Abstract
1,3-propanediol (1,3-PDO) is a valuable basic chemical, especially in the polymer industry to produce polytrimethylene terephthalate. Unfortunately, the production of 1,3-PDO mainly depends on petroleum products as precursors. Furthermore, the chemical routes have significant disadvantages, such as environmental issues. An alternative is the biobased fermentation of 1,3-PDO from cheap glycerol. Clostridium beijerinckii DSM 6423 was originally reported to produce 1,3-PDO. However, this could not be confirmed, and a genome analysis revealed the loss of an essential gene. Thus, 1,3-PDO production was genetically reinstalled. Genes for 1,3-PDO production from Clostridium pasteurianum DSM 525 and Clostridium beijerinckii DSM 15410 (formerly Clostridium diolis) were introduced into C. beijerinckii DSM 6423 to enable 1,3-PDO production from glycerol. 1,3-PDO production by recombinant C. beijerinckii strains were investigated under different growth conditions. 1,3-PDO production was only observed for C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis], which harbors the genes of C. beijerinckii DSM 15410. By buffering the growth medium, production could be increased by 74%. Furthermore, the effect of four different promoters was analyzed. The use of the constitutive thlA promoter from Clostridium acetobutylicum led to a 167% increase in 1,3-PDO production compared to the initial recombinant approach.
1. Introduction
The demand for 1,3-propanediol (1,3-PDO) has been increasing in the last few years and will continue to rise in the coming years. With a “Compound Annual Growth Rate” of 14.2% as of 2018, an optimistic market expectation for 1,3-PDO is US$ 1,442.77 million in 2027 [1]. 1,3-PDO is an important basic chemical and is widely used as an organic solvent in the food, cosmetics, and pharmaceutical industries. However, the main application of 1,3-PDO is in the polymer industry as a raw material or intermediate, especially as a key monomer in the production of polytrimethylene terephthalate [2,3]. In the past, ethylene oxide hydroformylation and acrolein hydration-hydrogeneration were the two processes mainly used for the chemical synthesis of 1,3-PDO. Both processes depend on petroleum products as precursors for 1,3-PDO production [4,5,6]. However, those processes have many disadvantages, such as high investment, technical difficulties, substrate toxicity, and environmental issues [2,7]. Therefore, a biobased synthesis of 1,3-PDO is desirable.
A biosynthetic 1,3-PDO production approach was developed by DuPont and Genencor in the early 2000s using recombinant Escherichia coli cells and glucose as substrate [8]. The process was commercialized by DuPont Tate & Lyle in Loudon (USA) in 2006, with a production capacity of 63,500 tons per year [9,10,11]. Another possibility is the use of glycerol or crude glycerol as a substrate for the microorganisms [12,13]. About 10% crude glycerol is generated as the main by-product from the biodiesel production. With the expanding biodiesel market, the availability of crude glycerol has increased over the last few years. As a result, the price of crude glycerol is dropping [14,15]. Cheap substrates are of particular importance since the cost of substrates accounts for 50–60% of the total production cost of 1,3-PDO [16]. Thus, the use of cheap glycerol from biodiesel as a substrate for the production of 1,3-PDO could be a good alternative to the original chemical production routes. Therefore, the establishment of a biological production of 1,3-PDO via fermentation of glycerol is desired for a sustainable 1,3-PDO production.
Several microorganisms are known to naturally reduce glycerol to 1,3-PDO including Klebsiella pneumoniae, Citrobacter freundii, and Enterobacter agglomerans [17,18,19]. A big disadvantage of some of these microorganisms is their pathogenicity and the associated restrictions on industrial use [7,19,20]. However, a few clostridial species are also able to use glycerol for the production of 1,3-PDO such as Clostridium pasteurianum, Clostridium butyricum, Clostridium diolis (reclassified now as Clostridium beijerinckii [21]), and some Clostridium beijerinckii strains [22,23,24,25,26]. These different clostridial species harbor different glycerol reduction pathways. In the case of C. pasteurianum, glycerol is dehydrated via a coenzyme B12-dependent glycerol dehydratase (DhaB, DhaC, DhaE) to 3-hydroxypropionaldehyde (3-HPA), followed by the conversion of 3-HPA via a NADH-linked 1,3-PDO dehydrogenase (DhaT) to 1,3-PDO [27]. The glycerol reductive pathway of C. butyricum and C. beijerinckii consists of a coenzyme B12-independent glycerol dehydratase (DhaB1, DhaB2) for the dehydration of glycerol to 3-HPA, also followed by the conversion of 3-HPA to 1,3-PDO via a NADH-linked 1,3-PDO dehydrogenase (DhaT) [28,29]. However, glycerol is not only reduced to 1,3-PDO but also oxidized and used for biomass and by products e.g., acetate, butyrate, ethanol, and butanol [30]. An overview of the glycerol consumption pathway is given in Figure 1.
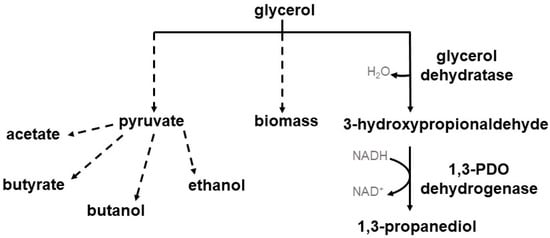
Figure 1.
Overview of the glycerol consumption pathway in clostridial species. Glycerol can either be converted reductively, forming 3-HPA and finally 1,3-PDO, or used for biomass and the production of byproducts.
In the past, most publications focused on the conversion of glycerol to 1,3-PDO with wild-type clostridial strains. Clostridia are difficult to manipulate genetically, due to the limited availability of genetic tools as well as the occurrence of native restriction-modification systems, resulting in major obstacles in obtaining recombinant strains [31,32]. Therefore, development of new genetic tools and methods to overcome restriction-modification systems are essential for the construction of an optimized production strain for the waste valorization from glycerol to 1,3-PDO in the future. One of these new developments is the newly published transformation protocol for C. beijerinckii DSM 6423 [31], which was used in this study to create recombinant 1,3-PDO production strains.
The two different 1,3-PDO production pathways form C. pasteurianum DSM 525 and C. beijerinckii DSM 15410 were expressed in C. beijerinckii DSM 6423 in a plasmid-based manner. Furthermore, the effect of different promoters on 1,3-PDO production was examined in this study. The results presented here demonstrate the heterologous production of 1,3-PDO with genetically engineered C. beijerinckii DSM 6423 strains for the first time.
2. Material & Methods
2.1. Strains and Cultivation
Bacterial strains used in this study are listed in Table 1. C. beijerinckii strains were cultivated under anaerobic conditions at 37 °C. YTG medium was used as a complex medium, for solid medium 2YTG medium containing 1.5% (w/v) agar was prepared. 2YTG medium contained (per L): tryptone, 16 g; yeast extract, 10 g; NaCl, 5 g; glucose, 20 g [31]. Growth experiments were performed in minimal medium referred to as glycerol medium. Glycerol medium contained (per L): K2HPO4, 4 g; KH2PO4, 1.5 g; (NH4)2SO4, 2 g; MgSO4 · 7H2O, 0.3 g; yeast extract, 1 g; cysteine-HCl · H2O, 1.2 g; resazurin, 1 mg; trace element solution, 1 mL. Composition of trace element solution (per L): CoCl2 · 2H2O, 0.2 g; CuCl2 · 2H2O, 0.02 g; H2BO3, 0.06 g; HCl (37%), 0.9 mL; MnCl2 · 4H2O, 0.1 g; Na2MoO4 · 2H2O, 0.035 g; NiCl2 · 6H2O, 0.025 g; ZnCl2, 0.07 g. In case of growth experiments with buffered medium, glycerol medium was prepared with 10.46 g/L 3-(N-morpholino)propanesulfonic acid (MOPS). For the construction and growth of recombinant C. beijerinckii strains, media were supplemented with the appropriate antibiotic. E. coli strains were cultivated aerobically under shaking conditions (180 rpm) at 37 °C in LB medium [33]. The solid medium contained 1.5% (w/v) agar. For cloning purposes, media were supplemented with the respective antibiotic.

