Antibiotic Prescriptions in Critically Ill Patients with Bloodstream Infection Due to ESBL-Producing Enterobacteriaceae: Compliance with the French Guidelines for the Treatment of Infections with Third-Generation Cephalosporin-Resistant Enterobacteriaceae—A Multicentric Retrospective Cohort Study
Abstract
:1. Introduction
2. Patients and Methods
2.1. Setting and Study Population
2.2. Data Collection
2.3. Objectives and Definitions
- In the absence of septic shock and a history of ESBL-E resistant to PIP-TAZ urinary infection/colonization <3 months;
- In the presence of septic shock and a history of ESBL-E urinary infection/colonization or antibiotic treatment within 3 months.
- A history of ESBL-E urinary infection/colonization or antibiotic treatment within 3 months.
- Treatment with PIP-TAZ or a cephalosporin active against P. aeruginosa within 1 month;
- A history of ESBL-E or PIP-TAZ resistant P. aeruginosa infection/colonization within 3 months.
- In the case of ESBL-E colonization:
- In the case of septic shock and a history of ESBL-E colonization/infection within 3 months.
- Acute pyelonephritis or complicated UTI with a susceptible strain in order of preference: trimethoprim–sulfamethoxazole, fluoroquinolone, cefoxitin (in case of E. coli), temocillin, amoxicillin–clavulanate, PIP-TAZ, and aminoglycosides;
- Intra-abdominal infection with a controlled source of infection and a strain with PIP-TAZ CMI ≤ 4: PIP-TAZ;
- Pneumonia and a strain with PIP-TAZ CMI ≤ 4: PIP-TAZ; otherwise, if susceptibility to quinolone: a fluoroquinolone. The use of temocillin or trimethoprim–sulfamethoxazole could be proposed.
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Data
3.2. Microbiological Data
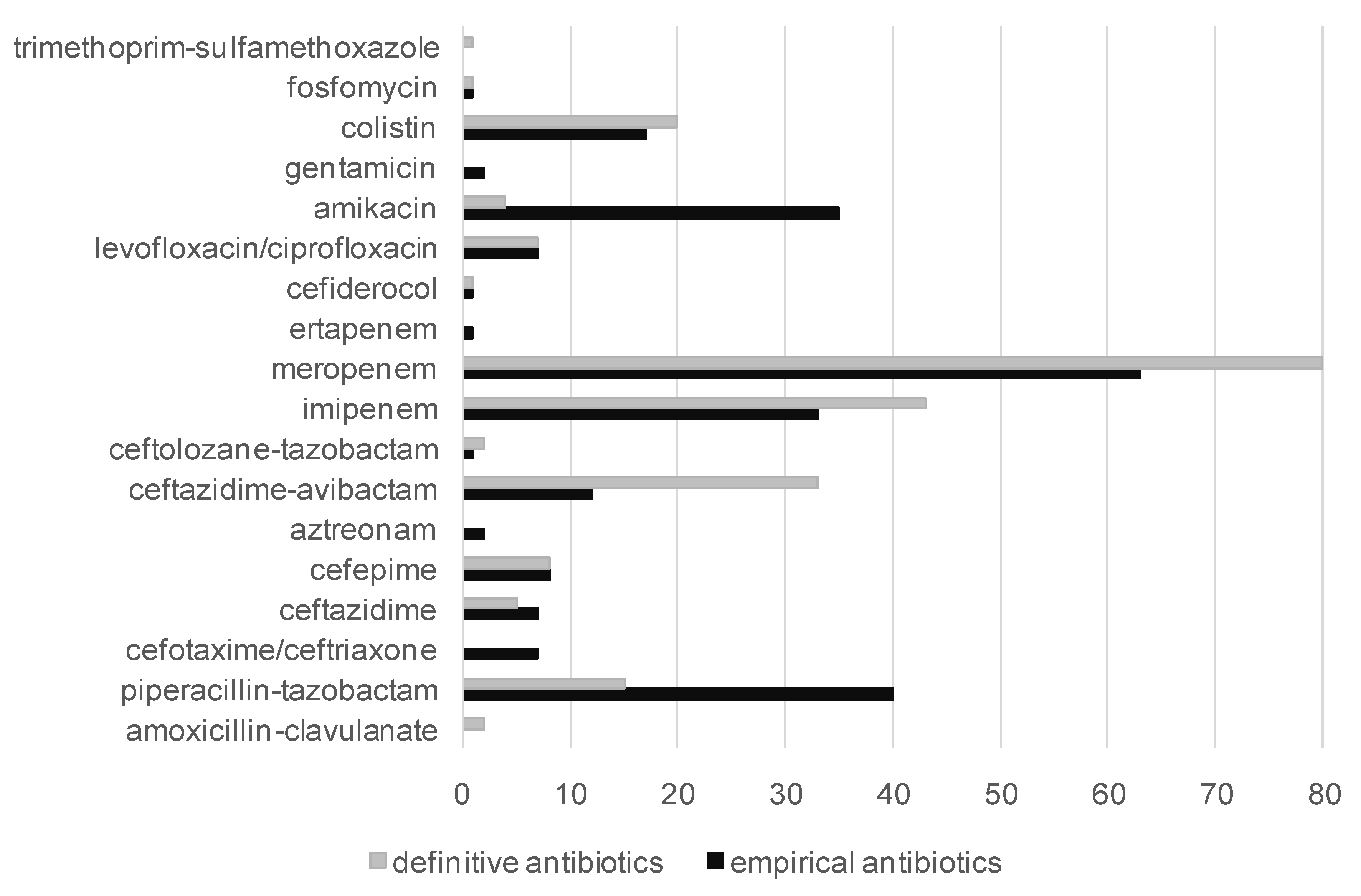
3.3. Empirical Antibiotic Treatment
3.4. Adequation and Appropriateness of Empirical Antibiotic Treatment
3.5. Factors Associated with an Empirical Prescription of a Carbapenem in Adequation with the Guidelines
