Iron Status and Supplementation during Tuberculosis
Abstract
1. Introduction
2. Iron Acquisition and Host Immune Response in Tuberculosis
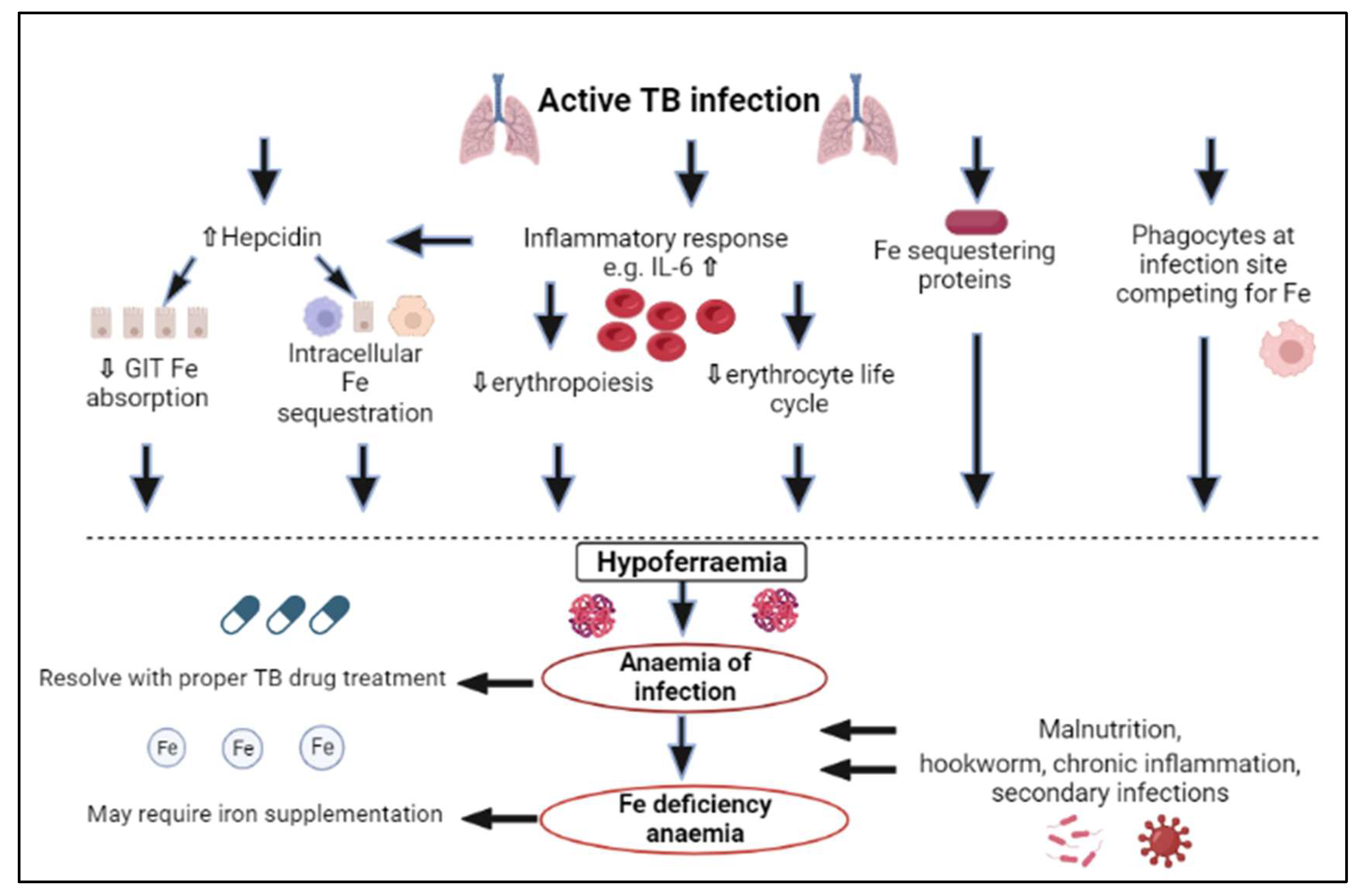
3. Iron Status in Tuberculosis
- Malnutrition because of insufficient dietary intake and a deterioration in appetite;
- Malabsorption because of hookworm infection, inflammation or other secondary infections;
- Haemoptysis resulting in blood loss via sputum.