Table 1.
Bacterial strains and plasmids used in this study.
2.2. Construction of Recombinant C. beijerinckii Strains
Standard molecular cloning techniques were performed according to established protocols [33]. All primers used for the construction of the different 1,3-PDO production plasmids are listed in Table 2. Primers were designed using the “NEBuilder®” online tool and synthesized by Biomers.net GmbH (Ulm, Germany). Genomic DNA or plasmid DNA served as a template for amplification via PCR. Amplification was performed using “CloneAmpTM HiFi polymerase” (Takara Bio USA, Inc., Mountain View, CA, USA) or “PhusionTM Green High-Fidelity DNA Polymerase” (Thermo Fischer Scientific Inc., Waltham, MA, USA). Linearization of vectors and plasmids was achieved using “FastDigestTM restriction enzymes” (Thermo Fischer Scientific Inc., Waltham, MA, USA). Purification of DNA fragments from agarose gels or solutions after PCR as well as after plasmid linearization were performed using the “ZymocleanTM Gel DNA Recovery Kit” (ZYMO Research Corp., Irvine, CA, USA) or the “DNA Clean &Concentrator® Kit” (ZYMO Research Corp., Irvine, CA, USA), respectively. The procedure was carried out as described by the manufacturer. Assembly of purified DNA fragments was performed with the “NEBulder® HiFi DNA Assembly Master Mix” (New England Biolabs® Inc., Ipswich, MA, USA), according to the manufacturer’s instructions. After assembly, 3.5-5 µL of cloning mixture were used to transform the chemically competent E. coli strain XL1-Blue MRF’. Plasmid preparation from E. coli cells was performed using the “ZyppyTM Plasmid Miniprep Kit” (ZYMO Research Corp., Irvine, CA, USA) following the manufacturer´s instructions. The accuracy of the desired plasmid was checked by an analytic digestion, again using “FastDigestTM restriction enzymes” (Thermo Fischer Scientific Inc., Waltham, MA, USA). Subsequently, plasmids that showed the expected DNA fragments were sent to Microsynth AG (Balgrach, Switzerland) for sequencing. An overview of the newly constructed plasmids and their relevant characteristics is shown in Table 1.

Table 2.
Primers used in this study.
To construct pMTL83251_Ppta-ack_1,3-PDO.diolis, the plasmid pMTL83251_Ppta-ack_1,3-PDO.CLOBI was digested using the restriction enzymes XbaI, HindIII, and NheI to remove the 1,3-PDO gene cluster from C. beijerinckii DSM 6423. The PCR-amplified 1,3-PDO gene cluster from C. beijerinckii DSM 15410 (locus tag K684DRAFT_00976-00979; primers: dhaB1/2CoT.diol_fwd and dhaB1/2CoT.diol_rev) was cloned into the digested pMTL83251_Ppta-ack_1,3-PDO.CLOBI, still harboring the pta-ack promoter from C. ljungdahlii. For cloning of pMTL83251_PthlA_dhaBCET.Cpas, plasmid pMTL83251 was linearized using restriction enzymes SalI and SmaI. Digested plasmid was ligated with PthlA (primers: PthlA.Cpas_fwd and PthlA.Cpas_rev) amplified from C. acetobutylicum as well as dhaBCET (locus tags: Ga0078015_112319-112317; primers: dhaBCE_fwd and dhaBCE_rev) and dhaT (locus tag: Ga0078015_112312; primers: dhaT_fwd and dhaT_rev) DNA fragments amplified from genomic DNA from C. pasteurianum DSM 525. Plasmids for testing the effects of different promoter sequences were based on the plasmid pMTL83251_Ppta-ack_1,3-PDO.diolis. The pta-ack promoter was removed using the restriction enzymes SmaI and XbaI. The PthlA fragment from C. acetobutylicum was amplified from the plasmid pMTL83251_PthlA_FAST (primers: PthlA_1,3-PDO_fwd and PthlA_1,3-PDO_rev) and ligated into the digested pMTL83251_Ppta-ack_1,3-PDO.diolis, resulting in the plasmid pMTL83251_PthlA_1,3-PDO.diolis. To construct the plasmid pMTL83251_Pbld_1,3-PDO.diolis, the PCR-amplified Pbld DNA fragment from C. saccharoperbutylacetonicum (amplified from pMTL83251_Pbld_FAST; primers: Pbld_1,3-PDO_fwd and Pbld_1,3-PDO_rev) was cloned into the digested pMTL83251_Ppta-ack_1,3-PDO.diolis plasmid. Pbgal form C. perfringens was amplified from the plasmid pMTL83251_PbgaL_FAST using primers PbgaL_1,3-PDO_fwd and PbgaL_1,3-PDO_rev, and ligated into the SmaI and XbaI digested pMTL83251_Ppta-ack_1,3-PDO.diolis plasmid to assemble the plasmid pMTL83251_PbgaL_1,3-PDO.diolis.
Prior to the transformation of C. beijerinckii, the newly constructed plasmids were transformed into electrocompetent E. coli SCS110 cells. Those E. coli cells are dcm and dam deficient, leading to unmethylated plasmid DNA after replication and isolation of plasmids. Transformation of C. beijerinckii was performed as described by Diallo et al., 2020 [31].
2.3. Strain Verification
Newly constructed C. beijerinckii strains and strains used for growth experiments were verified by 16S rRNA gene sequencing, and plasmids were retransformed in E. coli XL1-Blue MRF’ for further analysis. Therefore, genomic DNA was isolated using the “MasterPureTM Gram Positive DNA Purification Kit” (Lucigen Corp., Middleton, WI, USA). Genomic DNA served as a template for amplification of the 16S rRNA gene (primers: 16S-27F and 1492r) using the “ReproFast proofreading polymerase” (Genaxxon Bioscience GmbH, Ulm, Germany). The amplified 16S rRNA gene was sequenced by Microsynth AG (Balgrach, Switzerland), and the sequence was blasted using NCBI blastn with the RNA/ITS database. For plasmid verification, E. coli XL1-Blue MRF´ was transformed with 3 µL of genomic DNA. After growth, plasmids were isolated and checked via analytic digestion.
2.4. Growth Conditions of Batch Experiments
For batch growth in bottles, the different C. beijerinckii strains were inoculated in a glycerol medium. The medium was prepared as described above. The pH of the glycerol medium was adjusted to 7.5 with KOH. After preparing the medium, 50 mL aliquots were filled in bottles and closed airtight. The gas phase was exchanged with N2:CO2 (80:20), and bottles were autoclaved. Before inoculation, the medium was supplemented with 40 mM xylose and 100 mM glycerol. For recombinant C. beijerinckii strains, clarithromycin (5 µg/mL) was added to the medium. To analyze the influence of vitamin B12 supplementation, 5 mg/L sterile vitamin B12 was added to the autoclaved glycerol medium of C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas]. OD600 and pH were monitored during the growth experiments. 2-mL samples for the analysis of substrate consumption and product concentration were taken. The samples were withdrawn with syringes and frozen until analysis took place.