3.6. Definitive Antibiotic Treatment and Adequation with the Guidelines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodríguez-Baño, J.; Navarro, M.D.; Romero, L.; Muniain, M.A.; de Cueto, M.; Ríos, M.J.; Hernandez, J.R.; Pascual, A. Bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli in the CTX-M era: A new clinical challenge. Clin. Infect. Dis. 2006, 43, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Sanguinetti, M.; Montuori, E.; Trecarichi, E.M.; Posteraro, B.; Fiori, B.; Citton, R.; D’Inzeo, T.; Fadda, G.; Cauda, R.; et al. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae: Importance of inadequate initial antimicrobial treatment. Antimicrob. Agents Chemother. 2007, 51, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Jean, S.S.; Harnod, D.; Hsueh, P.R. Global threat of carbapenem-resistant Gram-negative bacteria. Front. Cell. Infect. Microbiol. 2022, 12, 823684. [Google Scholar] [CrossRef]
- Tamma, P.D.; Rodriguez, J. The use of noncarbapenem β-lactams for the treatment of extended-spectrum β-lactamase infections. Clin. Infect. Dis. 2017, 2017, 972–980. [Google Scholar] [CrossRef]
- Harris, P.N.A.; Tambyah, P.A.; Lye, D.C.; Mo, Y.; Lee, T.H.; Yilmaz, M.; Alenazi, T.H.; Arabi, Y.; Falcone, M.; Bassetti, M.; et al. MERINO Trial Investigators and the Australasian Society for Infectious Disease Clinical ResearchNetwork (ASID-CRN). Effect of piperacillin-tazobactam vs meropenem on 30-Day mortality for patients with E. coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: A randomized clinical trial. JAMA 2018, 320, 984–994. [Google Scholar]
- Antibiothérapie Des Infections à entérobactéries Et à Pseudomonas aeruginosa Chez L’adulte: Place Des Carbapénèmes Et de Leurs Alternatives. Mai 2019. Mise à Jour Mars 2023. Available online: https://has-sante.fr/jcms/c_2968915/fr/antibiotherapie-des-infections-a-enterobacteries-et-a-pseudomonas-aeruginosa-chez-l-adulte-place-des-carbapenemes-et-de-leurs-alternatives (accessed on 1 August 2023).