4. Biomarkers to Identify Anaemia of Infection and Iron Deficiency Anaemia in Tuberculosis patients
5. Clinical Outcomes Associated with Iron Biomarkers in Tuberculosis
6. Preclinical Studies of Iron Supplementation and Absorption in Tuberculosis
| Reference | TB Model | Iron intervention | Outcome |
|---|---|---|---|
| Byrd et al. [73] | Human monocytes infected with Mtb Erdman strain (ATCC 35801) and cultured in medium containing TNF-α, IFN-γ and calcitriol. | Ferric ammonium citrate, Fe-saturated transferrin, Fe-saturated lactoferrin. | Dose-dependent Fe restriction of growth of Mtb in monocytes that had been primed with TNF-α. Production of TNF-α by infected monocytes was inhibited by Fe. |
| Lounis et al. [74] | Balb/C mice infected intravenously with 7.2 × 103 H37Rv Mtb strain. | 50 mg/kg polymaltose ferric hydroxyde intraperitoneally, 3 times a week for 2 weeks before infection. | No significant differences in body weights between the Fe-loaded and control mice on day 42 after infection. Spleen weights were significantly higher in Fe-loaded mice at day 42 post-infection. Mtb CFU counts significantly higher in spleens and lungs of the Fe-loaded mice compared to controls. |
| Schaible et al. [75] | In vivo wild-type B6, β-2-microglobulin knockout and MHC-I knockout mice infected with 3–5 or 15–200 Mtb per lung. Some mice received 25 mg/mL ferric citrate in drinking water for duration of experiment to overload them with Fe. | Treated twice every week with intranasal 1 mg/mouse bovine lactoferrin or recombinant lactoferrin or intraperitoneally with deferoxamine in PBS or PBS alone. 25 mg/mL Fe3+Ci | Treatment with Fe3+Ci resulted in Fe overload and 10 times higher burden of lung Mtb. Treatment with lactoferrin ↓ bacterial load in β2m knockout but not B6 mice. Treatment with deferoxamine depleted Fe and ↓ bacterial load in both β2m knockout and B6 mice. |
| In vitro Mtb (Erdman) and M.bovis cultured in medium. | 1 mg/mL Fe3+Ci; or 1 mg/mL bovine lactoferrin; or 0.5 mg/mL deferroxamine; or deferroxamine and Fe3+Ci. | Excess Fe ↑ bacterial growth; deferoxamine chelated free Fe, resulting in ↓ growth of Mtb. | |
| Macrophages derived from bone marrow cells of B6 or β2m knockout mice were infected with Mtb and cultured with or without 0.5 mg/mL lactoferrin. | 0.5 mg/mL lactoferrin or 0.1 mg/mL rat anti-TfR antibody for 3 days. | Lactoferrin bound extracellular Fe, whereas anti-TfR antibody inhibited cellular Fe import, thus inhibiting growth of Mtb in infected macrophages. | |
| Serafin-Lopez et al. [54] | Murine macrophage-like cell line J774A.1 infected with Mtb H37Rv bacilli. Intracellular and extracellular growth of Mtb assessed. | Incubation of cell cultures with 5, 25 and 50 µM of ferric chloride for 0, 48 and 72 h. | Dose-dependent extracellular bacterial growth was observed after 48 h and 72 h but intracellular growth only at 72 h with Fe. Production of TNF-α was lower at 72 h compared with 48 h post-infection in macrophages with Fe. |
| Agoro et al. [77] | Male C57BL/6 mice infected intravenously with 2 × 106 M. Bovis BCG. | Mice on (1) Fe-rich diet (2500 mg Fe carbonyl/ kg food or (2) standard diet (280 mg Fe carbonyl/ kg food) for 4 weeks before infection plus duration of infection. | In vivo analysis Moderate Fe: ↓ proinflammatory cytokine levels, ↓ neutrophil recruitment, ↑ T-cell recruitment in granulomas and ↓ bacterial load. ↑ Bacterial clearance in liver correlated with upregulation of the gene encoding hepcidin and sequestration of Fe in tissues. In cultured macrophages Fe ↑ ROS and ↓ uptake and intracellular growth of M.Bovis BCG. |