2.5. Analytical Methods
The 2-mL samples withdrawn during growth experiments were thawed and subsequently centrifuged (18,000× g; 30 min; 4 °C). Acetate, butyrate, butanol, ethanol, acetoin, and isopropanol concentrations were determined using a “Clarus 600 gas chromatograph” (Perkin Elmer Inc., Waltham, MA, USA) equipped with a flame ionization detector heated to 300 °C and a flowrate of synthetic air of 450 mL min–1. H2 was used as the carrier gas (45 mL min–1). Prior to analysis, 480 μL supernatant was acidified with 20 µL 2 M HCl. 1 µL of acidified supernatant was injected onto an “Elite-FFAP” column (30 m × 0.32 mm; Perkin Elmer Inc., Waltham, MA, USA) with the injector heated to 225 °C. For analysis, the following temperature profile was used: 40 °C for 3 min, 40 °C to 250 °C by 40 °C min–1, 250 °C for 1 min. Xylose and 1,3-PDO concentrations were measured using an “Agilent 1260 Infinity Series HPLC” system (Agilent Technologies, Santa Clara, CA, USA) equipped with a refractive index detector and a diode array detector. 20 µL of supernatant were injected into a “CS organic acid” precolumn (40 × 8 mm) followed by a “CS organic acid” column (300 × 8 mm; CS-Chromatographie Service GmbH, Langerwehe, Germany). The column was heated to 40 °C, and a mobile phase consisting of 5 mM H2SO4 with a flow rate of 0.6 mL min–1 was used. Glycerol concentration was determined using the “Glycerol Assay Kit MAK117” (Sigma-Aldrich®, St. Louis, MO, USA) following the manufacturer’s manual. For glycerol standards, 1 mM, 0.8 mM, 0.6 mM, 0.4 mM, 0.2 mM, and 0.1 mM were used. Prior to analysis, the supernatant of the growth experiment samples was diluted (1:200 or 1:100) to fit the standard range used for the assay.
3. Results
3.1. Construction of Recombinant 1,3-PDO Production C. beijerinckii Strains
Two types of glycerol dehydratases are present in different clostridial strains. One is a coenzyme B12-dependent glycerol dehydratase, and the other is a coenzyme B12-independent glycerol dehydratase. The reported production of 1,3-PDO by C. beijerinckii DSM 6423 [37] could not be reproduced. Genome analysis showed that the essential gene encoding 1,3-PDO dehydrogenase was missing (Figure 2A). Therefore, we attempted to reconstitute the 1,3-PDO production ability. To examine whether C. beijerinckii DSM 6423 can use both glycerol dehydratases to produce 1,3-PDO, two different plasmids were constructed and transformed into C. beijerinckii. The strain C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas] contains a plasmid harboring the dhaB, dhaC, and dhaE genes (locus tags: Ga0078015_112319-112317) encoding the coenzyme B12-dependent glycerol dehydratase, as well as the dhaT gene (locus tag: Ga0078015_112312) encoding the 1,3-PDO dehydrogenase of C. pasteurianum DSM 525 [27,38]. The constitutively active thlA promoter of C. acetobutylicum was used to ensure expression. Figure 2B shows the 1,3-PDO gene cluster of C. pasteurianum. The 1,3-PDO gene cluster contains four more genes besides dhaB, dhaC, dhaE, and dhaT, which are not present on the plasmid. It is assumed that enzymes encoded by these genes are involved in the reactivation of coenzyme B12, due to the high similarity to genes also present in the 1,3-PDO gene cluster of C. freundii [39]. In contrast, the second recombinant strain of C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis] carries the plasmid pMTL83251_Ppta-ack_1,3-PDO.diolis harboring the whole 1,3-PDO gene cluster of C. beijerinckii DSM 15410 (formerly C. diolis DSM 15410). The 1,3-PDO gene cluster of C. beijerinckii DSM 15410 is shown in Figure 2C. The two genes dhaB1 and dhaB2 (locus tag: K684DRAFT_00976 and K684DRAFT_00977) encode a coenzyme B12-independent glycerol dehydratase, and dhaT (locus tag: K684DRAFT_00979) the 1,3-PDO dehydrogenase [28]. Furthermore, the 1,3-PDO gene cluster also includes the pduO gene (locus tag: K684DRAFT_00978). This gene is annotated as an ATP:cob(I)alamin adenosyltransferase, necessary for the conversion of vitamin B12 to coenzyme B12 [40] and is also present on the 1,3-PDO production plasmid. The 1,3-PDO gene cluster was cloned under the control of the constitutively active pta-ack promoter of C. ljungdahlii [41]. To examine the effect of different promoters on 1,3-PDO production, three additional plasmids were constructed. The plasmid pMTL83251_PthlA_1,3-PDO.diolis harbors the early growth phase-associated thlA promoter of C. acetobutylicum [42] instead of the pta-ack promoter, likewise pMTL83251_PthlA_dhaBCET.Cpas. For the plasmid pMTL38251_Pbld_1,3-PDO.diolis, the exponential growth phase-associated bld promoter of C. saccharoperbutylacetonicum [43] was used. Plasmid pMTL83251_PbgaL_1,3-PDO.diolis includes the lactose-inducible bgaL promoter of C. perfringens [44].
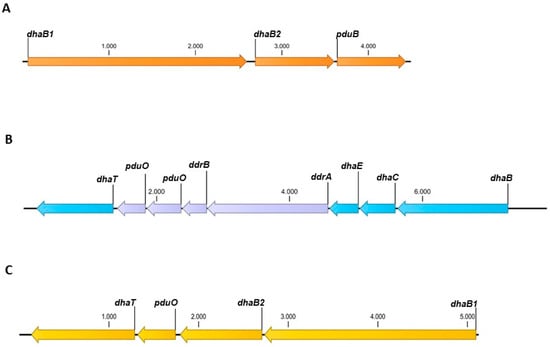
Figure 2.
Overview of the 1,3-PDO gene clusters from C. beijerinckii DSM 6423 (A), C. pasteurianum DSM 525 (B) and C. beijerinckii DSM 15410 (C). Genes in blue and yellow were incorporated into the plasmids pMTL83251_PthlA_dhaBECT.Cpas and pMTL83251_Ppta-ack_1,3-PDO.diolis, respectively. dhaB1/B2, genes for coenzyme B12-independent glycerol dehydratase; pduB, gene for propanediol utilization microcompartment protein; dhaBCE, genes for coenzyme B12-dependent glycerol dehydratase; ddrA, gene for glycerol reactivation factor large subunit; ddrB, gene for glycerol reactivation factor small subunit; pduO, gene for ATP:cob(I)alamin adenosyltransferase; dhaT, gene for 1,3-PDO dehydrogenase.
3.2. Recombinant 1,3-PDO Production
Different batch growth experiments were carried out to evaluate if the constructed recombinant C. beijerinckii strains produced 1,3-PDO and under what conditions the highest 1,3-PDO concentration could be achieved. Since C. beijerinckii DSM 6423 could not grow with glycerol as the sole carbon source, xylose was also added to the glycerol medium. The first batch growth experiment was performed with unbuffered glycerol medium for cultivation of C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis], C. beijerinckii [pMTL83251], and the wild-type C. beijerinckii (Figure 3). Compared to the control strains C. beijerinckii [pMTL83251] and the C. beijerinckii wild-type, the recombinant strain C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis] grew faster and reached the stationary phase after 24 h with an OD600 of 0.73. However, the three analyzed C. beijerinckii strains reached a final OD600 of about 1 (Figure 3A). The drastic pH drop during the first 24 h of cultivation was only detected in C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis] (Figure 3B). Unexpectedly, 1,3-PDO could only be detected in the growth medium of the recombinant strain C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis]. After almost 250 h of incubation, 18.9 mM 1,3-PDO was measured in case of C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis] (Figure 3C). The glycerol concentration in the case of C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis] decreased during the experiment by 33.3 mM. In contrast, the glycerol concentration of the wild-type strain and C. beijerinckii [pMTL83251] decreased by 7 mM and 13 mM, respectively (Figure 3D). Xylose was not consumed completely by any of the strains tested. The main product of the analyzed C. beijerinckii strains was butyrate (wild-type: 20.9 mM; C. beijerinckii [pMTL83251]: 21.3 mM; C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis]: 20.4 mM) (Figure 3E). Only traces of ethanol were detected for all tested C. beijerinckii strains (0.2 mM). The lowest acetate concentration (2.2 mM) was measured in case of the wild-type strain. However, this strain also produced the most butanol, with 4.5 mM during the growth experiment. C. beijerinckii [pMTL83251] produced 3.6 mM acetate and 2.9 mM butanol throughout the cultivation. The highest acetate concentration was measured in the growth medium of C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis] (6.4 mM). However, this strain produced only traces of butanol (0.2 mM).