- European Committee on Antimicrobial Susceptibility Testing (EUCAST 2012). Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 2.0. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST-files/Breakpoint-tables/Breakpoint-table-v-2.0-120222.pdf (accessed on 1 August 2023).
- McCabe, W.R.; Jackson, C.G. Gram-negative bacteremia: Etiology and ecology. Arch. Intern. Med. 1962, 110, 847–855. [Google Scholar] [CrossRef]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfonction/failure. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C.; Fischer, C.J.; Clemmer, T.P.; Slotman, G.J.; Metz, C.A.; Balk, R.A. The methylprednisolone severe sepsis study group. Sepsis syndrome: A valid clinical entity. Crit. Care Med. 1989, 17, 389–393. [Google Scholar] [CrossRef]
- Weiss, E.; Zahar, J.R.; Lesprit, P.; Ruppe, E.; Leone, M.; Chastre, J.; Lucet, J.C.; Paugam-Burtz, C.; Brun-Buisson, C.; Timsit, J.F.; et al. Elaboration of a consensual definition of de-escalation allowing a ranking of ß-lactams. Clin. Microbiol. Infect. 2015, 21, 649.e1–649.e10. [Google Scholar]
- Kern, W.V.; Rieg, S. Burden of bacterial bloodstream infection-a brief update on epidemiology and significance of multidrug-resistant pathogens. Clin. Microbiol. Infect. 2020, 26, 151–157. [Google Scholar] [CrossRef] [PubMed]
- McDanel, J.; Schweizer, M.; Crabb, V.; Nelson, R.; Samore, M.; Khader, K.; Blevins, A.E.; Diekema, D.; Chiang, H.Y.; Nair, R.; et al. Incidence of Extended-Spectrum β-Lactamase (ESBL)-Producing Escherichia coli and Klebsiella Infections in the United States: A Systematic Literature Review. Infect. Control Hosp. Epidemiol. 2017, 38, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Chu, F.Y.; Hsieh, C.C.; Hong, M.Y.; Chi, C.H.; Ko, W.C.; Lee, C.C. A simple scoring algorithm predicting extended-spectrum β-lactamase producers in adults with community-onset monomicrobial Enterobacteriaceae bacteremia: Matters of frequent emergency department users. Medicine 2017, 96, e6648. [Google Scholar] [CrossRef] [PubMed]
- Rottier, W.C.; Bamberg, Y.R.; Dorigo-Zetsma, J.W.; van der Linden, P.D.; Ammerlaan, H.S.; Bonten, M.J. Predictive value of prior colonization and antibiotic use for third-generation cephalosporin-resistant enterobacteriaceae bacteremia in patients with sepsis. Clin. Infect. Dis. 2015, 60, 1622–1630. [Google Scholar]
- Madrid-Morales, J.; Sharma, A.; Reveles, K.; Velez-Mejia, C.; Hopkins, T.; Yang, L.; Walter, E.; Cadenaa, J. Validation of Available Extended-Spectrum-Beta-Lactamase Clinical Scoring Models in Predicting Drug Resistance in Patients with Enteric Gram-Negative Bacteremia Treated at South Texas Veterans Health Care System. Antimicrob. Agents Chemother. 2021, 65, e02562-20. [Google Scholar] [CrossRef]
- Elligsen, M.; Pinto, R.; Leis, J.A.; Walker, S.A.N.; MacFaden, D.R.; Daneman, N. Using prior culture results to improve initial empiric antibiotic prescribing: An evaluation of a simple clinical heuristic. Clin. Infect. Dis. 2021, 73, e417. [Google Scholar] [CrossRef]
- Elligsen, M.; Pinto, R.; Leis, J.A.; Walker, S.A.N.; Daneman, N.; MacFadden, D.R. Improving decision making in empiric antibiotic selection (IDEAS) for Gram-negative bacteremia: A prospective clinical implementation study. Clin. Infect. Dis. 2021, 73, e630. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America 2022 Guidance on the Treatment of Extended-Spectrum β-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin. Infect. Dis. 2022, 75, 187–212. [Google Scholar]