| Kolloli et al. [76] | Pathogen-free white rabbits (Oryctolagus cuniculus) infected with Mtb CDC1551 via the aerosol route. | 25 mg Fe-dextran III or placebo (0.5 mL sterile dextran in water) intra-muscularly, 3 days a week. (1) For acute-phase results starting day 1 post-infection to 8 weeks and (2) for pre-established infection starting at 8 until 16 weeks. | No causal role for Fe in bacterial burden and tissue pathology, i.e., the reactivation of latent TB. Association between Fe supplementation and changes in host gene expression of Fe homeostasis and host immunity. |
| Nienaber et al. [78] | Male C3HeB/FeJ mice infected with 2.4 × 107 Mtb H37Rv via aerosol route. | AIN-93G control or AIN-93G diet supplemented with Fe (130 ppm Fe) from one-week post-infection for three weeks. | Fe lowered soluble transferrin receptor, ferritin and hepcidin, lung IL-1α, IL-1β, plasma IL-1, IL-6 and TNF-α. Fe did not affect lung bacterial loads. Fe ↑ T-cell, CD4+ T-cell, CD8+ T-cell, interstitial macrophage, alveolar macrophage, CD103 DC and CD11b DC counts in lungs and percentages of total lung cells in neutrophils, interstitial macrophages, monocyte-derived DCs, T cells, CD4+ T cells, CD8+ T cells, natural killer cells and CD11b DCs. |
7. Clinical Trials on Iron Supplementation and Absorption in Tuberculosis
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hajissa, K.; Marzan, M.; Idriss, M.I.; Islam, M.A. Prevalence of drug-resistant tuberculosis in Sudan: A systematic review and meta-analysis. Antibiotics 2021, 10, 932. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- World Health Organization. National Tuberculosis Prevalence Surveys 2007–2016; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Kumar, P. Adult pulmonary tuberculosis as a pathological manifestation of hyperactive antimycobacterial immune response. Clin. Transl. Med. 2016, 5, 38. [Google Scholar] [CrossRef]
- Kumar, N.P.; Moideen, K.; Banurekha, V.V.; Nair, D.; Babu, S. Plasma proinflammatory cytokines are markers of disease severity and bacterial burden in pulmonary tuberculosis. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2019; p. ofz257. [Google Scholar]
- Devi, U.; Rao, C.M.; Srivastava, V.K.; Rath, P.K.; Das, B.S. Effect of iron supplementation on mild to moderate anaemia in pulmonary tuberculosis. Br. J. Nutr. 2003, 90, 541–550. [Google Scholar] [CrossRef]
- Friis, H.; Range, N.; Kristensen, C.B.; Kæstel, P.; Changalucha, J.; Malenganisho, W.; Krarup, H.; Magnussen, P.; Andersen, Å.B. Acute-phase response and iron status markers among pulmonary tuberculosis patients: A cross-sectional study in Mwanza, Tanzania. Br. J. Nutr. 2009, 102, 310–317. [Google Scholar] [CrossRef]
- Hella, J.; Cercamondi, C.I.; Mhimbira, F.; Sasamalo, M.; Stoffel, N.; Zwahlen, M.; Bodmer, T.; Gagneux, S.; Reither, K.; Zimmermann, M.B. Anemia in tuberculosis cases and household controls from Tanzania: Contribution of disease, coinfections, and the role of hepcidin. PLoS ONE 2018, 13, e0195985. [Google Scholar] [CrossRef]
- Kerkhoff, A.; Meintjes, G.; Opie, J.; Vogt, M.; Jhilmeet, N.; Wood, R.; Lawn, S. Anaemia in patients with HIV-associated TB: Relative contributions of anaemia of chronic disease and iron deficiency. Int. J. Tuberc. Lung Dis. 2016, 20, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kang, Y.; Yoon, Y.S.; Um, S.-W.; Lee, S.M.; Yoo, C.-G.; Kim, Y.W.; Han, S.K.; Shim, Y.-S.; Yim, J.-J. The prevalence and evolution of anemia associated with tuberculosis. J. Korean Med. Sci. 2006, 21, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Minchella, P.A.; Donkor, S.; Owolabi, O.; Sutherland, J.S.; McDermid, J.M. Complex anemia in tuberculosis: The need to consider causes and timing when designing interventions. Clin. Infect. Dis. 2014, 60, 764–772. [Google Scholar] [CrossRef]
- Morris, C.D.; Bird, A.R.; Nell, H. The haematological and biochemical changes in severe pulmonary tuberculosis. Q. J. Med. 1989, 73, 1151–1159. [Google Scholar]