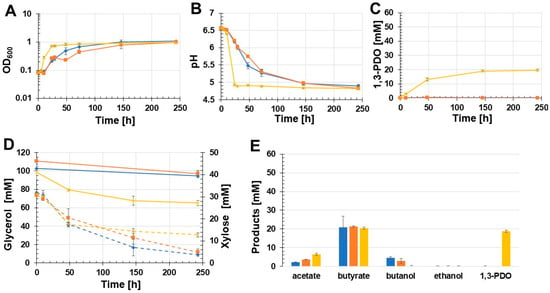
Figure 3.
Results of the batch growth experiment with unbuffered glycerol medium performed with the recombinant strains C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis], C. beijerinckii [pMTL83251], and the wild-type C. beijerinckii strain. OD600 (A), pH (B), 1,3-PDO production (C), substrate consumption (D), and concentration of products (E). Wild-type: blue rhombus; C. beijerinckii [pMTL83251]: orange squares; C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis]: yellow crosses; glycerol consumption: solid lines; xylose consumption: dashed lines; error bars represent standard deviations, n = 3.
3.3. Influence of Buffered Glycerol Medium on 1,3-PDO Production
Due to the drastic drop in pH values in the first growth experiment (Figure 3B), the effect of MOPS-buffered medium on the production of 1,3-PDO was examined. The following batch growth experiment was executed with the two recombinant strains C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas] and C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis]. As controls, the wild-type strain as well as C. beijerinckii [pMTL83251] were used. The results of this batch growth experiment are shown in Figure 4. Again, C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis] reached the stationary phase after 24 h of incubation (OD600: 0.85). In contrast, the OD600 of C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas] as well as the wild-type strain and C. beijerinckii [pMTL83251] decreased after 24 h and 48 h, respectively. Afterwards, the OD600 of all three strains rose again. All tested strains reached a similar final OD600 after 243 h (Figure 4A). Compared to the first growth experiment, the pH dropped in the case of C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis] during the first 48 h of incubation only to a pH value of 5.47. Afterwards, the pH value decreased to a final value of 5.39 (Figure 4B). Again, production of 1,3-PDO was only detected in the case of C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis]. The amount of 1,3-PDO was increased to 32.8 mM 1,3-PDO for the recombinant C. beijerinckii strain harbouring the 1,3-PDO production genes of C. beijerinckii DSM15410 (Figure 4C). Minor concentrations of 1,3-PDO production could be measured for the wild-type strain, C. beijerinckii [pMTL83251], and C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas] (0.7, 0.4, and 0.2 mM, respectively). A distinct decrease of 40.1 mM glycerol was only observed in the case of C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis]. The other three examined C. beijerinckii strains C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas], C. beijerinckii [pMTL83251], and the wild-type strain consumed only minor amounts of glycerol (3.5 mM, 14.1 mM, and 12.6 mM, respectively) (Figure 4D). The supplemented xylose was consumed completely by the strains C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas], C. beijerinckii [pMTL83251], and the wild-type strain during the growth experiment. In contrast, C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis] only consumed 18.1 mM of the added xylose (Figure 4D). As in the first growth experiment, butyrate was the main product. The highest butyrate production was detected in the culture broth of C. beijerinckii [pMTL83251] with 40.1 mM, followed by C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis] and C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas], which produced 39.6 mM and 38.1 mM butyrate, respectively. The wild-type strain produced 33.3 mM butyrate. The highest acetate production was also detected in the culture broth of the strain C. beijerinckii [pMTL83251] at 3.1 mM. The strains C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis], C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas], as well as the wild-type strain, produced 2.2 mM, 3.0 mM, and 1,3 mM acetate. Similar to the first growth experiment, the highest butanol concentration was obtained by the wild-type strain (4.1 mM butanol). In the case of C. beijerinckii [pMTL83251] and C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis], 1.5 mM and 0.5 mM butanol were produced. No butanol could be detected in the culture medium of C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas]. Only traces of ethanol were detected by all tested C. beijerinckii strains (0.4 mM). An overview of the synthesized products is shown in Figure 4E. The use of MOPS-buffered glycerol medium resulted in a 74% increase in 1,3-PDO production using C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis].
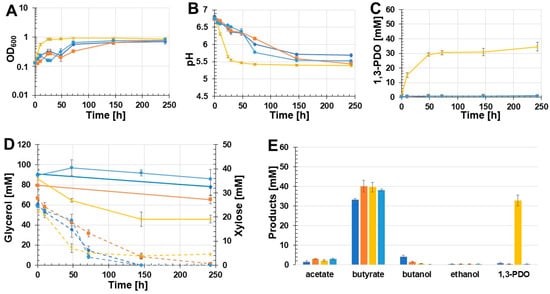
Figure 4.
Results of the batch growth experiment using MOPS buffered glycerol medium performed with the recombinant strains C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas], C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis], C. beijerinckii [pMTL83251], and the wild-type C. beijerinckii strain. OD600 (A), pH (B), 1,3-PDO production (C), substrate consumption (D), and concentration of products (E). Wild-type: blue rhombus; C. beijerinckii [pMTL83251]: orange squares; C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis]: yellow crosses; C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas]: light blue circles; glycerol consumption: solid lines; xylose consumption: dashed lines; error bars represent standard deviations; n = 3.
3.4. Effect of Vitamin B12 Supplementation
Since the used glycerol dehydratase of C. pasteurianum is coenzyme B12-dependent, the effect of supplementation of vitamin B12 in the MOPS-buffered growth medium of C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas] was studied. Therefore, the glycerol medium of C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas] was supplemented with vitamin B12. The results are shown in Figure 5. During this growth experiment, the recombinant strain C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis] grew slower than during previously described growth experiments. Cells reached the stationary phase after 48 h with an OD600 of 0.62. As reported before, a decrease of the OD600 after 24 h and 48 h was observed for C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas] as well as the wild-type strain and C. beijerinckii [pMTL83251]. After 243 h of incubation, the OD600 values of C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas], C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis], and the wild-type were similar; only the OD600 of C. beijerinckii [pMTL83251] was lower with a value of 0.69 (Figure 5A). In parallel to the slower growth, the pH of the culture broth of C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis] also dropped slower than described for the previous experiments (Figure 5B). Throughout the growth experiment, C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis] produced 43.1 mM 1,3-PDO. Again, the three other C. beijerinckii strains synthesized only traces of 1,3-PDO (wild-type and C. beijerinckii [pMTL83251]: 0.7 mM; C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas]: 0.6 mM) (Figure 5C). The 1,3-PDO-producing strain C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis] consumed 66.9 mM glycerol during batch growth. C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas] used 27.3 mM glycerol. The two control strains consumed only around 10 mM glycerol. In contrast, xylose was consumed completely by the wild-type strain, C. beijerinckii [pMTL83251], and C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas] (Figure 5D). In the case of C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis], 1,3-PDO was the main product. Compared to the other three tested strains, C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis] showed the lowest butyrate (19.9 mM) and acetate (1.1 mM) concentrations. The highest butyrate and acetate amounts were observed with the control strain C. beijerinckii [pMTL83251], i.e., 23.6 mM butyrate and 2.4 mM acetate. C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas] produced 20.9 mM butyrate and 1.6 mM acetate during growth. The wild-type strain produced butyrate (22.7 mM), acetate (1.5 mM) and butanol (1.4 mM). Traces of butanol were also observed in cultures of C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis] (0.4 mM). All examined C. beijerinckii strains synthesized traces of ethanol (0.3–0.2 mM). An overview of the products is provided in Figure 5E. Overall, a benefit of the vitamin B12 supplementation could not be observed.
