maghiar, A.; Zaha, D.C.; Pop, O.; Fritea, L.; Miere, F.; Cavalu, S. Evolution of Diagnostic Methods for Helicobacter pylori Infections: From Traditional Tests to High Technology, Advanced Sensitivity and Discrimination Tools. Diagnostics 2022, 12, 508. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- Llanes, R.; Feliciano, O.; Gutiérrez, O.; Gala, A.; Valdés, L.; Capó, V.; Llop, A.; Millán, L.; Rodríguez, A. Nuevos conocimientos sobre el diagnóstico y la resistencia antimicrobiana de Helicobacter pylori en Cuba. Rev. Ann. Acad. Cienc. Cuba 2014, 4, 1–9. Available online: http://www.revistaccuba.cu (accessed on 17 February 2023). ISSN:2304-0106.
- Galbán, E.; Arús, E.; Periles, U. Endoscopic findings and associated risk factors in primary health care settings in Havana, Cuba. MED Rev. 2012, 14, 30–37. [Google Scholar] [CrossRef]
- Rodríguez, A.; Llanes, R.; Bello, M.; Langaney, J.; Verdasquera, D.; Argüez, A.; Ruiz, S. Infección por Helicobacter pylori en pacientes atendidos en el Hospital General Docente Iván Portuondo. Panorama Cuba Salud 2014, 8, 26–32. Available online: https://revpanorama.sld.cu/index.php/panorama/article/view/16 (accessed on 17 February 2023).
- Gutiérrez, B.; Vidal, T.; Valmaña, C.E.; Camou-Juncas, C.; Santos, A.; Mégraud, F.; González, N.; Leonard, I.; Martínez, R.; Díaz-Canel, O.; et al. Helicobacter pylori infection in Havana, Cuba. Prevalence and cagA status of the strains. Vaccimonitor 2005, 14, 15–19. Available online: https://researchonline.nd.edu.au/med_article/151/ (accessed on 17 February 2023).
- Anuario Estadístico de Salud. República de Cuba. 2020, 1–192. Available online: https://temas.sld.cu/estadisticassalud/2021/08/11/anuario-estadistico-de-salud-2020/ (accessed on 17 February 2023).
- Rendón-Macías, M.E.; Villasís-Keever, M.A. Phases to determine the clinical utility of diagnostic tests. Rev. Alerg. Mex. 2020, 67, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Mathes, T.; Pieper, D. An algorithm for the classification of study designs to assess diagnostic, prognostic and predictive test accuracy in systematic reviews. Syst. Rev. 2019, 8, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Matsuzaki, J.; Hibi, T. What is the difference between Helicobacter pylori-associated dyspepsia and functional dyspepsia? J. Neurogastroenterol. Motil. 2011, 17, 124–130. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, N. Diagnosis of Helicobacter pylori by invasive test: Histology. Ann Transl Med 2015, 3, 1–8. [Google Scholar] [CrossRef]
- Dolak, W.; Bilgilier, C.; Stadlmann, A.; Leiner, J.; Püspök, A.; Plieschnegger, W.; Siebert, F.; Wewalka, F.; Schöfl, R.; Huber-Schönauer, U.; et al. A multicenter prospective study on the diagnostic performance of a new liquid rapid urease test for the diagnosis of Helicobacter pylori infection. Gut Pathog. 2017, 9, 78. [Google Scholar] [CrossRef]
- Corrales, R. Ensayo inmunoenzimático para la detección de anticuerpos IgG contra Helicobacter pylori. Master’s thesis, Postgraduate Center of Pedro Kourí Tropical Medicine Institute, School of Medicine of the University of Havana, Havana, Cube, 2016. [Google Scholar]