- Sahiratmadja, E.; Wieringa, F.T.; van Crevel, R.; de Visser, A.W.; Adnan, I.; Alisjahbana, B.; Slagboom, E.; Marzuki, S.; Ottenhoff, T.H.; van de Vosse, E. Iron deficiency and NRAMP1 polymorphisms (INT4, D543N and 3′ UTR) do not contribute to severity of anaemia in tuberculosis in the Indonesian population. Br. J. Nutr. 2007, 98, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Isanaka, S.; Mugusi, F.; Urassa, W.; Willett, W.C.; Bosch, R.J.; Villamor, E.; Spiegelman, D.; Duggan, C.; Fawzi, W.W. Iron deficiency and anemia predict mortality in patients with tuberculosis. J. Nutr. 2011, 142, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Eworo, R.E.; Nsonwu-Anyanwu, A.C.; Fabian, U.A.; Akpan, P.A.; Udo, P.E. Nutritional background of low-income pulmonary tuberculosis patients on anti-tuberculosis therapy at Infectious Disease Hospital, Calabar, Nigeria: A case-control study. Open Access Res. J. Sci. Technol. 2022, 5, 70–79. [Google Scholar] [CrossRef]
- Wondmieneh, A.; Gedefaw, G.; Getie, A.; Demis, A. Prevalence of undernutrition among adult tuberculosis patients in Ethiopia: A systematic review and meta-analysis. J. Clin. Tuberc. Other Mycobact. Dis. 2021, 22, 100211. [Google Scholar] [CrossRef]
- Bustamante-Rengifo, J.A.; Astudillo-Hernández, M.; del Pilar Crespo-Ortiz, M. Effect of Helicobacter pylori and Helminth Coinfection on the Immune Response to Mycobacterium tuberculosis. Curr. Microbiol. 2021, 78, 3351–3371. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.; Hussain, R.; Parsonnet, J. The impact of mucosal infections on acquisition and progression of tuberculosis. Mucosal Immunol. 2011, 4, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Taparia, M.P.; Yadav, D.; Koolwal, S. Study of Iron Metabolism in Pulmonary Tuberculosis Patients. Int. J. Health Sci. Res. 2018, 8, 70–77. [Google Scholar]
- Agoro, R.; Mura, C. Iron Supplementation Therapy, A Friend and Foe of Mycobacterial Infections? Pharmaceuticals 2019, 12, 75. [Google Scholar] [CrossRef]
- Cherayil, B.J. Iron and immunity: Immunological consequences of iron deficiency and overload. Arch. Immunol. Ther. Exp. 2010, 58, 407–415. [Google Scholar] [CrossRef]
- Ganz, T. Iron and infection. Int. J. Hematol. 2018, 107, 7–15. [Google Scholar] [CrossRef]
- Cole, S.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S.; Eiglmeier, K.; Gas, S.; Barry Iii, C. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998, 393, 537. [Google Scholar] [CrossRef]
- Ifeanyi, O.E. A Review on Iron Homeostasis and Anaemia in Pulmonary Tuberculosis. Int. J. Healthc. Med. Sci. 2018, 4, 84–89. [Google Scholar]
- Weiss, G.; Goodnough, L.T. Anemia of chronic disease. N. Engl. J. Med. 2005, 352, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Mayeur, S.; Spahis, S.; Pouliot, Y.; Levy, E. Lactoferrin, a pleiotropic protein in health and disease. Antioxid. Redox Signal. 2016, 24, 813–836. [Google Scholar] [CrossRef] [PubMed]
- Shields-Cutler, R.R.; Crowley, J.R.; Miller, C.D.; Stapleton, A.E.; Cui, W.; Henderson, J.P. Human metabolome-derived cofactors are required for the antibacterial activity of siderocalin in urine. J. Biol. Chem. 2016, 291, 25901–25910. [Google Scholar] [CrossRef]
- Sia, A.K.; Allred, B.E.; Raymond, K.N. Siderocalins: Siderophore binding proteins evolved for primary pathogen host defense. Curr. Opin. Chem. Biol. 2013, 17, 150–157. [Google Scholar] [CrossRef]
- Kurthkoti, K.; Amin, H.; Marakalala, M.J.; Ghanny, S.; Subbian, S.; Sakatos, A.; Livny, J.; Fortune, S.M.; Berney, M.; Rodriguez, G.M. The capacity of Mycobacterium tuberculosis to survive iron starvation might enable it to persist in iron-deprived microenvironments of human granulomas. MBio 2017, 8, e01092-17. [Google Scholar] [CrossRef] [PubMed]
- Bresnahan, K.A.; Chileshe, J.; Arscott, S.; Nuss, E.; Surles, R.; Masi, C.; Kafwembe, E.; Tanumihardjo, S.A. The acute phase response affected traditional measures of micronutrient status in rural Zambian children during a randomized, controlled feeding trial. J. Nutr. 2014, 144, 972–978. [Google Scholar] [CrossRef]