- Paul, M.; Carrara, E.; Retamar, P.; Tängden, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar]
- Kadri, S.S.; Adjemian, J.; Lai, Y.L.; Spaulding, A.B.; Ricotta, E.; Prevots, D.R.; Palmore, T.N.; Rhee, C.; Klompas, M.; Dekker, J.P.; et al. Difficult-to-treat resistance in Gram-negative bacteremia at 173 US Hospitals: Retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin. Infect. Dis. 2018, 67, 1803–1814. [Google Scholar] [CrossRef]
- Teitelbaum, D.; Elligsen, M.; Katz, K.; Lam, P.W.; Lo, J.; MacFadden, D.; Vermeiren, C.; Daneman, N. Introducing the escalation antibiogram: A simple tool to inform changes in empiric antimicrobials in the non responding patient. Clin. Infect. Dis. 2022, 75, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Guarascio, A.J.; Brickett, L.M.; Porter, T.J.; Lee, N.D.; Gorse, E.E.; Covey, J.R. Developmment of a statewide antibiogram to assess regional trends in antibiotic-resistant ESKAPE organisms. J. Pharm. Pract. 2019, 32, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Huson, M.A.M.; Stolp, S.M.; van der Poll, T.; Grobusch, P.M. Community-acquired bacterial bloodstream infections in HIV-infected patients: A systematic review. Clin. Infect. Dis. 2014, 58, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Gill, J.M.; Viczko, J.; Naugler, C.; Church, D. Risk factors and outcomes of bloodstream infections among people with human immunodeficiency virus: A longitudinal cohort study from 2000 to 2017. Open Forum Infect. Dis. 2022, 3, ofac318. [Google Scholar]
- Dellinger, P.; Rhodes, A.; Evans, L.; Alhazzani, W.; Beale, R.; Jaeschke, R.; Machado, F.R.; Masur, H.; Osborn, T.; Parker, M.M.; et al. Surviving sepsis campaign. Crit. Care Med. 2023, 51, 431–444. [Google Scholar] [CrossRef]
- Lien, F.; Lin, H.S.; Wu, Y.T.; Chiueh, T.S. Bacteremia detection from complete blood count and differential leukocyte count with machine learning: Complementary and competitive with C-reactive protein and procalcitonin tests. BMC Infect. Dis. 2022, 22, 287. [Google Scholar]
- Li, D.; Li, J.; Zhao, C.; Liao, X.; Xie, L.; Shang, W. Diagnostic value of procalcitonin, hypersensitive C-reactive protein and neutrophil-to-lymphocyte ratio for bloodstream infections in pediatric tumor patients. Clin. Chem. Lab. Med. 2023, 61, 366. [Google Scholar]
- Vincent, J.L.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.R.; Marshall, J.C.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA 2020, 323, 1478–1487. [Google Scholar] [CrossRef]
- Houard, M.; Rouze, A.; Ledoux, G.; Six, S.; Jaillette, E.; Poissy, J.; Preau, S.; Wallet, F.; Labreuche, J.; Nseir, S.; et al. Relationship between digestive tract colonization and subsequent ventilator-associated pneumonia related to ESBL-producing Enterobacteriaceae. PLoS ONE 2018, 13, e0201688. [Google Scholar] [CrossRef]
- Barbier, F.; Bailly, S.; Schwebel, C.; Papazian, L.; Azoulay, E.; Kallel, H.; Siami, S.; Argaud, L.; Marcotte, G.; Misset, B.; et al. Infection-related ventilator-associated complications in ICU patients colonised with extended-spectrum beta-lactamase-producing Enterobacteriaceae. Intensive Care Med. 2018, 44, 616–626. [Google Scholar] [CrossRef]