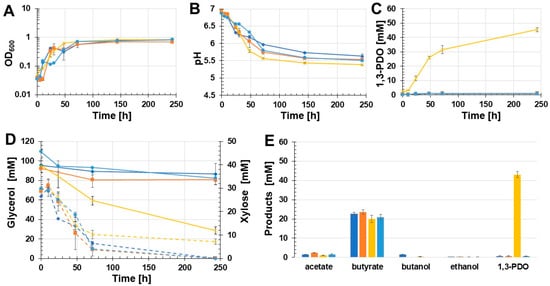
Figure 5.
Results of the batch growth experiment using MOPS buffered glycerol medium performed with the recombinant strains C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas], C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis], C. beijerinckii [pMTL83251], and the wild-type C. beijerinckii strain. The medium of C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas] was supplemented with vitamin B12. OD600 (A), pH (B), 1,3-PDO production (C), substrate consumption (D), and concentration of products (E). Wild-type: blue rhombus; C. beijerinckii [pMTL83251]: orange squares; C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis]: yellow crosses; C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas]: light blue circles; glycerol consumption: solid lines; xylose consumption: dashed lines; error bars represent standard deviations; n = 3.
3.5. Effect of Different Promoters on Recombinant 1,3-PDO Production
To exclude that the thlA promoter is not functional in C. beijerinckii and therefore C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas] could not produce 1,3-PDO in the previously performed batch growth experiments, and to examine if other promoters have a positive effect on the 1,3-PDO production, four different promoters were tested. The batch experiments were again performed with MOPS-buffered medium using the recombinant strains C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis], C. beijerinckii [pMTL83251_PbgaL_1,3-PDO.diolis], C. beijerinckii [pMTL83251_Pbld_1,3-PDO.diolis], C. beijerinckii [pMTL83251_PthlA_1,3-PDO.diolis], as well as the wild-type C. beijerinckii strain (Figure 6). C. beijerinckii [pMTL83251_PthlA_1,3-PDO.diolis] reached the stationary phase after 10 h, whereas C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis] reached the stationary phase after 24 h. 1,3-PDO gene expression of C. beijerinckii [pMTL83251_PbgaL_1,3-PDO.diolis] was induced via supplementation of 20 mM lactose after 10 h of cultivation (OD600: 0.19). Before induction, the C. beijerinckii [pMTL83251_PbgaL_1,3-PDO.diolis] cultures grew similarly. However, in contrast to the induced cultures, the OD600 of the uninduced strain did not increase but even decreased. After 48 h, the OD600 of the uninduced strain C. beijerinckii [pMTL83251_PbgaL_1,3-PDO.diolis] increased again. The strains containing the inducible bgaL promoter showed the lowest final OD600 (induced: 0.61; uninduced: 0.57) (Figure 6A). As observed in the growth experiments before, the pH of the 1,3-PDO production strains dropped faster than the pH values of the control cultures (Figure 6B). 1,3-PDO was produced by all recombinant production strains. Surprisingly, the 1,3-PDO production strain harbouring the thlA promoter and the 1,3-PDO genes of C. beijerinckii DSM 15410 produced at 50.4 mM the highest 1,3-PDO concentration after 240 h of incubation. Throughout the course of the experiment, C. beijerinckii [pMTL83251_Pbld_1,3-PDO.diolis] produced 37.4 mM 1,3-PDO. 36.3 mM 1,3-PDO were detected in induced cultures of C. beijerinckii [pMTL83251_PbgaL_1,3-PDO.diolis]. In contrast, the uninduced strain C. beijerinckii [pMTL83251_PbgaL_1,3-PDO.diolis] only produced 2.1 mM 1,3-PDO. During this growth experiment, C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis] produced only 27.3 mM 1,3-PDO. In the wild-type, traces of 1,3-PDO could be detected (0.3 mM). The 1,3-PDO values produced by the different tested strains are shown in Figure 6C. Throughout the growth experiment, C. beijerinckii [pMTL83251_PthlA_1,3-PDO.diolis] consumed 63.4 mM glycerol (Figure 6D). The induced C. beijerinckii [pMTL83251_PbgaL_1,3-PDO.diolis] as well as C. beijerinckii [pMTL83251_Pbld_1,3-PDO.diolis] showed similar glycerol consumption (53.2 mM and 52.1 mM, respectively). Surprisingly, the glycerol concentration in the culture broth of C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis] decreased by only 37.6 mM, matching the low 1,3-PDO production during growth (Figure 6D). Again, the non-1,3-PDO-producing strains metabolized the added xylose completely. Interestingly, the strain C. beijerinckii [pMTL83251_PthlA_1,3-PDO.diolis] consumed only 11 mM xylose (Figure 6D). 1,3-PDO was the main product in case of the induced C. beijerinckii [pMTL83251_PbgaL_1,3-PDO.diolis] strain and C. beijerinckii [pMTL83251_PthlA_1,3-PDO.diolis] (Figure 6E). In contrast to the approaches before, the by-product concentrations varied. Matching the low xylose consumption, C. beijerinckii [pMTL83251_PthlA_1,3-PDO.diolis] produced a low amount of butyrate (30 mM). However, more acetate (10.9 mM) was obtained than by most of the other tested strains. The highest acetate concentration was observed for the induced C. beijerinckii [pMTL83251_PbgaL_1,3-PDO.diolis] strain at 18.5 mM. The lowest acetate production was observed for the wild-type strain at only 1.6 mM. The other tested strains formed between 3.9 mM and 2.6 mM acetate. In contrast to the low acetate production, the wild-type strain showed the highest butyrate concentration (54.5 mM). In the culture broth of the induced C. beijerinckii [pMTL83251_PbgaL_1,3-PDO.diolis] strain, only 28.5 mM butyrate was measured. In contrast, the uninduced strain produced 35.8 mM butyrate. C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis] and C. beijerinckii [pMTL83251_Pbld_1,3-PDO.diolis] produced similar amounts of butyrate (48.6 mM and 48.3 mM, respectively). Traces of ethanol were detected in all studied strains (0.4–0.2 mM). The wild-type strain, C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis], and the uninduced C. beijerinckii [pMTL83251_PbgaL_1,3-PDO.diolis] strain synthesised traces of butanol (0.3–0.1 mM). 1,3-PDO production was increased with all tested promoters compared to the originally used pta-ack promoter.
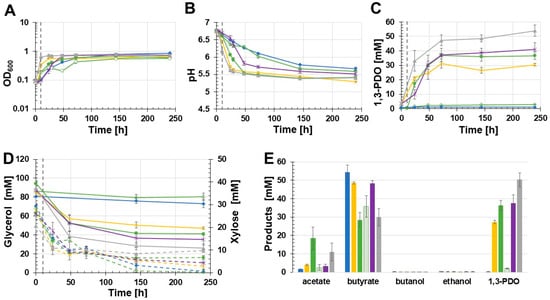
Figure 6.