- Lee, P.; Peng, H.; Gelbart, T.; Wang, L.; Beutler, E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc. Natl. Acad. Sci. USA 2005, 102, 1906–1910. [Google Scholar] [CrossRef]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.J. Regulation of iron metabolism by hepcidin under conditions of inflammation. J. Biol. Chem. 2015, 290, 18975–18983. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.D.; Altamura, S.; Devitt, E.; Mullins, S.; Lawless, M.W.; Muckenthaler, M.U.; Crowe, J. Pegylated interferon-α induced hypoferremia is associated with the immediate response to treatment in hepatitis C. Hepatology 2012, 56, 492–500. [Google Scholar] [CrossRef]
- Nicolas, G.; Chauvet, C.; Viatte, L.; Danan, J.L.; Bigard, X.; Devaux, I.; Beaumont, C.; Kahn, A.; Vaulont, S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J. Clin. Investig. 2002, 110, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Wrighting, D.M.; Andrews, N.C. Interleukin-6 induces hepcidin expression through STAT3. Blood 2006, 108, 3204–3209. [Google Scholar] [CrossRef]
- Millonig, G.; Ganzleben, I.; Peccerella, T.; Casanovas, G.; Brodziak-Jarosz, L.; Breitkopf-Heinlein, K.; Dick, T.P.; Seitz, H.-K.; Muckenthaler, M.U.; Mueller, S. Sustained submicromolar H2O2 levels induce hepcidin via signal transducer and activator of transcription 3 (STAT3). J. Biol. Chem. 2012, 287, 37472–37482. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Iron homeostasis in host defence and inflammation. Nat. Rev. Immunol. 2015, 15, 500. [Google Scholar] [CrossRef]
- Ganz, T. Anemia of inflammation. N. Engl. J. Med. 2019, 381, 1148–1157. [Google Scholar] [CrossRef]
- Weiss, G.; Ganz, T.; Goodnough, L.T. Anemia of inflammation. Blood 2019, 133, 40–50. [Google Scholar] [CrossRef]
- Atkinson, S.H.; Rockett, K.A.; Morgan, G.; Bejon, P.A.; Sirugo, G.; O’Connell, M.A.; Hanchard, N.; Kwiatkowski, D.P.; Prentice, A.M. Tumor necrosis factor SNP haplotypes are associated with iron deficiency anemia in West African children. Blood J. Am. Soc. Hematol. 2008, 112, 4276–4283. [Google Scholar] [CrossRef]
- Nairz, M.; Theurl, I.; Swirski, F.K.; Weiss, G. “Pumping iron”—How macrophages handle iron at the systemic, microenvironmental, and cellular levels. Pflügers Arch.-Eur. J. Physiol. 2017, 469, 397–418. [Google Scholar] [CrossRef]
- Chao, A.; Sieminski, P.J.; Owens, C.P.; Goulding, C.W. Iron Acquisition in Mycobacterium tuberculosis. Chem. Rev. 2019, 119, 1193–1220. [Google Scholar] [CrossRef]
- Madigan, C.A.; Martinot, A.J.; Wei, J.-R.; Madduri, A.; Cheng, T.-Y.; Young, D.C.; Layre, E.; Murry, J.P.; Rubin, E.J.; Moody, D.B. Lipidomic analysis links mycobactin synthase K to iron uptake and virulence in M. tuberculosis. PLoS Pathog. 2015, 11, e1004792. [Google Scholar] [CrossRef] [PubMed]
- Sritharan, M. Iron homeostasis in Mycobacterium tuberculosis: Mechanistic insights into siderophore-mediated iron uptake. J. Bacteriol. 2016, 198, 2399–2409. [Google Scholar] [CrossRef] [PubMed]
- Conrad, W.H.; Osman, M.M.; Shanahan, J.K.; Chu, F.; Takaki, K.K.; Cameron, J.; Hopkinson-Woolley, D.; Brosch, R.; Ramakrishnan, L. Mycobacterial ESX-1 secretion system mediates host cell lysis through bacterium contact-dependent gross membrane disruptions. Proc. Natl. Acad. Sci. USA 2017, 114, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, S.; Kashyap, A.; Bhagat, M.; Mahajan, R.; Sethi, S. Anemia and Nutritional Status in Tuberculosis Patients. Int. J. Appl. Basic Med. Res. 2021, 11, 226–230. [Google Scholar] [CrossRef]
- Boelaert, J.R.; Vandecasteele, S.J.; Appelberg, R.; Gordeuk, V.R. The effect of the host’s iron status on tuberculosis. J. Infect. Dis. 2007, 195, 1745–1753. [Google Scholar] [CrossRef]
- Nissenson, A.R.; Goodnough, L.T.; Dubois, R.W. Anemia: Not just an innocent bystander? Arch. Intern. Med. 2003, 163, 1400–1404. [Google Scholar] [CrossRef]