- Das, S.; Li, J.; Riccobene, T.; Carrothers, T.J.; Newell, P.; Melnick, D.; Critchley, I.A.; Stone, G.G.; Nichols, W.W. Dose Selection and Validation for Ceftazidime-Avibactam in Adults with Complicated Intra-Abdominal Infections, Complicated Urinary Tract Infections, and Nosocomial Pneumonia. Antimicrob. Agents Chemother. 2019, 63, e02187-18. [Google Scholar] [CrossRef] [PubMed]
- Isler, B.; Ezure, Y.; Garcia-Fogeda Romero, J.L.; Harris, P.; Stewart, A.G.; Paterson, D.L. Is ceftazidime/avibactam an option for serious infections due to extended-spectrum-β-lactamase- and AmpC-producing enterobacterales?: A systematic review and meta-analysis. Antimicrob. Agents Chemother. 2021, 65, e01052-20. [Google Scholar] [CrossRef] [PubMed]
- Dequin, P.F.; Aubron, C.; Faure, H.; Garot, D.; Guillot, M.; Hamzaoui, O.; Lemiale, V.; Maizel, J.; Mootien, J.Y.; Osman, D.; et al. The place of new antibiotics for Gram-negative bacterial infections in intensive care: Report of a consensus conference. Ann. Intensive Care 2023, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, J.; Xiao, Q.; Wang, Y.; Wang, J.; Zhu, M.; Cai, Y. Carbapenem-sparing beta-lactam/beta-lactamase inhibitors versus carbapenems for bloodstream infections caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae: A systematic review and meta-analysis. Int. J. Infect. Dis. 2023, 128, 194–204. [Google Scholar] [CrossRef]
- Umemura, T.; Kato, H.; Hagihara, M.; Hirai, J.; Yamagishi, Y.; Mikamo, H. Efficacy of combination therapies for the treatment of multi-drug resistant Gram-negative bacterial infections based on meta-analyses. Antibiotics 2022, 11, 524. [Google Scholar] [CrossRef]
- Benetazzo, L.; Delannoy, P.Y.; Houard, M.; Wallet, F.; Lambiotte, F.; Vachée, A.; Batt, C.; Van Grunderbeeck, N.; Nseir, S.; Robineau, O.; et al. Combination therapy with aminoglycoside in bacteremias due to ESBL-producing Enterobacteriaceae in ICU. Antibiotics 2020, 9, 777. [Google Scholar] [CrossRef]
- Kumar, A.; Zarychanski, R.; Light, B.; Parrillo, J.; Maki, D.; Simon, D.; Laporta, D.; Lapinsky, S.; Ellis, P.; Mirzanejad, Y.; et al. Early combination antibiotic therapy yields improved survival compared with monotherapy in septic shock: A propensity-matched analysis. Crit. Care Med. 2010, 38, 1773–1785. [Google Scholar] [CrossRef]
- Laterre, P.F.; Wittebole, X.; Van de Velde, S.; Muller, A.E.; Mouton, J.W.; Carryn, S.; Tulkens, P.M.; Dugernier, T. Temocillin (6g daily) in critically ill patients: Continuous infusion versus three times daily administration. J. Antimicrob. Chemother. 2015, 2015, 891–898. [Google Scholar] [CrossRef]
- Marin-Candon, A.; Rosso-Fernandez, C.M.; Bustos de Godoy, N.; Lopez-Cerero, L.; Gutierrez-Gutierrez, B.; Lopez-Cortes, L.E.; Barrera Pulido, L.; BorregueroBorreguero, I.; Leon, M.J.; Merino, V.; et al. Temocillin versus meropenem for the targeted treatment of bacteraemia due to third-generation cephalosporin-resistant Enterobacterales (ASTARTE): Protocol for a randomised, pragmatic trial. BMJ Open 2021, 11, e049481. [Google Scholar] [CrossRef]
- Luyt, C.E. Piperacillin-Tazobactam and Temocillin as Carbapenem-Alternatives for the Treatment of Severe Infections Due to Extended-Spectrum Beta-Lactamase Producing Gram-Negative Enterobacteriaceae in the Intensive Care Unit (PITAGORE). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05565222 (accessed on 4 September 2023).