Results of the batch growth experiment using MOPS buffered glycerol medium performed with the recombinant strains C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis], C. beijerinckii [pMTL83251_PbgaL_1,3-PDO.diolis], C. beijerinckii [pMTL83251_Pbld_1,3-PDO.diolis], C. beijerinckii [pMTL83251_PthlA_1,3-PDO.diolis], and the wild-type C. beijerinckii strain. OD600 (A), pH (B), 1,3-PDO production (C), substrate consumption (D), and concentration of products (E). Wild-type: blue rhombus; C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis]: yellow crosses; C. beijerinckii [pMTL83251_PbgaL_1,3-PDO.diolis] induced: green circles; C. beijerinckii [pMTL83251_PbgaL_1,3-PDO.diolis] uninduced: green empty circles; C. beijerinckii [pMTL83251_Pbld_1,3-PDO.diolis]: purple stars; C. beijerinckii [pMTL83251_PthlA_1,3-PDO.diolis]: gray triangle; glycerol consumption: solid lines; xylose consumption: dashed lines; induction of gene expression using 20 mM lactose: gray vertical dash lines; error bars represent standard deviations, n = 3.
Table 3 shows a summary of the performed batch growth experiments. A distinct increase in 1,3-PDO production could be obtained by using a buffered medium, resulting in an overall improvement of 82%. Furthermore, the use of buffered medium also resulted in a higher yield of 0.71 mol1,3.PDO/molglycerol. For those calculations, the mean of all experiments performed in buffered glycerol medium was used. The second improvement could be observed by exchanging the pta-ack promoter. The highest yield of 0.79 and 50.4 mM 1,3-PDO was obtained with the strain C. beijerinckii [pMTL83251_PthlA_1,3-PDO.diolis]. Comparing the 1,3-PDO production utilizing unbuffered medium and C. beijerinckii [pMTL83251_Ppta-ack_1,3-PDO.diolis], the 1,3-PDO production could be improved by 167% by using the strain C. beijerinckii [pMTL83251_PthlA_1,3-PDO.diolis] in MOPS-buffered glycerol medium.

Table 3.
Summary of all performed batch growth experiments.
4. Discussion
C. beijerinckii DSM 6423 (=C. beijerinckii NRRL B-593) is described as a natural 1,3-PDO producer [22,37,45]. However, the strain obtained from the DSMZ was unable to do so due to the loss of the 1,3-PDO dehydrogenase gene. In contrast to the published literature, the C. beijerinckii DSM 6423 wild-type strain only produced traces of 1,3-PDO. To reconstitute the 1,3-PDO production of the wild-type strain, a newly published transformation protocol of C. beijerinckii was employed to establish recombinant 1,3-PDO production in C. beijerinckii DSM 6423. Therefore, the heterologous gene expression of two different 1,3-PDO gene clusters, the effect of buffered growth media on the recombinant strains, and the use of different promoters for the gene expression were examined. The data presented clearly show that higher concentrations of 1,3-PDO could only be produced with recombinant C. beijerinckii DSM 6423 strains. In the published study of Gungormusler et al. the wild-type produced up to 131 mM 1,3-PDO (10 g/L) [37]. Genome analysis of C. beijerinckii DSM 6423 showed that the 1,3-PDO gene cluster is not complete. The dhaB1 and dhaB2 genes encoding a supposedly coenzyme B12-independent glycerol dehydratase could be identified. However, a dhaT gene encoding a 1,3-PDO dehydrogenase could not be found in the genome of C. beijerinckii DSM 6423. It is possible that another gene with a similar function can also convert 3-HPA to 1,3-PDO, resulting in low 1,3-PDO production. For example, alcohol dehydrogenases could also take over the same function due to their broad substrate specificity [46].
Higher concentrations of 1,3-PDO were only achieved using recombinant C. beijerinckii strains carrying the 1,3-PDO gene cluster of C. beijerinckii DSM 15410. The plasmid-based expression of the 1,3-PDO genes (dhaBCE and dhaT) of C. pasteurianum did not lead to an increase in 1,3-PDO production compared to the wild-type strain and C. beijerinckii [pMTL83251]. As mentioned before, the glycerol dehydratase complex of C. pasteurianum is coenzyme B12-dependent [27]. However, very little is known about the synthesis of coenzyme B12 in C. beijerinckii DSM 6423. In many cases, supplementation of vitamin B12 was necessary to produce 1,3-PDO when a coenzyme B12-dependent glycerol dehydratase was used [47]. In this study, even with supplementation of vitamin B12 (2.5 mg/L), C. beijerinckii [pMTL83251_PthlA_dhaBCET.Cpas] was unable to produce 1,3-PDO. The reason could be that C. beijerinckii DSM 6423 does not harbour the necessary genes for the conversion of vitamin B12 to coenzyme B12. Furthermore, Fokum et al. recently showed that the addition of vitamin B12 in high concentrations (7.5–10 mg/L) to the culture medium has a negative effect on the 1,3-PDO production of C. beijerinckii CCIC 22954 [48]. For industrial approaches, it is also more desirable to use a coenzyme B12-independent production strain, in order to keep cultivation costs low due to expensive vitamin B12 supplementation.
A further increase in 1,3-PDO production was accomplished by buffering the glycerol medium and preventing a fast pH decrease. The activities of enzymes as well as cofactors within the cell depend strongly on the pH. Therefore, a more stable pH throughout the bacterial cultivation is desired [49]. A positive effect of a controlled pH throughout the fermentation of glycerol was already described before [37]. Experiments with mixed cultures showed that the highest 1,3-PDO production yields could be measured at pH 7 and 8 [50]. Furthermore, most studies on the production of 1,3-PDO from glycerol are performed in fermenters with a controlled pH. Thus, it would be interesting to examine our recombinant production strains in a fermentation experiment with a steady pH.
The biggest improvements were made by exchanging the pta-ack promoter. Unfortunately, the direct activity test as described before for C. saccharoperbutylacetonicum and Eubacterium limosum [35,36] could not be applied for C. beijerinckii DSM 6423. In preliminary tests, C. beijerinckii carrying pMTL83251_PbgaL_FAST did not show differences in fluorescence compared to the wild-type strain. Thus, growth experiments were performed to examine the effects of different promoters. Although the lactose-inducible bgaL promoter of C. perfringens did not lead to the highest 1,3-PDO production, the inducible promoter system is almost tight in C. beijerinckii DSM6423 as indicated by the low 1,3-PDO concentration of uninduced cultures. Based on the presented results, the bgaL promoter can be described as the second inducible promoter for gene expression in C. beijerinckii DSM 6423. Another inducible system is based on the xylB promoter from Clostridium difficile strain 630, in which gene expression is induced by the addition of xylose [31,51]. The xlyB promoter was not examined in this study, since xylose was used as substrate in the growth experiments. Due to the close relationship of C. saccharoperbutylacetonicum and C. beijerinckii, both strains belong to the second clade of solvent-forming clostridia [52], which is why the bld promoter was also tested. Furthermore, the bld promoter showed the highest activity in C. saccharoperbutylacetonicum compared to promoters thlA, bgaL, and pta-ack [36]. Thus, we assumed high activity of the bld promoter in C. beijerinckii. However, the highest 1,3-PDO concentration and yield were detected in the case of C. beijerinckii [pMTL83251_PthlA_1,3-PDO.diolis] harboring the thlA promoter. This promoter also showed high activity in C. saccharoperbutylacetonicum [36].
The use of recombinant clostridial strains to produce 1,3-PDO was already reported before. In 2005, Gonzalez-Pajuelo et al. published an article using genetically modified C. acetobutylicum strains [53]. C. acetobutylicum was chosen as a host strain, as no genetic tools were available for C. butyricum at that time. The recombinant C. acetobutylicum strain was engineered for the heterologous production of 1,3-PDO using the genes from C. butyricum. Since the described fermentation approaches were fed-batch experiments, a comparison to this study is difficult. The modified C. acetobutylicum strain produced more 1,3-PDO compared to the tested strains of the present study due to the different cultivation method. However, all the recombinant C. beijerinckii strains presented here showed higher yields when grown in buffered glycerol medium. In comparison with other wild-type clostridial strains, the obtained concentrations are lower [7], but the yield is higher in the case of C. beijerinckii [pMTL83251_PthlA_1,3-PDO.diolis]. As mentioned before, a comparison of batch growth cultures and fermentation experiments under controlled conditions (pH) are hardly possible. However, continuous culture experiments with the newly constructed strains might even show higher 1,3-PDO production, thus proving the way for a cost-competitive bioprocess, when compared to chemical synthesis routes.