- Brock, J.H. Iron and the immune system. In Iron and Human Disease; CRC Press: Boca Raton, FL, USA, 2018; pp. 161–178. [Google Scholar]
- Jonker, F.A.; van Hensbroek, M.B. Anaemia, iron deficiency and susceptibility to infections. J. Infect. 2014, 69, S23–S27. [Google Scholar] [CrossRef]
- Cronjé, L.; Edmondson, N.; Eisenach, K.D.; Bornman, L. Iron and iron chelating agents modulate Mycobacterium tuberculosis growth and monocyte-macrophage viability and effector functions. FEMS Immunol. Med. Microbiol. 2005, 45, 103–112. [Google Scholar] [CrossRef]
- Gangaidzo, I.T.; Moyo, V.M.; Mvundura, E.; Aggrey, G.; Murphree, N.L.; Khumalo, H.; Saungweme, T.; Kasvosve, I.; Gomo, Z.A.; Rouault, T. Association of pulmonary tuberculosis with increased dietary iron. J. Infect. Dis. 2001, 184, 936–939. [Google Scholar] [CrossRef]
- Serafín-López, J.; Chacón-Salinas, R.; Muñoz-Cruz, S.; Enciso-Moreno, J.A.; Estrada-Parra, S.A.; Estrada-Garcia, I. The effect of iron on the expression of cytokines in macrophages infected with Mycobacterium tuberculosis. Scand. J. Immunol. 2004, 60, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Werner-Felmayer, G.; Werner, E.R.; Grünewald, K.; Wachter, H.; Hentze, M.W. Iron regulates nitric oxide synthase activity by controlling nuclear transcription. J. Exp. Med. 1994, 180, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Ekiz, C.; Agaoglu, L.; Karakas, Z.; Gurel, N.; Yalcin, I. The effect of iron deficiency anemia on the function of the immune system. Hematol. J. 2005, 5, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Kuvibidila, S.R.; Baliga, B.S.; Warrier, R.P.; Suskind, R.M. Iron deficiency reduces the hydrolysis of cell membrane phosphatidyl inositol-4, 5-bisphosphate during splenic lymphocyte activation in C57BL/6 mice. J. Nutr. 1998, 128, 1077–1083. [Google Scholar] [CrossRef]
- Oppenheimer, S.J. Iron and its relation to immunity and infectious disease. J. Nutr. 2001, 131, 616S–635S. [Google Scholar] [CrossRef]
- Jabara, H.H.; Boyden, S.E.; Chou, J.; Ramesh, N.; Massaad, M.J.; Benson, H.; Bainter, W.; Fraulino, D.; Rahimov, F.; Sieff, C. A missense mutation in TFRC, encoding transferrin receptor 1, causes combined immunodeficiency. Nat. Genet. 2016, 48, 74. [Google Scholar] [CrossRef]
- Johnson, E.E.; Wessling-Resnick, M. Iron metabolism and the innate immune response to infection. Microbes Infect. 2012, 14, 207–216. [Google Scholar] [CrossRef]
- Shimazaki, T.; Marte, S.; Saludar, N.; Dimaano, E.; Salva, E.; Ariyoshi, K.; Villarama, J.; Suzuki, M. Risk factors for death among hospitalised tuberculosis patients in poor urban areas in Manila, The Philippines. Int. J. Tuberc. Lung Dis. 2013, 17, 1420–1426. [Google Scholar] [CrossRef]
- Chu, K.-A.; Hsu, C.-H.; Lin, M.-C.; Chu, Y.-H.; Hung, Y.-M.; Wei, J.C.-C. Association of iron deficiency anemia with tuberculosis in Taiwan: A nationwide population-based study. PLoS ONE 2019, 14, e0221908. [Google Scholar] [CrossRef]
- Nagu, T.J.; Spiegelman, D.; Hertzmark, E.; Aboud, S.; Makani, J.; Matee, M.I.; Fawzi, W.; Mugusi, F. Anemia at the initiation of tuberculosis therapy is associated with delayed sputum conversion among pulmonary tuberculosis patients in Dar-es-Salaam, Tanzania. PLoS ONE 2014, 9, e91229. [Google Scholar] [CrossRef]
- Metanat, M.; Mashhadi, M.A.; Alavi-Naini, R.; Rezaie-Kahkhaie, L.; Sepehri-Rad, N.; Afshari, M. The Prevalence of Absolute and Functional Iron Deficiency Anemia in New Cases of Smear-positive Pulmonary Tuberculosis and Their Sputum Conversion Rate at the End of Intensive Tuberculosis Treatment Phase. Prague Med. Rep. 2020, 121, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Isanaka, S.; Aboud, S.; Mugusi, F.; Bosch, R.J.; Willett, W.C.; Spiegelman, D.; Duggan, C.; Fawzi, W.W. Iron status predicts treatment failure and mortality in tuberculosis patients: A prospective cohort study from Dar es Salaam, Tanzania. PLoS ONE 2012, 7, e37350. [Google Scholar] [CrossRef] [PubMed]