- Lo, C.L.; Lee, C.C.; Li, C.W.; Li, M.C.; Hsueh, P.R.; Lee, N.Y.; Ko, W.C. Fluoroquinolone therapy for bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Microbiol. Immunol. Infect. 2017, 2017, 355–361. [Google Scholar] [CrossRef]
- Meije, Y.; Pigrau, C.; Fernández-Hidalgo, N.; Clemente, M.; Ortega, L.; Sanz, X.; Loureiro-Amigo, J.; Sierra, M.; Ayestarán, A.; Morales-Cartagena, A.; et al. Non-intravenous carbapenem-sparing antibiotics for definitive treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum β-lactamase (ESBL) or AmpC β-lactamase: A propensity score study. Int. J. Antimicrob. Agents 2019, 54, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Mission Spares. Surveillance de la Consommation des Antibiotiques et des Résistances Bactériennes en Établissement de Santé. Mission SPARES, Résultats 2021. Saint-Maurice: Santé Publique France, 2023. Available online: https://www.santepubliquefrance.fr/maladies-et-traumatismes/infections-associees-aux-soins-et-resistance-aux-antibiotiques/resistance-aux-antibiotiques/documents/enquetes-etudes/surveillance-de-la-consommation-des-antibiotiques-et-des-resistances-bacteriennes-en-etablissement-de-sante.mission-spares-resultats-2021 (accessed on 22 October 2023).
- Bush, K.; Bradford, P.A. Epidemiology of β-lactamase-producing pathogens. Clin. Microbiol. Rev. 2020, 33, e00047-19. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.J.; Rawson, T.M.; Mookerjee, S.; Price, J.R.; Davies, F.; Otter, J.; Aylin, P.; Hope, R.; Gilchrist, M.; Shersing, Y.; et al. Changing patterns of bloodstream infections in the community and acute care across 2 coronavirus disease 2019 epidemic waves: A retrospective analysis using data linkage. Clin. Infect. Dis. 2022, 24, e1082–e1091. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.N.; Fowler, R.; Balada-Llasat, J.M.; Carroll, A.; Hanna Stone, H.; Akerele, O.; Buchan, B.; Windham, S.; Hopp, A.; Ronen, S.; et al. Multicenter Evaluation of the BioFire FilmArray Pneumonia/Pneumonia Plus Panel for Detection and Quantification of Agents of Lower Respiratory Tract Infection. J. Clin. Microbiol. 2020, 58, e00128-20. [Google Scholar] [CrossRef]
- Strich, J.R.; Ricotta, E.; Warner, S.; Lai, Y.L.; Demirkale, C.Y.; Hohmann, S.F.; Rhee, C.; Klompas, M.; Palmore, T.; Powers III, J.H.; et al. Pharmacoepidemiology of Ceftazidime-Avibactam Use: A Retrospective Cohort Analysis of 210 US Hospitals. Clin. Infect. Dis. 2021, 72, 611–621. [Google Scholar] [CrossRef]

| Variable | Total (n = 185) |
|---|---|
| Demographics | |
| Age (years), median (IQR) | 60 (19) |
| Male, n (%) | 126 (68.0) |
| Underlying diseases, n (%) | |
| McCabe > 1 | 53 (28.6) |
| Diabetes mellitus | 64 (34.6) |
| Heart failure | 25 (13.5) |
| COPD | 34 (18.4) |
| Chronic renal insufficiency | 21 (11.4) |
| Immunodeficiency, n (%) | 45 (24.3) |
| Immunosuppressive therapy | 22 (11.9) |