Author Contributions
Funding acquisition, P.D.; Investigation, T.S., J.K. and S.S.; Project administration, T.S., T.B. and P.D.; Visualization, T.S.; Writing—original draft, T.S.; Writing—review & editing, T.B., J.K., S.S. and P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Bioeconomy International submission platform within the project “Sustainable bio-based 1,3-propanediol production from C5/C3 sources by metabolically engineered clostridia (SupperC)”(Förderkennzeichen: 031B0814).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Maximilian Flaiz for providing the plasmids pMTL83251_PbgaL_FAST and pMTL83251_PthlA_FAST and for the help with the preliminary test of the FAST assay.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- da Silva Ruy, A.D.; de Brito Alves, R.M.; Hewer, T.L.R.; de Aguiar Pontes, D.; Teixeira, L.S.G.; Pontes, L.A.M. Catalysts for glycerol hydrogenolysis to 1,3-propanediol: A review of chemical routes and market. Catal. Today 2020, 381, 243–253. [Google Scholar] [CrossRef]
- Zhu, F.; Liu, D.; Chen, Z. Recent advances in biological production of 1,3-propanediol: New routes and engineering strategies. Green Chem. 2022, 24, 1390–1403. [Google Scholar] [CrossRef]
- Kaur, G.; Srivastava, A.K.; Chand, S. Advances in biotechnological production of 1,3-propanediol. Biochem. Eng. J. 2012, 64, 106–118. [Google Scholar] [CrossRef]
- Przystałowska, H.; Zeyland, J.; Szymanowska-Powałowska, D.; Szalata, M.; Słomski, R.; Lipiński, D. 1,3-Propanediol production by new recombinant Escherichia coli containing genes from pathogenic bacteria. Microbiol. Res. 2015, 171, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Xu, F.; Liu, H.; Liu, D. Downstream processing of 1,3-propanediol fermentation broth. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2006, 81, 102–108. [Google Scholar] [CrossRef]
- Cameron, D.C.; Altaras, N.E.; Hoffman, M.L.; Shaw, A.J. Metabolic engineering of propanediol pathways. Biotechnol. Prog. 1998, 14, 116–125. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Gao, H.; Wang, H.; Wan, Z.; Jiang, Y.; Xin, F.; Zhang, W.; Jiang, M. Current advances in microbial production of 1,3-propanediol. Biofuels Bioprod. Biorefining 2021, 15, 1566–1583. [Google Scholar] [CrossRef]
- Nakamura, C.E.; Whited, G.M. Metabolic engineering for the microbial production of 1,3-propanediol. Curr. Opin. Biotechnol. 2003, 14, 454–459. [Google Scholar] [CrossRef]
- Dürre, P. Technical alcohols and ketones. In Industrial Microbiology; Wilson, D.B., Sahm, H., Stahmann, K.P., Koffas, M., Eds.; Wiley-VCH: Weinheim, Germany, 2020; pp. 95–116. [Google Scholar]
- Biotechnology Innovation Organization. Advancing the biobased economy: Renewable chemical biorefinery commercialization, progress, and market opportunities, 2016 and beyond. Ind. Biotechnol. 2016, 12, 290–294. [Google Scholar] [CrossRef]
- Kurian, J.V. A new polymer platform for the future—Sorona® from corn derived 1,3-propanediol. J. Polym. Environ. 2005, 13, 159–167. [Google Scholar] [CrossRef]
- Clomburg, J.M.; Gonzalez, R. Anaerobic fermentation of glycerol: A platform for renewable fuels and chemicals. Trends Biotechnol. 2013, 31, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, S.S.; Gonzalez, R. Anaerobic fermentation of glycerol: A path to economic viability for the biofuels industry. Curr. Opin. Biotechnol. 2007, 18, 213–219. [Google Scholar] [CrossRef]
- Yang, F.; Hanna, M.A.; Sun, R. Value-added uses for crude glycerol—A byproduct of biodiesel production. Biotechnol. Biofuels 2012, 5, 13. [Google Scholar] [CrossRef]
- Anand, P.; Saxena, R.K. A comparative study of solvent-assisted pretreatment of biodiesel derived crude glycerol on growth and 1,3-propanediol production from Citrobacter freundii. New Biotechnol. 2012, 29, 199–205. [Google Scholar] [CrossRef]
- Hermann, B.G.; Patel, M. Today’s and tomorrow’s bio-based bulk chemicals from white biotechnology. Appl. Biochem. Biotechnol. 2007, 136, 361–388. [Google Scholar] [CrossRef] [PubMed]
- Biebl, H.; Zeng, A.P.; Menzel, K.; Deckwer, W.D. Fermentation of glycerol to 1,3-propanediol and 2,3-butanediol by Klebsiella pneumoniae. Appl. Microbiol. Biotechnol. 1998, 50, 24–29. [Google Scholar] [CrossRef]
- Barbirato, F.; Camarasa-Claret, C.; Grivet, J.P.; Bories, A. Glycerol fermentation by a new 1,3-propanediol-producing microorganism: Enterobacter agglomerans. Appl. Microbiol. Biotechnol. 1995, 43, 786–793. [Google Scholar] [CrossRef]
- Homann, T.; Tag, C.; Biebl, H.; Deckwer, W.D.; Schink, B. Fermentation of glycerol to 1,3-propanediol by Klebsiella and Citrobacter strains. Appl. Microbiol. Biotechnol. 1990, 33, 121–126. [Google Scholar] [CrossRef]
- Leja, K.; Czaczyk, K.; Myszka, K. Biotechnological synthesis of 1,3-propanediol using Clostridium ssp. Afr. J. Biotechnol. 2011, 10, 11093–11101. [Google Scholar]
- Kobayashi, H.; Tanizawa, Y.; Sakamoto, M.; Nakamura, Y.; Ohkuma, M.; Tohno, M. Reclassification of Clostridium diolis Biebl and Spröer 2003 as a later heterotypic synonym of Clostridium beijerinckii Donker 1926 (Approved Lists 1980) emend. Keis et al. 2001. Int. J. Syst. Evol. Microbiol. 2020, 70, 2463–2466. [Google Scholar] [CrossRef]
- Gungormusler, M.; Gonen, C.; Azbar, N. Continuous production of 1,3-propanediol using raw glycerol with immobilized Clostridium beijerinckii NRRL B-593 in comparison to suspended culture. Bioprocess Biosyst. Eng. 2011, 34, 727–733. [Google Scholar] [CrossRef]
- Biebl, H.; Spröer, C. Taxonomy of the glycerol fermenting clostridia and description of Clostridium diolis sp. nov. Syst. Appl. Microbiol. 2002, 25, 491–497. [Google Scholar] [CrossRef]
- Biebl, H. Fermentation of glycerol by Clostridium pasteurianum—Batch and continuous culture studies. J. Ind. Microbiol. Biotechnol. 2001, 27, 18–26. [Google Scholar] [CrossRef]
- Biebl, H.; Marten, S.; Hippe, H.; Deckwer, W.D. Glycerol conversion to 1,3-propanediol by newly isolated clostridia. Appl. Microbiol. Biotechnol. 1992, 36, 592–597. [Google Scholar] [CrossRef]
- Heyndrickx, M.; De Vos, P.; Vancanneyt, M.; De Ley, J. The fermentation of glycerol by Clostridium butyricum LMG 1212t2 and 1213t1 and C. pasteurianum LMG 3285. Appl. Microbiol. Biotechnol. 1991, 34, 637–642. [Google Scholar] [CrossRef]