- Feliciano, O.; Falcon, R.; Duquesne, A.; Fleitas, O.; Almaguer, T.; Gutierrez, O.; Orellana, A.; Capó, V.; Llanes, R. Helicobacter pylori antigen recognition patterns among cuban patients using Helicoblot 2.1 diagnostic system. Trop Gastroenterol. 2019, 40, 93–98. [Google Scholar] [CrossRef]
- Veijola, L.; Oksanen, A.; Sipponen, P.; Rautelin, H. Evaluation of a commercial immunoblot, Helicoblot 2.1 for diagnosis of Helicobacter pylori infection. Clin. Vaccine Immunol. 2008, 15, 1705–1710. [Google Scholar] [CrossRef]
- Hays, C.; Delerue, T.; Lamarque, D.; Burucoa, C.; Collobert, G.; Billöet, A.; Kalach, N.; Raymond, J. Molecular diagnosis of Helicobacter pylori infection in gastric biopsies: Evaluation of the Amplidiag® H. pylori + ClariR assay. Helicobacter 2019, 24, e12560. [Google Scholar] [CrossRef]
- Bravo Grau, S.; Cruz, J.P. Estudios de exactitud diagnóstica: Herramientas para su interpretación. Rev. Chil. Radiol. 2015, 21, 158–164. [Google Scholar] [CrossRef]
- Curado, M.P.; de Oliveira, M.M.; de Araújo Fagundes, M.; de Araújo Fagundes, M. Prevalence of Helicobacter pylori infection in Latin America and the Caribbean populations: A systematic review and meta-analysis. Cancer Epidemiol. 2019, 60, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Miftahussurur, M.; Yamaoka, Y. Diagnostic methods of Helicobacter pylori infection for epidemiological studies: Critical importance of indirect test validation. Biomed Res. Int. 2016, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Siddique, I.; Al-Mekhaizeem, K.; Alateeqi, N.; Memon, A.; Hasan, F. Diagnosis of Helicobacter pylori: Improving the sensitivity of CLOtest by increasing the number of gastric antral biopsies. J. Clin. Gastroenterol. 2008, 42, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Agah, S.; Khedmat, H.; Ghamar-Chehred, M.E.; Hadi, R.; Aghaei, A. Female gender and Helicobacter pylori infection, the most important predisposition factors in a cohort of gastric cancer: A longitudinal study. Caspian J. Intern Med. 2016, 7, 136–141, PMID: 27386067. [Google Scholar]
- Ibrahim, A.; Morais, S.; Ferro, A.; Lunet, N.; Peleteiro, B. Sex-differences in the prevalence of Helicobacter pylori infection in pediatric and adult populations: Systematic review and meta-analysis of 244 studies. Dig. Liver. Dis. 2017, 49, 742–749. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, H.; Zhang, M.; Li, C.; Tang, Y.; Li, X.; Yuan, S.; Wei, Q.; Wang, J.; Ning, X.; et al. Sex-Specific risk factors associated with Helicobacter pylori infection among individuals undergoing health examinations in China. Int. J. Gen. Med. 2022, 15, 5861–5868. [Google Scholar] [CrossRef]
- Rawla, P.; Barsouk, A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Prz. Gastroenterol. 2019, 14, 26–38. [Google Scholar] [CrossRef]
- Mégraud, F. The most important diagnostic modalities for Helicobacter pylori, now and in the future. Eur. J. Gastroenterol. Hepatol. 2012, 9, S13–S15, discussion S27-9. [Google Scholar] [CrossRef]
- Abou-Rached, A.; Jowana-Saba, C.; Yaghi, J.S.M.; Sanyour, A.E.H.; Abou-Kheir, S. Prospective study to evaluate the number and the location of biopsies in rapid urease test for diagnosis of Helicobacter pylori. Gastroenterol. Insights 2017, 8, 7223. [Google Scholar] [CrossRef]
- Midolo, P.; Marshall, B.J. Accurate diagnosis of Helicobacter pylori. Urease tests. Gastroenterol. Clin. N. Am. 2000, 29, 871–878. [Google Scholar] [CrossRef] [PubMed]