| Solid cancer | 22 (11.9) |
| Hematological malignancy | 18 (9.7) |
| Transplantation | 9 (4.9) |
| Admission, n (%) | |
| Medical | 162 (87.6) |
| Unscheduled surgical | 23 (12.4) |
| ESBL-E risk factors, n (%) | |
| Antibiotic treatment in the last 3 months | 153 (82.7) |
| Colonization with ESBL-E | 147 (79.5) |
| Hospital acquired infection | 166 (89.7) |
| Hospital stay before BSI onset, days, median (IQR) | 21 (24) |
| ICU stay before BSI onset, days, median (IQR) | 15 (22) |
| Source of BSI, n (%) | |
| Pneumonia | 103 (55.7) |
| Urinary tract | 26 (14.1) |
| Catheter related | 24 (13.0) |
| Intra-abdominal | 24 (13.0) |
| Unknown | 6 (3.2) |
| Disease severity at BSI onset, n (%) | |
| SOFA score, median (IQR) | 6 (5) |
| Pitt score, median (IQR) | 4 (4) |
| Shock | 97 (52.4) |
| Mechanical ventilation | 134 (72.4) |
| Clinical outcome, n (%) | |
| ICU stay, days, median (IQR) | 35 (47) |
| In-ICU mortality | 71 (38.4) |
| Antibiotics | Total n = 185 | Klebsiella sp. n = 126 | Escherichia coli n = 29 | Enterobacter sp. n = 22 | Others n = 8 |
|---|---|---|---|---|---|
| Amoxicillin–clavulanate | 19/185 (10) | 9/126 (7) | 10/29 (34) | 0/22 (0) | 1/8 (13) |
| Piperacillin–tazobactam | 54/185 (29) | 24/126 (19) | 24/29 (83) | 4/22 (18) | 2/8 (25) |
| CMI < 8 | 25/185 (14) | 7/126 (6) | 17/29 (59) | 0/22 (0) | 1/8 (13) |
| Temocillin | 47/83 (57) | 26/53 (49) | 12/19 (63) | 9/10 (90) | 0/1 (0) |
| Cefoxitin | 83/159 (52) | 69/115 (60) | 14/17 (82) | 0/20 (0) | 0/7 (0) |
| Cefepime | 59/185 (32) | 43/126 (34) | 4/29 (14) | 9/22 (41) | 3/8 (38) |
| Ertapenem | 153/185 (83) | 99/126 (76) | 29/29 (100) | 22/22 (100) | 3/8 (38) |
| Ceftazidime–avibactam | 114/120 (95) | 88/93 (95) | 7/8 (88) | 14/14 (100) | 5/5 (100) |
| Ceftolozan–tazobactam | 64/109 (59) | 47/83 (57) | 6/6 (100) | 7/15 (47) | 4/5 (80) |
| Levofloxacin | 54/185 (29) | 32/126 (25) | 11/29 (38) | 8/22 (36) | 3/8 (38) |
| Amikacin | 159/185 (86) | 111/126 (88) | 28/29 (97) | 18/22 (82) | 2/8 (25) |
| Colistin | 81/95 (85) | 61/74 (82) | 3/3 (100) | 10/11(91) | 7/7 (100) |
| Trimethoprim–sulfamethoxazole | 40/185 (22) | 23/126 (18) | 13/29 (45) | 1/22 (5) | 3/8 (38) |
| Tigecyclin | 81/95 (85) | 61/74 (82) | 3/3 (100) | 10/11 (91) | 7/7 (100) |
| Variables | Factors Associated with an Adequate Empirical Prescription of a Carbapenem | |||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| OR (CI 95%) | p | OR (CI 95%) | p | |
| Sex (Male) | 0.744 [0.256–2.162] | 0.587 | ||
| Age | 0.975 [0.938–1.012] | 0.186 | 0.979 [0.918–1.043] | 0.506 |
| Diabetes | 1.647 [0.539–5.030] | 0.381 | ||
| Chronic cardiac insufficiency | 1.776 [0.368–8.575] | 0.475 | ||
| Chronic respiratory insufficiency | 0.328 [0.109–0.990] | 0.048 | 0.551 [0.115–2.631] | 0.455 |
| Chronic renal insufficiency | 2.250 [0.267–18.929] | 0.456 | ||