- Luers, F.; Seyfried, M.; Daniel, R.; Gottschalk, G. Glycerol conversion to 1,3-propanediol by Clostridium pasteurianum: Cloning and expression of the gene encoding 1,3-propanediol dehydrogenase. FEMS Microbiol. Lett. 1997, 154, 337–345. [Google Scholar] [CrossRef]
- Sedlar, K.; Vasylkivska, M.; Musilova, J.; Branska, B.; Provaznik, I.; Patakova, P. Phenotypic and genomic analysis of isopropanol and 1,3-propanediol producer Clostridium diolis DSM 15410. Genomics 2021, 113, 1109–1119. [Google Scholar] [CrossRef]
- Raynaud, C.; Sarçabal, P.; Meynial-Salles, I.; Croux, C.; Soucaille, P. Molecular characterization of the 1,3-propanediol (1,3-PD) operon of Clostridium butyricum. Proc. Natl. Acad. Sci. USA 2003, 100, 5010–5015. [Google Scholar] [CrossRef]
- Celińska, E. Debottlenecking the 1,3-propanediol pathway by metabolic engineering. Biotechnol. Adv. 2010, 28, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Diallo, M.; Hocq, R.; Collas, F.; Chartier, G.; Wasels, F.; Wijaya, H.S.; Werten, M.W.T.; Wolbert, E.J.H.; Kengen, S.W.M.; von der Oost, J.; et al. Adaptation and application of a two-plasmid inducible CRISPR-Cas9 system in Clostridium beijerinckii. Methods 2020, 172, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Pyne, M.E.; Moo-Young, M.; Chung, D.A.; Chou, C.P. Development of an electrotransformation protocol for genetic manipulation of Clostridium pasteurianum. Biotechnol. Biofuels 2013, 6, 50. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor: New York, NY, USA, 2012. [Google Scholar]
- Heap, J.T.; Pennington, O.J.; Cartman, S.T.; Minton, N.P. A modular system for Clostridium shuttle plasmids. J. Microbiol. Methods 2009, 78, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Flaiz, M.; Ludwig, G.; Bengelsdorf, F.R.; Dürre, P. Production of the biocommodities butanol and acetone from methanol with fluorescent FAST-tagged proteins using metabolically engineered strains of Eubacterium limosum. Biotechnol. Biofuels 2021, 14, 117. [Google Scholar] [CrossRef]
- Baur, T.; Wentzel, A.; Dürre, P. Production of propionate using metabolically engineered strains of Clostridium saccharoperbutylacetonicum. Appl. Microbiol. Biotechnol. 2022, 106, 7547–7562. [Google Scholar] [CrossRef]
- Gungormusler, M.; Gonen, C.; Azbar, N. 1,3-Propanediol production potential by a locally isolated strain of Klebsiella pneumoniae in comparison to Clostridium beijerinckii NRRL B593 from waste glycerol. J. Polym. Environ. 2011, 19, 812–817. [Google Scholar] [CrossRef]
- Macis, L.; Daniel, R.; Gottschalk, G. Properties and sequence of the coenzyme B12-dependent glycerol dehydratase of Clostridium pasteurianum. FEMS Microbiol. Lett. 1998, 164, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Seifert, C.; Bowien, S.; Gottschalk, G.; Daniel, R. Identification and expression of the genes and purification and characterization of the gene products involved in reactivation of coenzyme B12-dependent glycerol dehydratase of Citrobacter freundii. Eur. J. Biochem. 2001, 268, 2369–2378. [Google Scholar] [CrossRef] [PubMed]
- Mera, P.E.; Escalante-Semerena, J.C. Multiple roles of ATP: Cob (I) alamin adenosyltransferases in the conversion of B12 to coenzyme B12. Appl. Microbiol. Biotechnol. 2010, 88, 41–48. [Google Scholar] [CrossRef]
- Hoffmeister, S.; Gerdom, M.; Bengelsdorf, F.R.; Linder, S.; Flüchter, S.; Öztürk, H.; Blümke, W.; May, A.; Fischer, R.J.; Bahl, H.; et al. Acetone production with metabolically engineered strains of Acetobacterium woodii. Metab. Eng. 2016, 36, 37–47. [Google Scholar] [CrossRef]
- Tummala, S.B.; Welker, N.E.; Papoutsakis, E.T. Development and characterization of a gene expression reporter system for Clostridium acetobutylicum ATCC 824. Appl. Environ. Microbiol. 1999, 65, 3793–3799. [Google Scholar] [CrossRef]
- Kosaka, T.; Nakayama, S.; Nakaya, K.; Yoshino, S.; Furukawa, K. Characterization of the sol operon in butanol-hyperproducing Clostridium saccharoperbutylacetonicum strain N1-4 and its degeneration mechanism. Biosci. Biotechnol. Biochem. 2007, 71, 58–68. [Google Scholar] [CrossRef]
- Hartman, A.H.; Liu, H.; Melville, S.B. Construction and characterization of a lactose-inducible promoter system for controlled gene expression in Clostridium perfringens. Appl. Environ. Microbiol. 2011, 77, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Gungormusler, M.; Gonen, C.; Azbar, N. Comparative evaluation of Clostridium beijerinckii (NRRL B-593) and Klebsiella pneumoniae for 1,3 propanediol production. J. Biotechnol. 2010, 150, 210–211. [Google Scholar] [CrossRef]
- Johnson, E.A.; Lin, E.C. Klebsiella pneumoniae 1,3-propanediol: NAD+ oxidoreductase. J. Bacteriol. 1987, 169, 2050–2054. [Google Scholar] [CrossRef]
- Nasir, A.; Ashok, S.; Shim, J.Y.; Park, S.; Yoo, T.H. Recent progress in the understanding and engineering of coenzyme B12-dependent glycerol dehydratase. Front. Bioeng. Biotechnol. 2020, 8, 500867. [Google Scholar] [CrossRef]
- Fokum, E.; Zabed, H.M.; Ravikumar, Y.; Elshobary, M.E.; Chandankere, R.; Zhang, Y.; Yun, J.; Qi, X. Co-fermentation of glycerol and sugars by Clostridium beijerinckii: Enhancing the biosynthesis of 1,3-propanediol. Food Biosci. 2021, 41, 101028. [Google Scholar] [CrossRef]
- Vivek, N.; Pandey, A.; Binod, P. Biological valorization of pure and crude glycerol into 1,3-propanediol using a novel isolate Lactobacillus brevis N1E9. 3.3. Bioresour. Technol. 2016, 213, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Moscoviz, R.; Trably, E.; Bernet, N. Consistent 1,3-propanediol production from glycerol in mixed culture fermentation over a wide range of pH. Biotechnol. Biofuels 2016, 9, 32. [Google Scholar] [CrossRef]
- Nariya, H.; Miyata, S.; Kuwahara, T.; Okabe, A. Development and characterization of a xylose-inducible gene expression system for Clostridium perfringens. Appl. Environ. Microbiol. 2011, 77, 8439–8441. [Google Scholar] [CrossRef] [PubMed]
- Poehlein, A.; Solano, J.D.M.; Flitsch, S.K.; Krabben, P.; Winzer, K.; Reid, S.J.; Jones, D.T.; Green, E.; Minton, N.P.; Daniel, R.; et al. Microbial solvent formation revisited by comparative genome analysis. Biotechnol. Biofuels 2017, 10, 58. [Google Scholar] [CrossRef]
- González-Pajuelo, M.; Meynial-Salles, I.; Mendes, F.; Andrade, J.C.; Vasconcelos, I.; Soucaille, P. Metabolic engineering of Clostridium acetobutylicum for the industrial production of 1,3-propanediol from glycerol. Metab. Eng. 2005, 7, 329–336. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).