- McNicholl, A.G.; Ducons, J.; Barrio, J.; Bujanda, L.; Forné-Bardera, M.; Aparcero, R.; Ponce, J.; Rivera, R.; Dedeu-Cuso, J.M.; Garcia-Iglesias, P.; et al. Accuracy of the ultra-rapid urease test for diagnosis of Helicobacter pylori infection. Gastroenterol. Hepatol. 2017, 40, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Piroozmand, A.; Soltani, B.; Razavizadeh, M.; Matini, A.H.; Gilasi, H.R.; Zavareh, A.N.; Soltani, S. Comparison of the serum and salivary antibodies to detect gastric Helicobacter pylori infection in Kashan (Iran). Electron. Physician 2017, 9, 6129–6134. [Google Scholar] [CrossRef] [PubMed]
- Burucoa, C.; Delchier, J.C.; Courillon-Mallet, A.; de Korwin, J.D.; Mégraud, F.; Zerbib, F.; Raymond, J.; Fauchère, J.L. Comparative evaluation of 29 commercial Helicobacter pylori serological kits. Helicobacter 2013, 18, 169–179. [Google Scholar] [CrossRef]
- Bosch, D.E.; Krumm, N.; Wener, M.H.; Yeh, M.M.; Truong, C.D.; Reddi, D.M.; Liu, Y.; Swanson, P.E.; Schmidt, R.A.; Bryan, A. Serology is more sensitive than urea breath test or stool antigen for the initial diagnosis of Helicobacter pylori gastritis when compared with histopathology. Am. J. Clin. Pathol. 2020, 154, 255–265. [Google Scholar] [CrossRef]
- Biranjia-Hurdoyal, S.D.; Seetulsingh-Goorah, S.P. Performances of four Helicobacter pylori serological detection kits using stool antigen test as gold standard. PLoS ONE 2016, 11, e0163834. [Google Scholar] [CrossRef]
- Ierardi, E.; Giorgio, F.; Losurdo, G.; Sorrentino, C.; Principi, M.; Di Leo, A. Detection of Helicobacter pylori DNA sequences in gastric biopsy samples to refine the diagnosis and therapy. J. Med. Microbiol. 2015, 64, 788–789. [Google Scholar] [CrossRef]
- Ramírez-Lazaro, M.J.; Lario, S.; Casalots, A.; Sanfeliu, E.; Boix, L.; Garcıa-Iglesias, P.; Sánchez-Delgado, J.; Montserrat, A.; Bella-Cueto, M.R.; Gallach, M.; et al. Real-time PCR improves Helicobacter pylori detection in patients with peptic ulcer bleeding. PLoS ONE 2011, 6, e20009. [Google Scholar] [CrossRef]
- Gastli, N.; Allain, M.; Lamarque, D.; Abitbol, V.; Billoët, A.; Collobert, G.; Coriat, R.; Terris, B.; Kalach, N.; Raymond, J. Diagnosis of Helicobacter pylori infection in a routine testing workflow: Effect of bacterial load and virulence factors. J. Clin. Med. 2021, 10, 2755. [Google Scholar] [CrossRef]
- AbdEllatif, M.E.; Shahin, R.; Shawqy, A.; Abbas, A.; Amer, M.A.; AbdelGhafarSaleh, A.; El-Ghadban, H.; Husien, A.M. Additional endoscopic corpus biopsy may increase accuracy of Helicobacter pylori detection. ARC J. Surg. 2016, 2, 1–9. [Google Scholar] [CrossRef]
- Akoglu, H. User’s guide to sample size estimation in diagnostic accuracy studies. Turk. J. Emerg. Med. 2022, 22, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Šimundić, A.M. Measures of diagnostic accuracy: Basic definitions. Electron. J. Int. Fed. Clin. Chem. Lab. Med. 2009, 19, 203–211. [Google Scholar]
- Canbek, G.; Taskaya Temizel, T.; Sagiroglu, S. PToPI: A comprehensive review, analysis, and knowledge representation of binary classification performance measures/metrics. SN Comput. Sci. 2023, 4, 13. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).