| Cancer | 0.584 [0.139–2.450] | 0.463 | ||
| Hemopathy | 2.250 [0.267–18.929] | 0.456 | ||
| Immunodepression | 1.989 [0.528–7.496] | 0.310 | ||
| Antibiotic allergy | 0.935 [0.098–8.879] | 0.953 | ||
| Antibiotics within 3 months | 1.083 [0.316–3.711] | 0.899 | ||
| ESBL colonization | 54.312 [10.192–289.417] | <0.0001 | 107.921 [9.303–1251.910] | 0.0002 |
| Length of stay in hospital | 1.009 [0.987–1.031] | 0.422 | ||
| SOFA at BSI onset | 54.312 [10.192–289.417] | 0.008 | 1.061 [0.787–1.431] | 0.696 |
| Septic shock | 3.885 [1.334–11.313] | 0.013 | 11.029 [0.936–129.888] | 0.056 |
| VM at BSI onset | 0.874 [0.300–2.548] | 0.805 | ||
| Respiratory source of BSI | 3.152 [1.087–9.134] | 0.035 | 3.456 [0.768–15.558] | 0.106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Berre, C.; Houard, M.; Vachée, A.; Georges, H.; Wallet, F.; Patoz, P.; Herbecq, P.; Nseir, S.; Delannoy, P.-Y.; Meybeck, A. Antibiotic Prescriptions in Critically Ill Patients with Bloodstream Infection Due to ESBL-Producing Enterobacteriaceae: Compliance with the French Guidelines for the Treatment of Infections with Third-Generation Cephalosporin-Resistant Enterobacteriaceae—A Multicentric Retrospective Cohort Study. Microorganisms 2023, 11, 2676. https://doi.org/10.3390/microorganisms11112676
Le Berre C, Houard M, Vachée A, Georges H, Wallet F, Patoz P, Herbecq P, Nseir S, Delannoy P-Y, Meybeck A. Antibiotic Prescriptions in Critically Ill Patients with Bloodstream Infection Due to ESBL-Producing Enterobacteriaceae: Compliance with the French Guidelines for the Treatment of Infections with Third-Generation Cephalosporin-Resistant Enterobacteriaceae—A Multicentric Retrospective Cohort Study. Microorganisms. 2023; 11(11):2676. https://doi.org/10.3390/microorganisms11112676
Chicago/Turabian StyleLe Berre, Camille, Marion Houard, Anne Vachée, Hugues Georges, Frederic Wallet, Pierre Patoz, Patrick Herbecq, Saad Nseir, Pierre-Yves Delannoy, and Agnès Meybeck. 2023. "Antibiotic Prescriptions in Critically Ill Patients with Bloodstream Infection Due to ESBL-Producing Enterobacteriaceae: Compliance with the French Guidelines for the Treatment of Infections with Third-Generation Cephalosporin-Resistant Enterobacteriaceae—A Multicentric Retrospective Cohort Study" Microorganisms 11, no. 11: 2676. https://doi.org/10.3390/microorganisms11112676
APA StyleLe Berre, C., Houard, M., Vachée, A., Georges, H., Wallet, F., Patoz, P., Herbecq, P., Nseir, S., Delannoy, P.-Y., & Meybeck, A. (2023). Antibiotic Prescriptions in Critically Ill Patients with Bloodstream Infection Due to ESBL-Producing Enterobacteriaceae: Compliance with the French Guidelines for the Treatment of Infections with Third-Generation Cephalosporin-Resistant Enterobacteriaceae—A Multicentric Retrospective Cohort Study. Microorganisms, 11(11), 2676. https://doi.org/10.3390/microorganisms11112676