- Kerkhoff, A.D.; Meintjes, G.; Burton, R.; Vogt, M.; Wood, R.; Lawn, S.D. Relationship between blood concentrations of hepcidin and anemia severity, mycobacterial burden, and mortality among patients with HIV-associated tuberculosis. J. Infect. Dis. 2015, 213, 61–70. [Google Scholar] [CrossRef]
- McDermid, J.M.; Hennig, B.J.; van der Sande, M.; Hill, A.V.; Whittle, H.C.; Jaye, A.; Prentice, A.M. Host iron redistribution as a risk factor for incident tuberculosis in HIV infection: An 11-year retrospective cohort study. BMC Infect. Dis. 2013, 13, 48. [Google Scholar] [CrossRef]
- Adedapo, K.; Arinola, O.; Ige, O.; Adedapo, A.; Salimonu, L. Combination of reduced levels of serum albumin and αlpha-2-macroglobulin differentiates newly diagnosed pulmonary tuberculosis patients from patients on chemotherapy. Afr. J. Biomed. Res. 2006, 9, 3. [Google Scholar] [CrossRef]
- Bapat, P.R.; Satav, A.R.; Husain, A.A.; Shekhawat, S.D.; Kawle, A.P.; Chu, J.J.; Purohit, H.J.; Daginawala, H.F.; Taori, G.M.; Kashyap, R.S. Differential levels of alpha-2-macroglobulin, haptoglobin and sero-transferrin as adjunct markers for TB diagnosis and disease progression in the malnourished tribal population of Melghat, India. PLoS ONE 2015, 10, e0133928. [Google Scholar] [CrossRef]
- Murray, M.J.; Murray, A.B.; Murray, M.B.; Murray, C. The adverse effect of iron repletion on the course of certain infections. Br. Med. J. 1978, 2, 1113–1115. [Google Scholar] [CrossRef] [PubMed]
- Sazawal, S.; Black, R.E.; Ramsan, M.; Chwaya, H.M.; Stoltzfus, R.J.; Dutta, A.; Dhingra, U.; Kabole, I.; Deb, S.; Othman, M.K. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: Community-based, randomised, placebo-controlled trial. Lancet 2006, 367, 133–143. [Google Scholar] [CrossRef]
- Koutroubakis, I.E.; Ramos-Rivers, C.; Regueiro, M.; Koutroumpakis, E.; Click, B.; Schwartz, M.; Swoger, J.; Baidoo, L.; Hashash, J.G.; Barrie, A. The Influence of Anti–tumor Necrosis Factor Agents on Hemoglobin Levels of Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 1587–1593. [Google Scholar] [CrossRef]
- Byrd, T.F. Tumor necrosis factor alpha (TNFalpha) promotes growth of virulent Mycobacterium tuberculosis in human monocytes iron-mediated growth suppression is correlated with decreased release of TNFalpha from iron-treated infected monocytes. J. Clin. Investig. 1997, 99, 2518–2529. [Google Scholar] [CrossRef]
- Lounis, N.; Truffot-Pernot, C.; Grosset, J.; Gordeuk, V.R.; Boelaert, J.R. Iron and Mycobacterium tuberculosis infection. J. Clin. Virol. 2001, 20, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Schaible, U.E.; Collins, H.L.; Priem, F.; Kaufmann, S.H. Correction of the iron overload defect in β-2-microglobulin knockout mice by lactoferrin abolishes their increased susceptibility to tuberculosis. J. Exp. Med. 2002, 196, 1507–1513. [Google Scholar] [CrossRef]
- Kolloli, A.; Singh, P.; Rodriguez, G.M.; Subbian, S. Effect of Iron Supplementation on the Outcome of Non-Progressive Pulmonary Mycobacterium tuberculosis Infection. J. Clin. Med. 2019, 8, 1155. [Google Scholar] [CrossRef] [PubMed]
- Agoro, R.; Benmerzoug, S.; Rose, S.; Bouyer, M.; Gozzelino, R.; Garcia, I.; Ryffel, B.; Quesniaux, V.F.; Mura, C. An iron-rich diet decreases the mycobacterial burden and correlates with hepcidin upregulation, lower levels of proinflammatory mediators, and increased T-cell recruitment in a model of mycobacterium bovis Bacille Calmette-Guerin infection. J. Infect. Dis. 2017, 216, 907–918. [Google Scholar] [CrossRef]
- Nienaber, A.; Baumgartner, J.; Dolman, R.C.; Ozturk, M.; Zandberg, L.; Hayford, F.E.; Brombacher, F.; Blaauw, R.; Parihar, S.P.; Smuts, C.M. Omega-3 fatty acid and iron supplementation alone, but not in combination, lower inflammation and anemia of infection in mycobacterium tuberculosis-infected mice. Nutrients 2020, 12, 2897. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Emmanouilidou, E.; Petraki, K.; Lydakis, C. Central nervous system tuberculosis reactivation following intravenous iron supplementation. Int. J. Mycobacteriol. 2019, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Cercamondi, C.I.; Stoffel, N.U.; Moretti, D.; Zoller, T.; Swinkels, D.W.; Zeder, C.; Mhimibra, F.; Hella, J.; Fenner, L.; Zimmermann, M.B. Iron homeostasis during anemia of inflammation: A prospective study of patients with tuberculosis. Blood 2021, 138, 1293–1303. [Google Scholar] [CrossRef]
| Biomarker | Levels in Iron Deficiency Anaemia | Levels in Anaemia of Infection |
|---|---|---|
| Red blood cells | Low | Low |
| White blood cells | Low to normal | Normal to high |
| Serum iron | Decreased | Decreased |
| Serum transferrin | High | Low |
| Transferrin saturation | Low | Low |
| Serum transferrin receptor | High | Normal to high |
| Hepcidin | Decreased | Increased |
| Haemoglobin | Decreased | Decreased to normal |
| Ferritin | Low | High |
| Mean corpuscular volume | Low | Normal |
| Mean corpuscular haemoglobin | Low | Normal |
| Reference | Participants | Iron intervention | Outcome |
|---|---|---|---|
| Devi et al. [6] | n = 117 male PTB patients 15–60 years old and Hb of 80–110 g/L n = 50 healthy male controls matched for age and socio-economic status. | 1 capsule twice/ day of (1) placebo containing 75 mg of sucrose; (2) ferrous fumarate containing 75 mg of elemental Fe; (3) ferrous fumarate containing 75 mg of elemental Fe with other haematinics for 2 months while hospitalised. | ↑ Hb, MCV and PCV at 1 month in both Fe-supplemented groups. This difference disappeared at 2 and 6 months. Serum Fe and Fe saturation of transferrin was ↑ in both Fe-supplemented groups for up to 2 months. Radiological and clinical improvements similar in all three groups. |
| Cercamondi et al. [80] | n = 18 mostly anaemic men and women aged 16–45 years old with a positive sputum smear confirmed by gene expert. | Test meal with 6mg of 57Fe iron as ferrous sulphate administered at baseline then 8 and 24 weeks post-treatment. At baseline intravenous infusion with 54Fe or 58Fe as Fe3+Ci, after a meal. | Significant ↓ in inflammation markers: AGP, CRP and IL-6, and 70% ↓ in hepcidin at 2 weeks. Haemoglobin ↑ and anaemia prevalence ↓ from 89% to 22%. TSAT ↑ and ferritin ↓ significantly but sTfR did not change from baseline until treatment completion. Negative correlation between hepcidin and serum iron, indicating Fe sequestration during infection, weakened upon treatment. At baseline, inflammation resulted in Fe sequestration and inhibited absorption and erythropoiesis. With treatment, hepcidin was suppressed and erythropoiesis upregulated for recovery of Hb. Fractional Fe absorption increased 10- and 20-fold as infection resolved. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nienaber, A.; Uyoga, M.A.; Dolman-Macleod, R.C.; Malan, L. Iron Status and Supplementation during Tuberculosis. Microorganisms 2023, 11, 785. https://doi.org/10.3390/microorganisms11030785
Nienaber A, Uyoga MA, Dolman-Macleod RC, Malan L. Iron Status and Supplementation during Tuberculosis. Microorganisms. 2023; 11(3):785. https://doi.org/10.3390/microorganisms11030785
Chicago/Turabian StyleNienaber, Arista, Mary A. Uyoga, Robin C. Dolman-Macleod, and Linda Malan. 2023. "Iron Status and Supplementation during Tuberculosis" Microorganisms 11, no. 3: 785. https://doi.org/10.3390/microorganisms11030785
APA StyleNienaber, A., Uyoga, M. A., Dolman-Macleod, R. C., & Malan, L. (2023). Iron Status and Supplementation during Tuberculosis. Microorganisms, 11(3), 785. https://doi.org/10.3390/microorganisms11030785







